Abstract
Our previous studies have identified that CD166 works as a cancer stem-like cell (CSC) marker in epithelial cancers with a large repertoire of cellular functions. However, the post-translational regulatory mechanisms underlying CD166 turnover remain elusive. Several independent studies have reported that E3 ubiquitin ligase CHIP revealed significant biological effects through ubiquitin proteasome pathway on some kinds of malignant tumors. With analyzing the effects of CHIP expressions on stem-like cell populations, we found that CHIP represses CSC characteristics mainly targeting the CSC related protein CD166 in head and neck cancer (HNC). To investigate the role and relationship between CD166 and CHIP, HNC tissues and cell lines were used in this study. A significant negative correlation was observed between the expression levels of CHIP and CD166 in HNC patient samples. We also found that CHIP directly regulates the stability of CD166 protein through the ubiquitin proteasome system, which was also identified participating in the regulation of CSC behaviors in HNCs. Our findings demonstrate that CHIP-CD166-proteasome axis participates in regulating CSC properties in HNCs, suggesting that the regulation of CD166 by CHIP could provide new options for diagnosing and treating in the patients with HNCs.
Keywords: CD166, CHIP, Ubiquitin E3 ligase, Cancer stem-like cell, Head and neck cancer
1. Introduction
CD166, also titled as activated leukocyte cell adhesion molecule (ALCAM), is a glycoprotein belonging to the immunoglobulin superfamily of adhesion molecules [1]. Unlike other adhesion molecules, which are usually down-regulated during malignant transformation, CD166 often shows increased expression in certain cancers [2], [3], which indicates that CD166 is under strict regulation during carcinogenesis. Our previous studies have identified that CD166 works well as a cancer stem-like cell (CSC) marker in head and neck cancers (HNCs) with a large repertoire of cellular functions [4]. However, the post-translational regulatory mechanisms underlying CD166 turnover remain elusive.
The ubiquitin-proteasome system is a major pathway that contributes to intracellular proteostasis. The carboxy-terminus of Hsc70 interacting protein (CHIP) has been identified as an E3 ubiquitin ligase and a potent regulator protein in maintaining protein homeostasis in diverse cellular processes [5], [6], [7]. Structurally, three tandem repeats of the tetratricopeptide (TPR) motif and a C-terminal U-box domain (U-box) contribute to the bioactivities of CHIP as a chaperone-associated E3 ubiquitin ligase involved in the ubiquitin-proteasome system [8], [9]. CHIP post-translationally controls the turnover of its substrate proteins and exerts regulatory roles in a myriad of biological processes [10], [11].
Until recently, accumulating evidences have suggested a role for CHIP in the initiation and progression of some cancers [11], [12], [13]. During the development of carcinogenesis, the production of mutated or aberrant cellular proteins must be gradually increased during malignant transformation and in response to genetic instability processes [14], [15]. As reported previously, CHIP induces ubiquitylation and degradation of several oncogenic proteins [11], [16], [17], [18], which has increased interest in understanding the mechanisms of CHIP in the context of carcinogenesis. Accordingly, the diversities of CHIP substrate proteins present heterogeneity among different tissues derived cancers. A comprehensive understanding of the function of CHIP depends on the in-depth study of its target proteins in different cancer types.
In the present study, we found that CHIP functions as an E3 ligase for CD166 by increasing the ubiquitination and degradation, thus contributing to the regulation of the CSC properties of HNCs. Our work also suggests that the regulation of CD166 could provide new options for diagnosing and treating HNCs.
2. Methods and materials
2.1. Cell culture and reagents
HNC cell lines (HN13, HN30, Cal27 and UMSCC12) and 293T cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Basal Media, China) supplemented with 10% fetal bovine serum (Gibco, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco, USA), at 37 °C in the presence of 5% carbon dioxide.
MG-132 and alvespimycin (17-DMAG) were purchased from Selleck (USA). Cycloheximide (CHX) was purchased from Sigma (USA). Antibodies used in this study are summarized in Supplementary Table S1.
2.2. Plasmid constructs and transfections
Plasmids and siRNAs were transfected into cells using Lipofectamine 2000 (Invitrogen, USA) and OPTI-MEM® I (Gibco, USA) according to the of manufacturer's instructions. The mammalian expression vectors used were pcDNA3.1(+)-myc-CHIP and pcDNA3.1-Flag-CD166; the plasmids pcDNA3.1(+)-myc and pcDNA3.1-Flag were used as control. The loss-of-function of CHIP mutants at the TPR (K30A) and U-box (H260Q) domain were generated using the QuickChange XL Site-directed Mutagenesis kit (Stratagene, USA). The primer sequences used for site-directed mutagenesis are shown in Supplementary Table S2 [10], [19].
UMSCC12 stable cells displaying CHIP knockdown were generated by transfecting UMSCC12 cells with CHIP-specific short hairpin RNA ( shCHIP: 5′-GGA CGA CAT CCC CAG CGC TCT-3′) lentivirus after which 10 μg/ml of puromycin (Calbiochem, Germany) was added to select for positive clones [17], and scrambled short hairpin RNA lentivirus were used for UMSCC12-Control. HN13 stable cells of CD166 knockdown were preserved from previous study [20]. Validated siRNAs specific to CHIP and CD166 were transfected into cells (Supplementary Table S3).
2.3. Spheroid formation assay
Live cells were dissociated and suspended in spheroid medium consisting of serum-free DMEM/F12 (Gibco, USA), human bFGF (20 ng/ml, Sino Biological Inc., China), human EGF (20 ng/ml, Sino Biological Inc., China), and B-27 supplement (Life Technologies, USA) in 6-well ultra-low attachment dishes (Corning, USA), as previously described [4]. The spheroid morphology was observed microscopically, and the number of spheroids in three duplicates was counted.
2.4. Colony formation assay
Cells were suspended, counted, diluted, and seeded at 1×103/well into 6-well plate in triplicates culturing for 7 days. Then, cellular colonies were fixed with paraformaldehyde and stained with coomassie brilliant blue subsequently. The number of large colonies, which containing over 50 cells for one colone, were counted and analyzed.
2.5. Immunofluorescence assay
For immunofluorescence studies, cells were fixed in paraformaldehyde and permeabilized with 0.15% Triton X-100, after which the cells were blocked with 3% BSA. The samples were incubated with primary antibodies overnight at 4 °C followed by secondary antibody (Life, USA). Cellular nuclei were counter stained with DAPI (Roche, USA). All of the labeled cells were examined using a Leica confocal fluorescence imaging microscope with LAS AF software, version 2.0 (Leica Microsystems).
2.6. Immunoblotting and co-immunoprecipitation
For immunoblotting, cellular extracts were acquired by using whole cell lysis buffer containing proteinase inhibitor cocktail (Roche, USA). After subjecting the lysates to 10% SDS-PAGE electrophoresis, proteins were transferred onto a polyvinylidene difluoride membrane by electroblotting. The membranes were then blocked in 5% skimmed milk for 1 h and incubated overnight at 4 °C with specific primary antibodies. Specific antibody-bound protein bands were visualized using an Odyssey Infrared Imaging System (LI-COR Bioscience, USA).
For co-immunoprecipitation (Co-IP) analysis, cells were lysed on ice by EBC buffer (50 mM Tris-HCl, pH 8.0, 125 mM NaCl, 0.5% NP-40, and protease inhibitor cocktail). Totally, 500 μg proteins were incubated overnight at 4 °C under rotation with primary antibodies or anti-Flag magnetic Beads (Sigma, USA). Immunoprecipitates bound to the primary antibodies were harvested by protein A+G agarose (Bioworld, USA). Samples of eluted immunoprecipitates were analyzed by immunoblotting.
2.7. Quantitative real-time PCR assay
Total RNA was extracted and reversely transcribed using the PrimerScript RT reagent Kit (TaKaRa, Japan) according to the protocols recommended by the manufacturer. The cDNA was subjected to quantitative real-time PCR (qRT-PCR) analysis using an SYBR Green Premix Kit (TaKaRa, Japan). The primer sequences are described in Supplementary Table S2. The relative expression was calculated using the 2-ΔΔCT method.
2.8. Animal experiments
Male BALB/c nude mice (nu/nu, aged 4–5 weeks and weighing approximately 20 g) were purchased from Shanghai Laboratory Animal Center (Shanghai, China) and were housed under specific-pathogen-free conditions in the experimental animal care center of the Ninth People's Hospital of the Shanghai Jiao Tong University School of Medicine. Animal welfare and experimental procedures were conducted in compliance with the Guide for Care and Use of Laboratory Animals (The Ministry of Science and Technology of China, 2006) and the related ethical regulations of the hospital. The Animal Care and Use Committees of the hospital approved all experimental procedures.
Briefly, the nude mouse xenograft tumor models were established by subcutaneous injection of 5×106 cells/per site. To evaluate the effect of 17-DMAG on the tumorigenesis of Cal27 cells, mice implanted with cells were randomly divided into two groups after the xenografts reaching a mean diameter of 5 mm (two sites were inoculated in one mouse, n=4), after which the mice were treated with 17-DMAG (10 mg/kg, i.p. 5 days per week) or DMSO as control. Tumor volumes (length × width2/2) were monitored.
2.9. Immunohistochemistry
Fresh tissues were fixed in 4% formaldehyde, embedded in paraffin and prepared into 5-micrometer sections. After deparaffinization, rehydration, and antigen retrieval, immunohistochemistry (IHC) staining was performed and endogenous peroxidase activity was quenched. The slides were incubated with primary antibody overnight at 4 °C. Then, the samples were incubated with a biotinylated secondary antibody for 50 min at 37 ℃, which was followed by staining with a DAB kit (GTVision, China).
2.10. Statistical analysis
The data were compiled using the software package SPSS. All experiments were performed in triplicate; and representative results were displayed. The values displayed correspond to the means±SD. Significant differences between two groups were determined based on Student's t-test and a p-value<0.05 was considered statistically significant.
3. Results
3.1. CHIP regulates CSC properties related CD166 alteration in HNCs
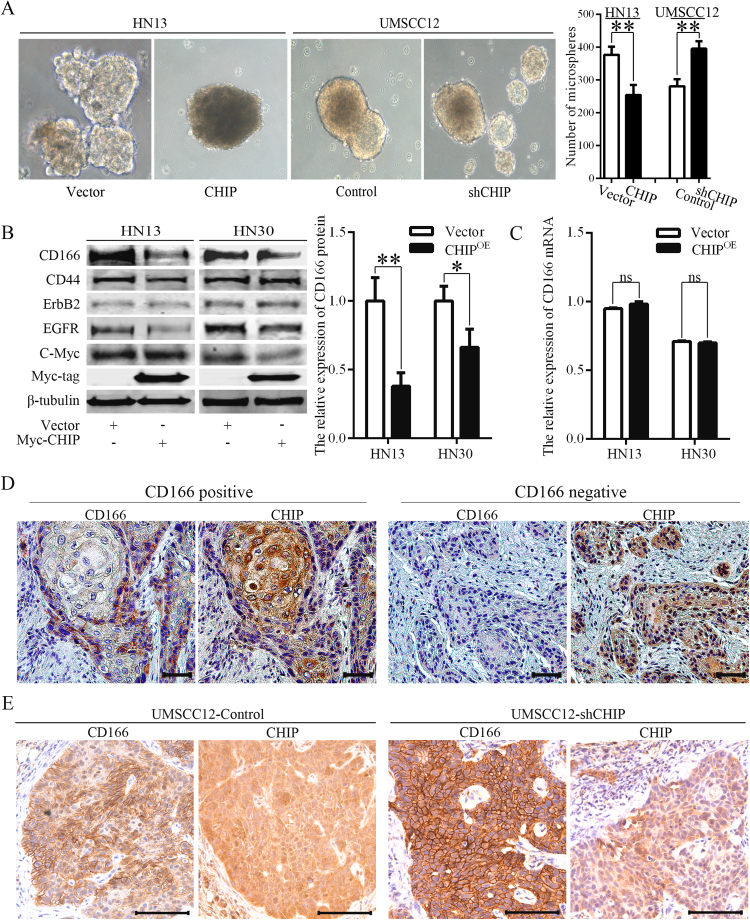
We examine the potential effects of CHIP on CSC properties through in vitro spheroid cultures to enrich CSCs from HNC cell lines. As shown in the Fig. 1A, over expression of CHIP (CHIPOE) in HN13 cells caused a decrease in the rate of microsphere formation and reduced the budding formation of microspheres. Moreover, knockdown of CHIP expression in UMSCC12 cells greatly increased the rate of microsphere formation (Fig. 1A), indicating that CHIP expression negatively affects the CSC characteristics in HNC cell lines. Next, we examined the expression levels of EGFR and c-Myc, which are CHIP target proteins, and found decreased levels in CHIPOE HN13 or HN30 cells (Fig. 1B). Then, we analyzed the expression levels of the CSC markers for HNCs, CD44 and CD166 [4], [21]. Although CD44 expression levels did not change significantly, we observed an obvious negative correlation between CHIP and CD166 protein expression in HNC cells (Fig. 1B) without changes in CD166 mRNA level (Fig. 1C).
Fig. 1.
CHIP regulates CSC properties by affecting CD166 expression in HNCs. (A) The effect of altered CHIP expression on the microsphere formation ability of HN13 (**p=0.007) and UMSCC12 cells (**p=0.003). (B) The protein levels of CHIP targeting oncogene-related proteins and candidate CSC markers were determined in CHIPOE HNC cells by immunoblotting assays. CD166 expression was decreased in CHIPOE HN13 cells (**p=0.0053) and HN30 cells (*p=0.0256). (C) Real-time qPCR analysis was conducted to determine the mRNA level of CHIP and CD166 in HN13 and HN30 cells 24 h after transfection. (D) Immunohistochemistry staining for CD166 and CHIP was conducted using consecutive sections of HNC samples, bar=200 µm. (E) CHIP and CD166 expression levels were examined in consecutive sections of UMSCC12-Control and UMSCC12-shCHIP xenografts by immunohistochemistry staining, bar=100 µm.
To determine the expression relationship between CHIP and CD166 in HNC samples, consecutive pathological sections were subjected to IHC staining for CD166 and CHIP. Comparatively, CD166-positive cells were mostly located at the frontier of invasive cancer nest, and strong negative correlations were observed for the positive distributions of CHIP and CD166 in HNC samples (Fig. 1D). In the CHIP positive areas, low levels of CD166 expression were observed and vice versa. Besides, in UMSCC12-shCHIP xenografts, elevated CD166 expression levels were also observed (Fig. 1E). These data suggest a post-translational modification relationship between CHIP and CD166.
3.2. CHIP decreases the protein stability of CD166 in HNCs
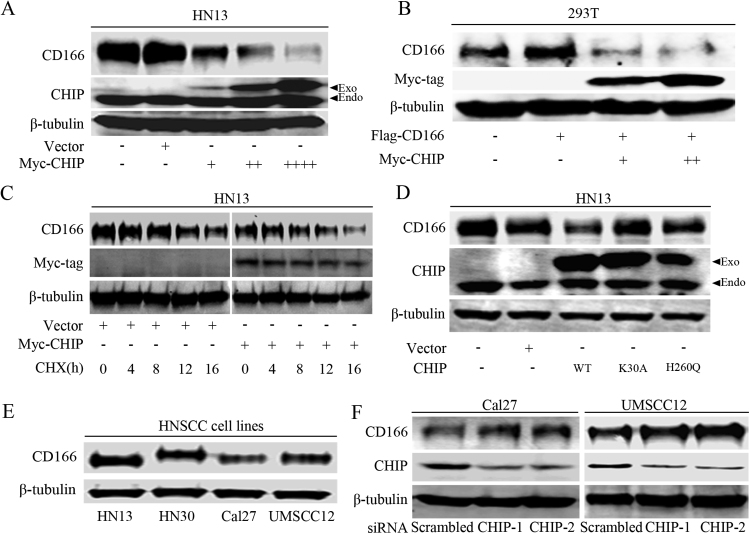
To further explore how CD166 expression is regulated by CHIP, we transfected a CHIP overexpression plasmid into HN13 and 293T cells, and we found that CHIPOE decreased both endogenously and exogenously expressed CD166 protein in a dose-dependent manner (Fig. 2A-B). After blocking de novo protein synthesis by CHX treatment, CHIPOE greatly promoted the degradation of CD166 protein in HN13 cells (Fig. 2C). Furthermore, we found that wild-type CHIP but not its enzymatically inactive mutants affected the CD166 protein levels (Fig. 2D). In the examined HNC cell lines, Cal27 and UMSCC12 cells expressed relatively lower endogenous CD166 levels (Fig. 2E), and we observed a clear increase in endogenous CD166 protein expression after knocking down CHIP expression (Fig. 2F). These data indicate that CHIP expression decreases the stability of CD166 protein via post-translational modification.
Fig. 2.
CHIP regulates the protein stability of CD166 in HNCs. (A) The effect of increasing the CHIP expression level on endogenous CD166 expression in HN13 cells (Exo, exogenous; Endo, endogenous). (B) The expression levels of endogenous and exogenous CD166 in 293 T cells with increasing exogenous CHIP expression. (C) The effect of CHIP on endogenous CD166 levels in HN13 cells in a time dependent manner upon treatment with cycloheximide (50 μg/ml). (D) The effect of CHIP mutants constructs on the expression levels of endogenous CD166 in HN13 cells (Exo: exogenous; Endo: endogenous). (E) CD166 protein expression levels were detected in HN13, HN30, Cal27 and UMSCC12 cells. (F) CD166 expression levels were determined in Cal27 and UMSCC12 cells after transfection with CHIP specific and scrambled siRNAs.
3.3. CHIP-CD166 axis regulates cancer stem-like properties in HNCs
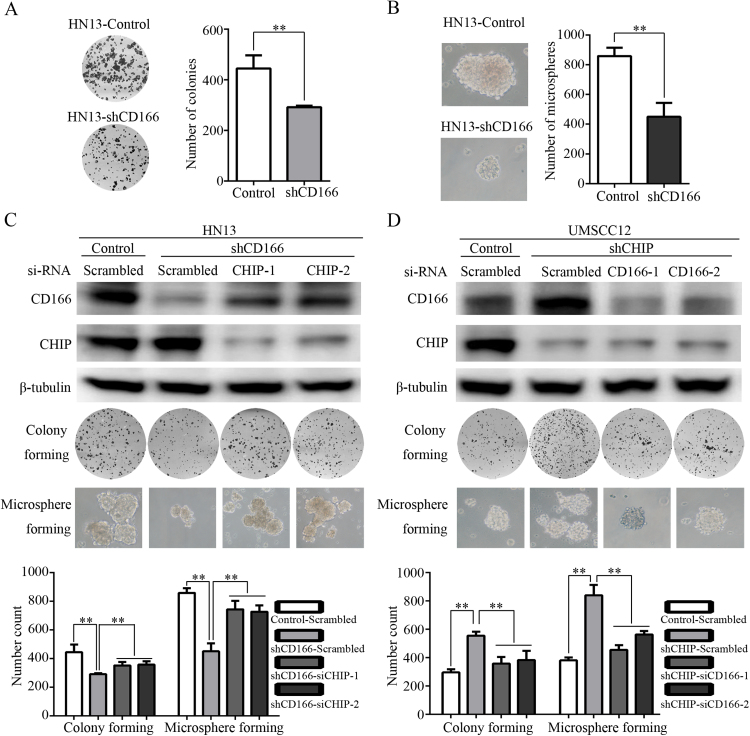
In order to identify the effect of CHIP-CD166 interaction on CSC properties of HNCs, HN13-shCD166 cell line with reduced CSC properties (colony forming and microsphere forming) was introduced (Fig. 3A-B). Then, rescue assays were designed by suppressing the expression level of CHIP in HN13-shCD166 cells and by reducing the expression level of CD166 in UMSCC12-shCHIP cells. Consequently, we managed to increase CD166 expression level in HN13-shCD166 cells by knocking down CHIP expression (Fig. 3C). Compared with HN13-Control cells, the decreased colony and microsphere forming abilities of HN13-shCD166 cells were obviously enhanced with two pairs of siRNA sequences targeting CHIP (Fig. 3C). In UMSCC12-shCHIP cells, its increased CD166 expression was reduced by two pairs of siRNA sequences targeting CD166 (Fig. 3D). UMSCC12-shCHIP cells presented a higher CD166 expression and CSC properties than UMSCC12-Control cell, and knocking down CD166 expression in UMSCC12-shCHIP significantly decreased its colony and microsphere formation (Fig. 3D). These data strengthen the point that CHIP-CD166 axis plays important roles in regulating CSC properties of HNCs.
Fig. 3.
CHIP-CD166 axis in the regulation of cancer stem-like properties of HNC cells. (A) Colony formation capacity of HN13-shCD166 cells compared with HN13-Control cells (**p=0.006). (B) Microsphere formation ability of HN13-shCD166 cells compared with HN13-Control cells (**p=0.003). (C) Western blotting results showed CD166 and CHIP expression levels in HN13-Control cells transfected with Scrambled siRNA seqeuence and in HN13-shCD166 cells transfected with Scrambled and two pair of CHIP specific siRNA sequences. Colony forming and miscrosphere forming abilities were detected and compared under the indicated treatment (**p<0.01). (D) Western blot results showed CD166 and CHIP expression levels in UMSCC12-Control cells transfected with Scrambled siRNA seqeuence and in UMSCC12-shCHIP cells transfected with Scrambled and two pair of CD166 specific siRNA sequences. Colony forming and miscrosphere forming abilities were detected and compared under the indicated treatment (**p<0.01).
3.4. CHIP mediates the degradation of CD166 through ubiquitin-proteasome pathway
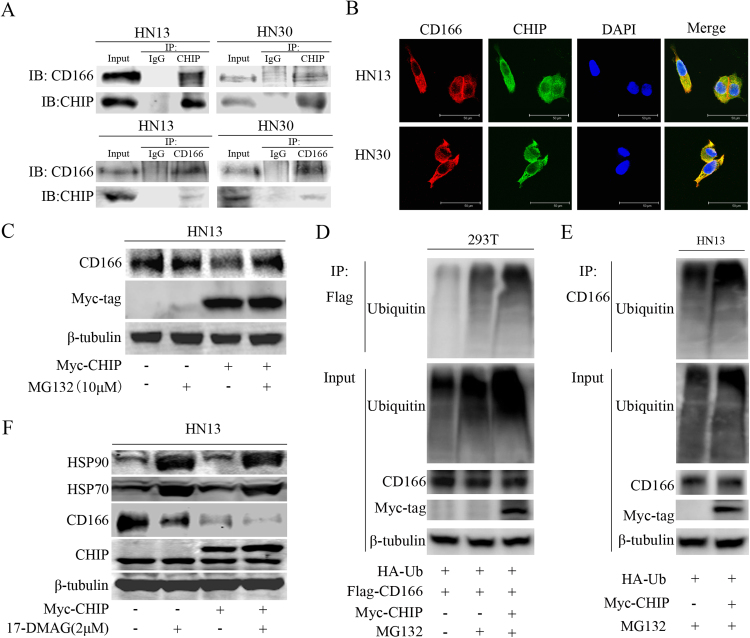
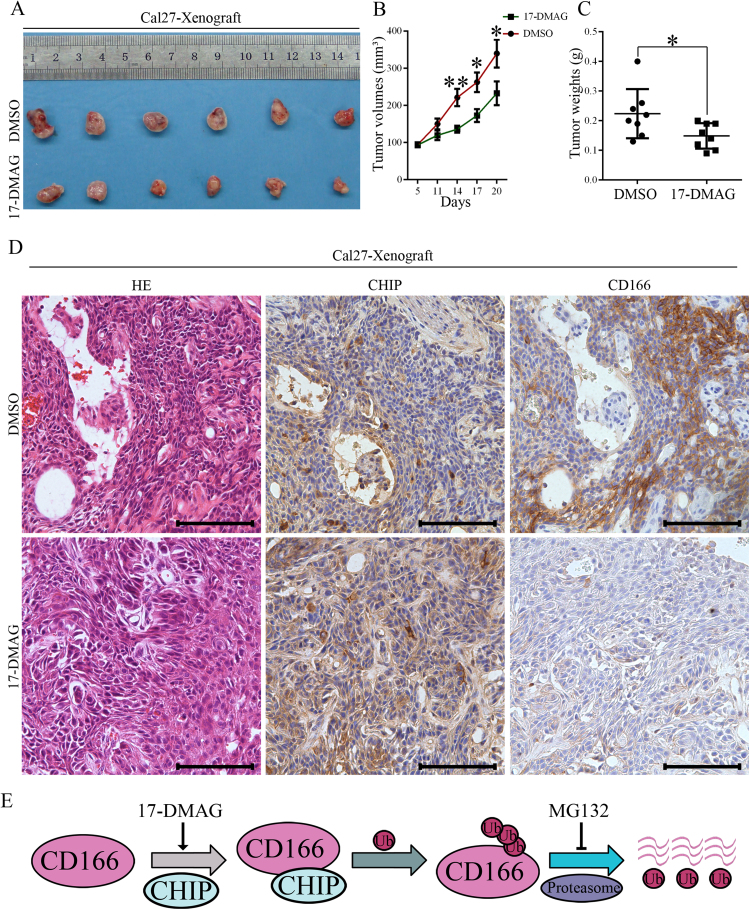
As CHIP is an E3 ubiquitin ligase that interacts with and induces the ubiquitylation and degradation of its substrates [11], [22], we first investigated a potential direct interaction between CHIP and CD166 in HNCs. In the co-IP assay, endogenous CHIP and CD166 interacted with each other in HN13 and HN30 cells (Fig. 4A). Based on co-localization immunofluorescent staining, CHIP and CD166 were observed in similar focal patterns by confocal microscopy of HN13 and HN30 cells (Fig. 4B). Next, we incubated HN13 cells with the proteasome inhibitor MG-132 to block the ubiquitin-proteasome system. We observed that the protein level of CD166 was increased in CHIPOE HN13 cells under the treatment of MG132 (Fig. 4C). As shown in Fig. 4D, MG132 treatment blocked the degradation of ubiquitinated exogenous CD166. When combined exogenous CHIP transfection and MG132 treatment, the ubiquitin levels were greatly enhanced for exogenous CD166 in 293 T cells and endogenous CD166 in HN13 cells (Fig. 4D-E). These data indicates that CHIP mediates the poly-ubiquitin-dependent degradation of CD166 through the proteasome. Because the roles of HSP90 and HSP70 in CHIP mediated proteasome degradation have been elucidated in previous studies [22], [23], we observed an up-regulation of HSP70 and HSP90 levels in the presence of the HSP90 inhibitor 17-DMAG. Upon treatment with 17-DMAG, we observed an increase in CHIP-mediated CD166 degradation in HN13 cells (Fig. 4F), suggesting that degradation of CD166 by CHIP depends on HSP90 and HSP70. In Cal27 xenografts, 17-DMAG treatment significantly suppressed the tumor growth in vivo (Fig. 5A-C). In the subsequently histological examination, the CD166 expression levels were decreased in the tissues from 17-DMAG-treated Cal27 cell xenografts (Fig. 5D).
Fig. 4.
CHIP degrades CD166 through the ubiquitin-proteasome pathway in HNC cells. (A) The potential direct interaction of endogenous CD166 and CHIP was determined by co-IP analysis in HN13 and HN30 cells. (B) The direct co-localization of CD166 and CHIP in HN13 and HN30 cells was detected by confocal microscopy (red for CD166, green for CHIP). Nuclei were counter stained with DAPI (blue), bar=50 µm. (C) MG132 (10 μM) blocked the reduction in CD166 expression levels in HN13 cells transfected with exogenous CHIP. (D) The effects of MG132 and CHIP on the ubiquitination of exogenous CD166 were determined using a co-IP assay in 293 T cells. (E) The effects of CHIP on the ubiquitination of endogenous CD166 were determined by co-IP in HN13 cells co-transfected with HA-Ub. (F) The effect of 17-DMAG (2 μM) and CHIP on the protein expression levels of HSP90, HSP70 and CD166 in HN13 cells.
Fig. 5.
HSP90 inhibitor 17-DMAG suppresses the growth of Cal27 xenografts in vivo. (A) Representative images of Cal27 xenografts in each group of mice after 20 days (8 sites in 4 mice). Mice received i.p. injection of 10 mg/kg 17-DMAG, or equivalent DMSO in PBS 5 days per week, respectively. (B) Tumor volumes changing of Cal27 xenograft in DMSO, or 17-DMAG treated mice. Tumor volumes were calculated with length×width2/2 of the xenograft (*p<0.05, **p<0.01). (C) Weights of the xenografts were compared between DMSO and 17-DMAG treated mice (*p<0.05). (D) H&E staining and immunohistochemistry staining of CHIP and CD166 in consecutive sections of Cal27 xenografts in DMSO and 17-DMAG treated mice; bar=100 µm. (E) Schematic model for the degradation of CD166 through the ubiquitin-proteasome pathway mediated by CHIP.
4. Discussion
Our previous studies have identified that CD166 participates in the regulation of CSC properties of HNCs. However, the over-expression reason of CD166 in CSCs is unknown by now. How to suppress CD166 expression effectively in CSCs? The post-translational regulatory mechanism underlying CD166 turnover also remains elusive. So, to further explore the role and mechanism of CD166 expression in regulating CSCs behaviors is so urgent.
In the present study, we observed a tight relationship between CHIP expression and CSC regulation. Enriched oral CSCs have also been reported to display induced differentiation abilities and enhanced migration/invasion/malignancy capabilities in vitro and in vivo [24], [25]. Cancer stem-like cells have been identified in various cancers, and some molecular systems have been characterized for regulating tumor initiation and stemness [25]. Although previous reports showed the CHIP suppresses CSC properties in cancer cells, no target substrates have been identified to explain this phenomenon [26]. Interestingly, we observed that CHIP but not its inactive mutants regulated the protein stability of CD166 in HNC cells, and CHIP-CD166 axis was identified to regulate CSC properties of HNCs.
Yan et al. reported that CD166 is a valuable marker for the enrichment of HNC stem cells by plasma membrane proteomic analysis [4]. Altered expression of CD166 is associated with cancer progression in various cancer types [27], [28]. Because altered CD166 expression correlates with the development of cancers [4], understanding the mechanisms underlying CD166 turnover is vital for clarifying CSC regulation. To validate that CHIP acts as an E3 ligase for CD166 in HNC, key steps in the ubiquitin-proteasome pathway were investigated and shown experimentally [22], [29]. We observed that CHIP directly interacts with CD166 in HNC cells. Moreover, when we inhibited the proteasome with MG132, the CD166 protein level was stabilized, and increased poly-ubiquitination of CD166 could be immuno-precipitated in CHIPOE HNC cells. As previously reported, HSP90 played a pivotal role in the stabilization of many labile oncogenic client proteins, and CHIP functions in the molecular chaperone system with HSP90 and HSP70 [8], [9]. We illustrated the synergistic effect of CHIP and 17-DMAG in degrading CD166 in vitro and substantially decreasing in vivo CD166 expression by 17-DMAG, suggesting that HSP90 is involved in CD166 stabilization. Besides, we could also conclude that 17-DMAG showed the potential to inhibit the CSC behaviors through promoting the ubiquitin degradation of CD166 mediated by CHIP in HNCs.
The above data suggest that CHIP promotes the protein degradation of CD166 through the ubiquitin-proteasome pathway and that the inhibition of HSP90 could enhance CHIP-mediated degradation of CD166 (Fig. 5E). The acquirement of CD166 expression resulting from CHIP decreased expression gained further insights into the molecular regulations in the initiation of HNCs.
5. Conclusions
The present study showed that CHIP regulates CSC properties of HNC through the CD166-CHIP-proteasome axis. Our results could provide new strategies for decreasing CD166 expression and suppressing CSC properties through the manipulation of E3 ligase CHIP in HNCs.
Declaration of interest
The authors declare that they have no competing interests.
Authors' contributions
Wantao Chen and Xu Wang conceived and designed the experiments. Meng Xiao performed the experiments. Meng Xiao, Xu Wang and Shengcai Qi summarized and analyzed the data. Meng Xiao, Xu Wang, Ming Yan, Jianjun Zhang and Qin Xu contributed to writing and revising the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The patients involved in this study signed written informed consent, and the study was approved by the Medical Ethics Committee of the Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine. Animal welfare and experimental procedures were conducted in compliance with the Guide for Care and Use of Laboratory Animals (The Ministry of Science and Technology of China, 2006) and the related ethical regulations of the hospital. The Animal Care and Use Committees of the hospital approved all experimental procedures.
Acknowledgements
We thank Professor Zeguang Han, Professor Ping Zhang and Professor Li Mao for critically reading the manuscript and providing insightful comments for this study. We also thank members of the W. Chen and W. Wei laboratories for useful discussions. This work was supported by the National Program on Key Research Project of China (2016YFC0902700), the National Natural Science Foundation of China (81572646 and 91229103), Shanghai Municipal Science and Technology Commission Funded Project (15DZ2292300) and the Innovation Fund for Doctoral Program of Shanghai Jiao Tong University, School of Medicine (BXJ201628).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.yexcr.2017.03.005.
Contributor Information
Xu Wang, Email: wangx312016@sh9h.org.
Wantao Chen, Email: chenwantao196323@sjtu.edu.cn.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Swart G.W., Lunter P.C., Kilsdonk J.W., Kempen L.C. Activated leukocyte cell adhesion molecule (ALCAM/CD166): signaling at the divide of melanoma cell clustering and cell migration? Cancer Metastasis Rev. 2005;24:223–236. doi: 10.1007/s10555-005-1573-0. [DOI] [PubMed] [Google Scholar]
- 2.Ofori-Acquah S.F., King J.A. Activated leukocyte cell adhesion molecule: a new paradox in cancer. Transl. Res. 2008;151:122–128. doi: 10.1016/j.trsl.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Ihnen M., Muller V., Wirtz R.M., Schroder C., Krenkel S., Witzel I., Lisboa B.W., Janicke F., Milde-Langosch K. Predictive impact of activated leukocyte cell adhesion molecule (ALCAM/CD166) in breast cancer. Breast Cancer Res Treat. 2008;112:419–427. doi: 10.1007/s10549-007-9879-y. [DOI] [PubMed] [Google Scholar]
- 4.Yan M., Yang X., Wang L., Clark D., Zuo H., Ye D., Chen W., Zhang P. Plasma membrane proteomics of tumor spheres identify CD166 as a novel marker for cancer stem-like cells in head and neck squamous cell carcinoma. Mol. Cell Proteom. 2013;12:3271–3284. doi: 10.1074/mcp.M112.025460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar P., Pradhan K., Karunya R., Ambasta R.K., Querfurth H.W. Cross-functional E3 ligases Parkin and C-terminus Hsp70-interacting protein in neurodegenerative disorders. J. Neurochem. 2012;120:350–370. doi: 10.1111/j.1471-4159.2011.07588.x. [DOI] [PubMed] [Google Scholar]
- 6.Yan S., Sun X., Xiang B., Cang H., Kang X., Chen Y., Li H., Shi G., Yeh E.T., Wang B., Wang X., Yi J. Redox regulation of the stability of the SUMO protease SENP3 via interactions with CHIP and Hsp90. EMBO J. 2010;29:3773–3786. doi: 10.1038/emboj.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo J., Lee E.W., Sung H., Seong D., Dondelinger Y., Shin J., Jeong M., Lee H.K., Kim J.H., Han S.Y., Lee C., Seong J.K., Vandenabeele P., Song J. CHIP controls necroptosis through ubiquitylation- and lysosome-dependent degradation of RIPK3. Nat. Cell Biol. 2016;18:291–302. doi: 10.1038/ncb3314. [DOI] [PubMed] [Google Scholar]
- 8.Paul I., Ghosh M.K. The E3 ligase CHIP: insights into its structure and regulation. Biomed. Res. Int. 2014;2014:918183. doi: 10.1155/2014/918183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edkins A.L. CHIP: a co-chaperone for degradation by the proteasome. Subcell. Biochem. 2015;78:219–242. doi: 10.1007/978-3-319-11731-7_11. [DOI] [PubMed] [Google Scholar]
- 10.Wei Q., Sha Y., Bhattacharya A., Abdel Fattah E., Bonilla D., Jyothula S.S., Pandit L., Khurana Hershey G.K., Eissa N.T. Regulation of IL-4 receptor signaling by STUB1 in lung inflammation. Am. J. Respir. Crit. Care Med. 2014;189:16–29. doi: 10.1164/rccm.201305-0874OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kajiro M., Hirota R., Nakajima Y., Kawanowa K., So-ma K., Ito I., Yamaguchi Y., Ohie S.H., Kobayashi Y., Seino Y., Kawano M., Kawabe Y., Takei H., Hayashi S., Kurosumi M., Murayama A., Kimura K., Yanagisawa J. The ubiquitin ligase CHIP acts as an upstream regulator of oncogenic pathways. Nat. Cell Biol. 2009;11:312–319. doi: 10.1038/ncb1839. [DOI] [PubMed] [Google Scholar]
- 12.Gaude H., Aznar N., Delay A., Bres A., Buchet-Poyau K., Caillat C., Vigouroux A., Rogon C., Woods A., Vanacker J.M., Hohfeld J., Perret C., Meyer P., Billaud M., Forcet C. Molecular chaperone complexes with antagonizing activities regulate stability and activity of the tumor suppressor LKB1. Oncogene. 2012;31:1582–1591. doi: 10.1038/onc.2011.342. [DOI] [PubMed] [Google Scholar]
- 13.Hatakeyama S., Watanabe M., Fujii Y., Nakayama K.I. Targeted destruction of c-Myc by an engineered ubiquitin ligase suppresses cell transformation and tumor formation. Cancer Res. 2005;65:7874–7879. doi: 10.1158/0008-5472.CAN-05-1581. [DOI] [PubMed] [Google Scholar]
- 14.Ruckova E., Muller P., Nenutil R., Vojtesek B. Alterations of the Hsp70/Hsp90 chaperone and the HOP/CHIP co-chaperone system in cancer. Cell. Mol. Biol. Lett. 2012;17:446–458. doi: 10.2478/s11658-012-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciocca D.R., Calderwood S.K. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T., Yang J., Xu J., Li J., Cao Z., Zhou L., You L., Shu H., Lu Z., Li H., Li M., Zhang T., Zhao Y. CHIP is a novel tumor suppressor in pancreatic cancer through targeting EGFR. Oncotarget. 2014;5:1969–1986. doi: 10.18632/oncotarget.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Ren F., Wang Y., Feng Y., Wang D., Jia B., Qiu Y., Wang S., Yu J., Sung J.J., Xu J., Zeps N., Chang Z. CHIP/Stub1 functions as a tumor suppressor and represses NF-kappaB-mediated signaling in colorectal cancer. Carcinogenesis. 2014;35:983–991. doi: 10.1093/carcin/bgt393. [DOI] [PubMed] [Google Scholar]
- 18.Wang S., Wu X., Zhang J., Chen Y., Xu J., Xia X., He S., Qiang F., Li A., Shu Y., Roe O.D., Li G., Zhou J.W. CHIP functions as a novel suppressor of tumour angiogenesis with prognostic significance in human gastric cancer. Gut. 2013;62:496–508. doi: 10.1136/gutjnl-2011-301522. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed S.F., Deb S., Paul I., Chatterjee A., Mandal T., Chatterjee U., Ghosh M.K. The chaperone-assisted E3 ligase C terminus of Hsc70-interacting protein (CHIP) targets PTEN for proteasomal degradation. J. Biol. Chem. 2012;287:15996–16006. doi: 10.1074/jbc.M111.321083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia G., Wang X., Yan M., Chen W., Zhang P. CD166-mediated epidermal growth factor receptor phosphorylation promotes the growth of oral squamous cell carcinoma. Oral Oncol. 2016;59:1–11. doi: 10.1016/j.oraloncology.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Prince M.E., Sivanandan R., Kaczorowski A., Wolf G.T., Kaplan M.J., Dalerba P., Weissman I.L., Clarke M.F., Ailles L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith M.C., Scaglione K.M., Assimon V.A., Patury S., Thompson A.D., Dickey C.A., Southworth D.R., Paulson H.L., Gestwicki J.E., Zuiderweg E.R. The E3 ubiquitin ligase CHIP and the molecular chaperone Hsc70 form a dynamic, tethered complex. Biochemistry. 2013;52:5354–5364. doi: 10.1021/bi4009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchiya M., Nakajima Y., Waku T., Hiyoshi H., Morishita T., Furumai R., Hayashi Y., Kishimoto H., Kimura K., Yanagisawa J. CHIP buffers heterogeneous Bcl-2 expression levels to prevent augmentation of anticancer drug-resistant cell population. Oncogene. 2015;34:4656–4663. doi: 10.1038/onc.2014.387. [DOI] [PubMed] [Google Scholar]
- 24.Chiou S.H., Yu C.C., Huang C.Y., Lin S.C., Liu C.J., Tsai T.H., Chou S.H., Chien C.S., Ku H.H., Lo J.F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 25.Boumahdi S., Driessens G., Lapouge G., Rorive S., Nassar D., Le Mercier M., Delatte B., Caauwe A., Lenglez S., Nkusi E., Brohee S., Salmon I., Dubois C., del Marmol V., Fuks F., Beck B., Blanpain C. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya M., Nakajima Y., Hirata N., Morishita T., Kishimoto H., Kanda Y., Kimura K. Ubiquitin ligase CHIP suppresses cancer stem cell properties in a population of breast cancer cells. Biochem. Biophys. Res. Commun. 2014;452:928–932. doi: 10.1016/j.bbrc.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Ihnen M., Kress K., Kersten J.F., Kilic E., Choschzick M., Zander H., Muller V., Mahner S., Janicke F., Woelber L., Milde-Langosch K. Relevance of activated leukocyte cell adhesion molecule (ALCAM) in tumor tissue and sera of cervical cancer patients. BMC Cancer. 2012;12:140. doi: 10.1186/1471-2407-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hein S., Muller V., Kohler N., Wikman H., Krenkel S., Streichert T., Schweizer M., Riethdorf S., Assmann V., Ihnen M., Beck K., Issa R., Janicke F., Pantel K., Milde-Langosch K. Biologic role of activated leukocyte cell adhesion molecule overexpression in breast cancer cell lines and clinical tumor tissue. Breast Cancer Res. Treat. 2011;129:347–360. doi: 10.1007/s10549-010-1219-y. [DOI] [PubMed] [Google Scholar]
- 29.Sarkar S., Brautigan D.L., Parsons S.J., Larner J.M. Androgen receptor degradation by the E3 ligase CHIP modulates mitotic arrest in prostate cancer cells. Oncogene. 2014;33:26–33. doi: 10.1038/onc.2012.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material