Abstract
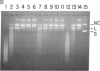
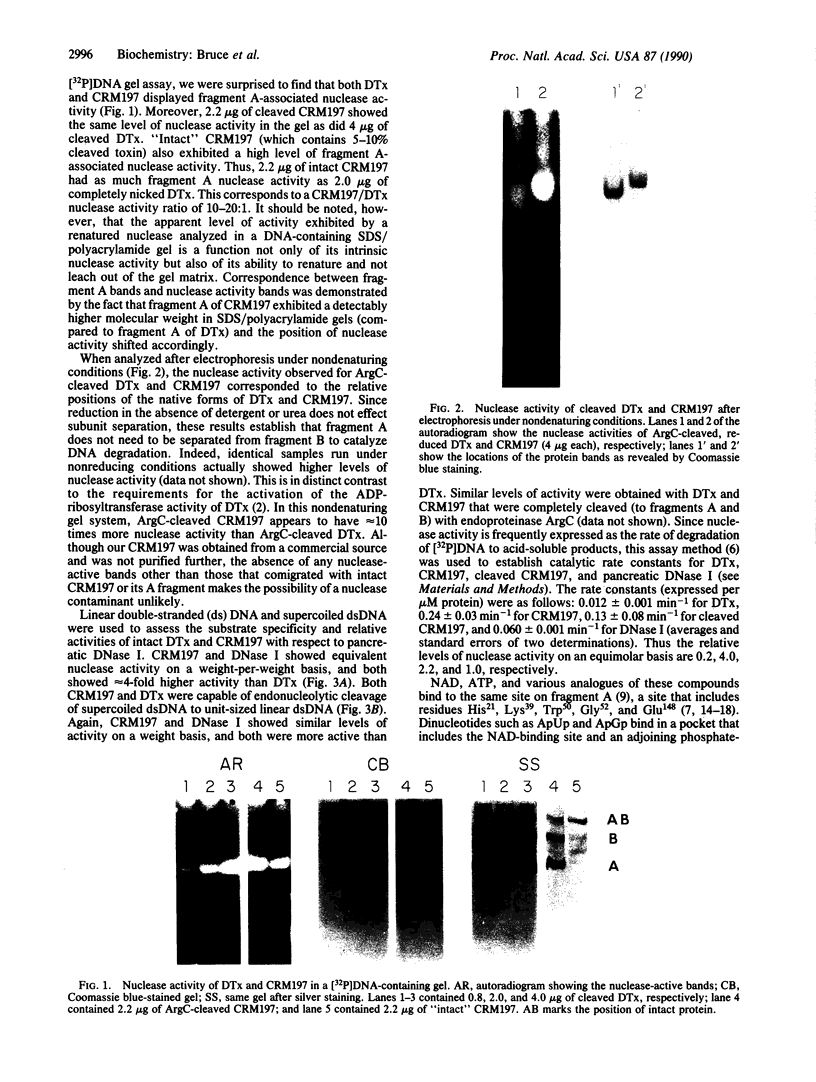
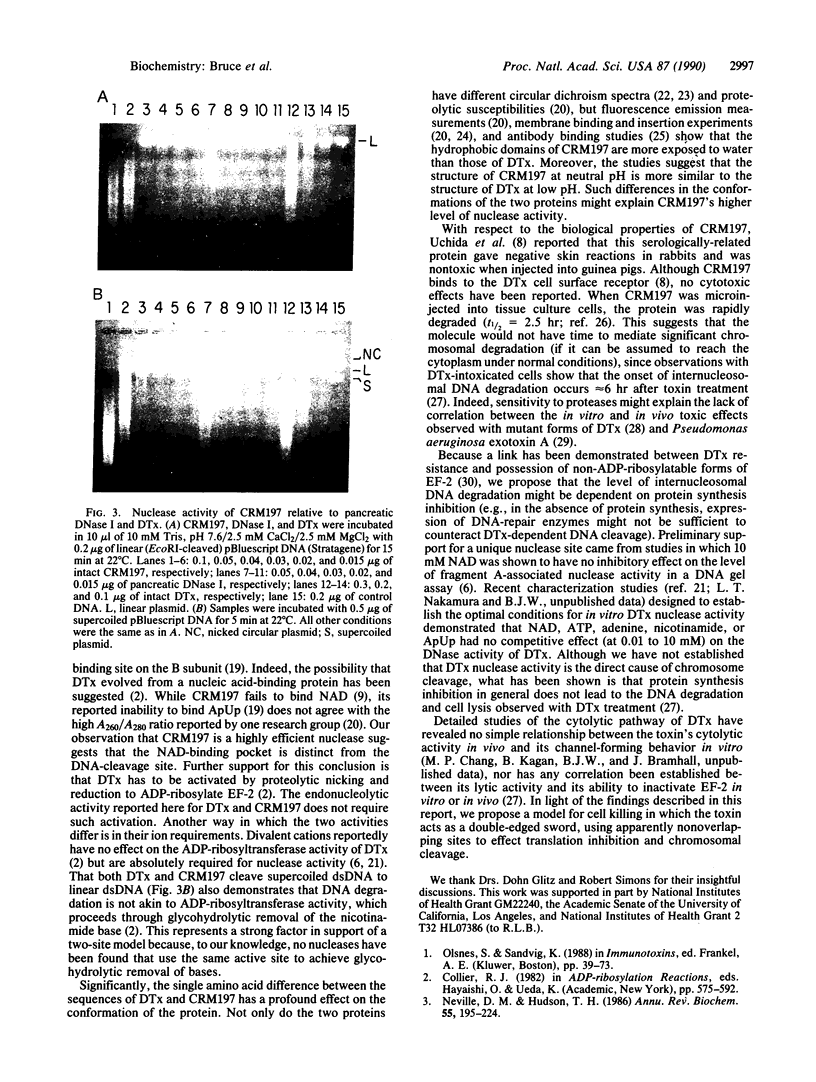
The cytotoxic mechanism of diphtheria toxin (DTx) is associated with its ability to inhibit protein synthesis by ADP-ribosylation of elongation factor 2. Although DTx intoxication leads to internucleosomal DNA cleavage and cell lysis, these events do not occur when protein synthesis is inhibited by alternative treatments (e.g., cycloheximide). Here we show that endonucleolytic degradation of DNA is an intrinsic activity of DTx and also of the crossreactive mutant protein CRM197. Assays using DNA-impregnated gels as well as linear and supercoiled DNA in solution revealed not only that CRM197 has nuclease activity but also that its specific activity is actually significantly greater than that of the wild-type molecule. Since CRM197 contains a single amino acid substitution that renders it incapable of ADP-ribosylation, we propose that the active sites for ADP-ribosyltransferase and nuclease activities are distinct.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigio M., Rossi R., Nucci D., Antoni G., Rappuoli R., Ratti G. Conformational changes in diphtheria toxoids. Analysis with monoclonal antibodies. FEBS Lett. 1987 Jun 29;218(2):271–276. doi: 10.1016/0014-5793(87)81060-8. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Collier R. J. NAD binding site of diphtheria toxin: identification of a residue within the nicotinamide subsite by photochemical modification with NAD. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3307–3311. doi: 10.1073/pnas.81.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. P., Baldwin R. L., Bruce C., Wisnieski B. J. Second cytotoxic pathway of diphtheria toxin suggested by nuclease activity. Science. 1989 Dec 1;246(4934):1165–1168. doi: 10.1126/science.2531465. [DOI] [PubMed] [Google Scholar]
- Chang M. P., Bramhall J., Graves S., Bonavida B., Wisnieski B. J. Internucleosomal DNA cleavage precedes diphtheria toxin-induced cytolysis. Evidence that cell lysis is not a simple consequence of translation inhibition. J Biol Chem. 1989 Sep 15;264(26):15261–15267. [PubMed] [Google Scholar]
- Collins C. M., Collier R. J. Circular dichroism of diphtheria toxin, Pseudomonas aeruginosa exotoxin A, and various derivatives. Biochim Biophys Acta. 1985 Apr 5;828(2):138–143. doi: 10.1016/0167-4838(85)90049-4. [DOI] [PubMed] [Google Scholar]
- Collins C. M., Collier R. J. Interaction of diphtheria toxin with adenylyl-(3',5')-uridine 3'-monophosphate. II. The NAD-binding site and determinants of dinucleotide affinity. J Biol Chem. 1984 Dec 25;259(24):15159–15162. [PubMed] [Google Scholar]
- Douglas C. M., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: substitution of glutamic acid 553 with aspartic acid drastically reduces toxicity and enzymatic activity. J Bacteriol. 1987 Nov;169(11):4967–4971. doi: 10.1128/jb.169.11.4967-4971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Giannini G., Rappuoli R., Ratti G. The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res. 1984 May 25;12(10):4063–4069. doi: 10.1093/nar/12.10.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J. E., Wisnieski B. J. An endosomal model for acid triggering of diphtheria toxin translocation. J Biol Chem. 1988 Oct 25;263(30):15257–15259. [PubMed] [Google Scholar]
- Hu V. W., Holmes R. K. Single mutation in the A domain of diphtheria toxin results in a protein with altered membrane insertion behavior. Biochim Biophys Acta. 1987 Aug 7;902(1):24–30. doi: 10.1016/0005-2736(87)90132-5. [DOI] [PubMed] [Google Scholar]
- Kandel J., Collier R. J., Chung D. W. Interaction of fragment A from diphtheria toxin with nicotinamide adenine dinucleotide. J Biol Chem. 1974 Apr 10;249(7):2088–2097. [PubMed] [Google Scholar]
- Kohno K., Uchida T. Highly frequent single amino acid substitution in mammalian elongation factor 2 (EF-2) results in expression of resistance to EF-2-ADP-ribosylating toxins. J Biol Chem. 1987 Sep 5;262(25):12298–12305. [PubMed] [Google Scholar]
- Lory S., Carroll S. F., Collier R. J. Ligand interactions of diphtheria toxin. II. Relationships between the NAD site and the P site. J Biol Chem. 1980 Dec 25;255(24):12016–12019. [PubMed] [Google Scholar]
- Mekada E., Uchida T. Binding properties of diphtheria toxin to cells are altered by mutation in the fragment A domain. J Biol Chem. 1985 Oct 5;260(22):12148–12153. [PubMed] [Google Scholar]
- Michel A., Dirkx J. Occurrence of tryptophan in the enzymically active site of diphtheria toxin fragment A. Biochim Biophys Acta. 1977 Mar 28;491(1):286–295. doi: 10.1016/0005-2795(77)90064-2. [DOI] [PubMed] [Google Scholar]
- Nakamura L. T., Wisnieski B. J. Characterization of the deoxyribonuclease activity of diphtheria toxin. J Biol Chem. 1990 Mar 25;265(9):5237–5241. [PubMed] [Google Scholar]
- Neville D. M., Jr, Hudson T. H. Transmembrane transport of diphtheria toxin, related toxins, and colicins. Annu Rev Biochem. 1986;55:195–224. doi: 10.1146/annurev.bi.55.070186.001211. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Sandvig K. How protein toxins enter and kill cells. Cancer Treat Res. 1988;37:39–73. doi: 10.1007/978-1-4613-1083-9_4. [DOI] [PubMed] [Google Scholar]
- Papini E., Colonna R., Schiavo G., Cusinato F., Tomasi M., Rappuoli R., Montecucco C. Diphtheria toxin and its mutant crm 197 differ in their interaction with lipids. FEBS Lett. 1987 May 4;215(1):73–78. doi: 10.1016/0014-5793(87)80116-3. [DOI] [PubMed] [Google Scholar]
- Papini E., Schiavo G., Sandoná D., Rappuoli R., Montecucco C. Histidine 21 is at the NAD+ binding site of diphtheria toxin. J Biol Chem. 1989 Jul 25;264(21):12385–12388. [PubMed] [Google Scholar]
- Rosenthal A. L., Lacks S. A. Nuclease detection in SDS-polyacrylamide gel electrophoresis. Anal Biochem. 1977 May 15;80(1):76–90. doi: 10.1016/0003-2697(77)90627-3. [DOI] [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973 Jun 10;248(11):3838–3844. [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Harper A. A. Diphtheria toxin and related proteins. II. Kinetic studies on intoxication of HeLa cells by diphtheria toxin and related proteins. J Biol Chem. 1973 Jun 10;248(11):3845–3850. [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Takamatsu K., Okada Y. Intracellular stability of diphtheria toxin fragment A in the presence and absence of anti-fragment A antibody. Proc Natl Acad Sci U S A. 1982 Jan;79(2):461–465. doi: 10.1073/pnas.79.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalman L. S., Wisnieski B. J. Mechanism of insertion of diphtheria toxin: peptide entry and pore size determinations. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3341–3345. doi: 10.1073/pnas.81.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. M., London E. Localization of the active site of diphtheria toxin. Biochemistry. 1988 May 3;27(9):3398–3403. doi: 10.1021/bi00409a041. [DOI] [PubMed] [Google Scholar]