Abstract
Chronic asymptomatic worm infection, often in combination with tuberculosis (TB), is common in low-income countries. Indeed, a life without worm infestation, as is now the case in most high-income countries, is a recent condition for humankind. Worms and Mycobacterium tuberculosis give rise to different immune response patterns (Th2 vs. Th1 driven), and we have studied whether chronic worm infection affects the susceptibility to and control of TB in the low income country of Ethiopia. Our results, as well of those in the general literature, are inconclusive, although we have some rather strong data in support of adult deworming in relation to vaccination with bacillus Calmette-Guérin (BCG) against TB. In addition, we discuss briefly the putative relationship between chronic worm infestation and autoimmunity/allergy.
Keywords: autoimmunity, confection, deworming, helminths/worms, tuberculosis
I. INTRODUCTION
Parasitic worms (helminths) are ubiquitous creatures that invade mammals, birds, fish, and plants. The coevolution with their hosts indicates that there may be an element of symbiosis in the coexistence. If not excessive in number, they rarely have serious effects on the well being of their hosts. In high numbers, however, they may cause morbidity in adults and malnutrition, growth retardation, and anemia in children. The latter is why the WHO advocates regular de-worming in children from regions where the prevalence of helminths is high.1
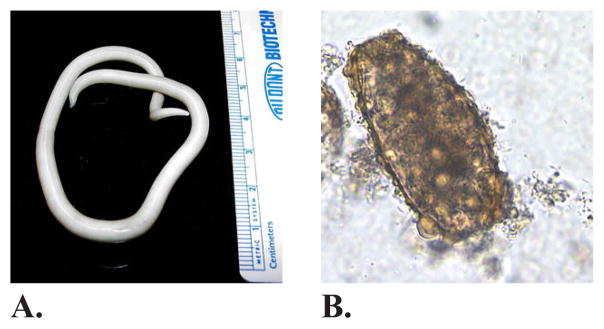
Worms are multicellular organisms that can vary in size from <1 mm to >10 m and may live very long lives, up to >10 years. Adult worms may be luminal such as Ascaris lumbricoides (roundworm) (Figure 1) and Trichuris trichuris (whipworm), which excrete their eggs in the soil from the feces of the infested individual or parenchymatous such as the Schistosoma species (snail fever worms), which excrete their eggs in fresh water through the feces or urine of the infested individual. It is only during the last century that humans (and their pets) living in high-income countries have started to separate from this coexistence; life without worms is a new and unique situation in vertebrate ecology.
FIG. 1.
(A) Adult female Ascaris lumbricoides. The roundworm Ascaris is by far the most common worm in Ethiopia. (B) Unfertilized egg of A. lumbricoides in an unstained wet mount, at 200× magnification. (Images reprinted, with permission, from DPDx: http://www.cdc.gov/dpdx/ascariasis/index.html.)
The peaceful and chronic coexistence between worm and host has required the deviation of a harmful immune response by the host and a reduction of its inflammatory consequences. These effects, or rather their absence, have been implied to be as one of the reasons explaining why autoimmune and allergic conditions have increased markedly in the rich world, whereas such conditions remain relatively rarer in the poor and still-worm-infested world. It is worth noting, however, that few comprehensive studies have been done on autoimmunity in Africa. The so-called dirt hypothesis2 is an off-shoot of this reasoning, suggesting that relative lack of environmental microbial and parasitic exposure may have as a consequence increased allergic-inflammatory reactions to nonmicrobial antigens, including self-antigens. Suffice it to say that there is little hard evidence to support that this hypothesis is the single or even most important reason to explain the asymmetry of allergic and autoimmune disease between high- and low-income countries. Indeed, the existence of this asymmetry in and of itself has been questioned. But it remains sufficiently exciting to attract a lot of scientific interest. Chronic worm infestation has also been implied to influence susceptibility and response to other infections, notably, TB, as the deviation of the immune profile toward a Th-2 type caused by worms contrasts the Th-1 type involved in control of Mycobacterium tuberculosis, for example.3
Here we report on experimental and clinical studies (performed mainly by our own group) that pertain to this issue, with emphasis on the effect of worms on vaccination against and susceptibility and resistance to mycobacterial infection.
Our interest in the field started when we learned that in worldwide veterinary preventive medicine it was customary to deworm both livestock and pets before they were vaccinated in order for the vaccine against infection (e.g., viral infection) to be effective. Apparently, the reason for this habit was mainly empiric. We could find neither major controlled studies that supported it nor any that explored the putative cellular or subcellular effects of deworming on the ensuing response to vaccination.
Working in Ethiopia at the time, it was intriguing to us that although the vast majority of individuals under the age of 20 in Ethiopia were infested (corresponding to infection with ecto/endoparasites, such as worms) with one or several species of worms, their response of protection against pulmonary TB by BCG vaccination was poor compared to the effect of the same vaccine in high-income, relatively worm-free countries such as England and Scandinavia.4 Several explanations have previously been given for the different protective effect of BCG, but we opted to look at this from the perspective of the role of worms.
II. EXPERIMENTAL STUDIES ON THE PROTECTIVE EFFECTS OF BCG IN MICE WITH OR WITHOUT SCHISTOSOMA INFECTION
We studied the effect of worm infestation on the protective effects of BCG in mice. To date, the most effective vaccine against M. tuberculosis infection is BCG (attenuated Mycobacterium bovis bacillus). It will very soon be 100 years since this vaccine was first tested in humans. A major drawback with BCG is that its effect against pulmonary TB, the most common and often lethal form of M. tuberculosis infection, is minimal in low-income countries, where the condition is most rampant. Although several other vaccine candidates have been produced and recently tested, none have shown a better protective effect than BCG in human and/or rodent models, in which most tests for efficacy are initially performed. The WHO still advocates general vaccination with BCG in newborns due to its relatively effective protection against pediatric TB. An additional reason is the newly discovered (but still not well understood) protective effects of BCG against leprosy.5 Although many high-income, low-TB-incidence countries, including the United States, have stopped general BCG vaccination of children, it is still one of the most widely used vaccines, with more than 300 million doses given annually.
Thus, despite its old age, BCG is still a highly relevant vaccine, which is why we have used it in our experimental studies. Mice were infested with Schistosoma mansoni, a human-specific worm (cestode) that hatches its eggs in specific snails from where its larvae, called cercarie, are released into the water. These cercariae penetrate human skin and travel to the liver. There, they develop into adult worms, mate, and produce eggs that ultimately end up in excreted stool.
The fur on rodents impedes cercariae from reaching the skin. However, in the laboratory, when the fur was shaved and cercariae were applied to the skin, this resulted in an infestation much like that described above in humans. Three weeks after application of cercariae on the skin of adult C57BL/6 rodents, the mice were vaccinated with BCG. Another 6 weeks later, they received live M. tuberculosis bacillus intravenously. Mice were sacrificed 8 weeks after M. tuberculosis exposure, and the numbers of M. tuberculosis-colony-forming units in the lungs and the liver were determined. In addition, splenic lymphocyte reactions to purified protein derivative (PPD) from M. tuberculosis) and the T-cell mitogen concanavalin-A were assessed. The degree of S. mansoni infestation of the mice was determined by counting the number of eggs in their stool.
The results from this experiment were very clear-cut.6 BCG had a strong protective effect against M. tuberculosis infection, provided that the mice were not S. mansoni infested. In those mice infected with S. mansoni, BCG failed to protect against the ensuing M. tuberculosis infection or did so to a much lower degree. We did not quite manage to prove that the extent of S. mansoni infestation (i.e., the number of excreted eggs) correlated with the number of colony-forming units that we scored in the lungs and liver. After cellular testing, we noted that S. mansoni–infested mice had a response strongly skewed toward a Th2 type, with splenocytes secreting increased amounts of IL-4 and IL-5 cytokines at the expense of IFN-γ, a major cytokine in splenocytes from non-S. mansoni–infested mice. S. mansoni in itself did not affect the general health of the mice, whereas M. tuberculosis killed the mice unless they were vaccinated with BCG, in the absence of worm infestation. With these experimental results, we were then ready to investigate the impact of BCG vaccination in humans, as reported below.
III. IMPORTANCE OF WORM INFESTATION FOR THE EFFECT OF BCG VACCINATION IN HUMANS
A. Studies in Urban High School Students
We performed two studies on the above issue, which resulted in partly different outcomes. In the first,7 we studied the effect of BCG vaccination on male high school students in Addis Ababa. Although this population was clinically healthy (without enteric problems), nearly 30% of the population carried one or more helminths in repeated stool examinations. There was no difference in tuberculin (PPD) skin reactivity between those with and without worms. The skin PPD-negative (<3 mm induration following intradermal injection of 2 TU [tuberculin] PPD), helminth-positive students were divided into groups treated with 400 mg albendazole (a broad-spectrum antihelminthic drug) or placebo. Six weeks later, isolated peripheral-blood mononuclear cells (MNCs) were tested for proliferation and IFN-γ secretion after exposure to PPD in vitro. The response to PPD in albendazole-treated, now worm-free students was significantly higher, in terms of both cellular proliferation and IFN-γ production. Both groups were then vaccinated with BCG. The essential finding was that 75% of students in both groups converted to having a skin-test positive reaction to PPD (i.e., a 2 TU PPD intradermal injection caused a >10-mm induration). However, unexpectedly this PPD skin positivity eventually waned in both groups, because when retested 4 months later, only 25% skin-tested positive.
However, testing MNC reactivity to PPD in vitro 1 month after BCG vaccination resulted in a clear difference. Albendazole-treated, helminth-free individuals had MNCs with additional increased proliferative capacity and IFN-γ production when exposed to PPD, whereas MNCs in worm-positive BCG-vaccinated students showed no significant increment, either in proliferation or IFN-γ production, in response to PPD. This difference remained 4 months after BCG vaccination, when PPD skin reactivity began to wane in both groups.
Thus, in this study population of male high school students, skin and lymphocyte reactivity to PPD dissociated completely, both before and after BCG vaccination. Although concomitant helminthic infestation did not affect skin reactivity before or after BCG vaccination, lymphocyte proliferation and IFN-γ secretion were affected. MNCs in worm-carrying students, in contrast to MNCs in worm-free students, were clearly hyporesponsive to PPD antigens, and BCG vaccination could not restore this anergy.
B. STUDIES WITH RURAL ADULTS
A second and larger study of the same issues was done in rural Ethiopia.8 Here, worm carriage was much higher, by ~70%, and double or multiple (two or more different worms) carriage was common. Essentially, the same design was used, insofar as worm-carrying tuberculin (PPD) skin-negative individuals were BCG vaccinated with or without prior albendazole (400 mg daily for 3 days) treatment. Separate experiments made it clear that albendazole alone, when given to worm-free individuals, had no effect on lymphocyte performance. In this study, we tested both Th-1 (IFN-γ) and Th-2 cytokines (IL-4 and IL-5) in isolated MNCs from subjects, using the single-cell enzyme-linked immunospot (ELISpot) assay. In addition, we tested TGF-β secretion, because indications from other studies9 showed that this cytokine could be involved as a down-regulating molecule in Th-1–driven responses.
Essentially, we found were in vivo skin reactivity converted to become positive in <75% of the BCG-vaccinated individuals, regardless of their worm-carrying status. However, unlike in the above study on Addis Ababa high school students, skin-test positivity did not wane rapidly but instead remained unchanged when tested 1 and 4 months after vaccination. In vitro studies on isolated MNCs showed clear differences, depending on whether cells were from worm-free (albendazole treated) or worm-carrying individuals. Cells from the latter group showed markedly decreased IFN-γ production when stimulated with PPD, along with much higher TGF-β production. There was no production of IL-4 or IL-5 in MNCs from either group when cells were stimulated with PPD, but there was a similar rate of production when MNCs were stimulated with concanavalin-A, a general T-cell mitogen. For technical reasons, we could not test for IL-10 production.
In summary, these two sets of experiments produced strong evidence that concomitant worm infestation affected the in vitro cellular response to PPD following BCG vaccination, when tested both in an urban and rural population in Ethiopia, a low-income country with a high annual incidence of TB (250/100,000). This difference in response pattern may indicate that worms can have a negative effect on the TB-preventive effect of BCG. Skin reactivity to PPD showed no such dichotomy of response and no data are available to show which of the two results is more appropriate in predicting the preventive outcome of vaccination. A 5-year long prospective study in a TB and high-helminth-prevalence country, in which BCG vaccination is administered with or without preceding albendazole treatment, would answer this question.
IV. CHRONIC WORM INFESTATION AND SUSCEPTIBILITY TO AND CLEARANCE OF OTHER INFECTIONS
A. Studies on Tuberculosis, HIV, and Malaria
Although chronic worm infestation is becoming increasingly rare with the reduction of poverty in a large part of the world, it is still a common condition among the poorest 2 billion individuals living in tropical as well as temperate parts of the world. In this part of the population, infections are still a major cause of morbidity and mortality and it therefore remains important to determine whether reducing worm burden would result in a reduction of morbidity and mortality of other infections, notably those of major public health importance such as TB, HIV, and malaria. This is particularly so because deworming is feasible, from both practical and economic points of view. Such longitudinal studies could also address another intriguing question:
Would the relative absence of worm infestation favor the appearance of autoimmune and/or allergic conditions? Such studies could be done in continuous demographic surveillance sites in Africa and Asia under the auspices of the INDEPTH Network. We and other investigators have studied this question, and the resulting data are variable and thus inconclusive. However, a uniform result cannot be expected. Type, duration, and extent of worm burden, type, duration, and frequency of deworming and type and extent of the intercurrent infection under study must be matched with other important variables such as nutritional conditions and genetics. Another critical question is whether worm infestation favors other infections or whether the reverse is true. This question has not been studied, let alone analyzed, by our group nor any other group, to our knowledge.
Our studies in Ethiopia, although equally as inconclusive as the general literature on the issue, still show some interesting points to which we refer below.
In a large 2001 study in a rural/semiurban area in Northern Ethiopia,10 we examined worm and HIV prevalence, respectively, in 230 patients with smear-positive pulmonary TB and 510 healthy household controls. We noted a highly significant difference between patients and controls regarding both worm frequency (71% vs. 35%) and HIV prevalence (46% vs. 11%). The association between TB and worm infestation, as well as between TB and HIV, was highly significant, and there was even a dose effect of the former. The higher the number of worm species, the stronger the association to TB, with odds ratios ranging from 4.7 to 12.2. We concluded that worm infestation may be a risk factor for acquiring both TB and HIV.
We did similar studies11,12 in the same area 10 years later, in which we found different and indeed nearly opposite results. During this period, HAART for HIV had been introduced on a large scale in the area, starting in 2005. At that time, prevalence of worms was much lower in both TB patients and household controls (e.g., 29% vs. 21%) but seroprevalence of HIV in TB patients was still high, at 47%,11 but declining to 29% and 23%,12 respectively. There was no difference in worm prevalence between HIV-positive and HIV-negative TB patients before TB treatment, but during anti-HIV treatment, worm prevalence, in the absence of antihelminthic treatment, fell significantly in the HIV-positive (from 33% to 8%) but not the HIV-negative group (29% to 22%). We interpreted this to be an antihelminthic effect of the concurrent HAART and cotrimexazole treatment of HIV-positive TB patients. At the time, 70% of these patients received such treatment. MNCs from helminth-positive TB patients had significantly increased the number of regulatory T and IL-10–producing cells and increased levels of eosinophils and IgE antibodies. However, none of the latter changes appeared to influence the clinical symptoms or the outcome of TB treatment. Reducing the worm burden with albendazole, which normalized eosinophil counts, IgE, and IL-10 equally, did not change the clinical appearance or outcome of anti-TB treatment.
In an early study13 done during the pre-HAART era in Ethiopia, we had found that HIV-positive patients with concomitant worm infestation had high plasma viremia that increased in a dose-dependent manner (i.e., the higher the egg load, the higher the number of HIV-RNA copies in plasma). Our studies done 10 years later in the same country11,12 did not confirm a correlation between worm burden and susceptibility and extent of HIV infection, which is in line with results of other studies performed in Africa during the same period.14
Finally, our studies in rural areas of Ethiopia with malaria patients who did or did not have asymptomatic worm infestation15 showed that malaria parasitemia, with or without clinical symptoms, was accompanied by a marked increase in IgE levels, which further increased in those with concomitant worm infestation. The level of malaria-parasite–infected red blood cells correlated with the intensity of worm-egg load in the stool, especially in those infested with Trichuris trichuris. We thus showed that concomitant worm infection may increase the risk of developing severe malaria.
V. CONCLUSION
Chronic asymptomatic worm infection is common in Ethiopia, but its prevalence appears to be decreasing, at least in urban areas, as a result of a reduction in poverty levels. Such worm infestation results in major immunological changes, including increased levels of IgE, TGF-β, and eosinophilia. Whether chronic worm infection affects the susceptibility and course of infections such as TB and HIV is still unclear from our studies, because they were not designed to detect at least subtle effects. On the other hand, we have no data to show that worms may not promote any of these infections, and it is well established that worms compete with their host for nutrients and iron.
We plan to embark on a large-scale prospective study, in which adults in a high-worm-prevalence area are regularly dewormed. We will follow over time the incidence of malaria, HIV, and TB, including self-reports of the incidence/prevalence of allergic/autoimmune conditions.
Acknowledgments
The majority of the work was done by two Ethiopian Ph.D. students, Daniel Elias and Ebba Abate from Gondar University in Ethiopia, supervised by Hannah Akuffo (DE) and Thomas Schön (EA). The students successfully defended their Ph.D. theses in Sweden in 2009 and 2013, respectively.
The authors thank our colleagues at DPDx, a web site developed and maintained by the Centers for Disease Control and Prevention’s Division of Parasitic Diseases and Malaria for the use of the images. I, Sven Britton, acknowledge John Fahey, with whom I was a postdoctoral fellow from 1972 to 1974 at the University of California at Los Angeles (UCLA). I intended to set up techniques of cellular immunology at UCLA; until then, John up had been involved in seroimmunology. I am not so sure that John got what he wanted out of me, but I got a lifelong friendship with and from John. His ever-growing interest in promoting the knowledge of immunology to young scientists from low-income countries tied us even closer together during the last decades of his life.
Dr. John L. Fahey’s international collaborations were supported by funding from the Fogarty Center of the National Institutes of Health (T22-TW-000003, D43-TW-000013).
ABBREVIATIONS
- AIDS
Acquired immune deficiency syndrome
- BCG
bacillus Calmette-Guérin
- CD
cluster of differentiation
- HAART
highly active antiretroviral therapy
- HIV
human immunodeficiency virus
- IFN-γ
interferon-gamma
- IL
interleukin
- TB
tuberculosis
- MNC
mononuclear cells
- TGF-β
transforming growth factor-beta
- Th1
T-helper response type 1
- Th2
T-helper response type 2
- UCLA
University of California, Los Angeles
- WHO
World Health Organization
References
- 1.WHO. Global Tuberculosis Report. Geneva, Switzerland: 2012. [Google Scholar]
- 2.Evans H, Mitre E. Worms as therapeutic agents for allergy and asthma: Understanding why benefits in animal studies have not translated into clinical success. J Allergy Clin Immunol. 2015;135:343–53. doi: 10.1016/j.jaci.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14:1118–26. doi: 10.1038/ni.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen P, Doherty TM. Learning from BCG: Designing a better tuberculosis vaccine. Discov Med. 2005;5(28):383–7. [PubMed] [Google Scholar]
- 5.Richardus JH, Oskam L. Protecting people against leprosy: Chemoprophylaxis and immunoprophylaxis. Clin Dermatol. 2015;33:19–25. doi: 10.1016/j.clindermatol.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Elias D, Akuffo H, Pawlowski A, Haile M, Schön T, Britton S. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine. 2005;23:1326–34. doi: 10.1016/j.vaccine.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 7.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guérin (BCG) vaccination. Clin Exp Immunol. 2001;123:219–25. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elias D, Britton S, Aseffa A, Engers H, Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-β production. Vaccine. 2008;26(31):3897–902. doi: 10.1016/j.vaccine.2008.04.083. [DOI] [PubMed] [Google Scholar]
- 9.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: Implications for parasite persistence. J Immunol. 2006;176:3248–56. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 10.Elias D, Mengistu G, Akuffo H, Britton S. Are intestinal helminths risk factors for developing active tuberculosis? Trop Med Int Health. 2006;11:551–8. doi: 10.1111/j.1365-3156.2006.01578.x. [DOI] [PubMed] [Google Scholar]
- 11.Abate E, Belayneh M, Gelaw A, Idh J, Getachew A, Alemu S, Diro E, Fikre N, Britton S, Elias D, Aseffa A, Stendahl O, Schön T. The impact of asymptomatic helminth co-infection in patients with newly diagnosed tuberculosis in northwest Ethiopia. PLoS One. 2012;7:e42901. doi: 10.1371/journal.pone.0042901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abate E, Elias D, Getachew A, Alemu S, Diro E, Britton S, Aseffa A, Stendahl O, Schön T. Effects of albendazole on the clinical outcome and immunological responses in helminth co-infected tuberculosis patients: A double blind randomised clinical trial. Int J Parasitol. 2015;45(2–3):133–40. doi: 10.1016/j.ijpara.2014.09.006. pii: S0020-7519(14)00290–2. [DOI] [PubMed] [Google Scholar]
- 13.Wolday D, Mayaan S, Mariam ZG, Berhe N, Seboxa T, Britton S, Galai N, Landay A, Bentwich Z. Treatment of intestinal worms is associated with decreased HIV plasma viral load. J Acquir Immune Defic Syndr. 2002;31:56–62. doi: 10.1097/00126334-200209010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Hübner MP, Killoran KE, Rajnik M, Wilson S, Yim KC, Torrero MN, Morris CP, Nikonenko B, Blanco JC, Hemming VG, Mitre E. Chronic helminth infection does not exacerbate Mycobacterium tuberculosis infection. PLoS Negl Trop Dis. 2012;6:e1970. doi: 10.1371/journal.pntd.0001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulu A, Legesse M, Erko B, Belyhun Y, Nugussie D, Shimelis T, Kassu A, Elias D, Moges B. Epidemiological and clinical correlates of malaria-helminth co-infections in Southern Ethiopia. Malar J. 2013;12:227. doi: 10.1186/1475-2875-12-227. [DOI] [PMC free article] [PubMed] [Google Scholar]