Abstract
Current conditioning regimens provide insufficient disease control in relapsed/refractory acute leukemia (AL) patients undergoing hematopoietic stem cell transplantation (HSCT) with active disease. Intensification of chemotherapy and/or total body irradiation (TBI) is not feasible because of excessive toxicity. Total marrow and lymphoid irradiation (TMLI) allows for precise delivery and increased intensity treatment via sculpting radiation to sites with high disease burden or high risk for disease involvement, while sparing normal tissue. We conducted a phase I trial in 51 patients (age: 16–57 years) with relapsed/refractory AL undergoing HSCT (matched related, matched unrelated, or one-allele mismatched unrelated) with active disease, combining escalating doses of TMLI (range 1200–2000 cGy) with cyclophosphamide (CY) and etoposide (VP16). The maximum tolerated dose was declared at 2000 cGy since TMLI simulation studies indicated that >2000 cGy might deliver doses toxic for normal organs at or exceeding those delivered by standard TBI. The post transplant non-relapse mortality (NRM) rate was only 3.9% (95% CI: 0.7–12.0) at day +100 and 8.1% (95% CI: 2.5–18.0) at one year. The cumulative incidence of grade II–IV acute GVHD was 43.1% (95% CI: 29.2–56.3) and for grade III–IV was 13.7% (95% CI: 6.9–27.3). The day +30 complete remission rate for all patients was 88% and was 100% for those treated at 2000 cGy. The overall one-year survival was 55.5% (95% CI: 40.7–68.1). The TMLI/CY/VP16 conditioning regimen is well tolerated at TMLI doses up to 2000 cGy with a low 100 day and 1 year NRM rate and no increased risk of GVHD with higher doses of radiation.
Keywords: Total marrow and lymphoid irradiation, acute myeloid leukemia, acute lymphoblastic leukemia, relapsed/refractory
Introduction
Acute leukemia patients who fail induction therapy or relapse after achievement of first complete remission (CR) require allogeneic hematopoietic stem cell transplantation (alloHSCT) as the primary curative option. Standard myeloablative alloHSCT regimens use high-dose chemotherapy, frequently in combination with total body irradiation (TBI), but provide insufficient disease control for patients who undergo alloHSCT with active disease, with a 3-year overall survival (OS) of only 19% for acute myeloid leukemia (AML) and 16% for acute lymphoblastic leukemia (ALL).1
Increasing the TBI dose has the potential to decrease post-transplant relapse rate as demonstrated in a randomized trial comparing 1200 cGy vs. 1575 cGy in AML patients in first CR with a 3-year relapse rate of 35% vs. 12%;2 however, disease-free survival (DFS) was similar in the two arms because of the increased mortality from toxicity and/or graft-versus-host disease (GVHD) in patients treated on the higher dose arm. Furthermore, in a retrospective study,3 the relapse rate was improved for patients with AML and chronic myelogenous leukemia (CML) treated with TBI doses >990 cGy. In a Center for International Blood and Marrow Transplant Research (CIBMTR) study, Marks et al. reported that patients with ALL beyond first CR receiving TBI/cyclophosphamide (CY) conditioning regimens had a lower relapse rate and increased DFS if treated with a TBI dose >1300 cGy4. Kal et al. compared results of different TBI regimens5 and showed that TBI regimens with higher biologically effective doses (BED) were associated with lower relapse rates and improved DFS and overall survival (OS). BED was used as an endpoint to normalize regimens for differences in dose-per-fraction, number of fractions, and dose-rates.
Despite the evidence of a dose-dependent anti-leukemia activity of TBI, more intense dosing is difficult to deliver to high risk patients because of significant increases in toxicities and long-term morbidities, which eventually offset any potential clinical advantage.2, 6–12 As a result and despite the potential for disease control from higher doses of radiation, patients with co-morbid conditions, older than 50 years, or with disease refractory to salvage chemotherapy are often ineligible for TBI-containing regimens.
Targeted forms of TBI delivery such as total marrow and lymphoid irradiation (TMLI) that selectively target diseased tissue while sparing healthy tissue are being pursued with the goal to reduce radiation-associated side effects and maximize the radiation therapeutic index.13–17 TMLI as part of alloHSCT conditioning regimens may make radiotherapy-containing regimens available to a broader spectrum of patients and allow safe dose intensification with curative intent for patients undergoing treatment with active disease.14–16 We report here a novel high intensity alloHSCT conditioning regimen combining TMLI with CY and etoposide (VP16) for patients with acute leukemia who are treatment-refractory or beyond second remission and therefore undergoing transplant with active disease. In a Phase I dose-escalation trial, we demonstrated that the TMLI/CY/VP16 conditioning regimen for alloHSCT is feasible, well tolerated at TMLI-targeted doses of up to 2000 cGy, and provides encouraging anti-leukemic activity.
Methods
This phase I clinical trial was registered with clinicaltrials.gov (NCT00576979, NCT02094794) and approved by the City of Hope Institutional Review Board. An assurance was filed with and approved by the Department of Health and Human Services. Informed consent was obtained for all study participants in compliance with the Declaration of Helsinki.
Eligibility criteria
Between 02/2008 and 10/2014, 51 eligible adult patients aged <60 years with relapsed/refractory AML or ALL and resistant to salvage conventional chemotherapy regimens were accrued on this trial. All patients had active disease at start of transplant preparative treatment. Stem cell donors were either HLA identical (siblings or unrelated) or 9/10 allele matched unrelated donors. The number of patients accrued to each dose level is indicated in Table 1; patient and disease characteristics are shown in Table 2.
Table 1.
Dose Levels for dose escalation of TMLI in TMLI/CY/VP16 alloHSCT conditioning regimen.
| Level | # Patients | Fraction and Schedule | Total Dose |
|---|---|---|---|
| 1 | 3 | 150 cGy bid × Days 1–4 | 1200 cGy |
| 2 | 3 | 150 cGy bid Day 1–4,150 cGy qd Day 5 | 1350 cGy |
| 3 | 9 | 150 cGy bid Days 1–5 | 1500 cGy (ribs, sternum, liver, brain limited to 1200cGy) |
| 4 | 6 | 150 cGy bid Days 1–5 | 1500 cGy (liver, porta hepatis, brain limited to 1200cGy) |
| 5 | 6 | 160 cGy bid Days 1–5 | 1600 cGy (liver, porta hepatis, brain limited to 1200cGy) |
| 6 | 6 | 170 cGy bid Days 1–5 | 1700 cGy (liver, porta hepatis, brain limited to 1200cGy) |
| 7 | 6 | 180 cGy bid Days 1–5 | 1800 cGy (liver, porta hepatis, brain limited to 1200cGy) |
| 8 | 6 | 190 cGy bid Days 1–5 | 1900 cGy (liver, porta hepatis, brain limited to 1200cGy) |
| 9 | 6 | 200 cGy bid Days 1–5 | 2000 cGy (liver, porta hepatis, brain limited to 1200cGy) |
Table 2.
Patient and disease characteristics.
| Variable | Median (range) or N |
|---|---|
| Age at transplant (years) | 34 (16–57) |
|
| |
| Disease diagnosis | |
| AML | 33 |
| ALL Ph− | 14 |
| ALL Ph+ | 2 |
| biphenotypic | 1 |
| undifferentiated | 1 |
|
| |
| Disease status at time of alloHSCT | |
| 1 RL | 14 |
| 2 RL | 3 |
| IF | 34 |
|
| |
| Cytogenetic risk (SWOG criteria), AML | |
| favorable | 0 |
| intermediate | 19 |
| unfavorable | 14 |
|
| |
| Cytogenetic risk (SWOG criteria), ALL | |
| favorable | 1 |
| intermediate | 7 |
| unfavorable | 5 |
| unknown significance | 3 |
|
| |
| Risk score* | 3 (0–6) |
|
| |
| KPS at HSCT | 80 (60–100) |
|
| |
| Donor source | |
| sibling | 25 |
| HLA matched unrelated | 5 |
| mismatched (1 allele) unrelated | 21 |
|
| |
| WBC at HSCT | 1.4 (0.1–14.9) |
|
| |
| % Blasts in blood at transplant** | 5 (0–85) |
|
| |
| % Blasts in marrow at transplant** | 52 (5–98) |
|
| |
| Extramedullary disease at time of HSCT | 9 |
AML=acute myeloid leukemia, ALL=acute lymphoblastic leukemia, Ph=Philadelphia chromosome, alloHSCT=allogeneic hematopoietic stem cell transplantation, RL=relapse, IF=induction failure, KPS=Karnofsky Performance Status, HLA=human leukocyte antigen, WBC=white blood cell count.
Scoring based on criteria by Duval et al.1
Excludes patients with solely extramedullary disease (Blasts in BM < 5%), n=4
TMLI
Details of the technique have been previously published.13 Briefly, all patients receiving TMLI underwent computed tomography (CT) simulation and were treated on a TomoTherapy® system. For treatment planning purposes, the target regions identified included the bone and bone marrow, major lymph node chains, spleen, testes, liver and brain. Mesenteric and Waldeyer Ring lymph nodes, as well as the mandible, were excluded as target regions to minimize dose to the oral cavity and GI tract. All other organs (such as lungs, heart, small and large intestine, kidneys, eyes, lenses, oral cavity, bladder, parotid glands, stomach, and esophagus) were identified as organs at risk (OARs), and efforts were made to minimize dose to these organs.
Preparative regimen and GVHD prophylaxis
All patients underwent bone marrow (BM) or peripheral blood stem cells (PBSC) alloHSCT with a conditioning regimen that combined escalating doses of TMLI with VP16 (60 mg/kg) and CY (100 mg/kg) (see Figure 1 for treatment schema). Eight to ten planned doses of TMLI ranging from 1200 to 2000 cGy were administered in twice-daily fractions over 4 or 5 days (Table 1). BM, major lymph node chains (excluding mesenteric lymph nodes and Waldeyer ring), and testes were escalated up to 2000 cGy with liver, porta hepatis, and brain kept at 1200 cGy (Table 1). Palifermin was administered to reduce the risk of mucositis.18 GVHD prophylaxis consisted of tacrolimus and sirolimus.19 No post-transplant maintenance therapy was part of the planned therapy.
Figure 1. Treatment Schema.
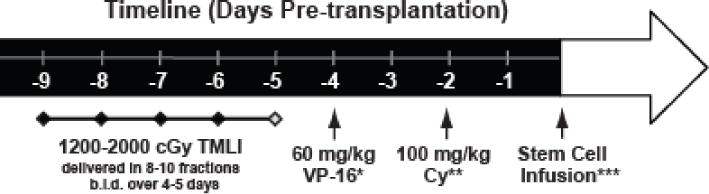
TMLI was delivered in 8–10 fractions b.i.d. over 4–5 days with total targeted dose ranging from 1200–2000 cGy. VP16 = etoposide; CY = cyclophosphamide.
*Adjusted body weight, **Ideal body weight, ***A window of 1–2 days is allowed for stem cell availability. Interval between CY and stem cell infusion must be >48 hrs.
Study Design and Statistical Analysis
This single-institution phase I trial tested escalating doses of TMLI in a TMLI/CY/VP16 alloHSCT conditioning regimen. The primary objectives were to establish the maximum tolerated dose (MTD) of TMLI in this regimen and to describe the toxicities at each dose level. Secondary objectives included estimation of non-relapse mortality (NRM), CR rate, OS, and an assessment of radiation dose to target and off-target organs.
Dose-limiting toxicity (DLT) was defined as any grade 3 or 4 non-hematological toxicity per the modified Bearman scale. Hematologic DLT was defined as grade 4 neutropenia associated with fever or infection lasting beyond 3 weeks, or grade 4 neutropenia lasting for more than 28 days per CTCAE 3.0 toxicity criteria. In addition, septic DLT was defined per CTCAE 3.0 as any grade 5 sepsis-related toxicity attributable to the protocol treatment/conditioning regimen.
Dose levels (DL) escalation/de-escalation/expansion proceeded using a standard 3+3 design for DL 1–4. Patients were treated in cohorts of 3 on each dose level. If 0/3 patients experienced a DLT, 3 patients were treated at the next dose level. If a DLT attributable (definite, possible, probable) to the study treatment was experienced in exactly 1/3 patients, 3 more patients (for a total of 6) were treated at that dose level. If no additional DLTs were observed at the expanded dose level (i.e. 1/6 with DLT), the dose was escalated.
On the basis of our myeloma trial which declared an MTD of 1600 cGy,16 DL 5–9 (at ≥1600 cGy) employed a modified rolling 3+2 design (a more conservative version of the 3-at-risk design20) to allow for up to 6 patients on a given DL for further evaluation of toxicity. Six patients were accrued to DL 5 and higher. At most, 3 patients were observed for DLT on the current DL at any time. Once each patient was evaluable for toxicity and passed without a DLT, an additional patient could be accrued on the tested DL. Once 3 patients were evaluable at a DL and none experienced dose-limiting toxicities, 3 additional patients were enrolled at that or an escalated DL. If a DLT was documented with fewer than 6 evaluable patients for a given DL, accrual continued at that dose level until 6 patients were evaluable. MTD was declared at the highest dose level at which 6 patients were treated and at most 1/6 patients experienced a DLT.
Patient Evaluation
The modified Bearman Scale was used to define non-hematologic DLT events,21 and the CTCAE 3.0 scale was used to define hematologic DLT events as well as to report all adverse events.22 To be evaluable for toxicity, a patient had to start treatment and been observed for at least 30 days following the completion of the transplant procedure or had experienced a DLT. Hematological toxicities were evaluated from day 0, whereas other DLTs were evaluated from Days −9 to +30. Engraftment was defined as the first of 3 consecutive days in which the absolute neutrophil count exceeded 0.5 × 109/L. GVHD grading was scored according to published criteria.23–25 Clinical response was assessed according to National Cancer Institute (NCI) criteria26 with blood draws at regularly scheduled visits and bone marrow biopsies at days 30, 100, and years 1 and 2 post-transplant. Patients were also followed longer term on companion protocols assessing radiation-related toxicity for TMLI patients and late effects of transplantation for HSCT patients.
Results
Patient characteristics
Fifty-one patients with a median age of 34 years (range, 16–57) and active disease refractory to salvage chemotherapy were enrolled for alloHSCT after receiving a conditioning regimen of TMLI in combination with CY and VP16 (Figure 1). Of the 51 patients, 33 patients had AML and 16 ALL (2 Philadelphia chromosome-positive). Twenty-one patients had >10% blasts in peripheral blood at time of transplant (17 AML, 4 ALL), and 42 patients had >10% blasts in bone marrow (28 AML, 13 ALL, 1 other). One patient had acute biphenotypic leukemia and one undifferentiated acute leukemia, respectively. BM (n=3) or PBSC (n=48) were given on day 0. Donors were HLA-identical siblings (n=25), matched unrelated (n=5), and mismatched (single allele) unrelated (n=21). The number of patients accrued to each DL is indicated in Table 1, and patients’ demographics, diagnosis, disease status, cytogenetic risk and risk per the criteria of Duval et al.,1 and treatment characteristics are summarized in Table 2.
TMLI radiation therapy
TMLI was delivered to sculpt radiation dose to lymph nodes and BM in each patient. Figure 2 exemplifies the dose distribution map in an AML patient who received a TMLI dose of 2000 cGy. Figure 3 depicts the median organ dose (D50) at each dose level for each OAR, for all 51 patients. The D50 dose for an organ was the dose received by no more than 50% of the whole organ. The D50 doses of TMLI compared favorably with those reported with standard TBI (see Figure 3). The D50 dose to a lung in a patient treated with standard TBI and shielded with 50% transmission lung blocks is usually between 850 to 900 Gy, whereas in this study the mean lung D50 was 680 cGy. (See Table 3 for D50 organ doses.) To normalize for variations with the prescribed dose, we also show, overall, that non-targeted organs received only 15 to 55% of the D50 received by BM (i.e., lung 42%, esophagus 31%, and oral cavity 24%) (Table 3).
Figure 2. Dose Distribution Map for Patient Treated at MTD.
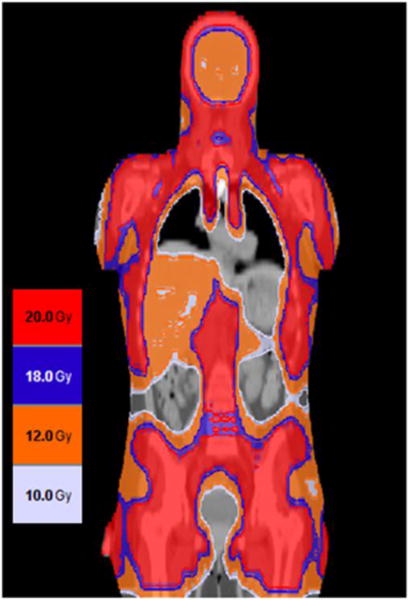
TMLI was delivered at 2000 cGy to bone and lymph nodes (excluding mesenteric lymph nodes and Waldeyer ring) and 1200 cGy to liver, spleen, and brain. Doses delivered are coded according to the colors indicated in the legend.
Figure 3. TMLI Median Organ Dose (D50) by Phase I Dose Levels.
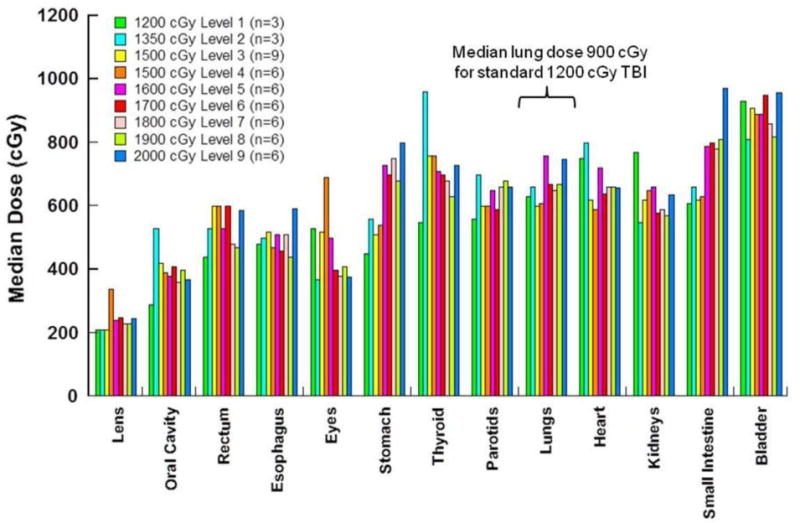
Dose levels and number of patients at each dose level are indicated in the legend. The doses plotted are averaged for the patients at each dose level. N=51 total patients treated.
Table 3.
D50 Organ Dose (Gy) (n = 51).
| Organs | D50 Organ Dose Mean ± 1 SD |
Range of the D50 Dose |
Percent of the Prescribed Target Dose | Range of the Percent Prescribed Target Dose |
|---|---|---|---|---|
| Lens | 2.4 +/− 0.6 | 1.7 – 5.1 | 15.0 +/− 4.3 | 10.0 – 34.0 |
| Oral Cavity | 3.9 +/− 1.1 | 2.5 – 7.7 | 24.3 +/− 8.4 | 14.0 – 51.3 |
| Rectum | 5.4 +/− 1.1 | 3.4 – 9.2 | 33.1 +/− 8.2 | 17.9 – 54.1 |
| Esophagus | 5.0 +/− 0.8 | 3.1 – 7.1 | 30.8 +/− 5.8 | 16.3 – 44.2 |
| Eyes | 4.5 +/− 1.8 | 2.1 – 11.5 | 28.4 +/− 13.0 | 13.1 – 71.9 |
| Stomach | 6.6 +/− 1.5 | 4.1 – 10.5 | 39.7 +/− 7.4 | 27.1 – 58.3 |
| Thyroid | 7.2 +/− 1.6 | 2.9 – 12.0 | 44.6 +/− 12.7 | 15.3 – 88.9 |
| Parotids | 6.4 +/− 1.0 | 4.6 – 9.0 | 39.6 +/− 7.5 | 26.0 – 60.0 |
| Lungs | 6.8 +/− 0.7 | 5.1 – 8.6 | 41.5 +/− 6.3 | 32.0 – 55.0 |
| Heart | 6.8 +/− 1.0 | 4.8 – 9.6 | 42.2 +/− 10.3 | 28.8 – 69.2 |
| Kidneys | 6.1 +/− 0.9 | 3.7 – 8.1 | 37.9 +/− 9.2 | 21.8 – 67.5 |
| Small Intestine | 7.5 +/− 1.6 | 4.9 – 11.6 | 45.4 +/− 6.9 | 26.8 – 61.1 |
| Bladder | 8.8 +/− 1.7 | 4.8 – 12.2 | 54.5 +/− 12.5 | 25.3 – 89.2 |
SD = standard deviation
Treatment and Toxicities
Bearman toxicities for each DL are shown in Table 4. Nine planned TMLI DLs were tested. At dose level 4, 1500 cGy, one patient developed grade 3 mucositis (stomatitis per Bearman toxicity scale21 for allogeneic transplantation) attributed to radiation and chemotherapy, requiring intubation for airway protection. The same patient also had grade 3 pulmonary toxicity and grade 3 renal toxicity, which was not attributed to TMLI but was secondary to organ dysfunction related to critical illness. The only other grade 3 toxicity was a renal toxicity. Renal failure in this patient was secondary to septic shock and was not considered to be a DLT, as this infection was not directly attributed to the TMLI/conditioning regimen. An additional 3 patients were treated at 1500 cGy with no additional dose-limiting toxicities. At the highest dose level (2000 cGy), no DLTs were observed in the six patients treated. No patients in any DL developed veno-occlusive disease.
Table 4.
Toxicities by dose level.
| Organ Assessed | DL1 (n=3) 1200 cGy | DL2 (n=3) 1350 cGy | DL3 (n=9) 1500 cGy | DL4 (n=6) 1500 cGy | DL5 (n=6) 1600 cGy | DL6 (n=6) 1700 cGy | DL7 (n=6) 1800 cGy | DL8 (n=6) 1900 cGy | DL9 lead-in (n=6) 2000 cGy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | Grade | Grade | Grade | Grade | Grade | Grade | Grade | ||||||||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 3 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 3 | |
|
| ||||||||||||||||||||
| Bladder toxicity | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
|
| ||||||||||||||||||||
| Cardiac toxicity | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
|
| ||||||||||||||||||||
| CNS toxicity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
|
| ||||||||||||||||||||
| GI toxicity | 2 | 0 | 3 | 0 | 6 | 0 | 3 | 0 | 0 | 5 | 1 | 1 | 1 | 6 | 0 | 5 | 0 | 4 | 2 | 0 |
|
| ||||||||||||||||||||
| Hepatic toxicity | 0 | 0 | 0 | 1 | 0 | 3 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
|
| ||||||||||||||||||||
| Pulmonary toxicity | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
|
| ||||||||||||||||||||
| Renal toxicity | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1** |
|
| ||||||||||||||||||||
| Stomatitis | 2 | 0 | 1 | 0 | 1 | 7 | 2 | 3 | 1* | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 1 | 0 |
This patient had the only dose-limiting toxicity in the trial.
Renal failure was secondary to septic shock secondary to infection not related to the conditioning regimen and therefore not considered a DLT.
No early deaths (death before day +30) were observed in the entire group of patients. Between days +30 and +100, two deaths occurred and were not related to primary disease or GVHD: One patient died from a Klebsiella infection (day +56), and one from toxic epidermal necrolysis and disseminated HHV-6 infection (day +61). The calculated day 100 NRM was 3.9% (95% CI: 0.7–12.0). One death occurred beyond day +100 and was not due to primary disease or GVHD; this patient died from pneumonia (day +1056). At 1 year the estimate of NRM was 8.1% (95% CI: 2.5–18.0). Causes of death by category are listed in Table 5.
Table 5.
Causes of death by category.
| Cause | N |
|---|---|
| Disease progression/persistent disease | 29 |
| Infection | 3 |
| Chronic graft versus host disease | 3 |
Engraftment
All 51 patients achieved a neutrophil recovery at a median of 15 days (range 11–23 days). Platelet engraftment was defined as the first of 7 consecutive days in which the platelet count was more than 20 × 109/L without transfusion support. Forty-five patients achieved platelet engraftment at a median of 17 days (range 11–77 days). Six patients did not achieve platelet transfusion independence; contributing factors were infections and medications.
Acute GVHD and Chronic GVHD
Seven patients (14%) experienced grades III and IV acute GVHD (aGVHD). The cumulative incidence of aGVHD at day +100 was 43.1% (95% CI: 29.2–56.3), with a median time to onset of 30 days (range: 6–75). None of the non–relapse-related deaths was attributed to complications of aGVHD. Chronic GVHD (cGVHD) occurred in 26 of the 42 patients surviving beyond 100 days. Twenty-four of these 26 patients had extensive disease. By day +365, the cumulative incidence of cGVHD was 37.9% (95% CI: 24.2–51.5), and the median time to onset was 138 days (range: 63–491). Three patients died of complications of cGVHD beyond day +100. No increase in acute or chronic GVHD was seen with increasing dose of radiation (Table 6).
Table 6.
Acute and chronic GVHD.
| Dose Level | aGVHD max grade | cGVHD max grade | ||||||
|---|---|---|---|---|---|---|---|---|
| None | I | II | III | IV | None | Lmtd | Ext | |
| 1: 1200 cGy | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| 2: 1350 cGy | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 2 |
| 3: 1500 cGy | 6 | 0 | 0 | 3 | 0 | 2 | 0 | 5 |
| 4: 1500 cGy | 0 | 1 | 3 | 1 | 1 | 1 | 1 | 3 |
| 5: 1600 cGy | 5 | 0 | 1 | 0 | 0 | 1 | 1 | 3 |
| 6: 1700 cGy | 2 | 1 | 2 | 1 | 0 | 2 | 0 | 4 |
| 7: 1800 cGy | 2 | 2 | 2 | 0 | 0 | 2 | 0 | 3 |
| 8: 1900 cGy | 3 | 0 | 2 | 1 | 0 | 4 | 0 | 1 |
| 9: 2000 cGy | 1 | 1 | 4 | 0 | 0 | 2 | 0 | 1 |
| All | 23 | 5 | 16 | 6 | 1 | 16 | 2 | 24 |
Lmtd = limited, Ext=extensive
Clinical Activity
Patients were evaluated at day +30 for response. At this landmark time-point, 45 of 51 patients (88%) achieved complete remission (CR). Of the 33 patients who were treated at a dose level ≤1700 cGy, 28 (85%) achieved CR, as did 17 of 18 patients (94%) who were treated at a dose level >1700 cGy. All 6 patients (100%) treated at the declared MTD (2000 cGy) also achieved CR. Persistent disease was observed in 6 patients at day +30. With a median follow-up of 24.6 months, 33 patients experienced disease relapse (BM only, 26; extramedullary disease only, 6; concurrent BM/extramedullary, 1). See Table S1 for the sites of extramedullary disease prior to and post alloHSCT. The 1-year and 2-year OS rates were 55.5% (95% CI: 40.7–68.1) and 41.5% (95% CI: 27.3–55.1), respectively. As of the data lock date (10/26/2015) twelve patients were alive and continuously in remission. Characteristics of these 12 patients, including age, disease type and status, and GVHD grade are listed in Table 7.
Table 7.
Characteristics of patients alive and in remission.
| Patient | Age at alloHSCT | Primary Disease at alloHSCT | Disease Status at alloHSCT | Blasts in PB* | Blasts in BM* | Duval Score | aGVHD Max Grade | cGVHD Max Grade | Days from alloHSCT to Last Contact |
|---|---|---|---|---|---|---|---|---|---|
| 01 | 34.4 | Biphenotypic leukemia |
Induction failure |
0% | 1% | N/A | None | None | 2190 |
| 02 | 22.4 | AML | Induction failure |
0% | 2% | 1 | None | None | 850 |
| 03 | 31.3 | ALL, Ph+ | Induction failure |
0% | 50% | 3 | III | Extensive | 1803 |
| 04 | 49.2 | AML | Induction failure |
0% | 42% | 1 | None | Extensive | 1457 |
| 05 | 47.6 | ALL | Induction failure |
N/A | 25% | 4 | II | Extensive | 735 |
| 06 | 50.2 | AML | 1st relapse |
0% | 5% | 1 | II | None | 362 |
| 07 | 56.5 | AML | Induction failure |
16% | 70% | 3 | II | Extensive | 762 |
| 08 | 53.8 | AML | Induction failure |
N/A | 10% | 1 | None | Extensive | 715 |
| 09 | 34.3 | AML | Induction failure |
30% | 80% | 3 | II | Extensive | 761 |
| 10 | 16.5 | ALL | Induction failure |
49% | 95% | 3 | II | Extensive | 392 |
| 11 | 40.5 | AML | Induction failure |
5% | 20% | 4 | I | None | 104 |
| 12 | 21.2 | Undifferentiated leukemia |
Induction failure |
2% | 30% | N/A | II | None | 100 |
AML=acute myeloid leukemia, ALL=acute lymphoblastic leukemia, BM = bone marrow, N/A = not available, PB = peripheral blood, Ph=Philadelphia chromosome.
At time of transplant.
Discussion
We report here a phase I dose escalation trial of TMLI in combination with VP16/CY as a preparative regimen for alloHSCT in patients with refractory/relapsed acute leukemia. We demonstrated that TMLI can be safely escalated to 2000 cGy, sparing non-hematopoietic and uninvolved organs, which received 15–55% of the BM dose, and is associated with a relatively low 30- and 100-day and 1 year NRM.
The declared MTD of 2000 cGy was clinically tolerable, with median doses delivered to the non-targeted healthy organs remaining below the corresponding doses delivered by TBI. However, the increase in doses to critical OARs approaching that of TBI (Figure 3 and Table 3), led us to the decision of not escalating the TMLI beyond 2000 cGy. Notably, the comparable incidence of GVHD with regard to published data27 and the relatively low rate of sepsis-related complications observed in the present trial may be related to the relatively low radiation dose to the gastrointestinal tract (see Table 3). Despite sparing of non-targeted organs, the extramedullary relapse rates in our study appeared to be no higher (only 7 of 51 patients) than those reported in patients undergoing TBI, which is consistent with our earlier experience.28, 29
TMLI is a targeted form of total body irradiation which utilizes intensity modulated radiation therapy (IMRT) delivered either through a helical tomographic (HT) or volumetric arc therapy (VMAT) approach. The initial patient was treated using an HT approach on a TomoTherapy device (Accuray, Inc.)13, 17 approximately 10 years ago. More recently, our group and others have demonstrated the feasibility of delivering TMLI using VMAT approaches on non-TomoTherapy devices.30–32 Therefore the delivery of TMLI is now device agnostic, and it is exportable and feasible in most centers with these technologies. Clinical trials have been completed or are ongoing using these different technology platforms.
Our first trial in multiple myeloma patients tested an initial autologous HSCT with melphalan (MEL) (200 mg/m2) conditioning, followed 6–10 weeks later by a second autologous HSCT using only TMI for conditioning. The regimen was generally safe, and DLTs (based on CTCAE criteria, not Bearman) were not observed until a TMI dose of 1800 cGy.16 The established MTD of 1600 cGy in that trial prompted us to be cautious in dosing patients above 1500 cGy and to gain more experience by switching to a rolling-6 design with dose increments of 100 cGy instead of 150 cGy. Subsequently, we evaluated TMLI in combination with a reduced intensity chemotherapy regimen for patients with advanced hematologic malignancies (primarily AML) who were over age 50 or had co-morbidities. Because fludarabine is a radiosensitizer, TMLI at only 1200 cGy was combined with a reduced-intensity regimen of fludarabine (FLU) and melphalan (MEL). The 1-year NRM was 19%, comparing favorably with results observed with FLU/MEL alone in published reports.14 When TMI was combined with the radiosensitizer busulfan and with VP16, DLTs occurred at 1350 cGy, precluding dose escalation above 1200 cGy.15 The dose of TMLI delivered and feasibility of dose escalation in combination with chemotherapy is dependent upon the chemotherapeutic agents delivered together with radiation.
Patients with relapsed/refractory acute leukemia (ALL/AML) have a dismal outcome even after HSCT, with a long-term survival of 16–19%.1 Unfortunately, most patients with acute leukemia who relapse after first remission are never able to achieve a second remission even with salvage chemotherapy, and they have very few therapeutic options outside of clinical trials.33 Since CR status at transplantation is a primary predictor of more favorable outcomes, the vast majority of patients who fail salvage treatment with active disease remaining are not usually considered for alloHSCT; only a few sparse studies have reported the outcomes of patients undergoing alloHSCT with active disease. A Southwest Oncology Group (SWOG) study comparing alloHSCT preparative regimens (TBI/VP16 vs busulfan/CY) in patients with poor-risk leukemia who were not in first CR at the time of transplant reported a 2-year DFS of ~20% for both regimens.34 A CIBMTR study found that patients with refractory acute leukemia undergoing alloHSCT between 1994 and 2005 had equally poor outcomes (3-year OS of 19% for AML and 16% for ALL); this study has led to many transplant centers excluding these patients from consideration for alloHSCT.1 Although our Phase I trial was designed to assess toxicity and feasibility, we are encouraged by the observed preliminary clinical response for patients with advanced forms of acute leukemia.
A phase II trial is under way (NCT#02094794) to assess the clinical activity of 2000 cGy TMLI in combination with CY/VP16 in a similar patient population as reported here, with the goal of a more precise assessment of the clinical response to this regimen. Should this study be successful, we will consider moving this approach upfront in acute leukemia patients with high-risk disease and in first CR following initial chemotherapy treatment. Furthermore, it would be possible to explore incorporation of molecular (and image-guided) therapies tailored to individuals’ molecular features to provide better initial disease control and to give post transplant targeted therapies to further reduce the chance of relapse post-transplant.
Supplementary Material
Highlights.
The TMLI/CY/VP16 conditioning regimen is safe up to a dose of 2000 cGy.
Radiation to off-target critical organs is lower than typically delivered by TBI.
There is an acceptable risk for GVHD and encouraging results for disease control.
Acknowledgments
We would like to thank the City of Hope staff and nurses, as well as the patients and their families, without whom this work would not be possible. This clinical trial was partially supported by NIH R01 CA195519, NIH P30 CA033572 (Biostatistics and Pathology Cores), the Gateway Foundation, and Accuray, Inc.
AS provides consulting for Amgen and Stemline and is on the speakers’ bureau for the former company. SK receives honoraria from, consults for, and is on the speakers’ bureau for Alexion Pharmaceuticals and receives research funding from Sanofi. VP provides consulting for Novartis, Amgen, Baxalta, and Pfizer and is on the speakers’ bureau for Novartis and Amgen. JR receives honoraria from and consults for Shire and Bayer, receives research funding from Daiichi Sankyo and Kite Pharma. SF has license agreements with and receives research support from Mustang Therapeutics, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work has been presented in part at the 2012 and 2015 Meetings of the American Society of Hematology in Atlanta and Orlando, the 2013 Meeting of the European Group for Blood and Marrow Transplantation in London, and the 2015 Annual Meeting of the Japan Society for Hematopoietic Cell Transplantation.
Financial Disclosure Statement
All other authors declare no competing financial interests.
References
- 1.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76:1867–1871. [PubMed] [Google Scholar]
- 3.Scarpati D, Frassoni F, Vitale V, et al. Total body irradiation in acute myeloid leukemia and chronic myelogenous leukemia: influence of dose and dose-rate on leukemia relapse. Int J Radiat Oncol Biol Phys. 1989;17:547–552. doi: 10.1016/0360-3016(89)90105-3. [DOI] [PubMed] [Google Scholar]
- 4.Marks DI, Forman SJ, Blume KG, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant. 2006;12:438–453. doi: 10.1016/j.bbmt.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Kal HB, Loes van Kempen-Harteveld M, Heijenbrok-Kal MH, Struikmans H. Biologically effective dose in total-body irradiation and hematopoietic stem cell transplantation. Strahlenther Onkol. 2006;182:672–679. doi: 10.1007/s00066-006-1528-6. [DOI] [PubMed] [Google Scholar]
- 6.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood. 1991;77:1660–1665. [PubMed] [Google Scholar]
- 7.Clift RA, Buckner CD, Appelbaum FR, Sullivan KM, Storb R, Thomas ED. Long-term follow-Up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood. 1998;92:1455–1456. [PubMed] [Google Scholar]
- 8.Latini P, Aristei C, Aversa F, et al. Lung damage following bone marrow transplantation after hyperfractionated total body irradiation. Radiother Oncol. 1991;22:127–132. doi: 10.1016/0167-8140(91)90008-5. [DOI] [PubMed] [Google Scholar]
- 9.Boulad F, Bromley M, Black P, et al. Thyroid dysfunction following bone marrow transplantation using hyperfractionated radiation. Bone Marrow Transplant. 1995;15:71–76. [PubMed] [Google Scholar]
- 10.Michel G, Socie G, Gebhard F, et al. Late effects of allogeneic bone marrow transplantation for children with acute myeloblastic leukemia in first complete remission: the impact of conditioning regimen without total-body irradiation–a report from the Societe Francaise de Greffe de Moelle. J Clin Oncol. 1997;15:2238–2246. doi: 10.1200/JCO.1997.15.6.2238. [DOI] [PubMed] [Google Scholar]
- 11.Bradley J, Reft C, Goldman S, et al. High-energy total body irradiation as preparation for bone marrow transplantation in leukemia patients: treatment technique and related complications. Int J Radiat Oncol Biol Phys. 1998;40:391–396. doi: 10.1016/s0360-3016(97)00578-6. [DOI] [PubMed] [Google Scholar]
- 12.Berger C, Le-Gallo B, Donadieu J, et al. Late thyroid toxicity in 153 long-term survivors of allogeneic bone marrow transplantation for acute lymphoblastic leukaemia. Bone Marrow Transplant. 2005;35:991–995. doi: 10.1038/sj.bmt.1704945. [DOI] [PubMed] [Google Scholar]
- 13.Wong JY, Liu A, Schultheiss T, et al. Targeted total marrow irradiation using three-dimensional image-guided tomographic intensity-modulated radiation therapy: an alternative to standard total body irradiation. Biol Blood Marrow Transplant. 2006;12:306–315. doi: 10.1016/j.bbmt.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal J, Wong J, Stein A, et al. Phase 1/2 trial of total marrow and lymph node irradiation to augment reduced-intensity transplantation for advanced hematologic malignancies. Blood. 2011;117:309–315. doi: 10.1182/blood-2010-06-288357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong JY, Forman S, Somlo G, et al. Dose escalation of total marrow irradiation with concurrent chemotherapy in patients with advanced acute leukemia undergoing allogeneic hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2013;85:148–156. doi: 10.1016/j.ijrobp.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong JY, Rosenthal J, Liu A, Schultheiss T, Forman S, Somlo G. Image-guided total-marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2009;73:273–279. doi: 10.1016/j.ijrobp.2008.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultheiss TE, Wong J, Liu A, Olivera G, Somlo G. Image-guided total marrow and total lymphatic irradiation using helical tomotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1259–1267. doi: 10.1016/j.ijrobp.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 18.Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. New Engl J Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 19.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankel P, Longmate J, Sposto R, Newman E, Groshen S. Proceedings of the American Statistical Association Biopharmaceutical Section. Alexandria, VA: American Statistical Association; 2016. [Google Scholar]
- 21.Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6:1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Therapy Evaluation Program, National Cancer Institute, National Institues of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 3.02003.
- 23.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 24.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–276. [PubMed] [Google Scholar]
- 26.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 27.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. New Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Stein A, Tsai N, et al. Extramedullary relapse following total marrow and lymphoid irradiation in patients undergoing allogeneic hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2014;89:75–81. doi: 10.1016/j.ijrobp.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 29.Kogut N, Tsai NC, Thomas SH, et al. Extramedullary relapse following reduced intensity allogeneic hematopoietic cell transplant for adult acute myelogenous leukemia. Leuk Lymphoma. 2013;54:665–668. doi: 10.3109/10428194.2012.720375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aydogan B, Yeginer M, Kavak GO, Fan J, Radosevich JA, Gwe-Ya K. Total marrow irradiation with RapidArc volumetric arc therapy. Int J Radiat Oncol Biol Phys. 2011;81:592–599. doi: 10.1016/j.ijrobp.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 31.Patel P, Aydogan B, Koshy M, et al. Combination of linear accelerator-based intensity-modulated total marrow irradiation and myeloablative fludarabine/busulfan: a phase I study. Biol Blood Marrow Transplant. 2014;20:2034–2041. doi: 10.1016/j.bbmt.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Han C, Schultheisss TE, Wong JY. Dosimetric study of volumetric modulated arc therapy fields for total marrow irradiation. Radiother Oncol. 2012;102:315–320. doi: 10.1016/j.radonc.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Forman SJ, Rowe JM. The myth of the second remission of acute leukemia in the adult. Blood. 2013;121:1077–1082. doi: 10.1182/blood-2012-08-234492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blume KG, Kopecky KJ, Henslee-Downey JP, et al. A prospective randomized comparison of total body irradiation-etoposide versus busulfan-cyclophosphamide as preparatory regimens for bone marrow transplantation in patients with leukemia who were not in first remission: a Southwest Oncology Group study. Blood. 1993;81:2187–2193. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


