Abstract
The fate and transport of Escherichia coli (E. coli) in lotic waters through vegetated filter strips (VFSs) was evaluated in a field model pasture, utilizing VFSMOD Windows along with direct pathogen testing. This study assessed effects of VFS on transport and deposition rates of E. coli in lotic overland flow waters. The VFS measured 44 m long by 40 m wide, covering an area of 1584 m2 and land slope of 15 %. Cowpat was applied onto the model pasture and washed by overland flow into the VFS. The 4-methylumbelliferyl β-D-glucuronide substrate confirmed the identity of E. coli prior to cowpat application and after isolating them from soil using centrifugation and membrane filtration techniques. Napier grass root system recorded the highest recovery rates of E. coli at 99.9 % along the length of VFS III. This efficiency reduced significantly (p < 0.05; df = 29) to 95 % in Kikuyu grass and 75 % in Couch grass–Buffer grass. The data demonstrated similarity in transport of manure-borne E. coli and organic carbon (OC) through all the simulated VFS. These results indicated that OC could be used as a true natural tracer of manure-borne E. coli, a pollution indicator organism of lentic and lotic surface waters provided the OC release kinetics from cowpat were similar to that of E. coli kinetics. Thus, efficient filtering to reduce E. coli concentrations and load in overland flows requires managing combined grass species, agro-pastoral systems models and dispersed or preferential flows to enhance surface water quality standards.
Keywords: Mass balance, Overland flow rate, Escherichia coli, Lotic surface water, Fate and transport, VFS, Micro-biome
1 Introduction
Manure-borne pathogens transported into lentic and lotic surface waters can cause both devastating waterborne disease outbreaks (George and Manges 2010), as well as endemic gastrointestinal disease and chronic illness for vulnerable communities dependent upon these surface waters (Russo and Johnson 2003), yet identification and monitoring of these enteric pathogens from water samples remain a challenge (Guber et al. 2006). Traditional fecal indicators have not been sufficient to predict the presence of pathogenic organisms in agricultural-related waters (Pachepsky et al. 2015), and livestock management continues to face the major challenge of promoting animal health, production and welfare, while complying with water quality regulations in order to safeguard human health, animal health and environmental resources (Berry and Wells 2010; Ferens et al. 2011; LeJeune and Wetzel 2007; Lewis et al. 2009). Overland flow influences the transport of microbial contaminants into lentic (reservoirs, lakes and others) and lotic (streams, rivers and others) water bodies (Tyrrel and Quinton 2003; Tate et al. 2006; Muñoz-Carpena and Parsons 2011). The introduction of VFS has proven to be an excellent best management practice (BMP) to reduce and remove sediment and nutrient loading and shows similar promise for mitigation of microbial contamination and anthropogenic contaminants from overland flow of farmland and livestock facilities (Abu-Zreig et al. 2003; Russo and Johnson 2003; Guber et al. 2006, 2009, 2011; Berry and Wells 2010; Cardoso et al. 2012; Lafrance and Caron 2012; Larson and Safferman 2012; Pachepsky et al. 2015).
In the implementation of VFS to address nutrient and sediment loading, hydraulic roughness has been associated with decreased flow volume and reduced peak velocity of overland flow (Fiener and Auerswald 2003; Duchemin and Hogue 2008). As VFS can have variable efficacy in removing point and non-point source pollution (Duchemin and Hogue 2008; Davis et al. 2009), it is critically important to evaluate site-specific and local environmental parameters through validated models and carefully described field site parameters. As VFS is more critically evaluated with respect to microbial contamination and anthropogenic contaminants, such as pesticides and fungicides, overland flow models initially developed to determine nutrient and sediment deposition into relevant surface water bodies can also provide insight into soil hydraulics, overland flow transport and microbial and soluble compound deposition profiles (Martinez et al. 2013; Allaire et al. 2015; Rippy 2015). Site-specific parameters influencing VFS implementation and efficacy include soil hydraulic properties such as capillary rise, soil porosity, sediment type and concentration, vegetation type, slope and filter strip length among others (Tollner et al. 1976, 1977). During steady-state rainfall, the continuity equation and steady-state infiltration capacity of overland flow decrease linearly from upstream to downstream in the VFS (Tollner et al. 1976, 1977); thus, it becomes extremely important to define these parameters in both wet and dry seasons.
The VFS model for window (VFSMOD W) was first designed and developed to improve the understanding of soil hydraulics, sediment and overland flow transport and delivery through VFS (Muñoz-Carpena and Parsons 2011). It is a combined model system with numerical submodels, which are capable of describing overland flow and infiltration with developed algorithm for the suspended solids infiltration by grass. It was developed as an improvement over GRASSF and SEDIMOT II models (Muñoz-Carpena and Parsons 1999) because unlike these models the following were introduced: detailed description of flow through VFS; sediment deposition resulting from changes in overland flow; infiltration of soil water, which is physically time dependent; manipulates series of complex rainfall patterns, intensity and amount and handles varying surface conditions along the length of VFS. This model for the study of hydrology and sediment transport through VFS may prove useful in understanding the fate and transport of microorganisms, given its critical inclusion of various site parameters. Despite our knowledge of the interaction between sediments, nitrates, phosphates and pesticides in watercourses (Tyrrel and Quinton 2003; Pachepsky et al. 2006), the deposition, transport and fate of the microbial organisms (viral, bacterial, protozoan and helminthic) in overland flows are least understood (Schijven et al. 2004; Tyrrel and Quinton 2003; Ling et al. 2009). Previous reports have also indicated major fluctuations in filter strips microorganisms trapping efficiencies (Guber et al. 2009). While utilizing manure-borne phosphorus transport has provided a means to model pathogen field transport given the broad availability of nutrient data and transport phenomena (Pachepsky et al. 2006), recent work testing these models with average fecal coliform concentrations in applied manure and on the percentage of fecal coliforms reaching the edge of the field plot indicates the models overestimate the number of contaminants reaching the field plots (Guber et al. 2011; Martinez et al. 2013; Allaire et al. 2015).
The limited available data on manure-borne contaminants continue to imply that existing phosphorus transport models fail to predict manure-borne E. coli transport and deposition into surface water bodies (Kruger et al. 2007; Guber et al. 2011; Hurley and Forman 2011; Fletcher et al. 2013; Liu and Davis 2014; Tian et al. 2014; Lucas and Greenway 2015). As a result, understanding of transport phenomena for manure-borne contaminants must be enhanced, while simultaneously searching for methods by which to reduce loading of contaminants into surface water bodies. Recently, the Web GIS-based VFSMOD system has been developed to critically evaluate vegetative filter strip design for reduction in sediment and nutrients into riverine systems (Park et al. 2013). Preliminary data have suggested that the agro-hydrologic model soil and water assessment tool (SWAT) may accurately predict hourly variations of fecal contamination into riverine systems with E coli contamination being more directly related to rainfall than re-suspension methods in one small rural catchment (Bougeard et al. 2011).
To our knowledge, our study is the first of its kind to evaluate the potential application of VFSMOD W to evaluate fecal pathogen removal from an agricultural plot area impacted by manure-borne pathogen and nutrient loading and to further critically comparative contribution of native grasses to fecal pathogen removal within this field pasture model system. As the majority of field studies and data collected relative to manure-borne pathogen loading into these systems utilizes E coli as fecal indicators and rapid diagnostic tests are constantly emerging to obtain cost-effective and time-sensitive measurements of E coli contamination (Russo and Johnson 2003), we aimed to measure concentrations of E. coli (CFU 100 mL−1) as well as to define mechanisms for filtering of E. coli in overland flow by the surface vegetation, different grass species, plant morphology, root systems and subsoil horizon along the length of established VFS. However, E. coli along the length of VFS root systems and subsoil horizons tend to exist at higher background concentrations than overland flow, introducing a potential confounding factor (Pachepsky et al. 2006; Martinez et al. 2013; Allaire et al. 2015), while the data are severely limited for other manure-borne pathogens in the mass balance equations (Pachepsky et al. 2006; Akan 2013; Mohanty et al. 2013; Allaire et al. 2015). Concurrent measurements of hydrologic parameters and microbial indicator organisms’ concentrations have hardly been made despite hydrologic pathways driving the fate and transport of those microorganisms (Pachepsky et al. 2006). Since the existence of fate and transport models for microorganisms is extremely limited in the field agricultural plot setting, it is absolutely critical for investigators to continue to collect both hydrologic site-specific parameters along with microbial indicator organism concentrations to test these models locally (Pachepsky et al. 2006).
The overarching aim of this study was to understand the transport and fate of E. coli in overland flow in the eastern escarpments of the Mau Forest, Njoro River Watershed, Kenya, and filtering efficiency of different native grass species, grass morphology, root zone systems and subsoil horizon zone in this local environment. Locally, the TAP livestock and crop research area and demonstration facility located adjacent to Njoro River can help estimate regional pathogens loading from cowpat into the Njoro River, eventually reaching and impacting Lake Nakuru with its diverse biota populations. Globally, this study contributes significantly to understanding the transport dynamics and deposition of E coli indicator organisms released from manure from cattle pens or open range lands and contributing to non-point source contamination of surface water through overland flow systems pollution. In particular, this study has focused on characterizing the transport and deposition rates of E. coli in overland flows through VFS.
2 Materials and methods
2.1 Description of study site
The study site was located at field 18 of Tatton Agriculture Park (TAP) situated at 2297 m above sea level and draining into Lake Nakuru, 1754 m above sea level. TAP consists of livestock and crops research and demonstration units or facilities adjacent to and bordering the Njoro River, located 25 km from Lake Nakuru and 172 km west of Nairobi in the East African Rift Valley, Njoro campus, Egerton University, Kenya (Fig. 1). Topographically, the TAP area land slopes range from 5 to 45 %. It is located in the eastern escarpments of Mau Ranges and slightly above Njoro River and the shrubby vegetation that surrounds Lake Nakuru, Lake Baringo and Lake Victoria. The region and site experienced a short rainy season (September to December 2013; and September to December 2014), a dry season (January–March 2014) and wet rainy season (April–August 2014). This was over 16 months and spanned two short rainy seasons. In 2014, the mean annual precipitation was 935.65 mm, with over 60 % falling in April through August. The air temperatures ranged from a minimum of 17.6 °C to a maximum of 22.5 °C, with a mean temperature of 20.5 °C. Solar radiation ranged from a minimum of 500 to a maximum of 650 calorie cm−2 day−1, with a mean irradiation of 585 calorie cm−2 day−1. Evaporation ranged from 3.2 to 5.6 mm day−1, with a mean evaporation of 4.5 mm day−1.
Fig. 1.
Study sites at Tatton Agriculture Park, in the eastern escarpment of the Mau Forest, Njoro River Watershed, Kenya (courtesy of Zack Ogari, KMFRI, Kenya)
The humidity ranged from 42 to 79 %, with a mean humidity of 66.5 %. The wind speed ranged from 3.5 to 7.4 km per hour, with a mean speed of 5.05 km per hour. The experimental field and surrounding riparian forest area have a clay loamy soil type. The underlying material in this soil type is clay loamy and Miocene age material of low permeability. Predominant study site vegetation consisted of mixed indigenous African Couch grass, Cynodon dactylon (L.), Pers., Wire grass, Eleusine indica (L.) Gaertner, African bristle grass, Setaria sphacelata (Schumach) Stapf and C. E. Hubb ex M. B. Moss, Buffer grass, Cenchrus ciliaris L. and Rescue grass, Bromus catharticus Vahl.
2.2 Experimental plot design and treatment
This study was designed as described by Entry et al. (2000) with slight modifications. Each VFS field plot spanned 44 m by 4 m, with two sets of vegetation types, including a control field plot consisting of a random mixture of African Couch grass–Buffer grass, while the experimental fields consisted of two exotic grasses each, namely: Kikuyu grass (Pennisetum clandestinum Chiov.) and Napier grass (Pennisetum perpureum Schumach). The grasses were selected for the experiment because they have dense top growth to provide good, uniform soil cover and a fibrous root system for stability. These grasses are used locally as sources of fodder by livestock farmers (Fig. 2a Kikuyu grass; Fig. 2b Napier grass) and have a greater below ground root system compared to turf grass. Napier grass has an extensive deep root system. The strips were arranged in randomized complete block design (RCBD) consisting of three blocks with 10-m Napier grass–Buffer grass draining into 20-m Kikuyu grass zone vegetation (VFS II); 10-m Kikuyu grass–Buffer grass draining into 20-m Napier grass zone vegetation (VFS III); and the control site consisting of 30 m of mixed indigenous grass vegetation, comprised of African Couch grass–Buffer grass (VFS I). The study was replicated in each of the blocks A, B, C (Fig. 3). A 10 cm distance separated each VFS to minimize border effect. Treatments were made during rainy days as described by Kirk (1982). The experiments were performed during natural rainfall events, August 2013 to December 2014.
Fig. 2.

a Kikuyu grass field plot, b Napier grass field plot
Fig. 3.
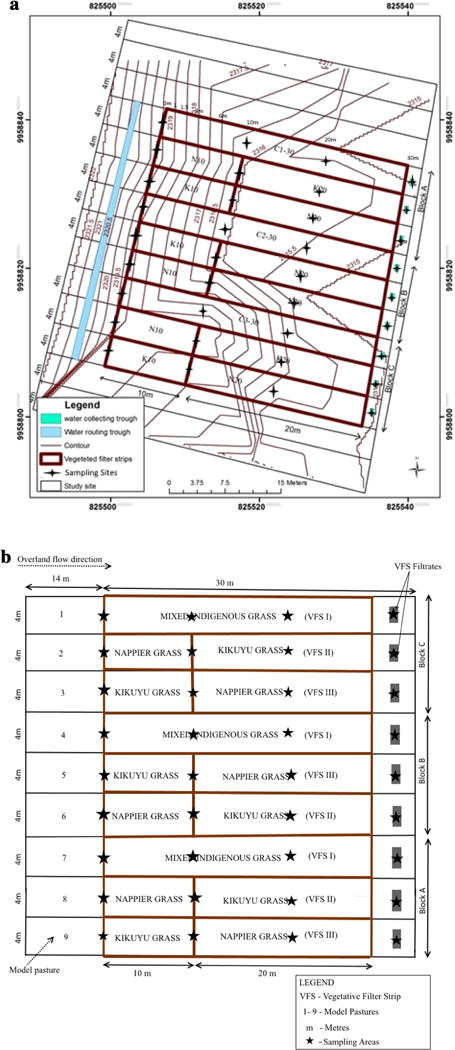
Site layout design contour grids, longitudes and latitudes coordinates and sampling sites for vegetated filter strip experimental field plots in a randomized complete block design; block A (C1-30: VFS I, N10K20: VFS II, K10N20: VFS III); block B (C2-30: VFS I, K10N20: VFS III, N10K20: VFS II); and block C (C3-30: VFS I, N10K20: VFS II, K10N20: VFS III). Key to Fig. 2 Kikuyu grass (K), Napier grass (N), subscript numbers in meters (10, 20) and control (mixed grass C1–C3)
The cattle manure (cowpat) used originated from free grazing dairy cattle within confines of the university grazing ground and fed on Kikuyu grass, Rhodes grass (Chloris gayana Kunth), Napier grass and a mixture of indigenous grasses. The fresh cowpat was collected by scooping it off a dairy cattle holding pen mud floor and included no urine. The cowpat was collected before the experiment and refrigerated (1 °C) until applied to the fields. The upper 14 m of each field represented pasture and was treated with dairy cowpat, while the remainder of each field acted as VFS. The cowpat application rate was 40 kg N Ha−1 (gross application of 5.2 kg cowpat). The experimental field area was 1584 m2, so the gross application was 5.2 kg of cowpat. This rate of cowpat application was equivalent to the cowpat that would be produced from a stocking density of six 450 kg animal units Ha−1 for a seven-day grazing duration. Cowpat was applied along the upper edge of the simulated pasture, for the high concentrations of cowpat constituents in overland flow to be produced as input to the VFS as described by Lim et al. (1998) and Guber et al. (2006). Sampling was done during short rainy season (August–December 2013; August–December 2014), dry season (January–March 2014) and wet season (April–August 2014).
2.3 Modeling overland flow transport and filtering of E. coli
The model used in this study was VFSMOD W (Muñoz-Carpena and Parsons 2011) (supplemental material attached, Eqs. 1–14; Foster 1982; Parlange et al. 1982; Deletic 2001; Bradford and Schijven 2002; Schijven et al. 2004).
2.3.1 Model calibration
The overland flow and E. coli transport model [Eqs. (1–14) in supplemental material] was determined using VFSMOD W (Muñoz-Carpena and Parsons 2011). The overland flow part was calibrated on data of the overland flow time series for each VFS strip and grass species separately. The boundary condition used in the VFS was h = 0 at x = 0. The Manning’s roughness coefficient utilized was 0.04 for VFS. Equations 2, 7, 8, 9 and 10 were fitted to the overland flow experimental data to estimate the values of the parameter α, saturated hydraulic conductivity (K) and net capillary drive (G). Escherichia coli breakthrough curves measured at the overland flow outlets were used to calibrate the transport part. The βm values were larger for Napier grass than Kikuyu grass and Couch grass mixtures in each VFS. Equation (11) was used to provide boundary condition and was used for the overland flow transport of either Kikuyu grass or Napier grass and Couch grass mixture in the VFS [Eqs. (10–14)]. For manure-borne E. coli, the release model of Bradford and Schijven (2002) performed much better than others for this study. The fitting parameters for E. coli used in the model were: the straining coefficient (kstr), the mass transfer rate (k) and the dispersivity (αL). Modeling kinetics of E. coli release from manure was used to derive the boundary condition for E. coli transport model.
2.3.2 Monte Carlo simulations to evaluate the different grass species efficiency
The percentage of E. coli that were filtered and retained by each of the different grass species in the VFS showed the effect of grass species efficiency. Several parameters have been reported to influence the VFS efficiency including the Manning coefficient, n, rainfall duration, tR, intensity of rainfall, initial soil water content, θi, E. coli release parameter, βm, net capillary drive, G, dispersivity, αL, conductivity, Ks. Twenty levels of probability were used to define values of each parameter at 0.025, 0.075,…, 0.975 (Guber et al. 2009). Iterations were carried out 350 times. For each sample, each model parameter was fixed only once at one of the 20 probability levels (Press et al. 1992; Guber et al. 2009).
2.3.3 Soil moisture content, total organic carbon and nutrients measurements
Gravimetric soil moisture was measured by taking soil samples with a sterile sampling auger (18 mm diameter). Ten sampling sites within each VFS field plot were designed to be used for sample collection at 0, 0.3, 1, 1.5, 2, 3, 6, 10, 20 and 30 m (Fig. 3a, b). At each location, samples were taken at four different depths, 0–5, 5–10, 10–15 and 15–20 cm, and stored in sterile plastic bags. In order to prevent moisture loss from collected samples, they were preserved in a cool box until they reached the laboratory, which was 500 m away from the experimental site. Overland flow samples were collected using sterilized 150-mL bottles and whirl park bags (Hach company Ltd, Loveland Colorado, USA) and stored in cool boxes at 4 °C. Grass leaves, stem and roots were collected into sterilized whirl park bags and kept in ice. The samples were collected at intervals of 5 min at 0, 0.3, 1, 1.5, 2, 3, 6, 10, 20 and 30 m of the VFS. The samples were held in ice and transported to the nearby soil science laboratory for processing and analyses. Samples were refrigerated until analyzed.
In the laboratory, samples were placed in metal containers and weight of the soil samples (wet weight) was measured using an electronic balance (Model GR 202, Wagtech International, Berkshire, UK). Then, samples were dried in an oven for 24 h at 105 °C. The weights of the soil samples were measured again after drying. Percent moisture content was calculated as described by Lowrance et al. (1998). The amount of precipitation was measured using one non-recording rain gauge at the university’s meteorological station, 500 m away from the experimental site. The distance covered by the deposited sediment was measured using a tape measure to the nearest 0.1 m.
In the laboratory, the samples were also analyzed for total suspended solids and suspended sediment concentration (g cm−3). Turbidity was measured using a portable turbidity meter in situ and results reported as nephelometric turbidity units (NTU) (Model 2100 P, Hach Company, Loveland Colorado, USA, and Model WQC-24, Tokyo, Japan). Temperature (°C) was measured in situ using oxygen meter. Oxygen (mg L−1) was measured in situ using oxygen meter. The overland flow rates in each of the fields were measured manually using a flow meter (Hach Company, Loveland Colorado, USA) during rainfall overland flows at 5-min intervals for the entire duration. Suspended organic carbon was calculated as the difference between TOC and DOC. Field samples were analyzed for environmental perturbations including total suspended solids (TSS); total nitrogen (TN); nitrate nitrogen (NO3−); nitrite nitrogen (NO2−); ammonium nitrogen (NH4−); organic nitrogen (ON); and total phosphorus (TP) (APHA 1992; Hay et al. 2006; Tate et al. 2006; Eisakhani and Malakahmad 2009). At the end of sampling day, samples to be analyzed for the above physical–chemical factors were centrifuged at 3500×g for approximately 8 min, filtered through 0.45-μm membrane filters, and the filtrates stored on ice. Particulate organic carbon and dissolved organic carbon were analyzed according to methods described by Strickland and Parsons, and APHA, respectively (Strickland and Parsons 1972; APHA 1992). Nitrate nitrogen was determined by hydrazine reduction methods (D’Elia et al. 1977). Nitrite nitrogen, ammonium nitrogen and total nitrate nitrogen were determined by per sulfate methods as described by APHA (APHA 1992). Organic nitrogen was determined from the difference of total nitrate and the ammonium nitrogen and nitrite nitrogen. Total phosphorus and dissolved reactive phosphorus were determined by per sulfate digestion and colorimetric methods as described by APHA (1992). Occurrence date, duration of the rainfall event (h), rate (cm h−1), amount (mm) of each rainfall event and volume (mL) realized were recorded.
2.3.4 E. coli settling velocity
The treatment efficiency of E. coli settling along the length of VFS was performed by the methods as described by Krishnappan et al. (2004) and Maus et al. (2008) for water elutriation system with slight modification in a laboratory experiment. In the experimental design setup, sediment and grain sizes were estimated. The samples were collected from the edge of the VFS (0 m), 0.3, 1, 1.5, 2, 3, 6, 10, 20 and 30 m at the outlet end of the VFS. The solids in different columns were analyzed by total suspended solids (TSS) analyses. Coarse material in the columns and fine particles in the outflow were sampled for measuring particle size distribution. Grain sizes and sediment settling velocity for sediment and E. coli were measured in the laboratory by flow rates analyses system, while particle size distribution was used to calculate the settling velocity distribution as described by Krishnappan et al. (2004) and Maus et al. (2008), respectively.
2.3.5 E. coli concentration
Prior to application of cowpat E. coli, concentrations were measured. Overland flow water and sediment samples were analyzed for E. coli concentration and load. Survival of total and fecal coliform, and E. coli bacteria in each VFS was assessed in the manure as surface flow on the day of application (Day 0), soil water; 2–4 days prior to application and after rainfall events following the application to the fields. Overland flow content of each field in each of the blocks A, B and C was analyzed for E. coli. Escherichia coli samples were collected into sterilized 5-mL bottles from the grass leaves, stems, roots systems and subsoil horizon and stored in cool boxes at 4 °C. For sediment samples, bacteria were elutriated by vortexing, for 2 min, 5 g of fresh substrate and 35 mL of sterile distilled water in a 50-mL centrifuge tube. After standing for 2 min, the suspension was serially diluted. The volumes of the appropriate dilutions (100 mL) were aseptically transferred into distribution trays (Quanti-Tray/2000), sealed and incubated in refrigerated incubator (Model FOC 225E, VELP Scientifica, Europe) at 35 °C for 18 h. After 18 h, colonies that indicated pink colors were counted to determine colony-forming units (CFU) 100 mL−1 of water. Escherichia coli counts from sediment samples were expressed in per gram of dry weight of the sample. All sets of analyses for E. coli determinations included suitable blanks and a standard E. coli culture (ATCC 25922) for quality control purposes.
The general protocol for E. coli identification was performed using DelAgua Water Testing Kit, battery operated at 37 and 44.5 °C for 22 h. Yellow-colored culture was initially swabbed onto membrane (medium) thermo-tolerant E. coli (mTEC) agar plates, incubated at 44.5 °C for 22 h, and yellow or yellow–brown colonies that developed on the membrane were confirmed to be E. coli by the substrate test (4-methylumbelliferyl β-D-glucuronide and indoacetyl β-D-galactoside—MI). Finally, the populations that were presumed to be E. coli were speciated by using the BBL crystal identification scheme (Becton–Dickinson Microbiology Systems, Sparks, Md., USA). For quality control and quality assurance, a standard isolate (E. coli, K-12 ATCC 25922) was included in the identification protocol. Three processes controlled conservation of E. coli mass balance, as described by Tian et al. (2002), namely fecal contaminant population dynamics, attenuation and diffusion. Overland flow samples were centrifuged and filtered by a 0.45-μm pore size Whatman filter paper. The filtrate was then run using the total organic carbon analyzer, and results reported as μg L−1 of water samples or μg mg−1 of soil samples.
2.4 Data analysis
A default value of (α, 0.1) was used for variable selection in multiple regressions. The E. coli recovery efficiency was estimated by the following relative recovery or percentage recovery equation (Morales-Morales et al. 2003; Guber et al. 2006):
| (1) |
where C was the concentration in overland flow and C0 was the concentration in the applied cowpat. The linear regression coefficient a and b
| (2) |
where was the relative organic carbon in the overland flow and was the relative E. coli concentrations in overland flow. For each regression, the determination of coefficient, R2, was estimated. The root-mean-square error (RMSE) equation was used for the goodness of fit for VFSMOD W application as follows (Muñoz-Carpena and Parsons 2011):
| (3) |
where Fi and Oi were fitted and observed values, respectively, for the same cowpat component and N was number of observations. The VFSMOD W modeling system version 6.x (Muñoz-Carpena et al. 2010), was used in this study. The coefficient of determination (R2) and Nash–Sutcliffe Index (NSI) (Nash and Sutcliffe 1970) were used to compare the observed and predicted VFS widths. Statistical analysis, including regression models, was fitted and checked for statistical significance using SYSTAT software (SYSTAT Institute Inc., USA, 2007) and PAST (Hammer et al. 2005). Probability for type I error α (rejecting a true null hypothesis) was chosen as 0.05 or 0.01 (α, 0.05; 0.01) including multiple regressions.
3 Results
3.1 Overland flow and release of cowpat constituents
Both the soil and cowpat characteristics within the overland flow pathway in the VFS I, II and III were determined using various techniques. The mean soil and dairy cowpat characteristics such as total phosphorus, total nutrients and organic carbon prior to rainfall and leaching of nutrients in the VFS were averaged across three replications, August 2013 to December 2014 (Table 1). Cowpat constituents were released at different rates and their downstream transport along the overland flow pathway. Not surprisingly, the cowpat soluble components dissolved and moved more rapidly than biopolymers and microbial colloidal particles. Adsorption of cowpat constituents including E. coli and other microbes, nutrients including total phosphorus, total nitrogen and organic carbon to macroscopic particles influenced their release rates. From the model, the release rates of cowpat constituents were slower along the length of VFS I than along the length of VFS III (Fig. 4). Release rates of cowpat constituents along the length of VFS II (Kikuyu grass) were the highest among these VFSs. The model parameter values effect on the simulated VFS filtering efficiency was evaluated in a set of simulations using VFSMOD W (Table 2). Manure was applied on a 14-m-long field and moved with overland flow water through 30-m-long and 4-m-wide VFS on clay loam soil profile. Napier grass showed the lowest dispersivity values, αL = 3:5. Dispersivity was the limiting parameter in this vegetation. Napier grass along the length of VFS III was the most efficient in filtering E. coli based on percentage attachment to different surface, namely: clay loam soil surfaces (25.5 %), vegetation surfaces (leaf, stem and litter) material (12.2 %) and manure waste particles (35 %), infiltrated (27 %) and overland flow exit (1.3 %). The mean soil hydraulic conductivity at the Couch grass–Buffer grass combination (control plots) was 4–8 times lower than at the Kikuyu grass and 3–9 times lower than Napier grass in the clay loam soil texture. The capillary drive parameter (G) values ranged from 6.6 to 26.2 cm. When the equations for mean soil texture class parameters and water retention by Brooks and Corey were used to estimate capillary drive parameter, the clay loam soil had a value of 27. The parameter σ was 0.75. The dispersivity values ranged from 0.1 to 52.3 cm. The straining parameter kstr values ranged from 0.91 to 0.94. The capacity of VFSMOD W model to simulate E. coli transport in the overland flow was indicated by relatively small RMSE, high values of the Nash–Sutcliffe efficiency and high correlation coefficients (Table 3). Principal components, eigenvalue and percentage variance of mean settling velocity of E. coli Couch grass–Buffer grass in VFS I, Kikuyu along the length of VFS II and Napier grass along the length of VFS III; and cumulative density function are averaged across three replications (A, B and C), August 2013 to Dec 2014 (Table 3).
Table 1.
Mean soil and dairy cowpat characteristics prior to rainfall and leaching of nutrients in the VFS averaged across three replications, August 2013–December 2014
| Soil/cowpat | Bulk density (g cm−3) |
Pore density (g cm−3) |
Solid (%) |
Pore space (%) |
Organic matter (%) |
Sand (%) |
Silt (%) | Clay (%) |
NO3-N (μg L−1) |
NH4-N (μg L−1) |
Total nitrogen (μg L−1)1 |
PO4-P (μg L−1) |
Total P (μg L−1) |
Total organic carbon (TOC) (μg L−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dairy cowpat | 0.50 ± 0.02 | 0 | 68.22 ± 9.21 | 78.22 ± 5.32 | 0 | 0 | 0 | 0.98 ± 0.03 | 11.90 ± 1.2 | 13 ± 1.2 | 11.90 ± 1.22 | 12.01 ± 1.3 | 34.05 ± 2.10 | |
| Clay loam | 1.08 ± 0.01 | 2.34 ± 0.02 | 46.1 ± 5.32 | 53.9 ± 5.21 | 2.86 ± 0.02 | 34 ± 2.32 | 32 ± 4.23 | 34 ± 3.38 | 21.49 ± 3.4 | 4.23 ± 0.2 | 8.01 ± 0.22 | 4.23 ± 0.02 | 52.0 ± 2.3 | 2.85 ± 0.01 |
Fig. 4.
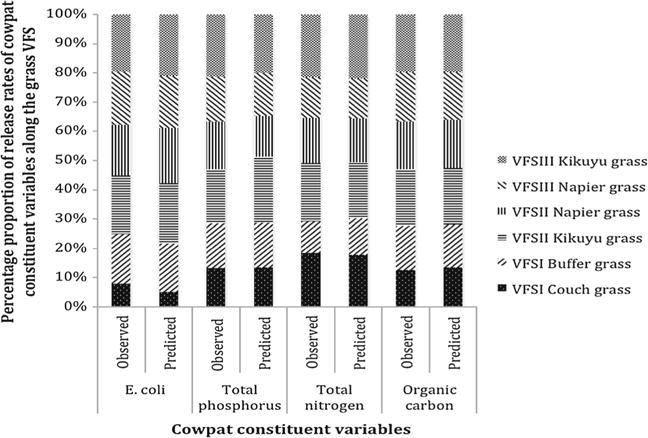
The observed and predicted mean release rates of nutrients and E. coli along the length of VF
Table 2.
The mean VFSMOD model performance parameters from model applications at slopes with different vegetation covers and mean release rates of three parameters averaged across three replications (A, B and C), August 2013 to December 2014
| VFS | Grass species | Net capillary drive G (cm) | Dispersivity value αL (cm) | Parameter σ | Saturated hydraulic conductivity, Ks (cm h−1) | Initial water content θL (cm3 cm−3) | Rainfall time, h | Rainfall intensity (mmh−1) |
|---|---|---|---|---|---|---|---|---|
| I | Couch | 10.1 ± 1.02 | 23.2 ± 2.4 | 0.12 ± 0.02 | 0.03 ± 0.001 | 0.29 ± 0.01 | 1.22 ± 0 | 26 ± 3.1 |
| Buffer | 7.2 ± 0.3 | 24.1 ± 2.1 | 0.12 ± 0.01 | 0.1 ± 0.002 | 0.31 ± 0.02 | 1.22 ± 0 | 26 ± 2.1 | |
| II | Kikuyu | 18.2 ± 2.6 | 1.4 ± 0.3 | 0.32 ± 0.01 | 2.69 ± 0.02 | 0.31 ± 0.01 | 1.22 ± 0 | 26 ± 2.33 |
| Napier | 26.1 ± 3.2 | 2.34 ± 0.1 | 0.36 ± 0.02 | 3.12 ± 0.05 | 0.3 ± 0.01 | 1.22 ± 0 | 26 ± 1.6 | |
| III | Kikuyu | 19.3 ± 2.7 | 5.21 ± 0.4 | 0.21 ± 0.01 | 1.96 ± 0.01 | 0.33 ± 0.02 | 1.22 ± 0 | 26 ± 3.1 |
| Napier | 26.2 ± 3.20 | 5.13 ± 0.2 | 0.16 ± 0.01 | 3.02 ± 0.02 | 0.32 ± 0.01 | 1.22 ± 0 | 26 ± 2.3 |
Table 3.
Mean performance of VFSs in retention of E. coli in the overland flow using VFSMOD W, and principal components, eigenvalue and % variance of mean settling velocity of E. coli at VFS I, VFS II and VFS III and cumulative density function averaged across three replications (A, B and C), August 2013 to December 2014
| Month/grass species | VFS factor averaged across replicates in blocks A, B and C of the VFS I, II and III
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overland flow (cm) RMSE | R: overland flow (p < 0.05) | E coli CFU 100 mL−1 RMSE | R: E. coli (p < 0.05) | Nash–Sutcliffe efficiency for E. coli | Mean settling velocity of E coli (eigenvalue) | Mean settling velocity of E coli (percentage variance) | Cumulative density | Degrees of freedom (df) | F | p value | |
| August 2013 | |||||||||||
| Couch–Buffer | 0.045 ± 0.001 | 0.592 ± 0.03 | 52.8 ± 3.30 | 0.592 ± 0.03 | 0.478 ± 0.02 | 0.275871 ± 0.01 | 6.8968 ± 0.7 | 0.0046163 ± 0.001 | 29 | 9.77 | <0.05 |
| Napier | 0.012 ± 0.001 | 0.992 ± 0.03 | 12.8 ± 3.30 | 0.892 ± 0.04 | 0.878 ± 0.03 | 3.69847 ± 0.01 | 82.462 ± 4.7 | 0.11541 ± 0.001 | 29 | 19.4 | <0.05 |
| Kikuyu | 0.015 ± 0.001 | 0.792 ± 0.03 | 22.8 ± 3.30 | 0.792 ± 0.05 | 0.678 ± 0.04 | 2.4554 ± 0.02 | 65.462 ± 2.7 | 0.01541 ± 0.001 | 29 | 14.5 | <0.05 |
| December 2013 | |||||||||||
| Couch–Buffer | 0.042 ± 0.002 | 0.546 ± 0.02 | 32.8 ± 23 | 0.583 ± 0.03 | 0.381 ± 0.01 | 0.29541 ± 0.12 | 6.8968 ± 0.453 | 0.0036163 ± 0.001 | 29 | 3.77 | <0.05 |
| Napier | 0.0142 ± 0.002 | 0.896 ± 0.02 | 11.8 ± 24 | 0.882 ± 0.04 | 0.730 ± 0.02 | 3.49842 ± 0.612 | 92.472 ± 5.009 | 0.12542 ± 0.002 | 29 | 19.4 | <0.05 |
| Kikuyu | 0.018 ± 0.002 | 0.672 ± 0.02 | 21.8 ± 25 | 0.789 ± 0.05 | 0.638 ± 0.03 | 2.49747 ± 0.123 | 72.462 ± 7.005 | 0.01441 ± 0.001 | 29 | 14.5 | <0.05 |
| April 2014 | |||||||||||
| Couch–Buffer | 0.0052 ± 0.0001 | 0.699 ± 0.04 | 62.4 ± 4.0 | 0.556 ± 0.04 | 0.466 ± 0.02 | 0.36980 ± 0.002 | 6.1112 ± 0.92 | 0.0046163 ± 0.003 | 29 | 4.77 | <0.05 |
| Napier | 0.0012 ± 0.0001 | 0.822 ± 0.04 | 12.4 ± 3.1 | 0.882 ± 0.05 | 0.755 ± 0.03 | 4.1234 ± 0.450 | 81.408 ± 8.97 | 0.11541 ± 0.002 | 29 | 19.4 | <0.05 |
| Kikuyu | 0.0017 ± 0.0001 | 0.742 ± 0.04 | 11.4 ± 4.2 | 0.764 ± 0.06 | 0.678 ± 0.04 | 3.6045 ± 0.0032 | 72.456 ± 4.98 | 0.11541 ± 0.001 | 29 | 14.5 | <0.05 |
| August 2014 | |||||||||||
| Couch–Buffer | 0.0031 ± 0.0002 | 0.534 ± 0.02 | 56.2 ± 4.32 | 0.469 ± 0.03 | 0.395 ± 0.03 | 0.69847 ± 0.002 | 5.8432 ± 0.12 | 0.003863 ± 0.002 | 29 | 6.77 | <0.05 |
| Napier | 0.0011 ± 0.0002 | 0.767 ± 0.01 | 12.2 ± 4.33 | 0.821 ± 0.04 | 0.723 ± 0.04 | 3.69841 ± 0.003 | 69.454 ± 6.89 | 0.11541 ± 0.003 | 29 | 19.4 | <0.05 |
| Kikuyu | 0.0024 ± 0.0002 | 0.645 ± 0.03 | 18.2 ± 4.34 | 0.612 ± 0.05 | 0.525 ± 0.05 | 2.3486 ± 0.004 | 57.361 ± 0.23 | 0.10523 ± 0.003 | 29 | 14.5 | <0.05 |
| December 2014 | |||||||||||
| Couch–Buffer | 0.0214 ± 0.003 | 0.412 ± 0.02 | 42.6 ± 5.74 | 0.211 ± 0.02 | 0.378 ± 0.02 | 0.2345 ± 0.004 | 6.8968 ± 0.67 | 0.00567 ± 0.001 | 29 | 5.77 | <0.05 |
| Napier | 0.001 ± 0.003 | 0.789 ± 0.02 | 18.6 ± 5.75 | 0.878 ± 0.03 | 0.7548 ± 0.03 | 3.9876 ± 0.231 | 62.5647 ± 11.2 | 0.11541 ± 0.002 | 29 | 19.4 | <0.05 |
| Kikuyu | 0.021 ± 0.003 | 0.567 ± 0.02 | 23.6 ± 5.76 | 0.689 ± 0.04 | 0.6741 ± 0.04 | 2.8765 ± 3452 | 54.3626 ± 8.43 | 0.02541 ± 0.003 | 29 | 14.5 | <0.05 |
Pearson’s product moment correlation coefficients (r), root-mean-square error (RMSE) for cumulative runoff along the length of VFS; r, RMSE and the Nash–Sutcliffe are given for E. coli concentrations in the overland flow
The performance of VFSs in retention of E. coli concentration in the overland flow using VFSMOD W model showed the effectiveness of this model in practice (Fig. 5a). Predicted and observed E. coli removal efficiency by vegetated filter strips was averaged across three replications, August 2013 to December 2014 (Fig. 5b). In this study, E. coli transport parameter values selected centered on clay loam soil field plots. The efficiency of Kikuyu grass, Napier grass and Couch grass–Buffer grass filter strip decreased after the rainfall intensity exceeded 2.0, 2.4 and 1.5 cm h−1, respectively. Thus, long rainfall could reduce the filtering capability of Kikuyu grass, Napier grass and Couch grass–Buffer grass. In addition, Kikuyu grass, Napier grass and Couch grass–Buffer grass efficiency could be decreased as a result of high initial soil water contents in the VFS. The grass removal efficiency of Kikuyu grass, Napier grass and Couch grass–Buffer grass VFS was correlated with an increased roughness of soil surface. The grass removal efficiency of Kikuyu grass, Napier grass and Couch grass–Buffer grass decreased as a result of low soil saturated hydraulic conductivity and small net capillary drive along the length of VFS. The grass removal efficiency of Kikuyu grass, Napier grass and Couch grass–Buffer grass was not influenced by the variations in the bacteria release parameter βm because there was no E. coli retention in the soil active layer. Kikuyu grass, Napier grass increase in dispersivity caused a small decrease in grass removal efficiency, but Couch grass–Buffer grass increase in dispersivity caused a large decrease in grass removal efficiency, indicating dispersivity is a limiting parameter in Couch grass–Buffer grass combination.
Fig. 5.
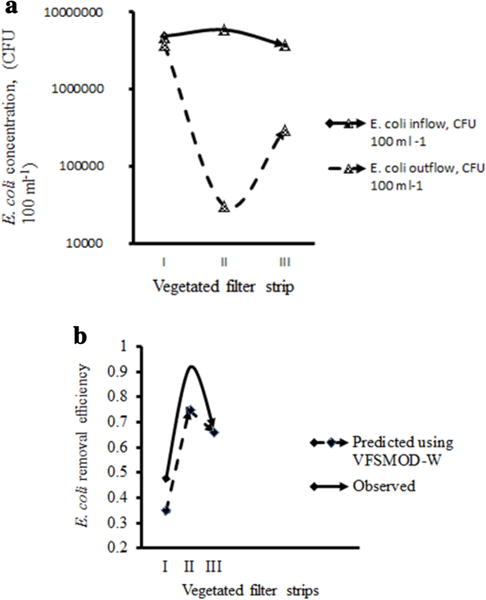
Predicted and observed E. coli removal effectiveness and efficiency by vegetated filter strips averaged across three replications, August 2013 to December 2014
3.2 Estimates of uncertainty along the length of VFS model inputs and parameters
In the uncertainty estimates of probability distribution functions, the following parameters and input values were defined for the model: initial water content, precipitation depth and intensity, the Manning’s roughness coefficient, net capillary drive, saturated hydraulic conductivity, dispersivity, αL, and release parameter of E. coli, βm. The initial water content was 0.28 cm3 cm−3, and saturated water content was 0.48 cm3 cm−3. The manning roughness coefficient was 0.042. The probability distribution functions of E. coli were found (Fig. 6). The coefficient of determination for Napier grass was R2 = 0.94, while the exponential equation for the probability function of E. coli settling velocity in Napier grass was:
| (4) |
where SE. coli is the settling velocity of E. coli in the overland flow and CDF is the cumulative density function. The coefficient of determination for Kikuyu grass was R2 = 0.96, while the exponential equation for the probability function of E. coli settling velocity in Kikuyu grass was:
| (5) |
Fig. 6.
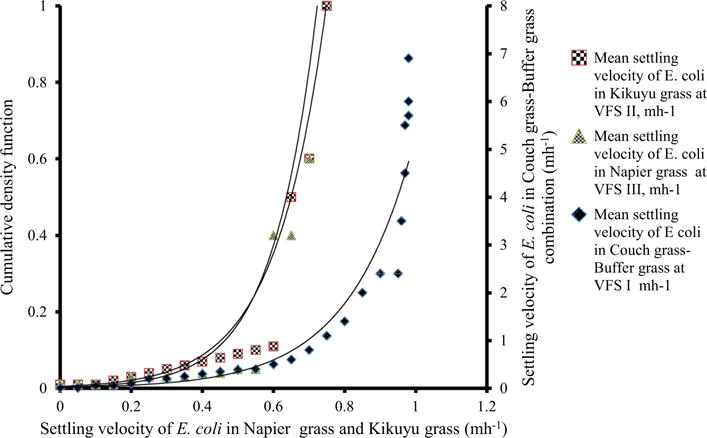
Relationship between settling velocity of E. coli and cumulative density function through Napier grass, Kikuyu grass and Couch grass–Buffer grass along the length of VFS I, II and III, respectively, averaged across three replications, August 2013 to December 2014
The coefficient of determination for Couch grass–Buffer grass was R2 = 0.95, while the exponential equation for the probability function of E. coli settling velocity in Couch grass–Buffer grass was:
| (6) |
The sediment delivery ratio measured was 1.8 % at 30-m exit of VFS and Manning’s roughness of 0.072 during rainfall event through VFS II during August 2013 to December 2014 (Fig. 7a). The hyetograph and hydrograph depicting the pooled quantity of overland flow and infiltration through VFS, August 2013 to Dec 2014, were estimated (Fig. 7b). The efficiency of simulated parameters nearly ranged from 0 to 100 %. Along the length of VFS II, Kikuyu grass had efficiency of <99 % in more than 25 % of the replications, followed by Napier grass at <95 % efficiency in more than 22 % of replications. The least efficiency simulated in Couch grass–Buffer grass simulations with a value of 5 % in all replications. Along the length of VFS III, Kikuyu grass showed an efficiency of <95 % in 25 % of the replications, followed by <75 % efficiency in 21 % of the replications. Along the length of VFS I, at least an efficiency of nearly 10 % was obtained in Couch grass–Buffer grass combinations in all replications. Relatively high overland flows, soil’s low levels of hydraulic conductivity, soil’s low levels of net capillary drive, high intensity precipitations and high soil moisture contents prior to precipitations influenced Napier grass and Kikuyu grass VFS. This effect led to low E. coli retention efficiencies. These low-level efficiency effects more than doubled in the Couch grass–Buffer grass combinations of VFS.
Fig. 7.
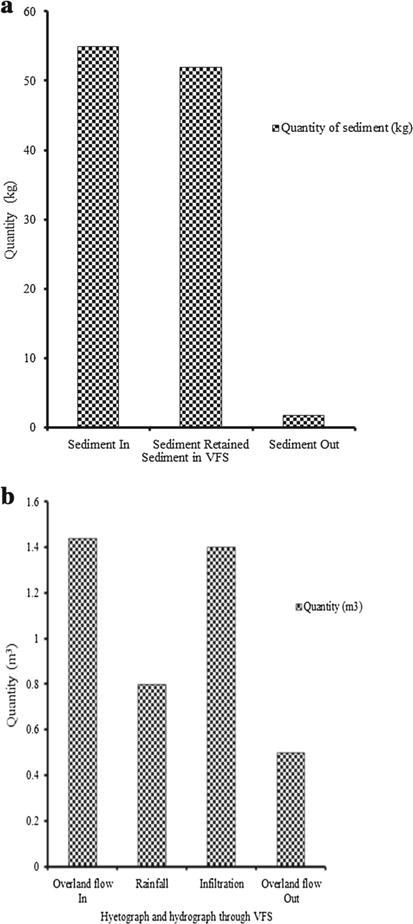
Hyetograph and hydrograph relationships and sediment delivery ratio during rainfall events through VFS averaged across three replications, August 2013 to December 2014
The mean overland flow rates (Q) measured at the outlet of vegetated filter strips in the experimental field plots showed that at 95 % confidence level, Couch grass–Buffer grass along the length of VFS I did not retard the moving water with time because of concentrated flow or preferential flow, while Kikuyu grass along the length of VFS II moving water was related to time, but Napier grass along the length of VFS III reduced the moving water the most (Fig. 8a–c).
Fig. 8.
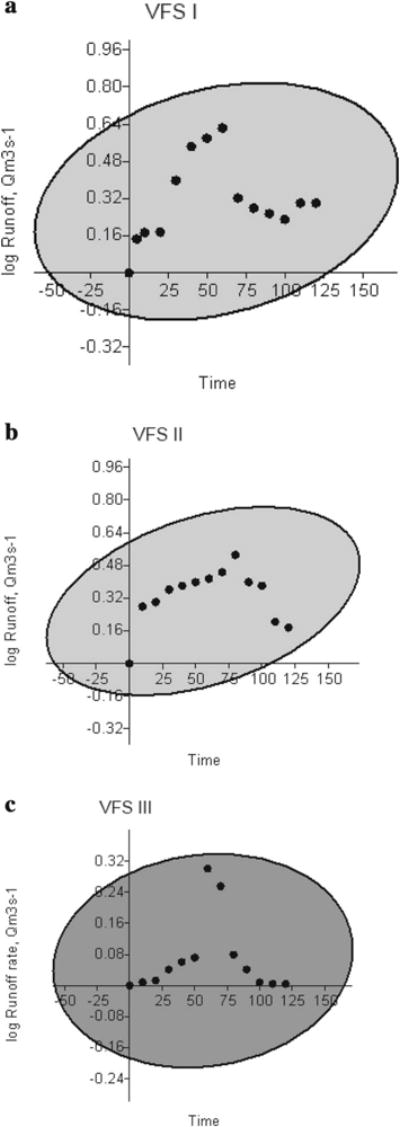
The mean overland flow rates (Q) through the Couch grass–Buffer grass along the length of VFS I, Kikuyu grass along the length of VFS II and Napier grass along the length of VFS III at 95 % confidence level averaged across three replications, August 2013 to December 2014
3.3 Effect of surface vegetation and subsoil horizon in filtering E. coli
The most impact was observed during dry season from the month of January to March 2014. It was observed that long time (up to 14 days) exposure of E. coli before natural rainfall in the field plots reduced bacteria’s populations’ attachment by up to 95 % onto soil particles, 75 % onto fecal particles or material and 50 % onto the organic particles from the plants exudates. There was highest impact in the VFS I, followed by VFS III and least effect on VFS II. The effects were higher in dry season as compared to the wet season.
The mean recovery of E. coli as a percentage of applied cowpat manure in overland flow between the inlet and position indicated (0 or 30 m) for three parallel VFS averaged across replications in three blocks (A, B and C) along the VFS, August 2013 to December 2014, showed that dispersivity rate was the limiting parameter in the Couch grass–Buffer grass combination, implying that this VFS contributed significantly (p < 0.05) to the preferential or concentrated flow. During ANOVA computations using F-distribution, the effect of different grass species, species morphology and root system and subsoil horizon in filtering E. coli showed variations at different levels of significance (α, 0.01 or 0.05; Table 4). Dispersivity was the limiting estimated parameter in the VFS. Spearman’s correlation coefficient rs showed that in Couch grass–Buffer grass combination (VFS I) field plots, suspended sediment concentration (mg L−1) was correlated with overland flow, Q (m3 s−1) (r = 0.8070) (Table 5a). Along the length of VFS II field plots, Kikuyu grass influenced suspended sediment concentration (SSC, mg L−1) and SSC was correlated with rate of overland flow, Q (m3 s−1) (r = 0.8492) (Table 5b). Along the length of VFS III field plots, Napier grass influenced suspended sediment concentration (SSC, mg L−1), and SSC was correlated with overland flow, Q (m3 s−1) (r = 0.5805) (Table 5c).
Table 4.
Mean recovery of E. coli (% of applied cowpat manure) in overland flow between the inlet and position indicated (0 or 30 m) for three parallel VFS averaged across replications in three blocks (A, B and C) along the length of VFS, August 2013 to December 2014
| Grass species | Distance (m) | Standard error of mean
of E. coli (% recovered as a proportion of original value in applied cowpat manure) at a slope of 15 %
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 0.5 | 1 | 1.5 | 2 | 3 | 6 | 10 | 20 | 30 | Degrees of freedom (df) | F | p value (α, 0.01; 0.05) | ||
| VFS I | Vegetation structure | |||||||||||||
| Couch | Overland flow | 28.6 ± 1.7 | 38 ± 6.2 | 33 ± 5.1 | 28 ± 0.6 | 28 ± 0.9 | 25 ± 4.2 | 26 ± 0.4 | 24 ± 5.1 | 24 ± 1.6 | 19.8 ± 0.8 | (2,9) | 4.24 | <0.05 |
| Leaf | 5.2 ± 2.4 | 4.9 ± 2.3 | 5.1 ± 0.3 | 6.4 ± 0.5 | 6.4 ± 2.4 | 7.6 ± 3.6 | 5.9 ± 0.1 | 6.7 ± 0.3 | 5.9 ± 1.2 | 5.7 ± 0.7 | (2,9) | 4.2 | <0.05 | |
| Stem | 4.1 ± 0.5 | 5.8 ± 3.2 | 9.0 ± 0.1 | 5 ± 0.3 | 6 ± 2.6 | 8 ± 2.7 | 7 ± 0.4 | 8.1 ± 0.5 | 8.0 ± 1.3 | 6.7 ± 0.5 | (2,9) | 4.73 | <0.05 | |
| Litter | 18 ± 0.3 | 19.2 ± 1.2 | 11 ± 0.2 | 17 ± 0.1 | 17 ± 1.1 | 19 ± 3.3 | 18.1 ± 0.5 | 17.2 ± 0.2 | 14.9 ± 2.1 | 12.6 ± 0.6 | (2,9) | 4.5 | <0.05 | |
| Roots system | 12 ± 0.4 | 13 ± 0.7 | 10 ± 0.3 | 8 ± 0.5 | 8.2 ± 2.2 | 9 ± 1.5 | 8.9 ± 1.2 | 10 ± 0.1 | 10 ± 4.2 | 10.1 ± 0.4 | (2,9) | 3.2 | <0.05 | |
| Subsoil horizon | 6 ± 0.2 | 7 ± 0.3 | 6 ± 0.1 | 5 ± 2.3 | 4 ± 0.6 | 2 ± 0.01 | 8 ± 0.3 | 6 ± 0.5 | 7.2 ± 2.3 | 6.2 ± 0.6 | (2,9) | 2.76 | <0.05 | |
| Buffer | Overland flow | 25 ± 2.0 | 33 ± 4.1 | 29 ± 2.2 | 31 ± 0.4 | 32 ± 0.7 | 28 ± 0.62 | 23 ± 0.21 | 19 ± 0.3 | 18 ± 0.21 | 14.2 ± 0.8 | (2,9) | 7.25 | <0.05 |
| Leaf | 6 ± 1.1 | 7 ± 0.3 | 6 ± 0.8 | 5 ± 0.5 | 5 ± 0.41 | 4 ± 0.54 | 4 ± 0.34 | 8 ± 0.2 | 7 ± 0.31 | 5 ± 0.3 | (2,9) | 3.67 | <0.05 | |
| Stem | 5 ± 2.1 | 3 ± 0.1 | 6 ± 0.7 | 4 ± 0.7 | 4 ± 0.54 | 5 ± 0.76 | 6 ± 0.22 | 7 ± 0.1 | 6 ± 0.31 | 6 ± 0.2 | (2,9) | 2.88 | <0.05 | |
| Litter | 19 ± 2.4 | 10 ± 0.2 | 12 ± 0.4 | 17 ± 2.3 | 16 ± 0.21 | 12 ± 0.65 | 11 ± 0.42 | 10 ± 0.22 | 8 ± 0.3 | 8 ± 0.1 | (2,9) | 4.43 | <0.05 | |
| Roots system | 18 ± 3.2 | 12 ± 2.2 | 13 ± 0.5 | 11 ± 2.1 | 0.65 | 8 ± 0.81 | 8 ± 0.44 | 9 ± 0.21 | 10 ± 0.12 | 12 ± 0.32 | (2,9) | 2.9 | <0.05 | |
| Subsoil horizon | 4 ± 0.7 | 5 ± 0.54 | 7 ± 0.66 | 9 ± 0.54 | 8 ± 0.8 | 9 ± 0.21 | 10 ± 0.32 | 11 ± 0.18 | 9 ± 0.17 | 11 ± 0.43 | (2,9) | 3.32 | <0.01 | |
| VFS II | ||||||||||||||
| Kikuyu | Overland flow | 13 ± 3.7 | 2.0 ± 0.8 | 1.2 ± 3.7 | (2,9) | 9.27 | <0.01 | |||||||
| Leaf | 3 ± 2.1 | 2 ± 0.6 | 0.5 ± 2.3 | (2,9) | 3.45 | <0.01 | ||||||||
| Stem | 4 ± 2.5 | 1.0 ± 0.8 | 1.0 ± 2.9 | (2,9) | 3.6 | <0.01 | ||||||||
| Litter | 6.02 ± 2.1 | 4.2 ± 01.2 | 1.5 ± 1.2 | (2,9) | 4.2 | <0.01 | ||||||||
| Roots system | 4.5 ± 2.3 | 1.02 ± 0.01 | 1.02 ± 1.0 | (2,9) | 4.8 | <0.01 | ||||||||
| Subsoil horizon | 1.5 ± 0.2 | 1.3 ± 01 | 0.09 ± 2.8 | (2,9) | 3.9 | <0.01 | ||||||||
| Napier | Overland flow | 38.5 ± 2.7 | 37 ± 4.5 | 32 ± 6.1 | 20 ± 3.2 | 17 ± 1.9 | 17 ± 1.1 | 15 ± 2.3 | 13 ± 1.6 | (2,9) | 50 | <0.01 | ||
| Leaf | 11.2 ± 2.1 | 12 ± 1.8 | 10 ± 2.5 | 12 ± 4.2 | 8 ± 0.3 | 1.8 ± 0.01 | 2 ± 0.01 | 3 ± 0.2 | (2,9) | 32 | <0.01 | |||
| Stem | 10.9 ± 2.4 | 6 ± 1.3 | 5 ± 0.9 | 6 ± 1.3 | 5 ± 0.6 | 3 ± 0.02 | 4 ± 0.02 | 2.7 ± 1.3 | (2,9) | 4.7 | <0.01 | |||
| Litter | 26.7 ± 1.2 | 25 ± 2.3 | 22 ± 2.3 | 16 ± 2.4 | 14 ± 0.5 | 8 ± 0.6 | 3 ± 0.02 | 0.1 ± 0.001 | (2,9) | 4.5 | <0.01 | |||
| Roots system | 23.3 ± 0.5 | 21 ± 0.7 | 19 ± 0.4 | 13 ± 0.3 | 3 ± 0.02 | 8.9 ± 0.7 | 5 ± 0.8 | 3 ± 0.2 | (2,9) | 7.1 | <0.01 | |||
| Subsoil horizon | 4.3 ± 0.3 | 5 ± 0.4 | 6 ± 0.5 | 4.8 ± 0.5 | 5 ± 0.6 | 2 ± 0.5 | 2 ± 0.6 | 2 ± 0.7 | (2,9) | 2.8 | <0.05 | |||
| VFS III | ||||||||||||||
| Kikuyu | Overland flow | 37.2 ± 1.6 | 35.3 ± 2.8 | 32.3 ± 4.1 | 28.1 ± 4.2 | 20 ± 4.2 | 14 ± 1.4 | 12 ± 1.3 | 10.2 ± 0.3 | (2,9) | 34.5 | <0.01 | ||
| Leaf | 12.3 ± 4.2 | 10 ± 1.3 | 10 ± 0.5 | 11 ± 0.7 | 9 ± 0.5 | 3 ± 1.3 | 2 ± 1.3 | 1.8 ± 0.2 | (2,9) | 12.3 | <0.01 | |||
| Stem | 6.5 ± 1.6 | 4 ± 0.6 | 7 ± 0.2 | 7 ± 0.6 | 6.3 ± 0.3 | 2 ± 0.9 | 5 ± 0.6 | 1.9 ± 0.2 | (2,9) | 3.7 | <0.01 | |||
| Litter | 22.4 ± 3.6 | 15 ± 0.2 | 20 ± 0.3 | 18 ± 0.3 | 15 ± 0.4 | 9 ± 0.2 | 6 ± 1.3 | 3.2 ± 0.1 | (2,9) | 5.2 | <0.01 | |||
| Roots system | 21.3 ± 2.8 | 12 ± 0.4 | 19 ± 0.4 | 15 ± 0.4 | 15 ± 0.4 | 10 ± 0.9 | 6 ± 0.5 | 2.9 ± 0.6 | (2,9) | 7 | <0.01 | |||
| Subsoil horizon | 5.0 ± 3.2 | 2 ± 0.3 | 5 ± 0.8 | 4 ± 0.02 | 4 ± 0.1 | 2 ± 2.1 | 1.9 ± 0.2 | 1.1 ± 0.2 | (2,9) | 6.2 | <0.01 | |||
| Napier | Overland flow | 12.7 ± 0.1 | 7.2 ± 4.2 | 2.2 ± 1.7 | (2,9) | 9.23 | <0.01 | |||||||
| Leaf | 10.4 ± 0.7 | 6.3 ± 1.5 | 1.7 ± 2.5 | (2,9) | 8.1 | <0.01 | ||||||||
| Stem | 5.3 ± 0.1 | 3.7 ± 1.6 | 1.2 ± 3.2 | (2,9) | 3.2 | <0.01 | ||||||||
| Litter | 7.2 ± 0.1 | 5.5 ± 4.5 | 0.1 ± 0.001 | (2,9) | 3.2 | <0.01 | ||||||||
| Roots system | 6.1 ± 0.1 | 6.2 ± 2.2 | 2.2 ± 1.5 | (2,9) | 2.88 | <0.05 | ||||||||
| Subsoil horizon | 1.3 ± 0.2 | 2.1 ± 3.1 | 0.8 ± 2.4 | (2,9) | 2.98 | <0.05 | ||||||||
Table 5.
Spearman’s rs correlation coefficient of variables in Couch grass–Buffer grass, Kikuyu grass and Napier grass in the VFS I, II and III, averaged across three replications (A, B and C), August 2013 to December 2014
| Runoff, Q (m3 s−1) | SSC (mg L−1) | Precipitation (mm) | Turbidity, NTU | Total coliform, CFU (mL−1) | Fecal coliform, CFU 100 mL−1 | E. coli, CFU 100 mL−1 | |
|---|---|---|---|---|---|---|---|
| a. VFS I Couch grass–Buffer grass combination | |||||||
| Runoff, Q (m3 s−1) | 0 | 0.001509* | 0.70226* | 0.3911* | 0.062511* | 0.054842* | 0.42656* |
| SSC (mg L−1) | 0.80702 | 0 | 0.41409* | 0.4086* | 0.30612* | 0.37682* | 0.92617* |
| Precipitation (mm) | −0.12346 | −0.26018 | 0 | 0.819* | 0.99132* | 0.38675* | 0.97378* |
| Turbidity, NTU | 0.27273 | 0.26316 | −0.07408 | 0 | 0.33059* | 0.11818* | 0.41994* |
| Total coliform, CFU (mL−1) | 0.55245 | 0.32281 | −0.00353 | −0.308 | 0 | 0.33059* | 0.5995* |
| Fecal coliform, CFU 100 mL−1 | 0.56643 | 0.2807 | −0.27514 | 0.4755 | 0.30769 | 0 | 0.0085* |
| E. coli, CFU 100 mL−1 | 0.25353 | −0.03004 | −0.01066 | 0.2571 | 0.16902 | 0.71833 | 0 |
|
| |||||||
| b. VFS II effect significantly (p < 0.05) attributed to Kikuyu grass | |||||||
| Runoff, Q (m3 s−1) | 0.00023* | 0.15082* | 0.4303* | 0.001635* | 6.83E−05* | 0.000483* | |
| SSC (mg L−1) | 0.84924 | 0.27066* | 0.44064* | 0.003905* | 0.003151* | 0.00132* | |
| Precipitation (mm) | 0.42207 | 0.33011 | 0.33848* | 0.95014* | 0.59259* | 0.8124* | |
| Turbidity, NTU | 0.23967 | 0.23448 | −0.28886 | 0.35801* | 0.041* | 0.020782* | |
| Total coliform, CFU 100 mL−1 | 0.78069 | 0.73895 | 0.019284 | 0.27785 | 0.006618* | 0.005126* | |
| Fecal coliform, CFU, 100 mL−1 | 0.88138 | 0.75 | 0.16391 | 0.57222 | 0.70937 | 1.49E−06* | |
| E. coli, CFU 100 mL−1 | 0.82735 | 0.78977 | 0.073104 | 0.63086 | 0.72414 | 0.94207 | |
|
| |||||||
| c. VFS III effect significantly (p < 0.05) attributed to Napier grass | |||||||
| Runoff, Q (m3 s−1) | 0 | 0.048* | 0.44173* | 0.84458* | 0.55644* | 0.003* | 0.082803* |
| SSC (mg L−1) | 0.5805 | 0 | 0.08041* | 0.05381* | 0.25297* | 0.18* | 0.4019* |
| Precipitation (mm) | −0.246 | −0.524 | 0 | 0.81903* | 0.91678* | 0.607* | 0.89969* |
| Turbidity, NTU | 0.0635 | 0.568 | −0.07408 | 0 | 0.7513* | 0.887* | 0.96535* |
| Total coliform, CFU (mL−1) | 0.189 | 0.358 | 0.033868 | 0.10248 | 0 | 0.076* | 0.041563* |
| Fecal coliform, CFU 100 mL−1 | −0.784 | −0.415 | 0.16578 | 0.04594 | −0.53036 | 0 | 2.52E−05* |
| E. coli, CFU 100 mL−1 | −0.52 | −0.267 | 0.040853 | 0.01409 | −0.59431 | 0.918 | 0 |
p value
3.4 The relationship between E. coli and organic carbon released from cowpat into overland flow along the length of VFS
The cowpat was a heterogeneous matrix containing microscopic and macroscopic dietary fiber, various polymers, microbial colloids and soluble components. The count of E. coli and mass of OC recovered in overland flow were estimated as a percentage of the initial values of these factors in applied cowpat. Retained percentages from VFS field plots varied from 28.6 to 99.99 for E. coli and from 29 to 100 for OC. This study suggests that the manure particulates that carried E. coli could be the same ones carrying OC, but should there be differences between particulates that carry E. coli and OC; that difference could manifest itself in distributions of time intervals between particulate attachment and its release back to the flow.
If these time intervals were nonlinearly dependent on velocity, then the increase in dispersion might cause changes in the E. colinormalized: OC ratio. This hypothesis could also be supported by the increase in standard errors of OC recoveries with distance as opposed to E. coli recoveries (Table 6). Concentrations of dissolved organic carbon (DOC) were different from particulate organic carbon (POC) just described above. This study indicates that E. coli and DOC in the overland flow were related (R2 = 0.781). Data analysis using ANOVA demonstrated that the ratio E. coli: DOC significantly decreased with distance and slope in Kikuyu grass and Napier grass but not in Couch grass–Buffer grass combination.
Table 6.
Mean recovery of E. coli and total organic carbon in overland flow
| VFS | Grass species | Percentage soil cover (%) | Recovery in overland flow % of initial E. coli concentration in cowpat before overland flow experiment
|
|
|---|---|---|---|---|
| E. coli | OC | |||
| I | Couch | 96.5 | 31 ± 6.2 | 34 ± 4.2 |
| Buffer | 97.3 | 30 ± 1.4 | 38 ± 1.9 | |
| II | Kikuyu | 97.5 | 7.4 ± 1.3 | 8.1 ± 8.1 |
| Napier | 96.4 | 6.8 ± 3.6 | 9.8 ± 1.7 | |
| III | Kikuyu | 97.5 | 8.2 ± 1.2 | 10.3 ± 6.3 |
| Napier | 96.4 | 5.2 ± 7.0 | 7.3 ± 2.4 | |
This study also compared the reduction in concentrations of E. coli and OC in overland flow in Kikuyu grass, Napier grass and Couch grass–Buffer grass combination. The ratio of E. coli and OC concentrations in overland flow was compared after normalizing E. coli concentrations in overland flow to initial concentrations in cowpat in Napier grass, Kikuyu grass and Couch grass–Buffer grass combinations.
E. coli concentrations in cowpat were 2.0 × 106 CFU g−1, while OC from E. coli cells was 3.16 × 10−5 g of manure dry weight. Some of OC in overland flow might be attributed to the E. coli cells as it contains over 95 % OC on dry mass basis. However, OC from E. coli can constitute only small part of the OC found in cowpat as the dry mass of E. coli cell varied mostly between 0.11 × 10−12 and 1.0 × 10−12 g. On dry mass basis, the microbial total biomass was an estimated <30 % of manure mass. On the assumption that every microbial biomass was transported in overland flow in the same way as E. coli, then using an estimate of 95 % OC in E. coli, we found that E. coli OC constituted about <30 % of cowpat dry weight. Normalized E. coli and OC concentrations in overland flow were related in Napier grass (VFS III; R2 = −0.941; Fig. 9a), Kikuyu grass (VFS II; R2 = −0.711; Fig. 9b), but not in Couch grass–Buffer grass combination (VFS I; R2 = −0.371; Fig. 9c). In Fig. 9, the scatters observed might be caused by the effects of time, hydraulic, distance and hydrologic on the ratio of E coli normalized: OC, in both Napier grass and Kikuyu grass VFS. ANOVA revealed that distance and time had a significant effect on the ratio E. coli: OC.
Fig. 9.
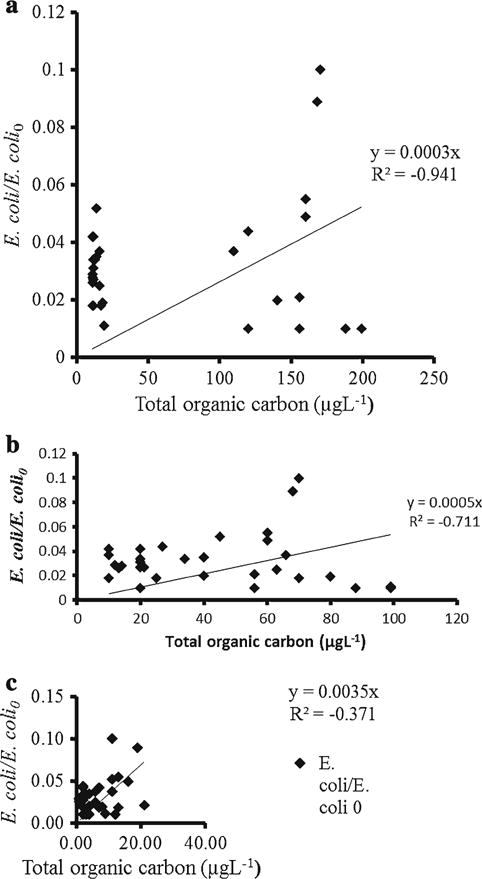
Relationship between normalized E. coli concentration and total organic carbon (OC) concentrations in overland flow through Napier grass, Kikuyu grass and Couch grass–Buffer grass; note E. coli—concentration of E. coli in overland flow; E. coli0—the initial concentration of E. coli in cowpat; also shown is the linear regression line
There were exponential curves obtained indicating approximate decreases in concentrations along different VFSs with time (Fig. 10a–f). The decrease in concentrations was estimated using the equation (Stout et al. 2005):
| (7) |
where C0 was the maximum concentration and k was the decrease rate constant. The equation was used to estimate the decrease in concentrations (C) of E. coli, fecal coliforms, total coliforms and overland flows. The mean values of the rate constants k for E. coli and OC ranged from 0.001 to 0.002 min−1 with a mean of 0.0016 min−1.
Fig. 10.
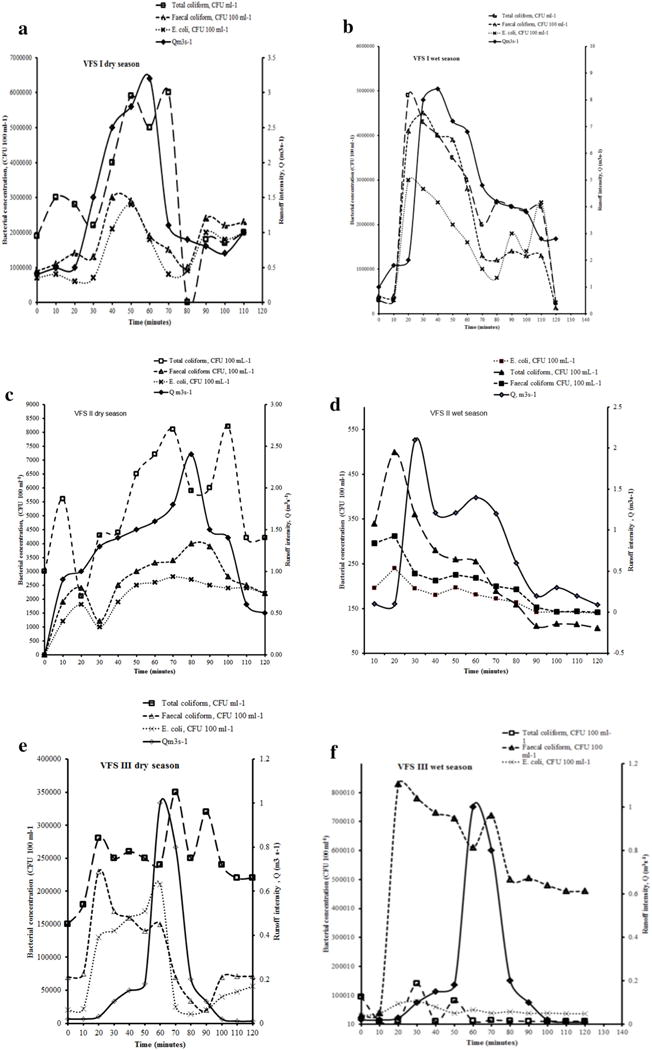
Temporal distribution of runoff intensity (m3s−1) in relation to total coliform, fecal coliform and E. coli concentrations in Couch grass–Buffer grass (VFS I), Kikuyu grass (VFS II) and Napier grass (VFS III) in dry (January–March 2014) season, wet (April–August 2014) season and short rainy seasons (September–March 2013) and (September–December 2014) seasons averaged across three replications, August 2013 to December 2014
4 Discussion
4.1 E. coli overland flow and transport model
Observed experimental data and predicted parameters of the model pasture significantly explained the transport and deposition rates of E. coli in the overland flow in relation to surface vegetation, hydrologic and soil hydraulics. Sediment delivery ratio showed that Napier grass was the most efficient in trapping particulate pollutants. This implied that E. coli attached to sediment particles, waste material and vegetation surfaces were trapped most efficiently as opposed to those entrained freely in overland flow. Sediment and overland flow properties influenced attachment of E. coli to solid particles because E. coli preferred cowpat waste particles to soil particles. This preference was probably because of the release of nutrients such as nitrates, phosphates and organic carbon from cowpat manure. These organic carbon compounds could be required by E. coli for metabolism to generate energy for survival, multiplication, growth and biomass production in the micro-biome. The hydraulic properties of soil in the Napier grass VFS could also contribute to the results measured because the production of humic acid from litters from dying plants’ leaves and stems could contribute large organic carbon in the clay loam soil surface, with considerable impacts on the E. coli survival and multiplication. The uncertainty analysis for Napier grass efficiency parameter provided the simulated VFS efficiency of nearly 100 %. Napier grass provided litter from the plants leaves as well as large stems. This finding contradicts previous reports on the status and challenges in modeling perspective of the water transport and fate of manure-borne pathogens and organisms indicators of fecal contamination at pedon, hillslope and watershed scales (Guber et al. 2006; Pachepsky et al. 2006). Dilution was least observed along the length of VFS III attributed to shielding of raindrops by the long distance (20 m) Napier grass leaves cover. This observation concurs with that of Schmitt et al. (1999) who reported that interception by tall prairie grasses and fully grown row crops could amount to 6 mm of a rainfall event. This further supports reports which show that younger crops and grasses that increase from zero to full cover during one growing season may intercept substantially less rain and may promote greater dilution of surface overland flow (Schmitt et al. 1999; Davis et al. 2009; Brown et al. 2013; Martinez et al. 2013; Allaire et al. 2015).
In the Kikuyu grass, there was low dispersivity value, αL = 7. The uncertainty analysis for Kikuyu grass VFS efficiency parameter provided the simulated VFS efficiency of 95.0 %.
However, Couch grass–Buffer grass combinations showed larger dispersivity values (αL = 65) than the rest of grasses in the VFS. When the equations for mean soil texture class parameters and water retention by Brooks and Corey were used to estimate capillary drive parameter, a value (27) obtained for the clay loam soil value estimate was close to estimates by Woolhiser et al. (1990). The smaller value of this parameter σ showed that this estimate approaches the Green–Ampt approximation (Woolhiser et al. 1990). This scenario meant a fast breakthrough occurrence of E. coli, which could be attributed to preferential overland flow pathways in the control. Similarly, the differences between estimated and observed concentrations in the Couch grass–Buffer grass studies could be attributed to preferential overland flow pathways. The integrity of VFS could have been compromised by the differences in grass species, disparities in vegetation densities and patchiness, and grass type and sizes, which allowed the bypass flow through the control VFS, which decreased its efficiency. Several challenges exist in obtaining parameters for these types of flow and transport models with bypass (Helmers et al. 2005; Guber et al. 2009). Different velocities in several portions of the overland flows in this study were represented by hydrodynamic dispersion in the model; however, for cases with concentrated or preferential and/or dominant bypass flows, this could have been insufficient. Couch grass–Buffer grass had the lowest efficiency in trapping E. coli. Parameters of Couch grass–Buffer grass combination provided low VFS efficiency (75 %) possibly because of concentrated flow or preferential flow produced by these VFSs.
4.2 Effect of different grass species and morphology in filtering E. coli
The soil bulk density in the VFS ranged from 0.079 to 0.22 g cm−3 with a mean of 0.17 g cm−3. This indicated that buffer efficiency (log 10 reduction) diminished as hydrologic transport capacity increased. This study hypothesized that the interception effect of different grass species affected the availability of E. coli for overall survival and losses to other pathways. In the VFS, different grass species influenced the cowpat applied to the model pasture, and raindrops and overland flow impacted upon the cowpat. The impact resulted in the E. coli being detached from the cowpat. It was then released from the cowpat and got entrained into overland flow pathway. The movements of E. coli upon being released from the cowpat micro-biome were suspected to follow several possible pathways. Initially, they could be intercepted by surface vegetation onto the leaves, stems and soil surface litter; secondly, they could be intercepted by the rough clay loam soil surface; thirdly, they could infiltrate through soil macro-pores into grass root zone system where they could move through interflow system back into the overland flow pathway; fourth, they could infiltrate into soil crevices of subsoil horizon zone; and fifth, they could compete for available nutrients and predation in the surface vegetation litter zone of soil surface, root zone and the subsoil horizon zone micro-biome. The processes of interceptions of E. coli along all these possible pathways influenced their availability for overall survival and multiplication rates in the overland flow and VFS field plots.
Napier grass showed the highest reduction efficiency of 99.97 %. In Napier grass surface vegetation, the rate of overland flow was significantly reduced as compared to other grass systems. The reason for this could be that Napier grass had larger leaves and stems than other existing grasses. The large leaves and stems reduced the velocity of these overland flows. This implied that E. coli velocity in the overland flow was also reduced and the discharge also reduced. This shows that E. coli concentration in the overland flow was positively related to rainfall in the Napier grass portion of the experimental field plots. The total overland flow volume interaction with Napier grass significantly influenced E. coli discharge. Napier grass significantly slowed down the surface hydrologic transport capacity. This illustrated that hydrologic transport was the limiting factor in the discharge of E. coli from Napier grass VFS. Large erratic torrential rainfall transport events accounted for the majority of E. coli discharge during the rainfall season. The role of 10-m Napier grass in reducing E. coli concentration in the overland flow during most rainfall events could be attributed to the limiting effect of surface hydrologic transport capacity. A cool, moist and nutrient-rich environment was the ecological mechanism behind increase in E. coli biomass in the surface vegetation litter, which accumulated on the soil surface. The plants litters helped moderate fluctuations in soil moisture and temperature where E. coli managed to survive and multiply. The increased infiltration capacity of overland flow was accompanied by reduced concentration of E. coli at the site. This was offset by improved E. coli survival and replication in the high humic environment, leading to the increase in the amount of E. coli discharged off the site. Apart from effects resulting into competition for available nutrients in the micro-biome, other pathways helped E. coli in being either protected from predation or sheltered for overall survival rates purposes. These effects were more significant in surface vegetation and specifically in Napier grass leaves, stems and litter than in other grass species because Napier grass leaves and stems were higher above the ground than the rest of the vegetation. The height of Napier grass above the ground helped in sheltering E. coli population, which attached themselves in the leaves and stems because majority of the micro-biome populations were more in the litter zone above the soil than the leaves and stems of this particular grass species.
Light intensity decrease in the overland flow depended largely on turbidity. Very little light penetrated the overland flow up to the soil surface. Oxygen concentrations on the surface of overland flow decreased reaching its minimum. The level of total phosphorus on the surface of overland flow was lower than the concentration in the soil surface. As the length of Napier grass VFS increased, total phosphorus levels reduced. Total nitrogen concentration was higher in soil surface than overland flow, showing that more nutrients were liberated in the soil surface than overland flow. Escherichia coli concentration was higher in the soil surface than in the overland flow. Firstly, this observation could be explained from the settling velocity or deposition perspective of E. coli to the soil surface or sediment from overland flow. Secondly, E. coli could be returning to the lotic overland flow water at the soil surface by small-localized upwelling currents at very slow rates. The return process could last for several minutes or hours depending on the duration of the overland flow on soil surface. Consequently, in the overland flow downslope, E. coli population survival and multiplication were severely limited by the lack of mineral nutrients, while at the beginning of VFS, soil surface had rich mineral nutrients. This resulted in significantly higher E. coli survival and multiplication in very specialized micro-biomes of soil macro-pore lentic water films. Soil macro-pore lentic water films served as new fresh mineral nutrients pumps supplying and sustaining the survival and multiplication of E. coli population. The minor upwelling water currents in the overland flow, which originated from soil macro-pores lentic water films, were formed as a result of either saturation excess or infiltration excess overland flows. This study hypothesized that the upwelling currents from the soil macro-pore lentic water films assisted E. coli populations, which were surviving and multiplying in these micro-biomes and returning to the lotic surface overland flow waters at very slow rates. When the mineral nutrients molecules returned to the lotic surface overland flow from lentic macro-pore water films by upwelling currents, the E. coli in overland flow would be supplied with those nutrients to sustain their survival and multiplication. Similarly, E. coli and other microbes to survive and multiply would utilize nutrients in the macro-pore lentic water films.
Kikuyu grass indicated reduction efficiency of 95.0 % in the VFS. In Kikuyu grass species, the most likely mechanism for the observed high efficiency of Kikuyu grass in the first 3 m in reducing E. coli concentration in the overland flow during most rainfall events could be attributed to the limiting effect of surface hydrologic transport capacity. However, for Kikuyu grass, leaves and stems touched the ground and were not in any way different from the litters, where the micro-biome populations were abundant, therefore causing more competition for the available nutrients and predation.
Couch grass–Buffer grass combination had the least reduction efficiency (75 %) because of concentrated or preferential flow in this VFS. In Couch grass–Buffer grass combination, however, the leaves and stems touched the ground and were not in any way different from the litters, where the micro-biome populations were abundant, therefore causing more competition for the available nutrients and predation. The Couch grass–Buffer grass (control) had one factor that helped increase the loss of E. coli population from the design in that there was the creation of preferential overland flow in these plots. Therefore, these control plots were the poorest in the retention of E. coli population within the entire design system because of the continued creation of preferential flow for every rainfall event. On the soil surface, the plants’ litters concentrated the percolating water onto micro-surfaces, which formed microhabitats with unique temperatures, which was slightly above the surrounding soil temperatures.
Different morphology of the different grass species such as leaves, stem and the dropped litter influenced the filtering mechanism of E. coli in the overland flow pathway. Such finding concurs with those that showed that volunteer grasses and forbs created better ground cover (Schmitt et al. 1999; Allaire et al. 2015).
4.3 Effect of surface vegetation in filtering E. coli
The mechanisms of E. coli transport were influenced by VFS functioning, which was connected to the decreasing edge of field losses including detachment and release rates, particulate transport rates, adsorption, infiltration and deposition processes. Initially, the E. coli in the cowpat was detached from the cowpat upon being impacted by rainfall drops or overland flow. The released E. coli was entrained into the infiltration excess overland flow and/or saturation excess overland flow. In this study, data showed that among the cowpat constituents, in the presence of organic and inorganic particles, including organic carbon particles, release rates of E. coli colloids tended to be slower than the release rates of other particles.
This study found that surface vegetation had four significant functions including first the decrease in overland flow velocity resulting in the loss of transport capacity, which led to the deposition of E. coli and adsorption onto clay loam soil particles. Secondly, E. coli could be adsorbed either onto the vegetation leaf, stem and litter or onto clay loam soil particles. Thirdly, surface vegetation improved percolation of E. coli entrained in overland flow into the root zone systems, which could be moved through interflow system back to the overland flow. Fourth, the surface vegetation also increased loss of the E. coli through infiltration rate of the overland flow into subsoil horizon zone. Factors controlling partitioning of different forms of E. coli (E. coli attached to either waste, or soil particles, or free unattached populations) during transport once they were entrained into the overland flow along the length of VFS were: interactions between environmental; soil hydraulics such as viscosity of water and other solutes in the soil; water hydrologic; and different vegetative factors. Moreover, Tyrrel and Quinton (2003) suggested that the fate and transport of microorganisms was influenced by rate of microbial detachment from manure, soil or epiphyte such as the amount of microbes attached to the soil surface; biological sheltering mechanisms; the strength of attachment to each other and the particle surfaces; the amount of rainfall kinetic energy; and the strength and velocity of overland flow. The transport and deposition of E. coli could be influenced by their density and size of particles. Paul and Clark (1996) suggested that small-sized particles of low density could remain in suspension when entrained in overland flow because they settled more slowly than particles of large and high density. This implies that E. coli cell that was strongly attached to a soil particle could not travel further than the one floating freely in sediment suspension. The low E. coli intercepting efficiency observed in some VFS suggested that deposition was less likely to occur in free-floating E. coli once entrained in the overland flow because of their lower density and small sizes than clay loam soil particles of similar sizes. The observed reduction in E. coli populations in overland flows passing through some VFS suggests that those plants were capable of intercepting E. coli cells transported freely with the overland flows. Escherichia coli in overland flow could either be deposited in sediment particles or be infiltrated alongside all the infiltrating water, or they were intercepted by the surface vegetation or in case of any deposition occurring on the soil surface; then, those E. coli were associated with soil particles. Coyne et al. (1998) reported of variability in trapping efficiencies of VFS and suggested the existence of variation in partitioning of microbes between water and suspended soil particles.
In the Napier grass litter zone of the surface vegetation, two possible effects could be deduced: First, it was possible that E. coli were immobilized by surface soil macro-pore–microbial interaction; secondly, there could be bacterial die-off on the surface due to a drop in overland flow temperature, creating temperature inversion on the soil surface, up to less than 10 °C at night. The temperature inversion hypothesis suggests low temperatures, which could create variation in the survival and multiplication resulting from environmental variation of the surface soil micro-biome of VFS. This condition could create temporary and short-lived, but lethal effects on the survival and multiplication of E. coli population. The temperature inversion concept in the soil surface micro-biome could be a new phenomenon to the E. coli microhabitat far different from the primary animal host (gastroduodenum–ileum–colon interface) environment, which could henceforth enhance their rate of die-off.
This study hypothesized that lentic waters on the surface of plants litters provided the warm, moist and cool microhabitat, which helped the population of E. coli in dissolving existing nutrients such as nitrates, phosphates and organic carbon. This hypothesis suggests that different grass species morphology and zones in the VFS influenced successful sustainable survival and multiplication of E. coli population. This hypothesis supports some of the most important processes, which have been reported to have a significant influence on the successful survival and multiplication of E. coli not only in vegetation litters micro-biome, but also in other ecosystems, which include: (1) E. coli strains establishing encoding regulation mechanisms, such as the attaching and effacing regulators in pathogenic and commensals E. coli strains and E. coli’s quorum-sensing mechanism, which transforms it to initiate intercellular communication, and which allows the unicellular organism to behave like multicellular organisms (Kaper et al. 2004); (2) E. coli’s multistep scheme nature of pathogenesis in occupying the new niche using adhesion and colonization morphological structures such as fimbriae (pili) and/or flagellin to attach to mucosal site; evade host defenses, multiply and damage the host cells (Kaper et al. 2004); (3) E. coli’s ability to establish high metabolic niche and to utilize available dissolved organic carbon and other nutrients more efficiently than other existing resident microbial species that enables it to compete better than other resident microbes (Sweeney et al. 1996) in the root system and to establish itself in the new environment and (4) finally, enhanced biofilm formation in the lentic water film in the plants litters (Quero et al. 2015).
4.4 Effect of roots system in filtering E. coli
The root system of different plant species including soil particles, nutrients, organic carbon or humus and interflow influenced the filtering of E. coli in overland flow pathway. In the Napier grass root zone system, infiltration of overland flow was highest (VFS III) due to the effect of Napier grass compared to VFS II and VFS I. The Napier grass (20 m) design showed that infiltration was highest in this VFS because Napier grass has structures similar to that of trees with a height that can be as high as 3-meter-long stem and deep roots system. However, the Napier grass (20 m) design contrasts the hypothesis that grass–shrub–tree design may outperform grass alone (Schmitt et al. 1999; Allaire et al. 2015). This was because Napier grass had characteristics of a tree such as long stem, deep roots and heights of up to 3 meters or more.
The kinetics or dynamics of the release of extracellular organics from the roots of Napier grass was utilized by E. coli to provide the optimal environmental ability to survive and multiply because of prolonged release of cowpat nutrients and organic constituents on the vegetated field plots. This study hypothesized that the root zone system temperature inversion at night for over 6 h significantly impacted on some of the most important lifestyle processes of E. coli including decrease in the biomass; low metabolic niche; substrate limiting particularly nitrates; and increased dormancy or persistence of the population in the micro-biome. E coli population abundance and attachment on surfaces were positively influenced by particles originating from the plants roots that had some level of organic carbon as compared to particles originating from soils such as clay, silt and sand. This occurred highest along the length of VFS III from Napier grass, followed by VFS II and finally least impacts on VFS I. The roots of Napier grass that majorly comprised of organic carbon particles in field plots attracted higher percentage of E. coli in the overland flow. However, non-organic carbon particles such as sand, silt and clay particles attracted only a lower percentage of E. coli. In the Napier grass, the attachment of E. coli depended on the type and source of particle in the overland flow. In the rhizosphere of Napier grass, which was the thin layer of clay loam soil around the root system of the vegetation, several major effects were significant. These effects included increased rate of die-off of E coli due to predation; secondly, higher rate of competition for the available nutrients with resident microbes in the micro-biome; and thirdly, immobilization of E. coli through adsorption onto clay loam soil particles, where most of the E. coli were removed from interaction with surface overland flow, therefore reducing E. coli population available for loss through infiltration into soil matrix. Three reasons are suggested for the attachment of E. coli, namely: Soil particles protected them from predation by resident microbes in the soil particle microhabitat; they obtained nutrient substrates from soil microhabitat; these microhabitats provided them optimum temperature on which to multiply. Another issue was that the overall survival rates of E. coli were enhanced because of the sheltering of this population from desiccation due to the available soil moisture content, protection from die-off due to the absence of direct sunlight radiation and the transition and protection from harsh temperature in the soil surface to the availability of optimum temperature in the soil matrix. In the root zone system, the available humic acid and fulvic acids and the presence of mucilage layer from Napier grass could favor the multischeme step process in this zone. In this zone, E. coli interacted with the aromatic core of humic acid consisting of aromatic heterocyclic and quinoidal rings, which bore carboxyl, phenolic hydroxyl and carbonyl groups attached to amino acids, peptides, sugars and phenols. These cross-linkages resulted in a sponge-like three-dimensional structure, which readily absorbed water, ions and organic molecules, and these reactive functional parts could bind to the E. coli double membrane. In this zone, the significantly high amount of E. coli population could be attributed to the capability of E. coli population to utilize higher metabolic niche than the resident microbes in this micro-biome. This process of high metabolic niche effect was enhanced by the modifications of the chemical environment of clay loam soil by the resident microbes through processes including water uptake by the grasses root systems; release of organic chemicals to the clay loam soil by the plants roots; release and production of important plants’ growth hormones such as auxins and gibberellins; and significant plants mineral nutrients mediated by microbial organisms in the root zone micro-biome. Large population of E. coli occurred within the grooves of the epidermal Napier grass cells, on the root surface and within the mucilaginous layers surrounding the root system. The release of this material stimulated the growth of E. coli alongside other microbes, but E. coli had a higher metabolic niche than the rest of microbes. This was because E. coli was capable of metabolizing these sugars more efficiently than the rest of the microbes in the micro-biome. There was also positive chemotactic response of E. coli due to materials released by the Napier grass roots microbes.
In the root zone of Napier grass, several environmental (humic acid, nitrate nitrogen, soil pH and soil temperature); hydraulic (soil moisture, initial water content of the soil, release rates); and hydrologic (overland flow rate, settling velocity of E. coli attached to soil particles) factors contributed significantly to the high amount of E. coli observed in the clay loam soil surface. This study hypothesized that these environmental, hydraulic and hydrologic factors significantly promoted reduced dormancy or developed persistence of non-viable units. Fast adaptation to these new niches and utilization of these processes to its own advantage suggests that E. coli population were versatile and were capable of establishing themselves in new habitats under harsh environmental conditions that could not be tolerated by several resident microbes in the micro-biomes. The root zone system optimum temperature initiated specific growth rate of E. coli that resulted in the increase in biomass in the micro-biome.
In the root zone of Kikuyu grass, the kinetics or dynamics at the roots release of extracellular organics from the roots of this grass were capable of being utilized by E. coli to provide the optimal environmental ability during the prolonged release of cowpat constituents on the vegetated field plots.
In the root zone of Couch grass–Buffer grass combination, the kinetics or dynamics at the roots release of extracellular organics from the roots of these plants roots were lowest compared to the rest of other grasses in this experimental plots studied, which were capable of being utilized by E. coli to provide the optimal environmental ability during the prolonged release of cowpat constituents on the vegetated field plots.
4.5 Effect of subsoil horizon zone in filtering E. coli
The clay loam soil particles macro-pores could have provided E. coli with a significant sheltering; protective; and large surface area for oxygen uptake and general gaseous exchange; and water film or moisture, which was moist, and cool on which to initiate the process of competition for the available nutrients with subsoil facultative anaerobes microbes and predator microorganisms in the micro-biome. The subsoil horizon influenced the filtering of E. coli through infiltration through soil macro-pores and adsorption on soil particles. In this habitat, E. coli tested had developed environmentally resistant stage by developing cysts and becoming dormant and took over 48 h to multiply to a half of its initial population.
4.6 Relationship between E. coli and OC in overland flow
The overland flow data were analyzed to identify any consistency in relationship between E. coli and OC in overland flow. The mean values of the ratio E. colinormalized: OC increased as the distance and slope increased. This implies that increase in distance and slope resulted in increased dispersion of both E coli and OC in the transport more in Kikuyu grass than in Napier grass, as indicated in differences in overland flow velocity and infiltration rate along different pathways along the VFS.
Higher manure dissolution rates with overland flow than infiltration were observed suggesting that the values recorded for the rate constants were higher for cowpat dissolution rates with low means for experiments with infiltration only without overland flows suggesting higher manure dissolution rates with overland flow than infiltration. These findings concur with those of other previous studies (Moor et al. 1988; Bradford and Schijven 2002; Shelton et al. 2003; Stout et al. 2005).
There were no significant average recovery percentages suggesting that the rates of E. coli and OC rates of release from cowpat and transport in overland flow were similar but not the same. Retention of E. coli in Kikuyu grass was highest in the first 1 m, followed by retention in Napier grass, but the least retention was observed in Couch grass–Buffer grass combination because of formation of preferential overland flow in the Couch grass–Buffer grass combination. This similarity in transport could show little to prove that the fast release and slow transport could influence the same retention as the slow transport and fast release. There was similarity of recovery from slopes of different lengths in Kikuyu grass, Napier grass but not in Couch grass–Buffer grass combination. In the first 1 m, the Kikuyu grass showed highest substantial loss by surface retention and infiltration of E. coli. At 3 m from the edge of Napier grass, substantial loss due to surface retention and infiltration started to overtake the losses observed in Kikuyu grass VFS. The 30-m Couch grass–Buffer grass combination did not show any substantial loss due to surface retention and infiltration, because of the preferential flow pathway. Stout et al. (2005) showed that similarity in transport of fecal coliform and phosphorus could not be proven with recovery data because fast release and slow transport had the same recovery effect as slow transport and fast release.
This suggests that over short and long distances, OC and E. coli are transported similarly and the transport of OC is substantially affected by deposition of solid phase (particulate organic carbon). This finding contrasts that of Stout et al. (2005) on fecal coliform: total phosphorus ratio, who reported that total phosphorus and fecal coliform were transported similarly without being affected substantially by deposition of solid phase over a short distance. They reported that total phosphorus to fecal coliform ratio was not significantly related to time, distance and slope. The current study had a constant slope, so there was no variation of the slope. This implies that DOC was transported exclusively in aqueous phase and was unaffected by sorption, particulate transport or deposition processes, which highly affected E. coli transport. Fajardo et al. (2001) and Stout et al. (2005), respectively, reported differences between E. coli and nitrate and between E. coli and chloride transport along the length of VFS. The correlation in OC and E. coli overland flow concentrations could be related to a large extent basically to the transport of OC in E. coli cells. Other dissolved organic carbon forming complexes binding to external cell sites and humic and fulvic acids could contribute to the observed correlations. Different authorities have also reported the contributions of E. coli to manure total biomass (Loferer-Kroßbacher et al. 1998; Neidhardt et al. 1990; Van Kessel et al. 2000).
5 Conclusions
The high overland flow velocity and volume enhanced the discharge of high concentration and load of E. coli. Napier grass provided the highest resistance of the overland flow velocity thus reducing the transport capacity leading to the improved filtering of E. coli in the root zone of this particular plant. Prolonged stay of E. coli in the Napier grass VFS due to the reduced transport capacity could lead to increased E. coli die-off as a result of exposure to high temperatures and ultraviolet radiation. This study reported that temperature inversion in the plants root system zone micro-biome influenced several important E. coli processes including persistence. The overland flow pathway was an external and a new environmental habitat different from the normal animal gastrointestinal tract where E. coli microbe normally resides. The particulate movement, sorption, deposition and infiltration processes into the surface vegetation, root system and subsoil horizon habitats partitioned cowpat-borne E. coli released into the overland flow pathway. Despite the new environmental conditions in its new habitats, E. coli was capable of residing and competing with other resident microbial species for the available nutrients resources. The data demonstrate a similarity in transport of cowpat manure-borne E. coli and OC through simulated Kikuyu grass, Napier grass and Couch grass–Buffer grass VFS. This kind of similarity was observed in the whole of the overland flow pathway under saturated conditions, which was close to saturated excess flow where infiltration was limited. These results may be different in representing E. coli and OC transport where there are unsaturated soils and high rates of infiltration, but they actually indicate that it could be possible to use OC as a true tracer of manure-borne E. coli in overland flow. Under new and unfavorable environmental condition, E. coli entered a dormant stage and lost its ability to compete resident microbial populations, which it would outcompete under normal environmental conditions. The present study suggests the existence of selective detachment and transport of waste materials and related E. coli were likely to occur because of their lower density than entrained soil particles of similar sizes. Thus, efficient filtering along the lengths of grassed VFS to reduce E. coli concentrations and load in overland flows requires managing combined grass species, agro-pastoral systems models and dispersed or preferential flows to enhance surface water quality standards. More studies need to be carried out in different types of hydraulic, hydrologic, watersheds and climatic regimes under different vegetation conditions to shed light on filtering mechanisms of pathogens of livestock manure by different vegetation types.
Supplementary Material
Acknowledgments
Professor Japheth O. Onyando (Chairman, Department of Agricultural Engineering and Technology), Dr. Wilkister N. Moturi (Chairperson, Department of Environmental Science) and Dr. Anastasia W. Muia (Department of Biological Sciences) at Egerton University (EU) initiated the experimental design. We also appreciate permission granted by the Dean Faculty of Agriculture to work in field 18 of Tatton Agriculture Park (TAP). We acknowledge the Microbiology Laboratory at Kenya Marine and Fisheries Research Institute (KMFRI) for microbiological work, including the identification of E. coli strains. We also wish to thank technicians at the EU Soil Science Laboratory for analyzing soil samples from the field, the library staff at EU for access to scientific literature within the Agricultural Electronic Library (TAEL), the staff at the EU Department of Water, Environmental and Civil Engineering for providing the meteorological data from the weather station, and Mr. Godfrey Maina from the EU Department of Environmental Science for site cartographic work. Dr. Renison Ruwa (the Director of KMFRI) under the EU-KMFRI Memorandum of Understanding (MOU) granted study permission. The Kenya National Commission of Science and Technology under the Science, Technology and Innovation PhD research grant number NCST/ST & I/RCD/4th Call Ph.D./181 provided funding.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s40974-016-0006-y) contains supplementary material, which is available to authorized users.
References
- Abu-Zreig M, Rudra RP, Whiteley HR, Lalonde MN, Kaushik NK. Phosphorus removal in vegetated filter strips. J Environ Qual. 2003;32(2):613–619. doi: 10.2134/jeq2003.6130. [DOI] [PubMed] [Google Scholar]
- Akan A. Preliminary design aid for bio retention filters. J Hydrol Eng. 2013 doi: 10.1061/(ASCE)HE.1943-5584.0000554. [DOI] [Google Scholar]
- Allaire SE, Sylvain C, Lange SF, Thériault G, Lafrance P. Potential efficiency of riparian vegetated buffer strips in intercepting soluble compounds in the presence of subsurface preferential flows. PLoS ONE. 2015;10(7):e0131840. doi: 10.1371/journal.pone.0131840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Public HealthAssociation, APHA. Standard methods for the examination of water and wastewater. 18th. Am Public health Assoc; New York: 1992. [Google Scholar]
- Berry ED, Wells JE. Escherichia coli O157:H7: recent advances in research on occurrence, transmission, and control in cattle and the production environment. Adv Food Nutr Res. 2010;60:67–117. doi: 10.1016/S1043-4526(10)60004-6. [DOI] [PubMed] [Google Scholar]
- Bougeard M, Le Saux Jc, Teillon A, Belloir J, Le Mennec C, Thome S, Durand G, Pommepuy M. Combining modeling and monitoring to study fecal contamination in a small rural catchment. J Water Health. 2011;9(3):467–482. doi: 10.2166/WH.2011.189. [DOI] [PubMed] [Google Scholar]
- Bradford SA, Schijven JF. Release of Cryptosporidium and Giardia from dairy calf manure: impact of solution salinity. Environ Sci Technol. 2002;36:3916–3923. doi: 10.1021/es025573l. [DOI] [PubMed] [Google Scholar]
- Brown R, Birgand F, Hunt W. Analysis of consecutive events for nutrient and sediment treatment in field-monitored bioretention cells. Water Air Soil Pollut. 2013 doi: 10.1007/s11270-013-1581-6. [DOI] [Google Scholar]
- Cardoso F, Shelton D, Sadeghi A, Shirmohammadi A, Pachepsky Y, Dulaney WJ. Effectiveness of vegetated filter strips in retention of Escherichia coli and Salmonella from swine manure slurry. Environ Manage. 2012;110:1–7. doi: 10.1016/j.jenvman.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Coyne MS, Gilffillen RA, Villalba A, Rhodes R, Dunn L, Blevins RL. Fecal bacteria trapping by grass filter strips during simulated rain. J Soil Water Conserv. 1998;53:140–145. [Google Scholar]
- D’Elia CF, Steudler PA, Corwin N. Determination of total nitrogen in aqueous samples using persulfate digestion. Limnol Oceanogr. 1977;22:760–764. [Google Scholar]
- Davis A, Hunt W, Traver R, Clar M. Bioretention technology: overview of current practice and future needs. J Environ Eng. 2009;135(3):109–117. doi: 10.1061/(ASCE)0733-9372(2009)135:3(109). [DOI] [Google Scholar]
- Deletic A. Modelling of water and sediment transport over grassed areas. J Hydrol (Amsterdam) 2001;248:168–182. [Google Scholar]
- Duchemin M, Hogue R. Reduction in agricultural non-point source pollution in the first year following establishment of an integrated grass/tree filter strip system in southern Quebec (Canada) 2008 doi: 10.1016/j.agee.2008.10.005. [DOI] [Google Scholar]
- Eisakhani M, Malakahmad A. Water quality assessment of Bertram River and its tributaries in cameron highlands, Malaysia. J World Appl Sci. 2009;7(6):769–776. [Google Scholar]
- Entry JA, Hubbard RK, Thies JE, Furman JJ. The influence of vegetation in riparian filter strips on coliform bacteria. 1. Movement and survival in water. J Environ Qual. 2000;29:1206–1214. [Google Scholar]
- Fajardo JJ, Bauder JW, Cash SD. Managing nitrate and bacteria in runoff from livestock contamination areas with vegetative filter strips. J Soil Water Conserv. 2001;56(3):185–191. [Google Scholar]
- Ferens DM, Habgood MD, Saunders NR, Tan YH, Brown DJ, Brock JA, et al. Stimulation of defecation in spinal cord-injured rats by a centrally acting ghrelin receptor agonist. Spinal Cord. 2011;49:1036–1041. doi: 10.1038/sc.2011.60. [DOI] [PubMed] [Google Scholar]
- Fiener F, Auerswald K. Effectiveness of grassed waterways in reducing runoff and sediment delivery from agricultural watersheds. J Environ Qual. 2003;32:927–936. doi: 10.2134/jeq2003.9270. [DOI] [PubMed] [Google Scholar]
- Fletcher T, Andrieu H, Hamel P. Understanding, management and modelling of urban hydrology and its consequences for receiving waters: a state of the art. Adv Water Resour. 2013 doi: 10.1016/j.advwatres.2012.09.001,261-279. [DOI] [Google Scholar]
- Foster GR. Modeling the erosion process. In: Baselman JA, editor. Hydrological modeling of small watersheds. Vol. 8. 1982. pp. 297–380. (ASAE Monograph). [Google Scholar]
- George DB, Manges AR. A systematic review of outbreak and non-outbreak studies of extraintestinal pathogenic Escherichia coli causing community-acquired infections. Epidemiol Infect. 2010;138(12):1679–1690. doi: 10.1017/S0950268810001639. [DOI] [PubMed] [Google Scholar]
- Guber AK, Shelton DR, Pachepsky YA, Sadeghi AM, Sikora LJ. Rainfall-induced release of faecal coliforms and other manure constituents: comparison and modelling. Appl Environ Microbiol. 2006;72:7531–7539. doi: 10.1128/AEM.01121-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guber AK, Yakirevich AM, Sadeghi AM, Pachepsky YA, Shelton DR. Uncertainty evaluation of coliform bacteria removal from vegetated filter strip under overland flow condition. J Environ Qual. 2009;38:1636–1644. doi: 10.2134/jeq2008.0328. [DOI] [PubMed] [Google Scholar]
- Guber AK, Pachepsky YA, Yakirevich RV, Shelton DR, Sadeghi AM, Goodrich DC, Unkrich CL. Uncertainty in modelling of fecal coliform overland transport associated with manure application in Maryland. Hydrol Proc. 2011;25:2393–2404. [Google Scholar]
- Hammer O, Harper DAT, Ryan PD. PAlaeontological STAtistics Ver. 2005;1:34. http://folk.uio.no/Ohammer/Past. [Google Scholar]
- Hay V, Pittroff W, Tooman EE, Meyer D. Effectiveness of vegetative filter strips in attenuating nutrient and sediment runoff from irrigated pastures. J Agric Sci. 2006;144:349–360. [Google Scholar]
- Helmers MJ, Eisenhauer DE, Franti TG, Dosskey MG. Modelling sediment trapping in a vegetative filter accounting for converging overland flow. Trans ASABE. 2005;48(2):541–555. [Google Scholar]
- Hurley S, Forman R. Stormwater ponds and biofilters for large urban sites: modeled arrangements that achieve the phosphorus reduction target for Boston’s Charles River, USA. Ecol Eng. 2011:850–863. doi: 10.1016/j.ecoleng.01.008. 2011. [DOI] [Google Scholar]
- Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental design: procedures for the behavioural Sciences. 2nd. Brooks Cole Publishing Co; Belmont: 1982. [Google Scholar]
- Krishnappan BG, Marasalek J, Exall K, Stephens RP, Rochfort Q, Un Seto P. A water elutriation apparatus for measuring settling velocity distribution of suspended solids in combined sewer overflows. Can J Water Qual Res. 2004;39:432–438. [Google Scholar]
- Kruger T, Freer J, Quinton JN, Macleod CJA. Processes affecting transfer of sediment and colloids, with associated phosphorus, from intensively farmed grasslands: a critical note on modelling of phosphorus transfers. Hydrol proc. 2007;21:557–562. [Google Scholar]
- Lafrance P, Caron E. Impact of vegetated filter strips on sorbed herbicide concentrations and sorption equilibrium in agricultural plots. J Environ Sci Health B. 2012;47(10):967–974. doi: 10.1080/03601234.2012.706565. [DOI] [PubMed] [Google Scholar]
- Larson RA, Safferman SI. Field application of farmstead runoff to vegetated filter strips: surface and subsurface water quality assessment. J Environ Qual. 2012;41(2):592–603. doi: 10.2134/jeq2011.0125. [DOI] [PubMed] [Google Scholar]
- LeJeune JT, Wetzel AN. Pre harvest of Escherichia coli 0157 in cattle. J Animal Sci. 2007;85:E73–E80. doi: 10.2527/jas.2006-612. [DOI] [PubMed] [Google Scholar]
- Lewis DJ, Atwill ER, Lennox MS, Pereira MDG, Miller WA, Conrad PA, Tate KW. Reducing microbial contamination in storm runoff from high use areas on California coastal dairies. Water Sci Tech WST. 2009;60(7):1731–1742. doi: 10.2166/wst.2009.561. [DOI] [PubMed] [Google Scholar]
- Lim TT, Edwards DR, Workman SR, Larson BT, Dunn L. Vegetated filter strip removal of cattle manure constituents in overland. Trans ASAE. 1998;41:1375–1381. [Google Scholar]
- Ling TY, Jong HJ, Apun K, Wan Sulaiman WH. Quantifying Escherichia coli release from soil under high -intensity rainfall. Trans ASAE. 2009;52(3):785–792. [Google Scholar]
- Liu J, Davis A. Phosphorus speciation and treatment using enhanced phosphorus removal bioretention. Environ Sci Tech. 2014 doi: 10.1021/es404022b,607-614. [DOI] [PubMed] [Google Scholar]
- Loferer-Kroβbacher M, Klima J, Psenner R. Determination of bacterial cell dry mass by transmission electron microscopy and densitometric image analysis. Appl Environ Microbiol. 1998;64:688–694. doi: 10.1128/aem.64.2.688-694.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrance R, Vellids G, Wauchope RD, Gay P, Bosch DD. Herbicide transport in a managed riparian forest buffer system. Trans ASAE. 1998;40:1047–1057. [Google Scholar]
- Lucas W, Greenway M. Nutrient retention performance of advanced bioretention systems results from three years of mesocosm studies. Low impact development technology: design methods and case studies. 2015:43–58. [Google Scholar]
- Martinez G, Pachepsky YA, Whelan G, Yakirevich AM, Guber A, Gish TJ. Rainfall-induced fecal indicator organisms transport from manured fields: model sensitivity analysis. Environ Int. 2013;63C:121–129. doi: 10.1016/j.envint.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Maus C, Holtrup F, Uhl M. Measurement method for in situ particle settling velocity. 11th international conference on urban drainage; Edinburgh, Scotland UK. 2008; 2008. pp. 1–10. [Google Scholar]
- Mohanty S, Torkelson A, Dodd H, Nelson K, Boehm A. Engineering solutions to improve the removal of fecal indicator bacteria by bioinfiltration systems during intermittent flow of stormwater. Environ Sci Tech. 2013 doi: 10.1021/es305136b,1-10798. [DOI] [PubMed] [Google Scholar]
- Moore JA, Smyth J, Baker S, Miner JR. Agricultural experiment station. Oregon State University; Corvallis, OR, USA: 1988. Evaluating coliform concentrations in runoff from various animal waste management systems. Special report 817. [Google Scholar]
- Morales-Morales HA, Vidal G, Olszewski J, Rock CM, Dasgupta D, Oshima KH, Smith GB. Optimization of a reusable hollow-fiber ultrafilter for simultaneous concentration of enteric bacteria, protozoa, and viruses from water. Appl Environ Microbiol. 2003;69(7):4098–4102. doi: 10.1128/AEM.69.7.4098-4102.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Carpena R, Parsons JE. Evaluation of VFSMOD, a vegetated filter strips hydrology and sediment filtration model. ASAE/CSAE-SCGR Annual International Meeting. 1999:6. [Google Scholar]
- Muñoz-Carpena MR, Parsons JE. Agricultural and biological engineering. University of Florida; 2011. VFSMOD-W, vegetated filter strips modelling system, model documentation and user’s manual version 6.x; p. 0570. [Google Scholar]
- Muñoz-Carpena R, Fox GA, Sabbagh GJ. Parameter importance and uncertainty in predicting overland pesticide reduction with filter strips. J Qual. 2010;39(1):1–12. doi: 10.2134/jeq2009.0300. [DOI] [PubMed] [Google Scholar]
- Nash JE, Sutcliffe JE. River flow forecasting through conceptual models: part I. A discussion of principals. J Hydrol. 1970;10:282–300. [Google Scholar]
- Neidhardt FC, Ingraham JL, Schaechter M. Physiology of the bacterial cell: a molecular approach. Sinauer Associates; Sunderland: 1990. [Google Scholar]
- Pachepsky YA, Sadeghi AM, Bradford SA, Shelton DR, Guber AK, Dao T. Transport and fate of manure-borne pathogens: modeling perspective. Agric Water Manag. 2006;86(1–2):81–92. doi: 10.1016/j.agwat.2006.06.010. [DOI] [Google Scholar]
- Pachepsky Y, Shelton D, Dorner S, Whelan G. Can E. coli or thermo tolerant coliform concentrations predict pathogen presence or prevalence in irrigation waters? Crit Rev Microbiol. 2015;10:1–10. doi: 10.3109/1040841X.2014.954524. [DOI] [PubMed] [Google Scholar]
- Park YS, Engel BA, Shin Y, Choi J, Kim NW, Kim SJ, Kong DS, Lim KJ. Development of web GIS-based VFSMOD System with three modules for effective vegetative filter strip design. Water. 2013;5:1194–1210. doi: 10.3390/w5031194. [DOI] [Google Scholar]
- Parlange JY, Lisle Y, Braddock RD, Smith RE. The three parameter infiltration equation. Soil Sci. 1982;133:337–341. [Google Scholar]
- Paul EA, Clark FE. Soil microbiology and biochemistry. 2nd. London: Academic Press; 1996. London. [Google Scholar]
- Press WW, Teukolsky SA, Vetterling WT, Flannery BP. Numerical recipes in Fortran: the art of scientific computing. 2nd. Cambridge Univ. Press; New York: 1992. [Google Scholar]
- Quero GM, Fasolato L, Vignaroli C, Luna GM. Understanding the association of Escherichia coli with diverse macro algae in the lagoon of Venice. Sci Rep. 2015;5(10969):1–33. doi: 10.1038/srep10969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippy M. Meeting the criteria: linking biofilter design to fecal indicator bacteria removal. Wiley Interdiscip Rev Water. 2015 doi: 10.1002/wat2.1096. [DOI] [Google Scholar]
- Russo TA, Johnson JR. Medical and economic impact of extra intestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5(5):449–456. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Schijven JF, Bradford SA, Yang S. Release of cryptosporidium and Giardia from dairy calf manure: physical factors. J Environ Qual. 2004;33:1499–1508. doi: 10.2134/jeq2004.1499. [DOI] [PubMed] [Google Scholar]
- Schmitt TJ, Dosskey MG, Hoagland KD. Filter strip performance and processes for different vegetation, widths, and contaminants. J Environ Qual. 1999;28:1479–1489. [Google Scholar]
- Shelton D, Pachepsky YA, Sadeghi AM, Stout WL, Karns JS, Gburek WJ. Release rates of manure-borne coliform bacteria from data on leaching through stony soil. Vadoze Zone J. 2003;2:34–39. [Google Scholar]
- Stout WL, Pachepsky YA, Shelton DR, Sadegh AM, Saporito LS, Sharpley AN. Runoff transport of fecal coliforms and phosphorus released from manure in grass buffer conditions. Lett Appl Microbiol. 2005;41:230–234. doi: 10.1111/j.1472-765X.2005.01755.x. [DOI] [PubMed] [Google Scholar]
- Strickland JDH, Parsons TR. A practical handbook of sea water analysis, 2nd edn. Bull Fish Res Bio Can. 1972;167:310. [Google Scholar]
- Sweeney NJ, Klemm P, McCormick BA, Moller-Nielsen E, Utley M, Schembri MA, Laux DC, Cohen PS. The Escherichia coli K-12 gntP gene allows E. coli F-18 to occupy a distinct nutritional niche in the streptomycin-treated mouse large intestine. Infect Immun. 1996;64(9):3497–3503. doi: 10.1128/iai.64.9.3497-3503.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate KW, Atwill ER, Bartolome JW, Nader G. Significant Escherichia coli attenuation by vegetative buffers on annual grasslands. J Environ Qual. 2006;35:795–805. doi: 10.2134/jeq2005.0141. [DOI] [PubMed] [Google Scholar]
- Tian YQ, Gong P, Radke JD, Scarborough J. Spatial and temporal modeling of microbial contaminants on grazing farmlands. J Environ Qual. 2002;31:860–869. doi: 10.2134/jeq2002.8600. [DOI] [PubMed] [Google Scholar]
- Tian J, Yi S, Imhoff P, Chiu P, Guo M, Maresca J, Beneski V, Cooksey S. Biochar-amended media for enhanced nutrient removal in stormwater facilities. World Environ Water Resour Congr. 2014;2014:197–208. [Google Scholar]
- Tollner EW, Barfield BJ, Haan CT, Kao TY. Suspended sediment filtration capacity of simulated vegetation. Trans ASAE. 1976;19(4):678–682. [Google Scholar]
- Tollner EW, Barfield BJ, Vachirakornwatane C, Haan CT. Sediment deposition patterns in simulated grass filters. Trans ASAE. 1977;20(5):940–944. [Google Scholar]
- Tyrrel SF, Quinton JN. Overland flow transport of pathogens from agricultural land receiving fecal wastes. J Appl Microbiol. 2003;94:87S–93S. doi: 10.1046/j.1365-2672.94.s1.10.x. [DOI] [PubMed] [Google Scholar]
- Van Kessel C, Nitschelm JJ, Howarth WR, et al. Carbon −13 input and turnover in a pasture soil exposed to long term elevated atmospheric CO2. Glob Change Biol. 2000;6:123–135. [Google Scholar]
- Woolhiser DA, Smith RE, Goodrich DC. KINEROS A kinematic runoff and erosion model: documentation and user manual ARS 77. USDA, ARS; Washington, DC, USA: 1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


