Abstract
Previous neuroimaging studies have revealed frontal and temporal functional abnormalities in patients with major depressive disorder (MDD) and a history of suicidal behavior. However, it is unknown whether multi-channel near-infrared spectroscopy (NIRS) signal changes among individuals with MDD are associated with a history of suicide attempts and a diathesis for suicidal behavior (impulsivity, hopelessness, and aggression). Therefore, we aimed to explore frontotemporal hemodynamic responses in depressed patients with a history of suicide attempts using 52-channel NIRS. We recruited 30 patients with MDD and a history of suicidal behavior (suicide attempters; SAs), 38 patient controls without suicidal behavior (non-attempters; NAs), and 40 healthy controls (HCs) matched by age, gender ratio, and estimated IQ. Regional hemodynamic responses during a verbal fluency task (VFT) were monitored using NIRS. Our results showed that severities of depression, impulsivity, aggression, and hopelessness were similar between SAs and NAs. Both patient groups had significantly reduced activation compared with HCs in the bilateral frontotemporal regions. Post hoc analyses revealed that SAs exhibited a smaller hemodynamic response in the left precentral gyrus than NAs and HCs. Furthermore, the reduced response in the left inferior frontal gyrus was negatively correlated with impulsivity level and hemodynamic responses in the right middle frontal gyrus were negatively associated with hopelessness and aggression in SAs but not in NAs and HCs. Our findings suggest that MDD patients with a history of suicide attempts demonstrate patterns of VFT-induced NIRS signal changes different from those demonstrated by individuals without a history of suicidal behaviors, even in cases where clinical symptoms are similar. NIRS has a relatively high time resolution, which may help visually differentiate SAs from NAs.
Introduction
Every year, one million individuals world over die from suicide [1]. This is a serious global health problem, but suicide is difficult to predict and prevent in majority of the people at risk. Although the pathogenesis of suicide is multifactorial, the strongest predictor of risk for a future attempt is a history of suicidal behavior and presence of a psychiatric disorder, particularly a major depressive disorder (MDD) [2, 3]. Although most patients with MDD never attempt suicide, those who do frequently exhibit a diathesis for suicidal behavior [4], a notion formalized in the diathesis–stress model of suicidal behavior [3]. According to this model, the proximal stressors leading to suicidal behavior are commonly psychiatric disorders together with acute psychosocial crises, whereas the components of the diathesis for suicidal behavior are impulsivity, aggression, and hopelessness.
In vivo brain imaging is a promising tool for identifying neurological correlates of the diathesis for suicidal behavior [5, 6]. A magnetic resonance imaging (MRI) study found that depressed patients at high suicide risk had a significantly thinner cortex in the left dorsolateral and ventrolateral prefrontal regions than those at a lower suicide risk [7]. A recent MRI study indicated that suicide attempters (SAs) with a past history of mood disorders have reduced activity in the left ventrolateral prefrontal cortices compared with healthy controls (HCs) and with patient controls with a past history of mood disorders but not of suicidal behavior [8]. Furthermore, functional MRI (fMRI) studies have shown that SAs can be distinguished from non-attempters (NAs) by specific frontal cortex activation patterns in response to angry and happy versus neutral faces [9] and by decreased activation in the medial prefrontal cortex while reading autobiographical accounts of recent suicide attempts compared with while reading neutral scripts [10]. Single-photon emission computed tomography (SPECT) studies have found that SAs have significant perfusion deficits in the prefrontal cortex [11] and bilateral superior frontal regions [12] compared with the control subjects and in the left frontal region compared with controls and depressed NAs [13]. In addition, a positron emission tomography (PET) study of patients with MDD revealed prefrontal hypofunction that was exacerbated by low serotonergic activity and was proportional to the lethality of past suicide attempts [14]. One recent investigation found that compared with depressed individuals with only suicidal thoughts, depressed individuals without suicide thoughts and plans, and healthy controls, depressed individuals with suicide plans showed relative hypometabolism in the right middle frontal gyrus and right inferior parietal lobe [15]. Thus, these neuroimaging studies have revealed altered brain structure and regional activity associated with vulnerability for suicide risk in psychiatric disorders [16, 17].
Multichannel near-infrared spectroscopy (NIRS) is a non-invasive optical technique that allows monitoring of hemodynamic changes related to cortical neural activity by measuring relative changes in oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb). NIRS has several advantages over other neuroimaging techniques for psychiatric research [18]. Near-infrared light is completely non-invasive, and therefore, there are no concerns regarding radiation exposure typically observed with other neuroimaging techniques, such as SPECT/PET. In addition, its easy applicability and high ecological validity make NIRS particularly suitable for psychiatric patients who may be afraid of tight enclosures (e.g., in MRI/PET scanners) or who exhibit motor restlessness that interferes with motion-sensitive imaging methods, such as MRI, SPECT, and PET. Furthermore, NIRS has a relatively high time resolution. Thus, it is a neuroimaging tool that can be applied clinically for assessing potential biomarkers in patients with psychiatric disorders. Many studies using NIRS have demonstrated that changes in mean oxy-Hb levels in frontotemporal regions induced by a verbal fluency task (VFT) are significantly lower in patients with MDD than in control subjects [19–23]. However, it is still unclear whether these NIRS signal changes are associated with a history of suicide attempts and a diathesis for suicidal behavior in MDD.
Here we hypothesized that patients with MDD and a history of suicidal behavior can be distinguished from patients with no such history and from non-psychiatric control subjects by measuring hemodynamic responses in frontotemporal regions using NIRS. We used phonemic verbal fluency as the cognitive task to stimulate changes in hemoglobin levels because it is easy to understand and execute for patients with psychiatric disorders who present with depressive symptoms [24], and poor performance in this task has been linked to suicidal behavior in patients with psychiatric disorders [25, 26]. In addition, we examined whether VFT-induced hemodynamic responses were associated with impulsivity, aggression, and hopelessness, which would support the validity of NIRS responses as a measure of vulnerability for suicide risk in MDD. Accordingly, the aim of this study was to explore frontotemporal hemodynamic responses in depressed patients with a history of suicide attempts using 52-channel NIRS.
Materials and methods
Participants
The study included 68 patients (including 44 females, comprising 64.7% of study patients) with MDD and 40 HCs matched by age, gender ratio, and estimated IQ. The patients were subdivided into two groups: 1) SAs (n = 30), with a documented history of suicidal behavior and 2) NAs (n = 38), with no known history of suicidal behavior and no family history of suicide attempts among first- or second-degree family members. Suicidal behavior was defined as any act performed with the intent to die [3]. The diagnosis of MDD was made according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, using the Mini International Neuropsychiatric Interview (MINI; Japanese version 5.0.0) [27]. The methods used by SAs were confirmed by clinical records and interviews and were as follows: jumping from heights (n = 2), hanging (n = 7), traffic accident (n = 1), neck cutting (n = 1), wrist cutting (n = 8), and drug overdose (n = 11). HCs were screened using MINI, and candidates with a history of psychiatric disorders or heritable neurological diseases among first- or second-degree family members were excluded. Exclusion criteria included the following: 1) a history of head trauma with loss of consciousness, 2) current or previous neurological diseases, 3) current or previous endocrine diseases, 4) alcohol/substance abuse or addiction within the past 12 months, 5) a history of electroconvulsive therapy, and 6) left-handedness [28]. After they were given a complete description of the study, written informed consent was obtained from all subjects. A series of questions was used to assess the person’s understanding of key issues, e.g., the purpose of the research, the foreseeable risks, and anticipated benefits of study participation. This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Kindai University Faculty of Medicine (No. 20–12).
Assessment of symptoms
Depression severity was evaluated using the 17-item Hamilton depression rating scale (HAM-D) administered using a structured interview guide [29]. IQ was estimated using the Japanese version of the National Adult Reading Test [30]. Daily doses of all antidepressants were converted to an equivalent dose of imipramine; those of antipsychotics, to that of chlorpromazine; and those of anxiolytics/hypnotics, to that of diazepam [31]. Impulsivity, aggression, and hopelessness were assessed using the Barratt Impulsiveness Scale 11th [32], Buss-Perry Aggression Questionnaire [33], and Beck Hopelessness Scale [34], respectively.
NIRS
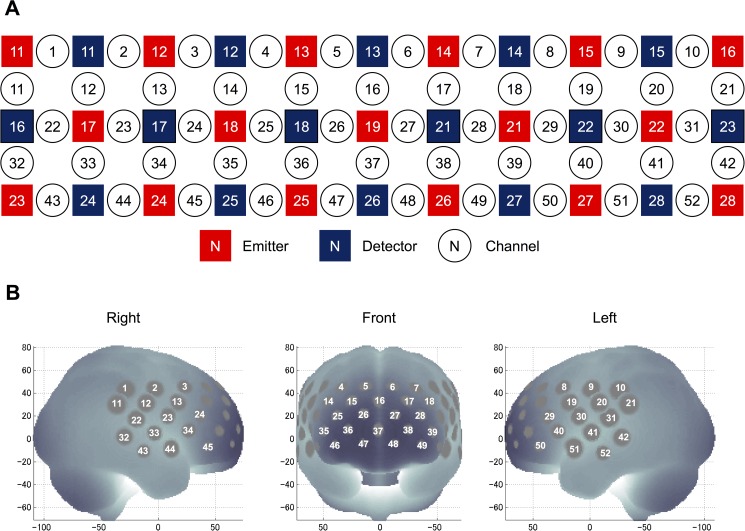
We used a 52-channel NIRS device (ETG-4000 Optical Topography System; Hitachi Medical Co., Tokyo, Japan) to estimate changes in regional cortical Hb concentration during the cognitive activation task, as described previously [35]. The probes (17 emitters and 16 detectors, alternating) were fixed using 3 × 11 thermoplastic shells with an inter-optode distance of 3.0 cm. Each adjoining pair of an emitter and detector was referred to as a “channel,” resulting in 52 channels in total (Fig 1A). The lowermost probes were positioned along the Fp1–Fp2 line according to the International 10–20 system (Fig 1B). The probes can measure Hb values bilaterally from the prefrontal and temporal surface regions at a depth of 20–30 mm from the scalp. This depth range corresponds roughly to the surface of the cerebral cortex.
Fig 1. Locations of the NIRS channels.
(A) Arrangement of the 17 emitters and 16 detectors and definition of the channels. (B) The anatomical site corresponding to each channel.
NIRS measures relative changes in oxy- and deoxy-Hb concentrations (in mM) using two wavelengths (695 and 830 nm) of near-infrared light based on the modified Beer–Lambert law [36]. However, NIRS cannot measure the absolute path length from the emitter to detector. We, therefore, recorded relative mean changes in Hb concentration from the baseline (in mM∙mm). NIRS signals were acquired with a time resolution of 0.1 s. Mean changes in task-related oxy/deoxy-Hb levels were calculated by a linear fit to two baseline periods, the final 10 s of the pre-task period and the final 5 s of the post-task period (integral mode). We set the moving average window to 5 s to remove high-frequency noise, such as from heartbeats and small movements. Data from channels were excluded due to excessive level of artifacts using a computer algorithm adopted in previous studies [37]; thus, the number of available channels varied between individuals, but the mean number of channels did not differ between the groups [SAs: mean ± SD, 49.3 ± 3.3; NAs: mean ± SD, 48.9 ± 4.6; and HCs: mean ± SD, 49.9 ± 3.5; Kruskal–Wallis non-parametric one-way analysis of variance (ANOVA), χ2 = 0.19, p = 0.91].
The spatial information for each channel was estimated using data from the Functional Brain Science Laboratory at the Jichi Medical University, Japan [38, 39]. According to the LONI Probabilistic Brain Atlas (LPBA40) [40], NIRS channels can record functional hemodynamics within the bilateral frontal, temporal, and parietal cortices. We anatomically labeled NIRS channels only after determining the LPBA40 region of highest probability.
For the analysis of NIRS data, we focused on oxy-Hb because it is thought that cortical activation is more directly reflected by a task-related change in this parameter than by a change in deoxy-Hb, as indicated by a stronger correlation with blood oxygenation level-dependent signals measured on fMRI [41].
Activation task
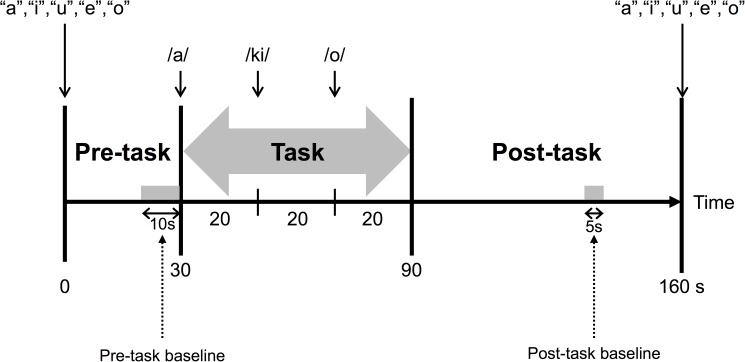
Changes in hemoglobin levels were stimulated using VFT because previous studies have shown measurable prefrontal activation during VFT in healthy subjects [19, 42]. The task procedure was similar to that described previously [35]. VFT used in the present study included a 30-s pre-task baseline period, 60-s task period comprising three 20-s blocks, and 70-s post-task baseline period. During the pre- and post-task baseline periods, the subjects were instructed to repeat a train of syllables (“a, i, u, e, and o”). During the 60-s task, subjects were asked to generate as many words as possible starting with that syllable (Fig 2). The possible syllables were as follows: block 1 (0–20 seconds), “a,” “to,” or “na”; block 2 (20–40 seconds), “i,” “ki,” or “se”; and block 3 (40–60 seconds), “o,” “ta,” or “ha.” The total the number of correct words represented the subject’s performance score.
Fig 2. Design of the verbal fluency task.
Statistical analysis
The threshold for statistical significance was set at p < 0.05 (two-tailed). Demographic and clinical variables were compared between the study groups using a χ2 test for categorical variables and t-test, Mann–Whitney U tests, or ANOVA (with Tukey’s post hoc test) for continuous variables.
To identify regional differences in VFT-induced frontotemporal hemodynamic responses, mean oxy-Hb changes among the study groups were compared using an ANOVA with a Bonferroni-corrected threshold of p = 0.00096 (i.e., 0.05/52), followed by Tukey’s post hoc test with a threshold of p = 0.05. To examine the relationships between mean oxy-Hb changes and demographic and clinical variables, we calculated Pearson’s correlation coefficients, again with the Bonferroni-corrected threshold of p = 0.00096.
All statistical tests were performed using IBM SPSS Statistics for Mac (version 22.0; IBM Corporation, Armonk, NY, USA).
Results
Demographic and clinical characteristics
Table 1 summarizes the demographic and clinical characteristics of the study groups. There were no significant differences between the three groups in any of the demographic variables. As expected, both MDD patient groups scored higher than HCs on impulsivity, aggression, and hopelessness, whereas no significant differences were observed between the SA and NA groups. In addition, both MDD patient groups showed poorer performance than HCs on VFT. Thus, SAs and NAs had similar clinical symptoms.
Table 1. Demographic, clinical, and neuropsychological characteristics of suicide attempters, non-attempters, and healthy controls.
| Patients with MDD | HCs (n = 40) | Analysis | |||
|---|---|---|---|---|---|
| SAs (n = 30) | NAs (n = 38) | Statistic | p value | ||
| Demographic characteristics | |||||
| Age (years) | 37.6 ± 10.0 | 38.8 ± 9.7 | 38.2 ± 10.5 | F (df = 2,105) = 0.13 | 0.88 |
| Females (n, %) | 22 (73.3) | 22 (57.9) | 25 (62.5) | χ2 = 1.76 | 0.41 |
| Estimated IQ | 104.5 ± 9.0 | 102.1 ± 8.6 | 105.9 ± 9.7 | F (df = 2,105) = 1.72 | 0.18 |
| Duration of illness (years) | 10.2 ± 6.3 | 8.8 ± 7.8 | U = 457.0 | 0.16 | |
| Comorbid axis 1 disorder (n, %) a | 14 (46.7) | 13 (34.2) | χ2 = 1.01 | 0.30 | |
| Clinical variables | |||||
| Depression severity | 14.9 ± 4.0 | 16.0 ± 4.6 | U = 507.5 | 0.44 | |
| Impulsivity | 101.5 ± 19.9 | 97.6 ± 19.4 | 81.8 ± 10.0 | F (df = 2,105) = 14.4 | <0.001b |
| Aggression | 66.0 ± 14.6 | 61.4 ± 9.9 | 51.6 ± 9.8 | F (df = 2,105) = 15.0 | <0.001 b |
| Hopelessness | 14.3 ± 4.4 | 13.6 ± 3.9 | 4.2 ± 2.8 | F (df = 2,105) = 88.6 | <0.001 b |
| Verbal fluency task performance | 12.9 ± 5.5 | 13.2 ± 4.4 | 16.3 ± 5.2 | F (df = 2,105) = 5.06 | 0.008 c |
| Antidepressant dosesd | 90.8 ± 91.9 | 83.1 ± 91.9 | U = 532.5 | 0.64 | |
| Antipsychotic dosese | 19.6 ± 51.7 | 50.4 ± 108.9 | U = 565.5 | 0.95 | |
| Benzodiazepine dosesf | 12.1 ± 17.2 | 8.3 ± 12.1 | U = 519.0 | 0.51 | |
Abbreviations: HCs, healthy controls; MDD, major depressive disorder; NAs, non-attempters; SAs, suicide attempters.
aComorbid anxiety axis 1 disorder included panic disorder, agoraphobia, social phobia, obsessive-compulsive disorder, post-traumatic stress disorder, generalized anxiety disorder, and eating disorder.
bSAs and NAs had higher scores than HCs, but no significant difference was observed between SAs and NAs.
cSAs and NAs had lower scores than HCs, but no significant difference was observed between SAs and NAs.
dAntipsychotic dosages were evaluated using chlorpromazine equivalent dosage.
eAntidepressant dosages were evaluated using imipramine equivalent dosage.
fAnxiolytic dosages were evaluated using diazepam equivalent dosage.
NIRS data
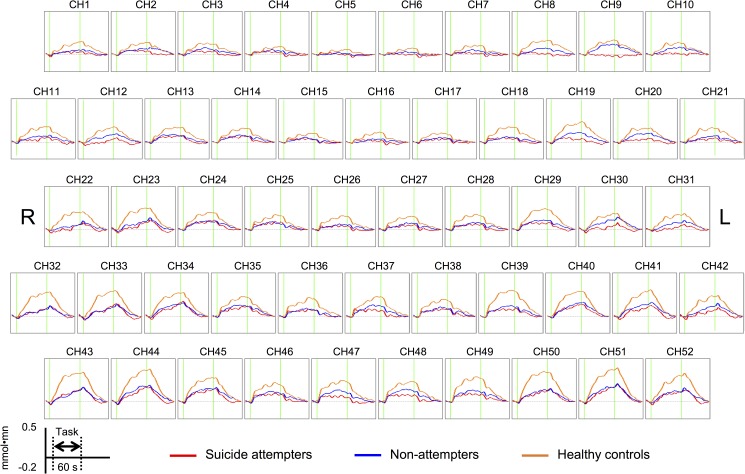
Fig 3 shows the grand-averaged waveforms of changes in the oxy-Hb signal in the SA, NA, and HC groups.
Fig 3. The grand-averaged waveforms of changes in the oxy-Hb signal in suicide attempters, non-attempters, and healthy controls.
Within-group differences
The HC group showed significantly increased mean oxy-Hb levels from the pre-task baseline to the VFT period in 49 channels (ch1–3 and ch7–52, t = 3.98–9.49, Bonferroni-corrected p < 0.00096). The NA group showed significant activations in 29 channels (ch9–11, ch13–14, ch19–20, ch24–25, ch28–30, ch34–41, and ch44–52; t = 3.60–7.06, Bonferroni-corrected p < 0.00096). In contrast, the SA group showed significant activations in 9 channels (ch13, ch24, ch29, ch35, ch38–39, ch45, and ch48–49; t = 3.72–5.46, Bonferroni-corrected p < 0.00096). Thus, VFT induced widespread frontotemporal cortical activation in HCs and NAs, whereas SAs showed significant activation only in the left superior frontal gyrus (ch48), left middle frontal gyrus (ch38 and ch49), bilateral inferior frontal gyrus (right: ch24, ch35, and ch45; left: ch29 and ch39), and right precentral gyrus (ch13).
Between-group differences
Table 2 shows a comparison between the groups of VFT-induced regional hemodynamic responses, with a Bonferroni-corrected significance threshold of p < 0.00096 applied.
Table 2. Comparison of regional hemodynamic responses of suicide attempters, non-attempters, and healthy control subjects threshold of p < 0.00096.
| MINI coordinates | Mean oxy-Hb changes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimated area | R/L | NIRS channel | x | y | z | SAs | NAs | HCs | F value | Post hoc |
| Middle frontal gyrus | L | 8 | −46 | 23 | 44 | 0.013 ± 0.068 | 0.039 ± 0.077 | 0.113 ± 0.118 | 11.2 | SA < HC, NA < HC |
| L | 18 | −42 | 42 | 32 | 0.038 ± 0.072 | 0.038 ± 0.067 | 0.125 ± 0.111 | 12.3 | SA < HC, NA < HC | |
| L | 19 | −55 | 18 | 31 | 0.035 ± 0.067 | 0.072 ± 0.066 | 0.160 ± 0.113 | 19.3 | SA < HC, NA < HC | |
| L | 28 | −35 | 58 | 20 | 0.046 ± 0.072 | 0.053 ± 0.081 | 0.126 ± 0.127 | 7.5 | SA < HC, NA < HC | |
| L | 49 | −35 | 63 | −4 | 0.072 ± 0.071 | 0.097 ± 0.109 | 0.194 ± 0.143 | 11.4 | SA < HC, NA < HC | |
| Inferior frontal gyrus | R | 34 | 59 | 27 | 8 | 0.065 ± 0.096 | 0.092 ± 0.104 | 0.198 ± 0.128 | 13.5 | SA < HC, NA < HC |
| R | 45 | 53 | 43 | −6 | 0.093 ± 0.100 | 0.108 ± 0.094 | 0.194 ± 0.148 | 7.7 | SA < HC, NA < HC | |
| L | 29 | −52 | 36 | 19 | 0.065 ± 0.083 | 0.076 ± 0.070 | 0.172 ± 0.121 | 13.7 | SA < HC, NA < HC | |
| L | 39 | −44 | 53 | 6 | 0.064 ± 0.075 | 0.084 ± 0.086 | 0.217 ± 0.144 | 21.2 | SA < HC, NA < HC | |
| L | 40 | −57 | 28 | 7 | 0.076 ± 0.113 | 0.091 ± 0.096 | 0.202 ± 0.164 | 9.2 | SA < HC, NA < HC | |
| L | 50 | −51 | 45 | −6 | 0.066 ± 0.132 | 0.088 ± 0.094 | 0.247 ± 0.174 | 18.0 | SA < HC, NA < HC | |
| Precentral gyrus | R | 23 | 64 | 8 | 20 | 0.025 ± 0.120 | 0.049 ± 0.109 | 0.171 ± 0.136 | 12.2 | SA < HC, NA < HC |
| L | 9 | −57 | −1 | 43 | −0.011 ± 0.094 | 0.073 ± 0.087 | 0.107 ± 0.116 | 11.4 | SA < NA, SA < HC | |
| L | 30 | −62 | 10 | 20 | 0.008 ± 0.101 | 0.063 ± 0.073 | 0.119 ± 0.139 | 7.8 | SA < HC | |
| Postcentral gyrus | R | 12 | 66 | −10 | 31 | −0.004 ± 0.070 | 0.047 ± 0.084 | 0.102 ± 0.112 | 11.1 | SA < HC, NA < HC |
| L | 20 | −64 | −8 | 31 | 0.019 ± 0.072 | 0.063 ± 0.101 | 0.113 ± 0.110 | 7.5 | SA < HC | |
| L | 31 | −67 | −17 | 19 | 0.005 ± 0.087 | 0.044 ± 0.099 | 0.111 ± 0.137 | 7.8 | SA < HC, NA < HC | |
| Middle temporal gyrus | R | 32 | 71 | −29 | 2 | 0.035 ± 0.094 | 0.040 ± 0.098 | 0.176 ± 0.189 | 12.1 | SA < HC, NA < HC |
| R | 43 | 69 | −13 | −10 | 0.070 ± 0.139 | 0.61 ± 0.107 | 0.234 ± 0.193 | 14.5 | SA < HC, NA < HC | |
| Superior temporal gyrus | R | 22 | 68 | −19 | 18 | 0.035 ± 0.094 | 0.040 ± 0.098 | 0.176 ± 0.189 | 12.3 | SA < HC, NA < HC |
| R | 33 | 66 | −4 | 5 | 0.056 ± 0.126 | 0.049 ± 0.112 | 0.192 ± 0.178 | 10.8 | SA < HC, NA < HC | |
| R | 44 | 60 | 11 | −8 | 0.075 ± 0.127 | 0.109 ± 0.104 | 0.249 ± 0.193 | 13.4 | SA < HC, NA < HC | |
| L | 51 | −57 | 14 | −8 | 0.074 ± 0.177 | 0.111 ± 0.139 | 0.239 ± 0.216 | 7.6 | SA < HC, NA < HC | |
Abbreviations: HCs, healthy controls; MDD, major depressive disorder; NAs, non-attempters; SAs, suicide attempters.
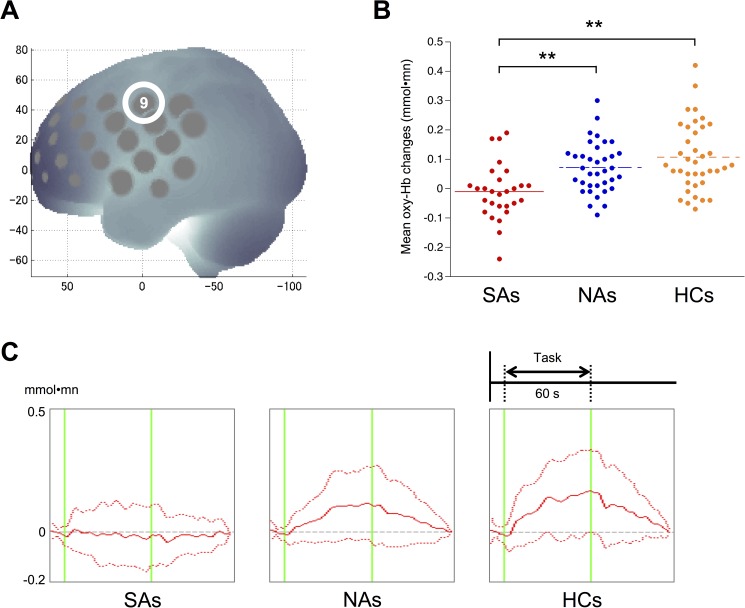
Compared with the HC group, both MDD patient groups exhibited significantly small mean oxy-Hb changes in the left middle frontal, bilateral inferior frontal, right precentral, bilateral postcentral, right middle temporal, and bilateral superior temporal gyri. Post hoc analyses revealed that the SA group exhibited a smaller hemodynamic response than the NA (p = 0.0038) and HC (p < 0.0000) groups in the left precentral gyrus (ch9; Fig 4A and 4B), whereas no difference was observed between the NA and HC groups (p = 0.31). In this region, patterns in the mean oxy-Hb signals differentiated the SA group from the NA and HC groups (Fig 4C): the time course of changes in the oxy-Hb signal gradually increased during the task and gradually decreased after it ended in the NAs and HCs, whereas SAs did not show this response.
Fig 4. The differential time course of the oxy-Hb signal in the left precentral gyrus in suicide attempters, non-attempters, and healthy controls.
(A) The anatomical site corresponding to channel 9 (left precentral gyrus). (B) Dot plots of mean oxy-Hb changes of the left precentral gyrus in SAs, NAs, and HCs. (C) The differential time course of oxy-Hb signal in SAs, NAs, and HCs in the left precentral gyrus. In NAs and HCs, the time course of changes in the oxy-Hb signal showed a gradual increase followed by a gradual decrease after the end of the task; SAs did not exhibit this response. The dashed red lines indicate standard deviations for each group. Abbreviations: HCs, healthy controls; NAs, non-attempters; SAs, suicide attempters **p < 0.01
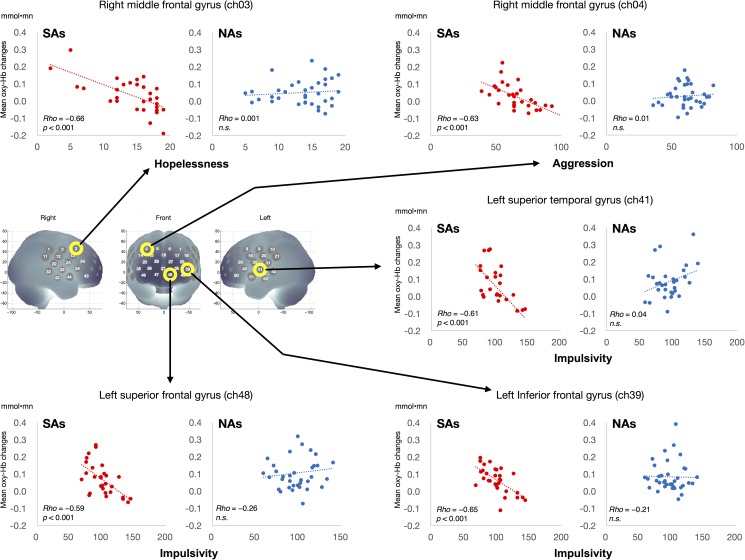
Correlation between NIRS data and symptoms
Significant negative correlations were found in SA group between impulsivity and the hemodynamic responses induced by VFT in the left inferior frontal gyrus (ch39; r = −0.64, p = 0.00013; Fig 5), the left superior frontal gyrus (ch48; r = −0.59, p = 0.00078), and the left superior temporal gyrus (ch41; r = −0.61, p = 0.00095). In addition, in this group there was a significant negative correlation between aggression and the hemodynamic response in the right middle frontal gyrus (ch4; r = −0.63, p = 0.00017) and between hopelessness and the response in the right middle frontal gyrus (ch3; r = −0.66, p = 0.00007). In contrast, the NA group showed no statistically significant correlations between any demographic or clinical variable and the hemodynamic responses.
Fig 5. Correlation between hemodynamic responses and impulsivity, aggression, and hopelessness in suicide attempters and non-attempters.
The reduced response in the left inferior frontal gyrus was negatively correlated with impulsivity level and the responses in the right middle frontal gyrus were negatively associated with hopelessness and aggression in suicide attempters (SAs) but not in non-attempters (NAs).
Furthermore, we did not find any significant correlations between the hemodynamic responses and doses of antidepressants in either MDD patient groups. In addition, only the HC group showed a significant positive correlation between VFT task performance and the hemodynamic responses in the left superior temporal gyrus (ch41; r = 0.57, p = 0.00015).
Discussion
This, to the best of our knowledge, is the first NIRS study to explore the distinct functional abnormalities of the left precentral gyrus in patients with MDD with a history of suicidal behavior. We observed a reduction in the VFT-induced hemodynamic responses in the bilateral frontotemporal regions in both MDD patient groups relative to the responses of HCs, with SAs displaying smaller hemodynamic responses in the left precentral gyrus than NAs and HCs. In this region, SAs exhibited virtually no activation from the pre-task baseline through the VFT period, in contrast to HCs and NAs. Our findings suggest that MDD patients with a history of suicide attempts demonstrate patterns of NIRS signal changes in the left precentral gyrus different from those demonstrated by individuals without a history of suicidal behaviors, even in cases where clinical symptoms are similar. NIRS has a relatively high time resolution, which may help visually differentiate SAs from NAs.
Recently, several neuroimaging studies have suggested the importance of the left precentral gyrus in the neuropathological processes underlying MDD. Left precentral gyrus thickening has been observed in healthy individuals with negative cognitive styles [43] and in patients with MDD [44]. One prospective longitudinal study has reported that progressive cortical thinning in the left precentral gyrus is associated with an increased risk for mood disorders [45]. The precentral gyrus may be of particular relevance for the etiology of suicide because of its potential role in impulsivity regulation. This region is involved in the executive functioning of response inhibition [46, 47] and is associated with other motor areas, such as the pre-supplementary motor area, which is crucial for inhibitory control [48–50]. Deficits in inhibitory control are associated with a greater propensity to act on suicidal or aggressive feelings [3]. This suggests that an important threshold for conversion to overt suicidal behavior may be functional abnormalities in the precentral region associated with impulsivity control. Furthermore, previous studies support the possibility that precentral cortical abnormalities are linked to vulnerability for suicide risk in patients with MDD [51, 52]. These lines of evidence support our results that NAs show the lack of an abnormal response. Our study suggests that the deficit in hemodynamic response measured using NIRS in the left precentral gyrus may play a distinct role in the vulnerability for suicide risk in patients with MDD.
We have reported a peculiar dysfunction of the left precentral gyrus in SAs; however, the choice of VFT as the cognitive task is debatable because thus far, a relationship between verbal fluency and suicidal behavior has not been confirmed. Recent NIRS studies have reported that social adaptation level, which might influence the risk of suicidal behavior [53], is associated with the dorsolateral prefrontal function [54], and the bilateral ventrolateral prefrontal function, and the anterior part of the temporal function [55]. However, the validity of NIRS as a measure of the vulnerability for suicide risk is supported by the associations that we observed between VFT-induced hemodynamic responses and diathesis for suicidal behavior (impulsivity, aggression, and hopelessness). We found significant negative correlations between aggression and hopelessness levels and mean oxy-Hb change in the right middle frontal gyrus. This region includes the dorsolateral prefrontal area, which also plays a critical role in the development of suicidal behavior [16, 17]. A previous investigation using NIRS for MDD also reported that the severity of suicidal ideation was associated with hemodynamic responses in dorsolateral prefrontal region [23]. In our study, SAs showed significant correlation between the severity of suicide ideation and hemodynamic responses in the right dorsolateral prefrontal region (ch45; r = −0.39, p = 0.037; uncorrected), but NAs did not show such correlation. Our results suggest that the relationship of levels of aggression and hopelessness with VFT-induced hemodynamic responses in the right dorsolateral prefrontal cortex in SAs is different from that in NAs and HCs; this region, in particular, may be related to the distinct pathophysiology of suicidal behavior in patients with MDD.
Furthermore, we demonstrated significant negative correlations between impulsivity level and hemodynamic responses in the left inferior frontal (including the dorsolateral prefrontal region), left superior frontal (including the orbitofrontal region), and left superior temporal gyri in SAs. Previous studies have reported that abnormalities in these regions are associated with suicidal behaviors in patients with MDD [11] and in those with schizophrenia [56]. Furthermore, a PET study concluded that the functional abnormality of the frontal cortex in SAs is related to the degree of impulsivity [14]. Neuroimaging studies have shown that impaired impulse control is associated with a whole-brain binding potential of the serotonin transporter in SAs [57, 58]. Thus, our results are supported by there being a regional serotonin deficiency in depressed SAs because a deficiency in serotonin input to the prefrontal cortex contributes to impulsive traits associated with a greater risk for suicidal behavior.
Limitations
There were several methodological limitations in our study. First, we neither assessed the suicidality of the subjects using a validated instrument nor stratified the group of patients by suicidal behavior (e.g., according to violent, highly lethal, or repeated attempts). We were unable to obtain collateral verification of the history of suicidal behavior from a family member for all subjects. Second, most participants with MDD were taking psychotropic medications, even on the day of the scan. Although, thus far, there have been no reports of clear evidence of the effects of medication on NIRS signals, a recent longitudinal NIRS study reported no differences in VFT-induced oxy-Hb changes between pre- and post-antidepressant treatment time points in a MDD cohort, despite significant improvements in symptoms of depression [22]. Third, we reported that one channel (ch9) showed a significant difference between the SA, NA, and HC groups. However, our study sample size was relatively small, and it might not be able to insist on the role of difference between SAs and NAs. If the sample size is increased, the difference between NAs and HCs in the left precentral gyrus might also become significant, which means the possibility of a type II error. Fourth, although the socioeconomic background could affect our results, we did not assess the subjects’ or parental socioeconomic status using a validated instrument. Finally, we did not formally evaluate personality traits or differences between males and females, and it will be important in future studies to examine these issues.
Conclusions
Our study demonstrated that patients with MDD and a history of suicide attempts exhibit patterns of NIRS signal changes in the left precentral gyrus that are different from those exhibited by individuals without a history of suicidal behavior and by healthy control subjects. NIRS has a relatively high time resolution, which may help visually differentiate depressed suicide attempters from non-attempters. Our results also suggest that the observed VFT-induced hemodynamic responses in frontotemporal regions are associated with the diathesis for suicidal behavior. Thus, we found unique pathophysiological features of suicidality in patients with MDD. Compared with other neuroimaging tools, the ease of applicability of NIRS may be particularly advantageous in longitudinal follow-ups that require repeated measurements to predict future suicidal behaviors in patients with MDD. Further NIRS studies of MDD are warranted. If confirmed, our findings may define a novel biomarker for suicide risk in MDD.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was partly supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 25461792, No. 25380965, No. 16K10229).
References
- 1.Hawton K, van Heeringen K. Suicide. Lancet. 2009;373(9672):1372–81. Epub 2009/04/21. 10.1016/S0140-6736(09)60372-X [DOI] [PubMed] [Google Scholar]
- 2.Hawton K, Casanas ICC, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147(1–3):17–28. 10.1016/j.jad.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 3.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4(10):819–28. 10.1038/nrn1220 [DOI] [PubMed] [Google Scholar]
- 4.Mann JJ. The serotonergic system in mood disorders and suicidal behaviour. Philos Trans R Soc Lond B Biol Sci. 2013;368(1615):20120537 10.1098/rstb.2012.0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann JJ. What does brain imaging tell us about the predisposition to suicidal behavior. Crisis. 2005;26(3):101–3. Epub 2005/11/10. 10.1027/0227-5910.26.3.101 [DOI] [PubMed] [Google Scholar]
- 6.van Heeringen K. Brain imaging: healthy networks for suicide prevention. Crisis. 2014;35(1):1–4. 10.1027/0227-5910/a000213 [DOI] [PubMed] [Google Scholar]
- 7.Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlosser RG. Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J Psychiatr Res. 2012;46(11):1449–55. 10.1016/j.jpsychires.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 8.Ding Y, Lawrence N, Olie E, Cyprien F, le Bars E, Bonafe A, et al. Prefrontal cortex markers of suicidal vulnerability in mood disorders: a model-based structural neuroimaging study with a translational perspective. Transl Psychiatry. 2015;5:e516 10.1038/tp.2015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jollant F, Lawrence NS, Giampietro V, Brammer MJ, Fullana MA, Drapier D, et al. Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. Am J Psychiatry. 2008;165(6):740–8. Epub 2008/03/19. 10.1176/appi.ajp.2008.07081239 [DOI] [PubMed] [Google Scholar]
- 10.Reisch T, Seifritz E, Esposito F, Wiest R, Valach L, Michel K. An fMRI study on mental pain and suicidal behavior. J Affect Disord. 2010;126(1–2):321–5. 10.1016/j.jad.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 11.Audenaert K, Goethals I, Van Laere K, Lahorte P, Brans B, Versijpt J, et al. SPECT neuropsychological activation procedure with the Verbal Fluency Test in attempted suicide patients. Nucl Med Commun. 2002;23(9):907–16. [DOI] [PubMed] [Google Scholar]
- 12.Willeumier K, Taylor DV, Amen DG. Decreased cerebral blood flow in the limbic and prefrontal cortex using SPECT imaging in a cohort of completed suicides. Transl Psychiatry. 2011;1:e28 10.1038/tp.2011.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aman MG, McDougle CJ, Scahill L, Handen B, Arnold LE, Johnson C, et al. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1143–54. 10.1097/CHI.0b013e3181bfd669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oquendo MA, Placidi GP, Malone KM, Campbell C, Keilp J, Brodsky B, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry. 2003;60(1):14–22. Epub 2003/01/07. [DOI] [PubMed] [Google Scholar]
- 15.van Heeringen K, Wu GR, Vervaet M, Vanderhasselt MA, Baeken C. Decreased resting state metabolic activity in frontopolar and parietal brain regions is associated with suicide plans in depressed individuals. J Psychiatr Res. 2017;84:243–8. 10.1016/j.jpsychires.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 16.van Heeringen C, Bijttebier S, Godfrin K. Suicidal brains: a review of functional and structural brain studies in association with suicidal behaviour. Neurosci Biobehav Rev. 2011;35(3):688–98. 10.1016/j.neubiorev.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 17.Jollant F, Lawrence NL, Olie E, Guillaume S, Courtet P. The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World J Biol Psychiatry. 2011;12(5):319–39. 10.3109/15622975.2011.556200 [DOI] [PubMed] [Google Scholar]
- 18.Ehlis AC, Schneider S, Dresler T, Fallgatter AJ. Application of functional near-infrared spectroscopy in psychiatry. Neuroimage. 2014;85 Pt 1:478–88. [DOI] [PubMed] [Google Scholar]
- 19.Kameyama M, Fukuda M, Yamagishi Y, Sato T, Uehara T, Ito M, et al. Frontal lobe function in bipolar disorder: a multichannel near-infrared spectroscopy study. Neuroimage. 2006;29(1):172–84. 10.1016/j.neuroimage.2005.07.025 [DOI] [PubMed] [Google Scholar]
- 20.Noda T, Yoshida S, Matsuda T, Okamoto N, Sakamoto K, Koseki S, et al. Frontal and right temporal activations correlate negatively with depression severity during verbal fluency task: a multi-channel near-infrared spectroscopy study. J Psychiatr Res. 2012;46(7):905–12. 10.1016/j.jpsychires.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 21.Suto T, Fukuda M, Ito M, Uehara T, Mikuni M. Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study. Biol Psychiatry. 2004;55(5):501–11. 10.1016/j.biopsych.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 22.Tomioka H, Yamagata B, Kawasaki S, Pu S, Iwanami A, Hirano J, et al. A longitudinal functional neuroimaging study in medication-naive depression after antidepressant treatment. PLoS One. 2015;10(3):e0120828 10.1371/journal.pone.0120828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pu S, Nakagome K, Yamada T, Yokoyama K, Matsumura H, Yamada S, et al. Suicidal ideation is associated with reduced prefrontal activation during a verbal fluency task in patients with major depressive disorder. J Affect Disord. 2015;181:9–17. 10.1016/j.jad.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 24.Takizawa R, Fukuda M, Kawasaki S, Kasai K, Mimura M, Pu S, et al. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage. 2014;85 Pt 1:498–507. [DOI] [PubMed] [Google Scholar]
- 25.Bartfai A, Winborg IM, Nordstrom P, Asberg M. Suicidal behavior and cognitive flexibility: design and verbal fluency after attempted suicide. Suicide Life Threat Behav. 1990;20(3):254–66. [PubMed] [Google Scholar]
- 26.Richard-Devantoy S, Berlim MT, Jollant F. A meta-analysis of neuropsychological markers of vulnerability to suicidal behavior in mood disorders. Psychol Med. 2014;44(8):1663–73. 10.1017/S0033291713002304 [DOI] [PubMed] [Google Scholar]
- 27.Otsubo T, Tanaka K, Koda R, Shinoda J, Sano N, Tanaka S, et al. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci. 2005;59(5):517–26. 10.1111/j.1440-1819.2005.01408.x [DOI] [PubMed] [Google Scholar]
- 28.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. Epub 1971/03/01. [DOI] [PubMed] [Google Scholar]
- 29.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45(8):742–7. [DOI] [PubMed] [Google Scholar]
- 30.Matsuoka K, Uno M, Kasai K, Koyama K, Kim Y. Estimation of premorbid IQ in individuals with Alzheimer's disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin Neurosci. 2006;60(3):332–9. 10.1111/j.1440-1819.2006.01510.x [DOI] [PubMed] [Google Scholar]
- 31.Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69(8):440–7. 10.1111/pcn.12275 [DOI] [PubMed] [Google Scholar]
- 32.Kobashi M, Ida M. Making the Revised Version of Barratt Impulsiveness Scale 11th in Japanese: A Study on Reliability and Validity. The Journal of Psychology Rissho University 2013;4:53–61. [Google Scholar]
- 33.Ando A, Soga S, Yamasaki K, Shimai S, Shimada H, Utsuki N, et al. [Development of the Japanese version of the Buss-Perry Aggression Questionnaire (BAQ)]. Shinrigaku Kenkyu. 1999;70(5):384–92. [DOI] [PubMed] [Google Scholar]
- 34.Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol. 1974;42(6):861–5. [DOI] [PubMed] [Google Scholar]
- 35.Tsujii N, Mikawa W, Tsujimoto E, Akashi H, Adachi T, Kirime E, et al. Relationship between prefrontal hemodynamic responses and quality of life differs between melancholia and non-melancholic depression. Psychiatry Res. 2016. Epub 2016/06/05. [DOI] [PubMed] [Google Scholar]
- 36.Cope M, Delpy DT, Reynolds EO, Wray S, Wyatt J, van der Zee P. Methods of quantitating cerebral near infrared spectroscopy data. Adv Exp Med Biol. 1988;222:183–9. [DOI] [PubMed] [Google Scholar]
- 37.Takizawa R, Kasai K, Kawakubo Y, Marumo K, Kawasaki S, Yamasue H, et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr Res. 2008;99(1–3):250–62. 10.1016/j.schres.2007.10.025 [DOI] [PubMed] [Google Scholar]
- 38.Singh AK, Okamoto M, Dan H, Jurcak V, Dan I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage. 2005;27(4):842–51. 10.1016/j.neuroimage.2005.05.019 [DOI] [PubMed] [Google Scholar]
- 39.Tsuzuki D, Jurcak V, Singh AK, Okamoto M, Watanabe E, Dan I. Virtual spatial registration of stand-alone fNIRS data to MNI space. Neuroimage. 2007;34(4):1506–18. 10.1016/j.neuroimage.2006.10.043 [DOI] [PubMed] [Google Scholar]
- 40.Shattuck DW, Mirza M, Adisetiyo V, Hojatkashani C, Salamon G, Narr KL, et al. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage. 2008;39(3):1064–80. 10.1016/j.neuroimage.2007.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strangman G, Boas DA, Sutton JP. Non-invasive neuroimaging using near-infrared light. Biol Psychiatry. 2002;52(7):679–93. [DOI] [PubMed] [Google Scholar]
- 42.Herrmann MJ, Ehlis AC, Fallgatter AJ. Frontal activation during a verbal-fluency task as measured by near-infrared spectroscopy. Brain Res Bull. 2003;61(1):51–6. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Yao S, Zhu X, Wang X, Zhu X, Zhong M. Gray matter volume abnormalities in individuals with cognitive vulnerability to depression: a voxel-based morphometry study. J Affect Disord. 2012;136(3):443–52. 10.1016/j.jad.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 44.Peng D, Shi F, Li G, Fralick D, Shen T, Qiu M, et al. Surface vulnerability of cerebral cortex to major depressive disorder. PLoS One. 2015;10(3):e0120704 Epub 2015/03/21. 10.1371/journal.pone.0120704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papmeyer M, Giles S, Sussmann JE, Kielty S, Stewart T, Lawrie SM, et al. Cortical Thickness in Individuals at High Familial Risk of Mood Disorders as They Develop Major Depressive Disorder. Biol Psychiatry. 2015;78(1):58–66. Epub 2014/12/24. 10.1016/j.biopsych.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 46.Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: an interference task specialized for functional neuroimaging—validation study with functional MRI. Hum Brain Mapp. 1998;6(4):270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154(12):1670–5. Epub 1997/12/16. 10.1176/ajp.154.12.1670 [DOI] [PubMed] [Google Scholar]
- 48.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 49.Jha A, Nachev P, Barnes G, Husain M, Brown P, Litvak V. The Frontal Control of Stopping. Cereb Cortex. 2015;25(11):4392–406. 10.1093/cercor/bhv027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9(11):856–69. 10.1038/nrn2478 [DOI] [PubMed] [Google Scholar]
- 51.Hwang JP, Lee TW, Tsai SJ, Chen TJ, Yang CH, Lirng JF, et al. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J Geriatr Psychiatry Neurol. 2010;23(3):171–84. 10.1177/0891988710363713 [DOI] [PubMed] [Google Scholar]
- 52.Sublette ME, Milak MS, Galfalvy HC, Oquendo MA, Malone KM, Mann JJ. Regional brain glucose uptake distinguishes suicide attempters from non-attempters in major depression. Arch Suicide Res. 2013;17(4):434–47. 10.1080/13811118.2013.801813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turecki G, Brent DA. Suicide and suicidal behaviour. The Lancet. 2016;387(10024):1227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pu S, Nakagome K, Yamada T, Yokoyama K, Matsumura H, Terachi S, et al. Relationship between prefrontal function during a cognitive task and social functioning in male Japanese workers: a multi-channel near-infrared spectroscopy study. Psychiatry Res. 2013;214(1):73–9. 10.1016/j.pscychresns.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 55.Ohtani T, Nishimura Y, Takahashi K, Ikeda-Sugita R, Okada N, Okazaki Y. Association between longitudinal changes in prefrontal hemodynamic responses and social adaptation in patients with bipolar disorder and major depressive disorder. J Affect Disord. 2015;176:78–86. 10.1016/j.jad.2015.01.042 [DOI] [PubMed] [Google Scholar]
- 56.Aguilar EJ, Garcia-Marti G, Marti-Bonmati L, Lull JJ, Moratal D, Escarti MJ, et al. Left orbitofrontal and superior temporal gyrus structural changes associated to suicidal behavior in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1673–6. 10.1016/j.pnpbp.2008.06.016 [DOI] [PubMed] [Google Scholar]
- 57.Lindstrom MB, Ryding E, Bosson P, Ahnlide JA, Rosen I, Traskman-Bendz L. Impulsivity related to brain serotonin transporter binding capacity in suicide attempters. Eur Neuropsychopharmacol. 2004;14(4):295–300. 10.1016/j.euroneuro.2003.11.001 [DOI] [PubMed] [Google Scholar]
- 58.Ryding E, Ahnlide JA, Lindstrom M, Rosen I, Traskman-Bendz L. Regional brain serotonin and dopamine transporter binding capacity in suicide attempters relate to impulsiveness and mental energy. Psychiatry Res. 2006;148(2–3):195–203. Epub 2006/11/07. 10.1016/j.pscychresns.2006.06.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.