Abstract
Background:
Serum concentrations of polybrominated diphenyl ethers (PBDEs) in U.S. women are believed to be among the world’s highest; however, little information exists on the partitioning of PBDEs between serum and breast milk and how this may affect infant exposure.
Objectives:
Paired milk and serum samples were measured for PBDE concentrations in 34 women who participated in the U.S. EPA MAMA Study. Computational models for predicting milk PBDE concentrations from serum were evaluated.
Methods:
Samples were analyzed using gas chromatography isotope-dilution high-resolution mass spectrometry. Observed milk PBDE concentrations were compared with model predictions, and models were applied to NHANES serum data to predict milk PBDE concentrations and infant intakes for the U.S. population.
Results:
Serum and milk samples had detectable concentrations of most PBDEs. BDE-47 was found in the highest concentrations (median serum: 18.6; milk: 31.5 ng/g lipid) and BDE-28 had the highest milk:serum partitioning ratio (2.1 ± 0.2). No evidence of depuration was found. Models demonstrated high reliability and, as of 2007–2008, predicted U.S. milk concentrations of BDE-47, BDE-99, and BDE-100 appear to be declining but BDE-153 may be rising. Predicted infant intakes (ng/kg/day) were below threshold reference doses (RfDs) for BDE-99 and BDE-153 but above the suggested RfD for BDE-47.
Conclusions:
Concentrations and partitioning ratios of PBDEs in milk and serum from women in the U.S. EPA MAMA Study are presented for the first time; modeled predictions of milk PBDE concentrations using serum concentrations appear to be a valid method for estimating PBDE exposure in U.S. infants.
Citation:
Marchitti SA, Fenton SE, Mendola P, Kenneke JF, Hines EP. 2017. Polybrominated diphenyl ethers in human milk and serum from the U.S. EPA MAMA Study: modeled predictions of infant exposure and considerations for risk assessment. Environ Health Perspect 125:706–713; http://dx.doi.org/10.1289/EHP332
Introduction
Polybrominated diphenyl ethers (PBDEs) are persistent flame retardant chemicals used in consumer products. PBDEs were produced in three technical mixtures: pentaBDE (tri-through hexa-brominated congeners), octaBDE (hexa- and hepta-brominated congeners), and decaBDE (primarily deca-brominated BDE-209). PentaBDE and octaBDE, used in polyurethane foam and electronics, respectively, were withdrawn from the U.S. market in 2004. DecaBDE, used in plastics, was phased out in 2013. However, products imported from other countries may still contain PBDEs. In addition, consumer products may be used for many years and cause increased body burdens well beyond the time of manufacture. Animal and in vitro studies suggest that PBDEs can cause adverse neurodevelopmental, reproductive, and thyroid effects; however, exposure and health outcomes in humans are not fully understood (Birnbaum 2007; Chao et al. 2011; Eriksson et al. 2001).
The United States was the largest consumer of pentaBDE, and concentrations in samples from the U.S. population (e.g., serum, breast milk) are 10- to 100-fold higher than those reported in Europe or Asia (Marchitti et al. 2013a; Sjödin et al. 2014). Highly lipophilic, these chemicals partition into lipid-rich compartments, including human milk (Marchitti et al. 2013b; Schecter et al. 2003), with the highest exposed population believed to be breastfeeding infants (Johnson-Restrepo and Kannan 2009; Toms et al. 2008). This has led to concern over potential health impacts during sensitive periods of growth and development (Chao et al. 2011; Gascon et al. 2012). Although breastfeeding is known to provide superior benefits to infants and should continue to be encouraged, the lack of nationally representative breast milk data has limited our understanding of the concentrations of environmental chemicals in breast milk and our ability to assess infant exposures (Lehmann et al. 2014). One approach involves developing models that extrapolate chemical concentrations from a surrogate or alternative matrix, such as maternal serum, to predict levels in breast milk using chemical-specific milk:serum partitioning ratios. The United States has several years of nationally representative serum data on many persistent chemicals from its National Health and Nutrition Examination Survey (NHANES), and these surveys are continuing to produce such data (http://www.cdc.gov/exposurereport/). However, limited partitioning information exists for environmental chemicals, and the use of PBDE concentrations in maternal blood as a surrogate for predicting maternal and infant exposure is still a developing field. The U.S. EPA (Environmental Protection Agency) Methods Advancement for Milk Analysis (MAMA) Study was initiated to better understand environmental chemical concentrations in women and the factors that influence chemical partitioning and infant exposure (Hines et al. 2009, 2015). The objectives of the present study were to measure and compare concentrations of PBDEs in paired samples of serum and milk donated by women in the MAMA Study. Serum was evaluated as a surrogate for predicting breast milk concentrations and infant exposure, and sources of PBDE exposure were explored using demographic and lifestyle information.
Methods
Human Subjects
A convenience sample of 34 healthy, breastfeeding women (21–39 years) were recruited by Westat Inc. (Chapel Hill, NC) and asked to provide informed consent before participating in this study, as described (Hines et al. 2009). Milk and serum samples were collected at 2–7 weeks (first visit) and 3–4 months (second visit) postpartum between December 2004 and July 2005. A demographic and lifestyle questionnaire was administered at the first visit (see Supplemental Material, “First Visit Questionnaire, U.S. EPA MAMA Study”). Human subject participation was approved by the institutional review boards of the University of North Carolina (IRB 03-EPA-207) and the Centers for Disease Control and Prevention (CDC; IRB 3961).
Sample Collection
Women were asked to fast for 1.5 hr before arrival. Milk (90 mL into PBDE-free polypropylene tubes) was collected using a breast pump (Medela). At the same visit, blood (20 mL) was collected via venipuncture into nonheparinized glass vacutainer tubes (Becton Dickinson). Blood was clotted (1 hr) and spun at 3,000 rpm (15 min) to collect serum. Samples were shipped on dry ice to the CDC (Atlanta, GA) and stored at –20°C.
Serum and Milk Analysis
Nine PBDE congeners [2,4,4´-tribromodiphenyl ether (BDE-28), 2,2´,4,4´-tetrabromodiphenyl ether (BDE-47), 2,3´,4,4´-tetrabromodiphenyl ether (BDE-66), 2,2´,3,4,4´-pentabromodiphenyl ether (BDE-85), 2,2´,4,4´,5-pentabromodiphenyl ether (BDE-99), 2,2´,4,4´,6-pentabromodiphenyl ether (BDE-100), 2,2´,4,4´,5,5´-hexabromodiphenyl ether (BDE-153), 2,2´,4,4´,5,6´-hexabromodiphenyl ether (BDE-154), and 2,2´,3,4,4´,5´,6-heptabromodiphenyl ether (BDE-183)] were measured in serum and milk (ng/g lipid), as described (Sjödin et al. 2004a, 2004b). 2,2´,4-Tribromodiphenyl ether (BDE-17) was measured in serum but not in milk. In addition to the PBDEs, the brominated flame retardant 2,2´,4,4´,5,5´-hexabromobiphenyl (BB-153) was also measured as part of the standard CDC panel of flame retardants. Serum samples were extracted, denatured, and analyzed using gas chromatography isotope dilution high-resolution mass spectrometry (GC-ID-HRMS) with a MAT95XP instrument (Thermo Electron). Serum lipid concentration was determined using available kits (Roche Diagnostics). Milk samples underwent solid-phase dispersion onto diatomaceous earth and were dried by pressurized nitrogen; lipids and target analytes were extracted with dichloromethane. Milk lipids were determined gravimetrically, and analysis performed by GC-ID-HRMS. Blank (n = 3) and quality control (n = 3) samples were included with each set of unknown specimens (batch n ≤ 24).
Laboratory quality assurance practices were regularly monitored (Sjödin et al. 2004a, 2004b). The limits of detection (LOD) in serum and milk were defined as three times the standard deviation of the blank samples; in the absence of a signal in the blank samples, the LOD was determined to be the lowest calibration standard having a signal-to-noise ratio > 10. LOD values varied for each PBDE and were dependent on sample size and potential interferences detected in blank samples.
Predictions of Milk PBDE Concentrations and Infant Exposure
Exposure models for predicting milk PBDE concentrations from serum concentrations have been developed (Marchitti et al. 2013b). These models rely on congener-specific predictions of PBDE milk:serum partitioning ratios and have been validated but not extensively tested (see Supplemental Material, “Additional modeling information”). To test them, model equations were applied to serum PBDE concentrations from MAMA Study participants to calculate predicted milk PBDE concentrations, which were then compared to observed values. Models were then applied to NHANES PBDE serum data available from 2003–2008 for women 20–39 years of age to obtain predicted representative milk PBDE concentrations for the U.S. population. NHANES serum PBDE concentrations represented individual samples (2003–2004) or pooled samples (2005–2006 and 2007–2008) for different ethnicities (non-Hispanic white, non-Hispanic black, and Mexican American); weighted arithmetic means (ng/g lipid) are intended to be representative of the U.S. population (Sjödin et al. 2014).
From predicted milk concentrations, average daily intakes of PBDEs in U.S. infants (DIi; ng/kg bw/day) were calculated using the formula: DIi = CmFMb (Johnson-Restrepo et al. 2007), where Cm is our predicted mean PBDE concentration in U.S. breast milk (ng/g lw), F is the average lipid content of breast milk (4.0 g lipid/100 g milk), and Mb is the average daily consumption of milk [150 mL/kg/day (milk density 1.03 g/mL)] for infants age birth to < 1 month, as recommended by the Exposure Factors Handbook of the U.S. EPA (2011).
Statistics
PBDE concentrations < LOD were assigned a value equal to the LOD divided by the square root of 2. To determine PBDE milk:serum partitioning ratios, only participants with both serum and milk concentrations > LOD were used (n = 5–34 women) for individual PBDEs. Correlations and statistical comparisons were performed only for compounds detected in ≥ 50% of samples. Spearman correlation coefficients (r s) assessed the relationship between milk and serum PBDE concentrations. Statistical comparisons were performed using paired t-tests.
Questionnaire data were examined for associations with PBDE concentrations; categorical variables were assessed using one-way analysis of variance while continuous variables were assessed with Spearman correlations. Given the exploratory nature of the study, adjustments were not made for multiple comparisons. Analyses were conducted using SAS Enterprise Guide 4.1 (SAS Institute Inc., Cary, NC). Significance was denoted at p ≤ 0.05.
Results
Concentrations of PBDEs in Serum
Serum PBDE detection rates ranged from 3–100% (Table 1). BDE-47 was measured at the highest concentration (median, 18.6 ng/g lipid). BDE-99, BDE-100, and BDE-153 were measured at median concentrations of 3.6, 3.9, and 5.5 ng/g lipid, respectively. Remaining analytes were measured at median concentrations of ≤ 1.1 ng/g lipid. Serum PBDE concentrations did not differ significantly between visits (Figure 1).
Table 1.
Concentrations of PBDEs in serum (ng/g lipid) from women in the U.S. EPA MAMA Study.
| PBDE | Weeks postpartuma | n | % detect | LOD (ng/g lipid) | GM (95% CI) | 5th percentile | 25th percentile | Median | 75th percentile | 95th percentile | Range |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BB-153 | 2–7 | 33 | 70 | 0.3–0.5 | 0.8 ± 0.3 | 0.3 | 0.4 | 1.0 | 1.7 | 2.5 | 0.2–4.6 |
| 12–16 | 30 | 67 | 0.3–0.7 | 0.9 ± 0.4 | 0.2 | 0.4 | 1.0 | 1.5 | 3.4 | 0.2–5.5 | |
| BDE-17 | 2–7 | 34 | 15 | 0.3–0.5 | 0.3 ± 0.1 | 0.3 | 0.3 | 0.3 | 0.4 | 0.8 | 0.2–1 |
| 12–16 | 30 | 3 | 0.3–0.7 | 0.3 ± 0.1 | 0.2 | 0.3 | 0.4 | 0.4 | 0.5 | 0.2–1.1 | |
| BDE-28 | 2–7 | 34 | 91 | 0.3–1 | 1.2 ± 0.6 | 0.4 | 0.8 | 1.1 | 1.8 | 7.1 | 0.3–8.2 |
| 12–16 | 30 | 63 | 0.3–1.3 | 1.1 ± 0.6 | 0.4 | 0.8 | 1.0 | 1.7 | 6.5 | 0.3–6.6 | |
| BDE-47 | 2–7 | 34 | 100 | 0.4–6 | 20.4 ± 16.3 | 4.8 | 12.2 | 18.6 | 28.7 | 173.0 | 4.5–227.0 |
| 12–16 | 30 | 100 | 0.3–7.9 | 20.4 ± 14.8 | 6.7 | 12.1 | 16.8 | 28.2 | 111.0 | 5.4–222.0 | |
| BDE-66 | 2–7 | 34 | 38 | 0.3–0.5 | 0.4 ± 0.2 | 0.3 | 0.3 | 0.4 | 0.5 | 1.9 | 0.3–2.4 |
| 12–16 | 30 | 13 | 0.3–0.7 | 0.4 ± 0.1 | 0.2 | 0.3 | 0.4 | 0.4 | 1.1 | 0.2–2.1 | |
| BDE-85 | 2–7 | 34 | 53 | 0.3–0.9 | 0.6 ± 0.4 | 0.2 | 0.3 | 0.5 | 0.7 | 4.5 | 0.2–5.3 |
| 12–16 | 30 | 47 | 0.3–1.2 | 0.7 ± 0.3 | 0.3 | 0.4 | 0.6 | 0.9 | 2.6 | 0.3–5.1 | |
| BDE-99 | 2–7 | 34 | 97 | 0.3–3.2 | 4.7 ± 5.4 | 1.0 | 2.1 | 3.6 | 6.8 | 61.9 | 1–62.7 |
| 12–16 | 30 | 83 | 0.4–4.2 | 4.8 ± 3.9 | 1.7 | 2.8 | 3.9 | 7.8 | 30.9 | 1.2–57.6 | |
| BDE-100 | 2–7 | 34 | 97 | 0.3–0.9 | 4.0 ± 3.2 | 0.9 | 2.3 | 3.9 | 5.7 | 29.3 | 0.4–39.1 |
| 12–16 | 30 | 100 | 0.3–1.2 | 3.9 ± 2.5 | 1.1 | 2.2 | 4.1 | 4.9 | 28.5 | 0.9–28.9 | |
| BDE-153 | 2–7 | 34 | 97 | 0.3–1.4 | 6.2 ± 8.4 | 1.0 | 2.6 | 5.5 | 15.3 | 63.5 | 1.0–134.0 |
| 12–16 | 30 | 100 | 0.3–1.9 | 6.0 ± 5.4 | 1.4 | 2.9 | 5.1 | 13.6 | 39.7 | 1.3–68.8 | |
| BDE-154 | 2–7 | 34 | 53 | 0.3–0.5 | 0.6 ± 0.4 | 0.2 | 0.3 | 0.4 | 0.7 | 4.2 | 0.2–5.2 |
| 12–16 | 30 | 53 | 0.3–0.7 | 0.5 ± 0.3 | 0.3 | 0.4 | 0.5 | 0.6 | 2.6 | 0.2–3.7 | |
| BDE‑183 | 2–7 | 34 | 29 | 0.3–0.5 | 0.4 ± 0.1 | 0.3 | 0.3 | 0.4 | 0.4 | 0.8 | 0.2–0.9 |
| 12–16 | 29 | 14 | 0.3–0.7 | 0.4 ± 0.1 | 0.2 | 0.3 | 0.4 | 0.4 | 0.7 | 0.2–1.1 | |
| ΣPBDEs | 2–7 | 34 | ND | ND | 43.2 ± 30.7 | 13.6 | 24.7 | 38.4 | 63.5 | 327.0 | 10.5–369.0 |
| 12–16 | 30 | ND | ND | 42.8 ± 23.5 | 15.3 | 26.5 | 41.0 | 57.6 | 172.5 | 11.7–341.1 | |
| Abbreviations: CI, confidence interval; GM, geometric mean; ND, not determined. aVisit 1, 2–7 weeks postpartum; visit 2, 12–16 weeks postpartum. | |||||||||||
Figure 1.
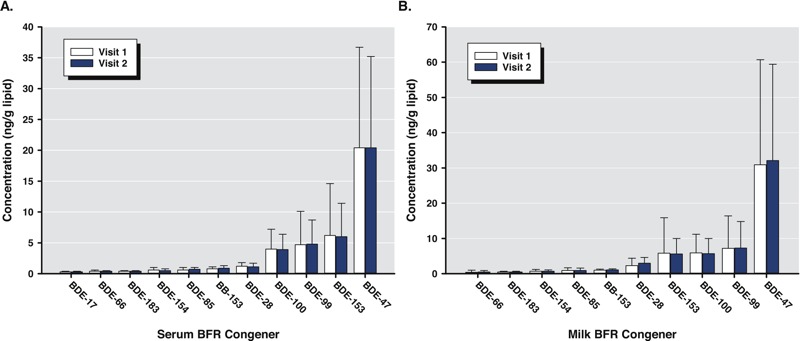
PBDE concentrations (ng/g lipid) in serum (A) and milk (B) at visits 1 and 2 from women in the U.S. EPA MAMA Study, 2004–2005. Data presented are geometric means ± 95% confidence intervals (n = 18–34). BFR, brominated flame retardant.
Concentrations of PBDEs in Human Milk
Milk PBDE detection rates ranged from 26–100% (Table 2). BDE-47 was measured at the highest concentration (median, 31.5 ng/g lipid). BDE-99, BDE-100, and BDE-153 were measured at median concentrations of 6.0, 5.3, and 5.0 ng/g lipid, respectively. The remaining analytes were measured at median concentrations of ≤ 2.4 ng/g lipid. Milk PBDE concentrations varied for individuals between visits (range, –89% to 700%), however, median changes (range, –4% to 6%) were not significantly different from zero, and no significant differences in milk concentrations between visits were detected (Figure 1).
Table 2.
Concentrations of PBDEs in milk (ng/g lipid) from women in the U.S. EPA MAMA Study.
| PBDE | Weeks postpartuma | n | % detect | LOD (ng/g lipid) | GM (95% CI) | 5th percentile | 25th >percentile | Median | 75th percentile | 95th percentile | Range |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BB-153 | 2–7 | 34 | 74 | 0.2–0.9 | 1.0 ± 0.3 | 0.3 | 0.6 | 1.0 | 1.6 | 3.1 | 0.3–3.4 |
| 12–16 | 30 | 87 | 0.1–2.3 | 1.1 ± 0.3 | 0.3 | 0.6 | 1.3 | 1.8 | 3.3 | 0.2–4.4 | |
| BDE-28b | 2–7 | 18 | 100 | 0.2–0.9 | 2.3 ± 2.1 | 0.8 | 1.5 | 2.0 | 2.9 | 14.7 | 0.6–17.6 |
| 12–16 | 22 | 100 | 0.1–2.3 | 3.0 ± 1.6 | 1.3 | 1.7 | 2.4 | 5.1 | 14.2 | 1.3–15.2 | |
| BDE-47 | 2–7 | 34 | 100 | 0.8–3.1 | 30.9 ± 29.8 | 6.0 | 15.7 | 31.5 | 49.2 | 236.4 | 5.9–462.5 |
| 12–16 | 30 | 100 | 0.5–8.1 | 32.1 ± 27.3 | 7.8 | 20.2 | 28.2 | 49.8 | 226.0 | 6.1–394.5 | |
| BDE-66b | 2–7 | 18 | 39 | 0.2–0.9 | 0.4 ± 0.6 | 0.2 | 0.3 | ND | 0.4 | 3.1 | 0.1–4.9 |
| 12–16 | 22 | 73 | 0.1–2.3 | 0.5 ± 0.4 | 0.1 | 0.3 | 0.4 | 0.6 | 2.2 | 0.1–4.0 | |
| BDE-85 | 2–7 | 34 | 59 | 0.2–0.9 | 0.9 ± 0.8 | 0.2 | 0.4 | 0.8 | 2.0 | 6.4 | 0.2–10.3 |
| 12–16 | 30 | 73 | 0.1–2.3 | 0.9 ± 0.7 | 0.3 | 0.5 | 0.8 | 1.5 | 5.6 | 0.2–8.8 | |
| BDE-99 | 2–7 | 34 | 100 | 0.7–2.6 | 7.2 ± 9.2 | 1.9 | 3.8 | 6.0 | 11.0 | 84.2 | 1.3–128.0 |
| 12–16 | 30 | 100 | 0.4–6.6 | 7.3 ± 7.5 | 2.2 | 4.1 | 6.7 | 11.0 | 68.2 | 1.5–104.5 | |
| BDE-100 | 2–7 | 34 | 100 | 0.2–0.9 | 5.9 ± 5.3 | 1.6 | 3.1 | 5.3 | 7.3 | 51.9 | 0.9–65.2 |
| 12–16 | 30 | 100 | 0.1–2.3 | 5.7 ± 4.3 | 1.3 | 3.6 | 4.9 | 7.8 | 45.0 | 1.0–47.3 | |
| BDE-153 | 2–7 | 33 | 100 | 0.3–1.2 | 5.8 ± 10.1 | 1.1 | 2.0 | 5.0 | 15.8 | 58.7 | 0.7–163.4 |
| 12–16 | 30 | 100 | 0.2–3.2 | 5.6 ± 4.4 | 1.0 | 2.8 | 5.2 | 13.6 | 39.1 | 0.9–45.1 | |
| BDE-154 | 2–7 | 34 | 65 | 0.2–0.9 | 0.7 ± 0.5 | 0.2 | 0.4 | 0.6 | 1.3 | 5.4 | 0.2–6.8 |
| 12–16 | 30 | 77 | 0.1–2.3 | 0.7 ± 0.4 | 0.3 | 0.4 | 0.6 | 0.9 | 4.0 | 0.2–4.5 | |
| BDE‑183 | 2–7 | 34 | 26 | 0.2–0.9 | 0.5 ± 0.2 | 0.2 | 0.3 | ND | 0.6 | 1.5 | 0.2–2.9 |
| 12–16 | 30 | 27 | 0.1–2.3 | 0.5 ± 0.2 | 0.2 | 0.3 | ND | 0.7 | 1.8 | 0.2–1.9 | |
| ΣPBDEs | 2–7 | 34 | ND | ND | 57.1 ± 50.1 | 17.6 | 32.8 | 50.8 | 77.2 | 453.1 | 10.3–676.6 |
| 12–16 | 30 | ND | ND | 59.4 ± 41.4 | 18.1 | 38.9 | 54.2 | 80.1 | 300.0 | 10.7–577.0 | |
| Abbreviations: CI, confidence interval; GM, geometric mean; ND, not determined. aVisit 1, 2–7 weeks postpartum; visit 2, 12–16 weeks postpartum. bConcentrations of BDE-28 and BDE-66 are reported from a subset of the study population (n = 18–22). | |||||||||||
PBDE Milk:Serum Partitioning
No significant differences in milk:serum partitioning ratios were found between visits, so data were combined. BDE-28 demonstrated the highest milk:serum partitioning ratio (2.1; Table 3). Milk concentrations of tri- through hexa-brominated congeners were significantly higher than corresponding serum concentrations, resulting in milk:serum partitioning ratios significantly > 1. Predicted milk:serum ratios using models in the study by Marchitti et al. (2013b) fell within confidence intervals of those observed in MAMA participants, except for BDE-100 and BDE-28, where observed values were slightly higher by 7–16% (Table 3).
Table 3.
Median PBDE Milk:serum partitioning ratios and chemical properties.
| PBDE | Observeda milk:serum ratios | Predictedb milk:serum ratios | Chemical properties | ||
|---|---|---|---|---|---|
| Mol wt | No. Br atoms | Log Kowc | |||
| BDE-28* | 2.1 ± 0.2 (n = 25) | 1.4 ± 0.2 | 406.9 | 3 | 5.94 |
| BDE-47* | 1.7 ± 0.1 (n = 34) | 1.5 ± 0.1 | 485.8 | 4 | 6.81 |
| BDE-66* | 1.9 ± 0.5 (n = 5) | ND | 485.8 | 4 | NR |
| BDE-85* | 1.4 ± 0.5 (n = 17) | 1.3 ± 0.2 | 564.7 | 5 | NR |
| BDE-99* | 1.4 ± 0.1 (n = 34) | 1.4 ± 0.2 | 564.7 | 5 | 7.32 |
| BDE-100* | 1.5 ± 0.1 (n = 34) | 1.3 ± 0.0 | 564.7 | 5 | 7.24 |
| BB-153 | 1.0 ± 0.1 (n = 24) | ND | 627.6 | 6 | 9.10 |
| BDE-153 | 0.9 ± 0.1 (n = 33) | 0.9 ± 0.0 | 643.6 | 6 | 7.90 |
| BDE-154 | 1.2 ± 0.4 (n = 20) | 0.8 ± 0.1 | 643.6 | 6 | 7.82 |
| BDE-183 | 0.6 ± 0.2 (n = 6) | ND | 722.5 | 7 | 8.27 |
| Abbreviations: Br, bromine; mol wt, molecular weight; ND, not determined; NR, not reported. aMilk:serum ratios were determined from MAMA participants with both milk and serum measurements > LOD (n = 5–34 women, depending on PBDE). Data from both visits were combined. bMilk:serum ratios were predicted using models developed by Marchitti et al. (2013b). cSource: Braekevelt et al. 2003; Hardy 2002. *Analytes with milk:serum partitioning ratios significantly > 1 for MAMA Study participants (p < 0.02). | |||||
Correlations
Milk PBDE concentrations and serum PBDE concentrations were highly correlated for individual PBDEs and Σ6PBDE (r s = 0.57–0.97; p < 0.0001; n = 62–63 samples). PBDE concentrations were not correlated with most questionnaire variables including weeks postpartum, parity, prior breastfeeding, body mass index (BMI), diet, or age of furniture in the home. However, BB-153 concentrations (milk and serum) were moderately correlated (as defined as rs = 0.25–0.5) with maternal age [rs = 0.35–0.47, p < 0.01 (see Table S1)] and inversely correlated (milk only) with vehicle age [rs = –0.30, p < 0.02 (see Table S2)]. Concentrations of some PBDEs were inversely correlated with both maternal age [rs = –0.26 to –0.40, p < 0.05 (see Table S1)] and home age [rs = –0.25 to –0.36, p < 0.05 (see Table S3)], but positively correlated with vehicle age [rs = 0.29–0.45, p < 0.05 (see Table S2)].
Modeled Predictions of Milk PBDE Concentrations
Models developed by Marchitti et al. (2013b) were tested using MAMA data; predicted and observed milk PBDE concentrations were highly correlated (Figure 2; see Figure S1). Applying the models to NHANES serum PBDE data for women of childbearing age (20–39 years) (Sjödin et al. 2014) allowed us to predict national concentrations of PBDEs in U.S. breast milk for three time periods [2003–2004, 2005–2006, and 2007–2008 (Table 4)]. Mean milk PBDE concentrations for MAMA participants, primarily non-Hispanic whites, were within confidence intervals for concentrations predicted for non-Hispanic whites in NHANES 2003–2005.
Figure 2.
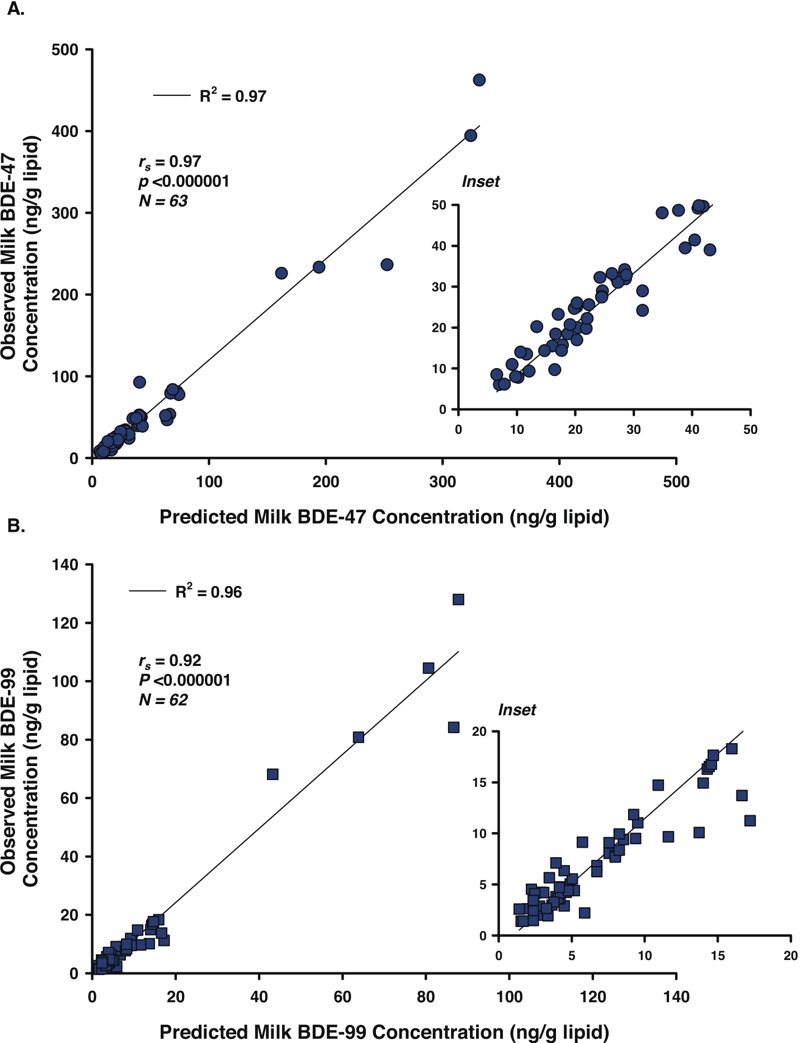
Predicted milk BDE-47 (A) and BDE-99 (B) concentrations versus those observed in MAMA Study participants. Solid lines are the least-squares fit of predicted and observed concentrations [R 2 = 0.97, slope = 1.24 (A); R 2 = 0.96, slope = 1.27 (B)]. Spearman correlation coefficients (r s) and related p-values are shown.
Table 4.
Predicted U.S. milk PBDE concentrations and estimated daily infant intakes.
| PBDE | Year | Predicted U.S. milka(ng/g lipid) | Estimated daily intakes for U.S. infantsb (ng/kg/day) | MAMA study participantsc | |||||
|---|---|---|---|---|---|---|---|---|---|
| NHW | NHB | MA | NHW | NHB | MA | Milk (ng/g lipid) | Infantd (ng/kg/day) | ||
| BDE-47 | >2003–2004 | 59.4 ± 24.5 | 78.1 ± 37.4 | 57.8 ± 29.1 | 356 ± 147 | 469 ± 224 | 347 ± 175 | 55.2 | 331 |
| 2005–2006 | 73.0 ± 54.5 | 89.1 ± 65.4 | 44.4 ± 13.0 | 438 ± 327 | 535 ± 392 | 266 ± 78 | |||
| 2007–2008 | 47.0 ± 22.3 | 47.0 ± 12.0 | 45.6 ± 29.9 | 282 ± 134 | 282 ± 72 | 274 ± 179 | |||
| BDE-99 | 2003–2004 | 15.4 ± 8.1 | 23.1 ± 15.7 | 11.5 ± 4.5 | 92 ± 49 | 139 ± 94 | 69 ± 27 | 14.9 | 89 |
| 2005–2006 | 18.8 ± 15.4 | 24.5 ± 22.4 | 9.1 ± 2.2 | 113 ± 92 | 147 ± 134 | 55 ± 13 | |||
| 2007–2008 | 10.8 ± 3.1 | 10.1 ± 3.2 | 9.0 ± 7.1 | 65 ± 19 | 61 ± 19 | 54 ± 43 | |||
| BDE-100 | 2003–2004 | 12.7 ± 5.0 | 10.8 ± 5.0 | 8.2 ± 3.8 | 76 ± 30 | 65 ± 30 | 49 ± 23 | 10.9 | 65 |
| 2005–2006 | 12.5 ± 7.2 | 14.7 ± 14.6 | 6.8 ± 2.4 | 75 ± 43 | 88 ± 88 | 41 ± 14 | |||
| 2007–2008 | 7.7 ± 2.6 | 9.3 ± 3.7 | 9.5 ± 5.9 | 46 ± 16 | 56 ± 22 | 57 ± 35 | |||
| BDE‑153 | 2003–2004 | 16.3 ± 5.8 | 7.8 ± 3.2 | 5.6 ± 2.2 | 97 ± 35 | 47 ± 19 | 34 ± 13 | 14.8 | 89 |
| 2005–2006 | 10.3 ± 3.7 | 8.9 ± 4.0 | 6.2 ± 3.5 | 62 ± 22 | 53 ± 24 | 37 ± 21 | |||
| 2007–2008 | 11.0 ± 3.3 | 10.2 ± 3.8 | 10.3 ± 5.8 | 66 ± 20 | 61 ± 23 | 62 ± 35 | |||
| Abbreviations: MA, Mexican American; NHB, non-Hispanic black; NHW, non-Hispanic white. aPredicted U.S. milk PBDE concentrations were determined by applying congener-specific models (Marchitti et al. 2013b) to NHANES mean serum data for women of child-bearing age [20–39 years (Sjödin et al. 2014)]. bEstimated intakes for U.S. infants were calculated from predicted U.S. milk PBDE concentrations (this table) using the formula DIi = CmFMb. cMAMA Study (2004–2005) mean milk concentrations (visit 1) from 34 women (21–39 years); 85% NHW, 9% NHB, and 3% MA. dInfant intakes for MAMA Study participants were estimated from MAMA milk concentrations (this table) using the formula DIi = CmFMb. | |||||||||
Daily Infant Intakes
Predicted mean U.S. milk PBDE concentrations (Table 4) were used to estimate average daily infant intakes (DIi; ng/kg/day) using the formula DIi = CmFMb, as described in “Methods.” For 2007–2008, estimated PBDE intakes for U.S. infants were 274–282 ng/kg/day (BDE-47), 54–65 ng/kg/day (BDE-99), 46–57 ng/kg/day (BDE-100), and 61–66 ng/kg/day (BDE-153), depending on ethnic group (Table 4). Estimated PBDE intakes for MAMA participant infants were 331 ng/kg/day (BDE-47), 89 ng/kg/day (BDE-99), 65 ng/kg/day (BDE-100), and 89 ng/kg/day (BDE-153) (Table 4).
Discussion
As concentrations of other persistent chemicals, such as PCBs and dioxins, in human serum and milk have decreased in recent decades, concentrations of PBDEs were found to be increasing, and human exposures are expected to continue for many years (Schecter et al. 2010; Sjödin et al. 2014). U.S. women of childbearing age have among the world’s highest milk PBDE concentrations, which has led to concern over potential health impacts to breastfeeding infants (Bradman et al. 2007; Park et al. 2011). However, a lack of comprehensive data on breast milk concentrations of PBDEs in the United States has precluded our ability to conduct more complete exposure assessments. Although national data on chemicals in milk would be preferred, nationally representative data on hundreds of chemicals in serum is available and estimating U.S. population-level breast milk concentrations from these serum concentrations using congener-specific milk:serum partitioning information would be desirable.
Historically, in the absence of data on milk:serum partitioning, risk assessors have assumed that, at steady state, lipophilic and persistent chemicals partition to lipid stores in the body equally, such that the lipid-based concentration in one matrix, such as serum, can be assumed to be equal to the lipid-based concentration in a different matrix, such as breast milk. The science has since advanced, and recent studies suggest this approach is too simplistic and does not accurately take into account what is now understood about the complexities of chemical partitioning (LaKind et al. 2009; Mannetje et al. 2012; Marchitti et al. 2013a, 2013b). Various factors and physicochemical properties have been shown to influence partitioning between maternal serum and breast milk including molecular weight, molecular size, steric hindrance, lipophilicity, and halogenation (Needham et al. 2011). Thus, partitioning can vary substantially among different congeners of the same chemical class, and those congeners with greater distribution into breast milk should be evaluated more prudently when determining infant exposures (Mannetje et al. 2012; Marchitti et al. 2013b). To measure accurate partitioning ratios, careful consideration should be given to study design with milk and serum samples taken sufficiently postpartum (after the milk supply has been adequately established), and as close in time as possible from the same woman (i.e., within 1–2 hr). Few studies met these criteria prior to 2006; however, recent quality data available for PBDEs (LaKind et al. 2009; Schecter et al. 2006, 2010) were used to develop the first congener-specific models and approach for estimating nationally representative breast milk PBDE concentrations in the U.S. population from NHANES serum concentrations (Marchitti et al. 2013b). Although these models were initially validated and found to be highly predictive, they had yet to be rigorously tested or extended to more recent NHANES data sets. In this study, we have presented for the first time serum and milk concentrations of PBDEs from paired samples taken from women enrolled in the U.S. EPA MAMA Study, which followed quality partitioning data guidelines, and the use of these data to further test and expand these exposure models.
Serum and milk concentrations of PBDEs in MAMA Study participants were highly correlated. Consistent with other reports (Marchitti et al. 2013b; Schecter et al. 2010), BDE-47 was present at the highest concentrations in milk and serum, and lower brominated PBDEs demonstrated higher milk:serum partitioning ratios than higher brominated congeners (PBDE milk:serum ratio: 3 Br > 4 Br > 5 Br >> 6 Br >> 7 Br). Although PBDE log Kow values generally increase with increasing halogenation, steric hindrance and overall size of the higher brominated congeners may substantially limit their ability to distribute into breast milk. A recent review of available chemical partitioning data from 13 studies for persistent lipophilic organic chemicals, including PCDD/Fs (polychlorinated dibenzo-p-dioxins and furans), PCBs (polychlorinated biphenyls), PBDEs, and organochlorine pesticides, reported that mean partitioning ratios can vary by approximately 30-fold among a comprehensive list of specific congeners (Mannetje et al. 2012). Although most congeners appeared to have partitioning ratios that ranged more narrowly, albeit with congener-specific differences, ratios determined for the higher halogenated congeners of each chemical class (e.g., the deca-brominated BDE-209) suggest they have a very limited capacity to distribute into breast milk (Mannetje et al. 2012).
Exposure models developed for predicting milk PBDE concentrations from serum concentrations (Marchitti et al. 2013b) demonstrated high accuracy and predictability when tested against MAMA serum and milk data. Further application of these models to more recent NHANES serum data (through 2008) suggest that BDE-47, BDE-99, and BDE-100 are declining in U.S. breast milk for non-Hispanic white and non-Hispanic black populations, although these results were not statistically significant. For some populations, however, milk concentrations of BDE-100 (Mexican Americans) and BDE-153 (all ethnic groups) may be increasing, which may reflect unknown exposure sources related to diet or lifestyle. Predicted U.S. infant PBDE intakes are expected to follow the same trends, and as of the 2007–2008 time period, estimated daily infant intakes were below threshold reference doses (RfDs) for BDE-99 [RfD, 100 ng/kg body weight (bw)/day (U.S. EPA 2008a)] and BDE-153 [RfD, 200 ng/kg bw/day (U.S. EPA 2008b)] but above that suggested for BDE-47 [RfD, 100 ng/kg bw/day (U.S. EPA 2008c)]. Other studies have reported similar findings (Johnson-Restrepo et al. 2007; Park et al. 2011). Studies evaluating exposure to concentrations of PBDEs in breast milk in the highest quartiles (3rd and 4th) suggest subtle associations between early-life PBDE exposure and increased anxiety, withdrawal, and activity/impulsivity behaviors in early childhood (Adgent et al. 2014; Hoffman et al. 2012). However, improvement in certain adaptive behaviors and cognitive outcomes were also associated, suggesting that the positive factors associated with breastfeeding may mitigate some of the potentially adverse outcomes associated with PBDEs.
RfDs are determined by the U.S. EPA’s Integrated Risk Information System (IRIS) and are estimates of daily oral human exposure to chemicals that are not likely to cause harmful effects during a lifetime. These daily values are based on dose–response assessments from available studies; uncertainty may span orders of magnitude and is based on the design of the studies providing the dose–response data and how well those studies model a lifetime human exposure scenario. The RfD for BDE-47 (100 ng/kg bw/day) was derived from dose–response data for behavioral impairments observed in adult male mice following a single oral dose administered 10 days after birth, which represents a period of maximum vulnerability for mouse brain development (Eriksson et al. 2001). Calculation of this RfD included the application of an uncertainty factor of 3,000, accounting for a) the possibility that some people may be more sensitive to the effects of BDE-47 than others, b) the possibility that humans may be more sensitive than mice, c) the fact that the study administered only a single dose of the chemical whereas human exposure may be expected to occur on a daily basis over a prolonged period of time, and d) deficiencies in the toxicological database for BDE-47, which, at the time the RfD was derived, lacked prenatal developmental neurotoxicity studies, reproductive toxicity studies, and standard chronic or subchronic studies of systemic toxicity (U.S. EPA 2008c). Although RfDs are derived for one chemical at a time, humans are exposed concomitantly to complex mixtures of chemicals, including PBDE congeners; thus, an active area of research is the collective impacts of chemical combinations on human health.
Milk PBDE concentrations from MAMA participants (residing in North Carolina) were comparable to those reported from many U.S. states but were substantially (58–76%) higher than those from New Hampshire, Massachusetts, and Texas (Table 5). This may reflect differences in study demographics or regional differences in PBDE use or legislation. It is unclear whether concentrations of PBDEs in breast milk decrease with breastfeeding duration (i.e., depuration). One study (n = 18) reported a significant decrease (12–18%) in milk PBDE concentrations during the first 6 months postpartum (Hooper et al. 2007), and another study (n = 303) reported a significant increase (18%) in milk BDE-153 concentrations from 3 to 12 months postpartum (Daniels et al. 2010). Other studies have reported no consistent pattern in milk PBDE concentrations over time (Dunn et al. 2010; LaKind et al. 2009). The median interval between milk collections in MAMA participants was 8.1 weeks, and no evidence of depuration was observed.
Table 5.
Median milk concentrations (ng/g lipid) from U.S. studies, 2002–2006.
| Study | State | Year | n | BDE-47 | BDE-99 | BDE-100 | BDE-153 |
|---|---|---|---|---|---|---|---|
| U.S. EPA MAMA studya | NC | 2004–2005 | 34 | 31.5 | 6.0 | 5.3 | 5.0 |
| Daniels et al. 2010 | NC | 2004–2006 | 303 | 28.0 | 5.0 | 5.0 | 6.0 |
| Dunn et al. 2010 | NH | 2005–2006 | 40 | 13.4 | 2.0 | 2.5 | 5.0 |
| Wu et al. 2007 | MA | 2004–2005 | 46 | 13.9 | 2.4 | 2.4 | 3.1 |
| Johnson-Restrepo et al. 2007 | MA | 2004 | 38 | 7.7 | 1.5 | 0.5 | 1.1 |
| LaKind et al. 2009 | PA | 2004–2005 | 10 | 26.0 | 4.6 | 4.0 | 3.5 |
| LaKind et al. 2008 | PA | 2004–2006 | 19 | 24.0 | 3.8 | 3.6 | 3.4 |
| Schecter et al. 2003 | TX | 2002 | 47 | 18.4 | 5.7 | 2.9 | 2.0 |
| Schecter et al. 2006 | TX | 2003 | 11 | 10.0 | 2.0 | 2.1 | 2.4 |
| Park et al. 2011 | CA | 2003–2005 | 82 | 29.7 | 6.4 | 5.7 | 6.3 |
| She et al. 2007 | PNWb | 2003 | 40 | 27.8 | 5.4 | 5.3 | 4.8 |
| aVisit 1 median values. bPNW, Pacific Northwest (MT, OR, and WA). | |||||||
Study Limitations
The MAMA Study was limited to a small convenience sample of primarily non-Hispanic white, well-educated mothers. Preliminary evaluation of demographic and lifestyle variables indicated that, as in other studies (Petreas et al. 2003), concentrations of PBDEs in milk and serum from MAMA participants decreased with maternal age. PBDE concentrations were not associated with either dietary preferences or parity, which is similar to other findings (Guvenius et al. 2003); however, our survey questions on diet may have been too limited and did not account for many subcategories such as various forms of dairy (e.g., cheese, ice cream, yogurt) nor discriminate among alternative types of milk (e.g., soy, almond milk). In addition, approximately half of the MAMA participants were first-time mothers, which limited our analysis of parity. Moreover, because of the small sample size, correlations between PBDE concentrations and demographics may be ancillary observations, and further research is necessary. This need is not unique to PBDEs. Indeed, the lack of nationally representative data for environmental chemicals in breast milk in general has substantially limited exposure assessments for infants and young children (Lehmann et al. 2014). As a solution to this problem, some countries (e.g., Sweden and Germany) have conducted breast milk monitoring programs; however, no such program exists in the United States. As a sustainable alternative, we have developed models to predict breast milk PBDE concentrations based on serum concentrations (Marchitti et al. 2013b). This study further validated these models and applied them to NHANES serum data from 2003–2008 to obtain nationally representative estimates of PBDE concentrations in breast milk in the U.S. population over time. From these data, we estimated daily infant intakes of PBDEs meant to be representative for U.S. breastfeeding infants. Before this study, no national intake data for U.S. infants were available. However, caution should be used when interpreting our results because two extrapolations have been performed in this study: a) from U.S. women of childbearing age in the NHANES survey to U.S. lactating women, and b) from U.S. breast milk concentrations to U.S. daily infant intakes. These extrapolations may increase uncertainty; however, until data are available to allow for more direct evaluations of early-life exposures, such extrapolations expedite the development of methods and tools to improve risk assessment for infants and young children.
Conclusions
Maternal serum, in conjunction with milk:serum partitioning models, appears to be an acceptable surrogate for predicting PBDE concentrations in human milk. Using NHANES serum data, concentrations of most PBDEs in U.S. breast milk appear to be declining but concentrations of BDE-153 may be rising, which may reflect an unknown source related to diet or lifestyle. Breastfeeding confers extensive developmental benefits to infants and should continue to be recommended despite the presence of environmental chemicals. However, continued research of the molecular complexities of chemical partitioning is necessary for performing accurate infant exposure and risk assessments.
Supplemental Material
Acknowledgments
We thank the study participants and Westat, Inc. (A. Ware, B. Bradford, and B. Karasek) and the technical and nursing staff (D. Levin, M.A. Bassett, T. Montilla, J. Reidy, E. Samandar, R. Wang, and J. Preau). We also would like to thank A. Sjödin [Centers for Disease Control and Prevention (CDC), Atlanta, GA] for performing analytical measurements.
Footnotes
Funding was provided by the U.S. EPA, the NIH, and the CDC, and S.A.M. was supported by a Postdoctoral Research Program appointment at the Ecosystems Research Division by the Oak Ridge Institute for Science and Education through Interagency Agreement No. (DW8992298301) between the U.S. Department of Energy and the U.S. EPA.
This article has been reviewed by the U.S. EPA and approved for publication. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. EPA.
The authors declare they have no actual or potential competing financial interests.
References
- Adgent MA, Hoffman K, Goldman BD, Sjödin A, Daniels JL. Brominated flame retardants in breast milk and behavioural and cognitive development at 36 months. Paediatr Perinat Epidemiol. 2014;28(1):48–57. doi: 10.1111/ppe.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum L. Health effects of brominated flame retardants (BFRs). Organohalogen Compounds. 2007;69:670–673. [Google Scholar]
- Bradman A, Fenster L, Sjödin A, Jones RS, Patterson DG, Jr, Eskenazi B. 2007. Polybrominated diphenyl ether levels in the blood of pregnant women living in an agricultural community in California. Environ Health Perspect 115 71 74, doi: 10.1289/ehp.8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braekevelt E, Tittlemier SA, Tomy GT. Direct measurement of octanol-water partition coefficients of some environmentally relevant brominated diphenyl ether congeners. Chemosphere. 2003;51(7):563–567. doi: 10.1016/S0045-6535(02)00841-X. [DOI] [PubMed] [Google Scholar]
- Chao HR, Tsou TC, Huang HL, Chang-Chien GP. Levels of breast milk PBDEs from southern Taiwan and their potential impact on neurodevelopment. Pediatr Res. 2011;70:596–600. doi: 10.1203/PDR.0b013e3182320b9b. [DOI] [PubMed] [Google Scholar]
- Daniels JL, Pan IJ, Jones R, Anderson S, Patterson DG, Jr, Needham LL, et al. 2010. Individual characteristics associated with PBDE levels in U.S. human milk samples. Environ Health Perspect 118 155 160, doi: 10.1289/ehp.0900759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn RL, Huwe JK, Carey GB. Biomonitoring polybrominated diphenyl ethers in human milk as a function of environment, dietary intake, and demographics in New Hampshire. Chemosphere. 2010;80:1175–1182. doi: 10.1016/j.chemosphere.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ Health Perspect. 2001;109:903–908. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Fort M, Martínez D, Carsin AE, Forns J, Grimalt JO, et al. 2012. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ Health Perspect 120 1760 1765, doi: 10.1289/ehp.1205266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Norén K. 2003. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect 111 1235 1241, doi: 10.1289/ehp.5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy ML. A comparison of the properties of the major commercial PBDPO/PBDE product to those of major PBB and PCB products. Chemosphere. 2002;46(5):717–728. doi: 10.1016/s0045-6535(01)00236-3. [DOI] [PubMed] [Google Scholar]
- Hines EP, Calafat AM, Silva MJ, Mendola P, Fenton SE. 2009. Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating North Carolina women. Environ Health Perspect 117 86 92, doi: 10.1289/ehp.11610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines EP, Mendola P, von Ehrenstein OS, Ye X, Calafat AM, Fenton SE. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod Toxicol. 2015;54:120–128. doi: 10.1016/j.reprotox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Adgent M, Goldman BD, Sjödin A, Daniels JL. 2012. Lactational exposure to polybrominated diphenyl ethers and its relation to social and emotional development among toddlers. Environ Health Perspect 120 1438 1442, doi: 10.1289/ehp.1205100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper K, She J, Sharp M, Chow J, Jewell N, Gephart R, et al. 2007. Depuration of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk from California first-time mothers (primiparae). Environ Health Perspect 115 1271 1275, doi: 10.1289/ehp.10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Restrepo B, Addink R, Wong C, Arcaro K, Kannan K. Polybrominated diphenyl ethers and organochlorine pesticides in human breast milk from Massachusetts, USA. J Environ Monit. 2007;9:1205–1212. doi: 10.1039/b711409p. [DOI] [PubMed] [Google Scholar]
- Johnson-Restrepo B, Kannan K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere. 2009;76:542–548. doi: 10.1016/j.chemosphere.2009.02.068. [DOI] [PubMed] [Google Scholar]
- LaKind JS, Berlin CM, Jr, Sjödin A, Turner W, Wang RY, Needham LL, et al. 2009. Do human milk concentrations of persistent organic chemicals really decline during lactation? Chemical concentrations during lactation and milk/serum partitioning. Environ Health Perspect 117 1625 1631, doi: 10.1289/ehp.0900876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaKind JS, Berlin CM, Jr, Stokes JL, Naiman DQ, Paul IM, Patterson DG, Jr, et al. Lifestyle and polybrominated diphenyl ethers in human milk in the United States: a pilot study. Toxicol Environ Chem. 2008;90:1047–1054. [Google Scholar]
- Lehmann GM, Verner MA, Luukinen B, Henning C, Assimon SA, LaKind JS, et al. Improving the risk assessment of lipophilic persistent environmental chemicals in breast milk. Crit Rev Toxicol. 2014;44(7):600–617. doi: 10.3109/10408444.2014.926306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannetje A, Coakley J, Mueller JF, Harden F, Toms LM, Douwes J. Partitioning of persistent organic pollutants (POPs) between human serum and breast milk: a literature review. Chemosphere. 2012;89(8):911–918. doi: 10.1016/j.chemosphere.2012.06.049. [DOI] [PubMed] [Google Scholar]
- Marchitti SA, Hines EP, LaKind JS, Berlin CM Jr, Fenton SE, Kenneke JF. Amsterdam, Netherlands: Elsevier; 2013a. Environmental chemicals in breast milk. In: Reference Module in Earth Systems and Environmental Sciences. Elias SA, ed. pp. 1–13. [Google Scholar]
- Marchitti SA, LaKind JS, Naiman DQ, Berlin CM, Kenneke JF. Improving infant exposure and health risk estimates: using serum data to predict polybrominated diphenyl ether concentrations in breast milk. Environ Sci Technol. 2013b;47:4787–4795. doi: 10.1021/es305229d. [DOI] [PubMed] [Google Scholar]
- Needham LL, Grandjean P, Heinzow B, Jørgensen PJ, Nielsen F, Patterson DG, Jr, et al. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 2011;45(3):1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, She J, Holden A, Sharp M, Gephart R, Souders-Mason G, et al. High postnatal exposures to polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) via breast milk in California: does BDE-209 transfer to breast milk? Environ Sci Technol. 2011;45:4579–4585. doi: 10.1021/es103881n. [DOI] [PubMed] [Google Scholar]
- Petreas M, She J, Brown FR, Winkler J, Windham G, Rogers E, et al. 2003. High body burdens of 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47) in California women. Environ Health Perspect 111 1175 1179, doi: 10.1289/ehp.6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Sjödin A, Needham L, Birnbaum L. Partitioning of polybrominated diphenyl ethers (PBDEs) in serum and milk from the same mothers. Chemosphere. 2010;78:1279–1284. doi: 10.1016/j.chemosphere.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Schecter A, Päpke O, Harris TR, Tung KC. Partitioning of polybromonated diphenyl ether (PBDE) congeners in human blood and milk. Toxicol Environ Chem. 2006;88:319–324. [Google Scholar]
- Schecter A, Pavuk M, Päpke O, Ryan JJ, Birnbaum L, Rosen R. 2003. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ Health Perspect 111 1723 1729, doi: 10.1289/ehp.6466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- She J, Holden A, Sharp M, Tanner M, Williams-Derry C, Hooper K. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk from the Pacific Northwest. Chemosphere. 2007;67:S307–S317. doi: 10.1016/j.chemosphere.2006.05.154. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Caudill SP, Wong LY, Turner WE, Calafat AM. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the National Health and Nutrition Examination Survey: 2003–2008. Environ Sci Technol. 2014;48:753–760. doi: 10.1021/es4037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, III, Patterson DG., Jr Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem. 2004a;76:1921–1927. doi: 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- Sjödin A, McGahee EE, III, Focant JF, Jones RS, Lapeza CR, Zhang Y, et al. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers and polybrominated and polychlorinated biphenyls in breast milk. Anal Chem. 2004b;76:4508–4514. doi: 10.1021/ac0495384. [DOI] [PubMed] [Google Scholar]
- Toms LM, Harden F, Paepke O, Hobson P, Ryan JJ, Mueller JF. Higher accumulation of polybrominated diphenyl ethers in infants than in adults. Environ Sci Technol. 2008;42:7510–7515. doi: 10.1021/es800719v. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) 2,2’,4,4’,5-Pentabromodiphenyl ether (BDE-99) (CASRN 60348-60-9). Integrated Risk Information System. 2008a https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/1008_summary.pdf [accessed 1 March 2017]
- U.S. EPA. 2,2’,4,4’,5,5’-Hexabromodiphenyl ether (BDE-153) (CASRN 68631-49-2). Integrated Risk Information System. 2008b https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/1009_summary.pdf [accessed 1 March 2017]
- U.S. EPA. 2,2’,4,4’-Tetrabromodiphenyl ether (BDE-47) (CASRN 5436-43-1). Integrated Risk Information System. 2008c https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/1010_summary.pdf [accessed 1 March 2017]
- U.S. EPA. Washington DC: U.S. EPA; 2011. Exposure Factors Handbook: 2011 Edition. EPA/600/R-09/052F. https://www.epa.gov/expobox/exposure-factors-handbook-2011-edition [accessed 1 March 2017] [Google Scholar]
- Wu N, Herrmann T, Paepke O, Tickner J, Hale R, Harvey LE, et al. Human exposure to PBDEs: associations of PBDE body burdens with food consumption and house dust concentrations. Environ Sci Technol. 2007;41:1584–1589. doi: 10.1021/es0620282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


