SUMMARY
Induction of type I interferon in response to microbial pathogens depends on a conserved cGAS-STING signaling pathway. The presence of DNA in the cytoplasm activates cGAS, while STING is activated by cyclic dinucleotides (cdNs) produced by cGAS or from bacterial origins. Here, we show that Group B Streptococcus (GBS) induces IFN-β production almost exclusively through cGAS-STING-dependent recognition of bacterial DNA. However, we find that GBS expresses an ectonucleotidase, CdnP, which hydrolyzes extracellular bacterial cyclic-di-AMP. Inactivation of CdnP leads to c-di-AMP accumulation outside the bacteria and increased IFN-β production. Higher IFN-β levels in vivo increase GBS killing by the host. The IFN-β overproduction observed in the absence of CdnP is due to the cumulative effect of DNA sensing by cGAS and STING-dependent sensing of c-di-AMP. These findings describe the importance of a bacterial c-di-AMP ectonucleotidase and suggest a direct bacterial mechanism that dampens activation of the cGAS-STING axis.
Keywords: Interferon-β, Streptococcus agalactiae, cGAS, c-di-AMP, ectonucleotidase
eTOC paragraph
Type I IFN induction is important to control Group B Streptococcus (GBS) infection. Andrade et al. show that type I interferon induction by GBS infection depends on the cGAS/STING pathway. GBS expresses an ectonucleotidase that degrades c-di-AMP produced by GBS and reduces extracellular c-di-AMP, thus dampening type I IFN responses.

INTRODUCTION
Type I interferon (IFN) production is a conserved pro-inflammatory response to microbial infections (McNab et al., 2015). However, the production of type I IFN can be beneficial or detrimental to the host in the case of bacterial infections (Monroe et al., 2010). For instance, the type I IFN response is detrimental for the host during infections by Listeria monocytogenes, Mycobacterium tuberculosis and Neisseria gonorrhoeae (Archer et al., 2014; Manzanillo et al., 2012, Andrade et al. Type I interferon induction by Neisseria gonorrhoeae: Dual requirement of cyclic GMP-AMP synthase and Toll-like receptor 4 http://dx.doi.org/10.1016/j.celrep.2016.05.030). In contrast, type I IFN is critical for host defenses against certain extracellular pathogens such as Streptococcus species (e.g., S. pneumoniae, S. pyogenes, S. agalactiae) and Staphylococcus aureus (Gratz et al., 2011; Mancuso et al., 2007; Parker et al., 2014). Induction of type I IFN in response to bacteria is dependent on two main pathways (Monroe et al., 2010). The first pathway is a Toll-like receptor 4 (TLR4)-dependent sensing of LPS molecules present on the surface of gram-negative bacteria. The second pathway is a cytosolic sensing of bacterial nucleic acids, mainly double-stranded DNA, present in the host cytoplasm.
Several cytoplasmic DNA receptors have been identified, among which the recently described cyclic GMP-AMP synthase (cGAS) has a critical role (Barber, 2014; Cai et al., 2014; Paludan, 2015). The cGAS enzyme is activated by DNA binding and produces a specific eukaryotic cyclic dinucleotide (cdN), abbreviated hereafter as 2′3′ cGAMP (Ablasser et al., 2013; Gao et al., 2013a) which acts as a second messenger to activate the endoplasmic reticulum (ER)-localized protein stimulator of IFN genes (STING). Once activated, STING recruits TANK-Binding Kinase 1 (TBK1) to phosphorylate interferon regulatory factor 3 (IRF3), ultimately leading to type I IFN production.
Prior to the discovery of 2′3′ cGAMP, only bacteria were known to synthesize cdNs. All bacteria synthesize at least one cdN, either c-di-AMP, c-di-GMP, and/or cGAMP, which acts as a secondary messenger in several processes such as biofilm formation, cell wall homeostasis, bacterial growth, or virulence gene expression (Corrigan and Grundling, 2013; Romling et al., 2013). In addition to their intracellular functions for bacterial physiology, cdNs are secreted or released outside bacteria and recognized by the innate immune system (Danilchanka and Mekalanos, 2013). Cytosolic sensing of bacterial cdN induces IFN-β production through activation of STING (Burdette et al., 2011; McWhirter et al., 2009; Woodward et al., 2010).
Given the overlapping induction by DNA through the cGAS-STING axis and by bacterial cdN through STING activation, the main bacterial inducer of the IFN-β response remains to be identified (Danilchanka and Mekalanos, 2013). On one hand, STING-dependent induction of IFN-β correlates with the amount of secreted bacterial cdN (Barker et al., 2013; Dey et al., 2015; Schwartz et al., 2012; Woodward et al., 2010; Yamamoto et al., 2012). On the other hand, the affinity of STING for bacterial cdN is lower compared to 2′3′ cGAMP (Gao et al., 2013b; Kranzusch et al., 2015; Zhang et al., 2013). The relative contribution of DNA and bacterial cdN sensing for IFN-β induction have been recently evaluated using cGAS- and STING-deficient cells infected with the intracellular pathogens M. tuberculosis, L. monocytogenes and Chlamydia trachomatis. Overall, bacterial DNA recognition by cGAS seems to be the main stimulus for IFN-β induction and the role of bacterial cdN remains controversial. Some studies suggest that bacterial cdN does not play a significant role in IFN-β induction (Collins et al., 2015; Hansen et al., 2014; Wassermann et al., 2015; Watson et al., 2015; Zhang et al., 2014), while others indicate the opposite or a species-dependent role (Barker et al., 2013; Dey et al., 2015; Schwartz et al., 2012; Woodward et al., 2010; Yamamoto et al., 2012).
In this study, we characterized the IFN-β response to S. agalactiae (the Group B Streptococcus, GBS), a commensal bacteria of the human intestinal and vaginal flora, but the leading cause of neonatal invasive infections in developed countries (Joubrel et al., 2015). We previously demonstrated that GBS induces IFN-β by a TBK1-IRF3-dependent and TLR-independent pathway in murine macrophages in response to live bacteria (Charrel-Dennis et al., 2008). Here we report that GBS induces type I IFN by a cGAS- and STING-dependent pathway, primarily upon recognition of bacterial DNA, in murine macrophages and human monocytes. However, we observed that GBS degrades c-di-AMP present outside the bacteria. We showed that c-di-AMP is produced by GBS, but the amount of extracellular c-di-AMP is kept low by the activity of the cell wall-anchored ectonucleotidase CdnP. The CdnP enzyme is unrelated to the currently known bacterial cdN phosphodiesterases and acts sequentially with a second ectonucleotidase, NudP (Firon et al., 2014), to degrade extracellular c-di-AMP into adenosine. We show that inactivation of CdnP leads to c-di-AMP accumulation outside the bacteria and an overproduction of IFN-β due to a STING-dependent, cGAS-independent, recognition of c-di-AMP. We thus report a direct mechanism used by a bacteria to dampen IFN-β production by degrading c-di-AMP to prevent STING overactivation.
RESULTS
cGAS is the main sensor for IFN induction in response to WT GBS
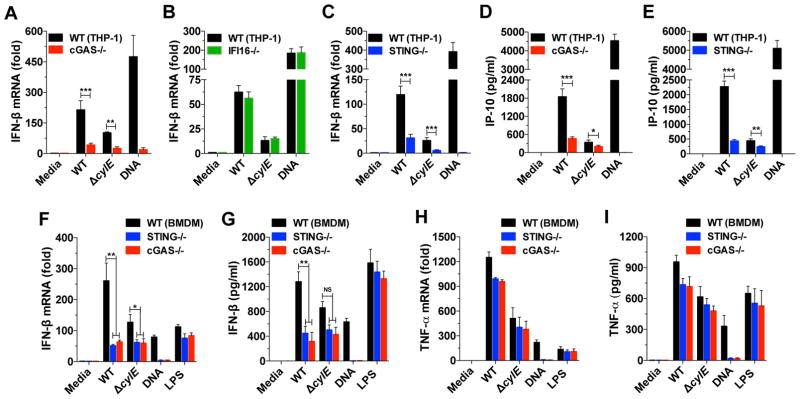
We previously demonstrated that type I IFN production in response to GBS relies on GBS phagocytosis, alteration of the phagolysosomal membrane by the bacterial pore-forming toxin β-hemolysin/cytolysin, and the release of bacterial DNA into the cytosol (Charrel-Dennis et al., 2008). To clarify the role of the DNA sensors cGAS and the DNA-binding PYHIN family member, IFI16, in GBS infection, human THP-1 cells were infected with GBS bacteria. We observed robust IFN-β induction by WT GBS infection in THP-1 cells (Figure 1A) consistent with our previous results in murine bone marrow-derived macrophages (BMDMs) (Charrel-Dennis et al., 2008). Using cGAS−/− or IFI16−/− THP-1 cell lines (Andrade et al. Type I interferon induction by Neisseria gonorrhoeae: Dual requirement of cyclic GMP-AMP synthase and Toll-like receptor 4 http://dx.doi.org/10.1016/j.celrep.2016.05.030), we demonstrate that IFN-β induction by WT GBS depends mostly, but not exclusively, on cGAS (Figure 1A), and is independent of IFI16 (Figure 1B). In agreement with cGAS acting upstream of the adapter STING, we observed a similar drop in IFN-β induction by WT GBS in STING−/− THP-1 cells (Figure 1C and Figure S1). The level of IFN-β induction observed at the mRNA level was confirmed by the quantification of IP-10, a surrogate cytokine for type I IFN expression, in WT, cGAS−/−, and STING−/− THP-1 cells (Figures 1D and 1E). Transfection of purified GBS DNA confirmed the critical function of the cGAS-STING axis for IFN-β production in response to cytoplasmic DNA (Figures 1A–E). Moreover, infections with GBS lacking cytolysin (ΔcylE) confirmed the important function of this toxin for type I IFN induction (Figures 1A–E).
Figure 1. cGAS is the main mediator of IFN-β production in response to WT GBS.
(A–C) Quantification of IFN-β induction by qRT-PCR in human THP-1 cells and cGAS−/− (A), IFI16−/− (B), and STING −/− (C) inactivated cell lines following 6 hrs of infection with GBS WT or the isogenic ΔcylE mutant (MOI-6). Transfection with DNA (3 μg/ml) was added as a control.
(D–E) Quantification of IP-10 production in THP-1 cGAS−/− (D) and STING −/− (E) by ELISA after 18 hrs of infection.
(F–G) Quantification of IFN-β induction using WT, cGAS−/− and STING−/− BMDMs at the mRNA level after 6 hrs of infection (F) or at the protein level after 18 hrs of infection (G) with GBS WT and ΔcylE mutant (MOI-6). An additional LPS control (100 ng/ml), independent of the cGAS-STING axis, was added.
(H–I) Same experiments as in (F–G) to quantify levels of TNF-α mRNA (H) and protein (I). Data are represented as mean ± SD of at least three independent experiments. Asterisks indicate statistically significant differences (*,p < 0.05; **, p < 0.01 and ***, p < 0.001). NS, not significant. See also Figure S1.
Similar results were obtained with murine BMDMs. Induction of IFN-β in BMDMs infected with GBS is strongly and similarly decrease in cGAS−/− and STING−/− cells (Figures 1F and 1G). The cGAS-STING axis has only a slight effect on TNF-α expression (Figures 1F and 1G) demonstrating the conserved and specific role of the cGAS-STING axis in response to GBS infection. Overall, we confirmed our previous study describing that WT GBS induces type I IFN by a DNA-dependent sensing pathway, and identified cGAS as the main upstream component mediating this response. In addition, the similar response observed in cGAS−/− and STING−/− cells was consistent with cGAS relaying the information to STING through the synthesis of 2′3′ cGAMP and suggests that activation of STING directly by GBS cdN, independently of cGAS, plays a minor role.
GBS hydrolyzes extracellular c-di-AMP
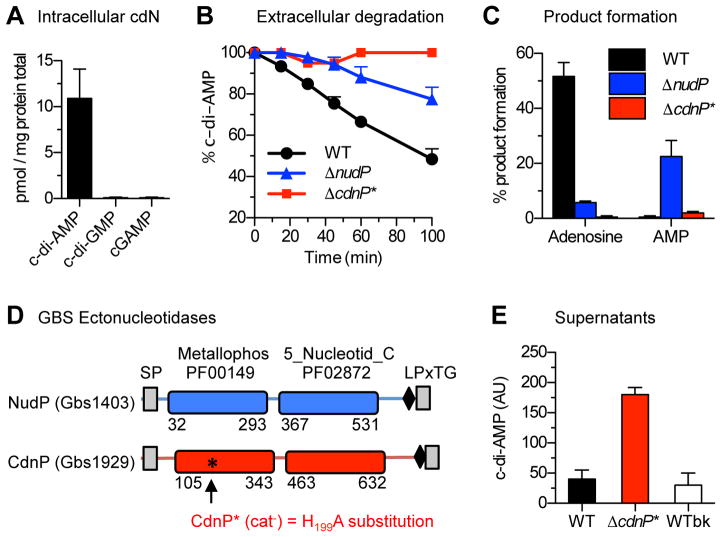
To understand the putative role of bacterial cdN in IFN-β induction by WT GBS, we looked for genes involved in cdN synthesis in the GBS genome. Homologues of the DacA di-adenylate cyclase (Gbs0902) and of the GdpP intracellular c-di-AMP phosphodiesterase (Gbs2100), the two conserved enzymes involved in c-di-AMP synthesis and intracellular degradation (Corrigan and Grundling, 2013) were identified, in contrast to genes encoding enzymes involved in c-di-GMP and cGAMP metabolism (Davies et al., 2012; Romling et al., 2013). Consistently, c-di-AMP, but not c-di-GMP or cGAMP, was detected in GBS extracts (Figure 2A).
Figure 2. CdnP degrades extracellular c-di-AMP.
(A) GBS synthesizes only c-di-AMP. The intracellular concentration of cyclic di-nucleotides was quantified by LC-MS/MS in total WT GBS extracts.
(B) GBS degrades extracellular c-di-AMP. At t = 0, 100 μM c-di-AMP was added to early stationary phase cultures of GBS previously washed and resuspend in Tris-HCl (pH 7.5) containing 5 mM MnCl2. Kinetics of extracellular c-di-AMP degradation (mean and S.D. from at least three independent bacterial cultures) were followed by RR-HPLC for the WT (black dots), the ΔnudP (blue triangles), and the ΔcdnP* (red squares) ectonucleotidase mutants.
(C) Extracellular c-di-AMP is degraded into adenosine by the sequential activity of the NudP and CdnP ectonucleotidases. Same experiments as in (B) monitoring the formation of the reaction products at the end of the experiment (t = 100 min) for the WT (black), the ΔnudP (blue), and the ΔcdnP* (red) ectonucleotidase mutants.
(D) Schematic representations of NudP and CdnP. The archetypal metallophosphatase (Metallophos, pfam domain 00149) and substrate-binding (5-Nucleotid_C, pfam domain 02872) domains of ectonucleotidases are colored (blue for NudP, red for CdnP). Numbers indicate the amino acid positions of the domains in the proteins. Transmembrane domains are indicated as grey boxes and are necessary for secretion (SP: signal peptide) and cell wall anchoring through the LPxTG motif (black rhombus). The black asterisk indicates the position of the conserved histidine residue at position 199 of CdnP essential for metallophosphodiesterase activity. The corresponding mutation to alanine in the catalytically inactive (cat−) mutant CdnP* is shown.
(E) CdnP activity limits extracellular c-di-AMP accumulation by GBS. Extracellular c-di-AMP in the GBS culture supernatants was quantified by an enzyme-linked assay followed by RR-HPLC. The two control GBS strains (black: WT and white: WTbk) are compared in parallel with the ΔcdnP* mutant (red). Mean and S.D. in arbitrary units (AU) are calculated from at least three independent experiments. See also Figure S2.
Cyclic-di-AMP is released outside bacteria by a mechanism involving non-specific transporters (Kaplan Zeevi et al., 2013; Woodward et al., 2010; Yamamoto et al., 2012), and could also occur through spontaneous lysis (Oliveira et al., 2012). However, the absence of STING activation independent of cGAS suggests a low level of c-di-AMP outside GBS. We therefore hypothesized that GBS may degrade extracellular c-di-AMP. To test this hypothesis, intact WT GBS cells were incubated with exogenously added c-di-AMP. In this condition, the concentration of extracellular c-di-AMP decreases linearly (Figure 2B) coinciding with an accumulation of extracellular adenosine (Figure 2C).
The formation of adenosine suggests the involvement of the NudP ectonucleotidase that degrades extracellular ADP and AMP into adenosine (Firon et al., 2014). Interestingly, the rate of c-di-AMP hydrolysis is slower with ΔnudP mutant cells compared to WT bacteria (Figure 2B) and AMP production is observed instead of adenosine formation (Figure 2C). Overall, these results demonstrate that GBS hydrolyzes extracellular c-di-AMP and suggest that an enzyme present at the bacterial surface hydrolyzes extracellular c-di-AMP into AMP, which is further processed into adenosine by NudP.
The ectonucleotidase CdnP degrades extracellular c-di-AMP
Among the approximately 30 GBS proteins with a cell wall anchoring motif (Glaser et al., 2002), we identified a second ectonucleotidase Gbs1929 (NCBI WP_000033934.1), hereafter named CdnP for cyclic dinucleotide phosphodiesterase. CdnP is an 800-aa protein containing a signal peptide, a canonical LPxTG cell surface localization motif, a 5′-nucleotidase domain, and a metallophosphoesterase domain (Figure 2D). CdnP has a conserved NHE motif (residues 198-200) in which the histidyl residue is essential for metallophosphodiesterase activity (Matange et al., 2015; Zimmermann et al., 2012). Therefore, we engineered the GBS chromosome to replace the His199 residue with an alanine (Figure 2D). The resulting inactivated CdnP mutant (Δcdnp*) is unable to hydrolyze exogenously added c-di-AMP (Figures 2B and 2C). This result indicates that CdnP is indeed the enzyme responsible for c-di-AMP extracellular degradation by GBS.
To further demonstrate that c-di-AMP is released extracellularly by GBS, we analyzed WT and Δcdnp* supernatants by HPLC. Almost no c-di-AMP was detected in the WT GBS supernatant, in contrast to the Δcdnp* supernatant (Figure S2A). To compare the amount of extracellular c-di-AMP, we adapted a c-di-AMP quantification assay based on the S. pneumoniae c-di-AMP binding protein CabP (Bai et al., 2014). Resin-bound CabP was used to bind c-di-AMP present in GBS supernatants and bound nucleotides were quantitated by HPLC after elution (Figure S2B). Supernatants of WT and WTbk control (the WT CdnP strain isogenic to ΔcdnP*) contain a similar low amount of extracellular c-di-AMP. In contrast, the ΔcdnP* supernatant contains 5- to 7-fold more extracellular c-di-AMP (Figure 2E). These results strongly suggest that the low level of extracellular c-di-AMP observed in WT supernatants is due to the CdnP activity.
CdnP has 2′,3′ cNMP activity
CdnP is annotated as a putative bifunctional 2′,3′-cyclic-nucleotide 2′-phosphodiesterase and 3′-nucleotidase (EC 3.1.4.16) based on homology with CpdB from Escherichia coli. CpdB-related proteins are predicted to hydrolyze the cyclic phosphoester linkage of 2′,3′ cyclic nucleotides (2′,3′ cNMP) to give 2′ or 3′ monophosphate nucleotides (2′ NMP or 3′ NMP), among which the 3′ NMP can be further hydrolyzed by the same enzyme to give the corresponding nucleoside and inorganic phosphate (Anraku, 1964).
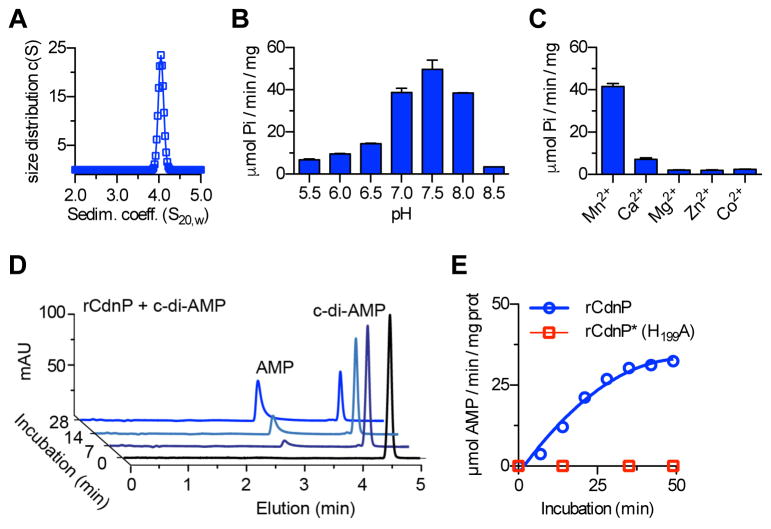
To characterize the enzymatic activity, we purified the recombinant rCdnP (Figure 2D: residues 28-798). The rCdnP protein is soluble and has an apparent molecular mass of about 80 kDa, in agreement with its theoretical 82 kDa mass. Sedimentation velocity experiments revealed an s20,w of 4.2 S and a frictional ratio of 1.6, giving a calculated mass of 80 kDa, corresponding to a monomeric protein displaying an elongated shape (Figure 3A). Purified rCdnP converts the predicted substrate 2′,3′ cNMP to nucleoside and phosphate (Figure S3A). The optimal enzymatic activity is at pH 7.5 (Figure 3B) and is dependent on manganese with an optimum near 0.5 mM (Figure 3C). There is a stringent Mn2+ requirement since only low activity was observed with Ca2+ and no activity was detected with other divalent ions such as Mg2+, Co2+, or Zn2+ (Figure 3C). The substrate specificity and kinetic parameters of rCdnP were determined in the presence of 2 mM Mn2+ at pH 7.5. The Michaelis constants of rCdnP are shown in Table 1 and the highest specific activity was obtained with 2′,3′ cUMP (Figure S3A). In contrast, CdnP has no activity on 3′,5′ cNMP, including the universal second messenger cAMP (Table 1 and Figure S3A). Since 2′,3′ cNMPs are instable in vivo (Rao et al., 2010), we hypothesized that they are not the CdnP physiological substrates.
Figure 3. The ectonucleotidase CdnP is a manganese-dependent c-di-AMP phosphodiesterase.
(A) Analytical ultracentrifugation analysis of rCdnP. The purified recombinant CdnP protein (residues 29–768) is a monomer with an elongated shape. Sedimentation coefficients are expressed in Svedberg units where 1 S =10−13 S.
(B and C) Influence of the pH and cations on the activity of CdnP. Quantification of inorganic phosphate release (Pi) was done with Biomol Green reagents after incubation of 3 nM rCdnP with 2 mM of 2′,3′ cUMP in the presence of 2 mM Mn2+ (B) or different cations at pH 7.5 (C). Samples were taken every 10 min and activities are expressed as μmol of Pi per min per mg of proteins.
(D) CdnP degrades c-di-AMP into AMP. rCdnP was incubated with c-di-AMP at 37°C. Kinetics of substrate degradation and product formation was followed by RR-HPLC. Representative chromatograms in arbitrary units (mAU) are shown.
(E) Same experiment as in (D) with the quantification of AMP formation by the rCdnP protein and the inactive rCdnP* mutant with the H199A substitution. See also Figure S3.
Table 1.
Kinetic parameters of CdnP on cyclic mono and dinucleotides.
| Substrate | Km (μM) | Kcat (s−1) | Kcat/Km (s−1/M−1) |
|---|---|---|---|
| Cyclic nucleotide | |||
| 2′,3′ cGMP | 22 ± 4 | 9.4 | 4.2 × 105 |
| 2′,3′ cUMP | 42 ± 10 | 19.8 | 4.7 × 105 |
| 3′,5′ cAMP | n.d. | n.d. | n.d. |
| 3′,5′ cGMP | n.d. | n.d. | n.d. |
|
| |||
| Cyclic dinucleotide | |||
| c-di-AMP | 14 ± 1 | 17.4 | 1.2 × 106 |
| c-di-GMP | 13.2 ± 6 | 2.6 | 1.9 × 105 |
| c-GAMP | 4.0 ± 1.6 | 4.5 | 1.1 × 106 |
| 2′3′ cGAMP | n.d. | n.d. | n.d. |
The Vmax and Km (mean ± S.D.) were obtained from double reciprocal plots of initial velocity measurements with at least five different concentrations of substrates. The kcat was calculated assuming a molecular mass of 81 kDa. (n.d. = substrate hydrolysis not detected.)
CdnP is a 3′5′ cyclic dinucleotide 3′ phosphodiesterase
Based on our observation with intact GBS cells, we further characterized the substrate specificity of CdnP. Remarkably, bacterial cdNs were hydrolyzed by rCdnP to give the corresponding 5′ NMP nucleotides (Figures 3D and 3E). We determined the Michaelis constants for c-di-AMP, c-di-GMP, and cGAMP (Table 1) and observed the highest specific activity (7 μmol Pi/min/mg prot) with c-di-AMP (Figure S3B). In agreement with the data obtained with GBS cells, we observed that CdnP and NudP cooperate to cleave c-di-AMP into adenosine. CdnP cleaves the two 3′ phosphodiester bonds of c-di-AMP to give two 5′ AMP molecules, which are further cleaved by NudP to give two adenosine and two inorganic phosphates (Figure S3B).
Bacterial cdNs are made of two 3′,5′ phosphodiester linkages connecting the base units. In contrast, the metazoan 2′3′ cGAMP (>G(2′,5′)pA(3′,5′)p>) contains one 2′,5′ and one 3′,5′ phosphodiester linkage (Ablasser et al., 2013; Gao et al., 2013a). While CdnP hydrolyzes the bacterial cGAMP containing two 3′,5′ linkages, no activity was detected on the metazoan 2′3′ cGAMP (Table 1 and Figure S3B). Interestingly, addition of 2′3′ cGAMP does not inhibit the activity of rCdnP on the other substrates (data not shown). On the other hand, non-hydrolysable c-di-NMP analogs, such as cyclic diadenosine monophosphorodithioate, Rp-isomers (Rp, Rp-c-diAMPSS), a di-thiophosphate analogue of c-di-AMP, inhibit rCdnP activity (Figure S3C). In addition, modifications of the 2′, 2″ positions of the ribose, by adding fluor or O-methyl-ribose, influence the catalytic efficiency of rCdnP (data not shown). It is thus likely that the metazoan 2′3′ cGAMP is not a substrate for rCdnP because the specific conformation imposed by the 2′,5′ linkage may hinder the substrate-enzyme interaction (Collins et al., 2015).
To confirm the enzymatic activity, we purified the recombinant rCdnP* where the CdnP critical His199 residue was mutated to Ala. In similar conditions, rCdnP* was totally inactive whatever the substrate used (Figure 3E and data not shown). These results demonstrate that CdnP is a 3′,5′ cyclic dinucleotide 3′ phosphodiesterase.
Degradation of c-di-AMP by CdnP limits STING activation
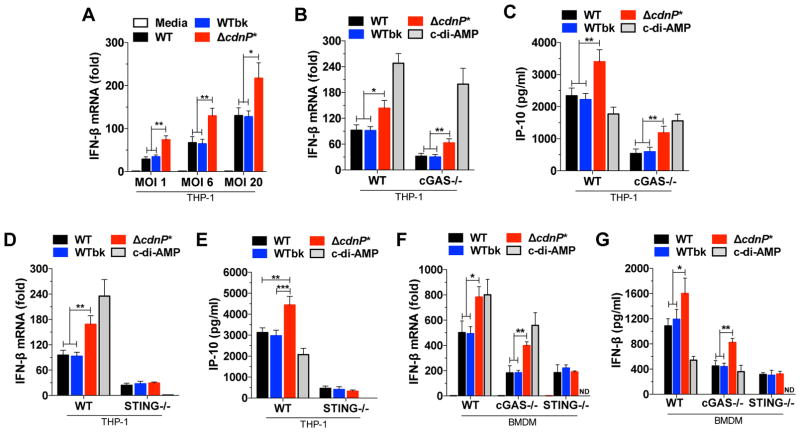
IFN-β induction in response to GBS appears mainly dependent on DNA recognition by cGAS while the direct activation of STING by c-di-AMP has at best a minor role when using WT bacteria (Figure 1). However, WT GBS degrades c-di-AMP present outside the bacteria due to the CdnP ectonucleotidase (Figures 2 and 3). To uncover the effect of CdnP, we compared IFN-β induction in THP-1 cells infected with WT GBS, ΔcdnP* mutant, or the isogenic WTbk control. At all MOIs tested, transcription of IFN-β is higher in THP-1 cells infected with the ΔcdnP* mutant as compared to those infected with the WT and WTbk controls (Figure 4A). This increase in IFN-β induction is not due to difference in bacterial growth rate, phagocytosis or intracellular killing, and is specific since TNF-α induction is similar between ΔcdnP* and WT bacteria (Figures S4A–D).
Figure 4. Increased type I interferon response by GBS ΔcdnP* mutant is dependent on STING.
(A) THP-1 cells were infected with GBS WT, WTbk or ΔcdnP* mutant for 4 hrs at different MOIs and IFN-β mRNA levels were quantified by qRT-PCR and normalized to GAPDH levels. Uninfected cells (media) served as controls.
(B) Quantification of IFN-β mRNA in WT and cGAS−/− THP-1 cells after 4 hrs of infection (MOI-6) with GBS WT, WTbk, or ΔcdnP* mutant or after transfection with c-di-AMP (100 nM).
(C) Quantification of IP-10 by ELISA in WT and cGAS−/− THP-1 cells supernatants after 18 hrs of infection.
(D) Same experiment as in (B) with STING−/− THP-1 cells.
(E) Same experiment as in (C) with STING−/− THP-1 cells.
(F) Same experiment as in (B) with WT, cGAS−/− and STING−/− BMDMs.
(G) Same experiment as in (F) with the quantification of mouse IFN-β production by ELISA after 18 hrs of infection.
All data are represented as mean ± SD of three experiments and asterisks indicate statistically significant differences (*, p < 0.05; **, p < 0.01 and ***, p < 0.001). ND, not detected. See also Figure S2 and S4.
To characterize the respective role of GBS DNA and c-di-AMP on type I IFN induction, cGAS−/− and STING−/− THP-1 cells were infected with the same strains. As observed previously (Figure 1), IFN-β induction and IP-10 production by the GBS WT and WTbk controls is similarly decreased in both cGAS−/− and STING−/− cell lines, with a 64–70% and 70–75% decrease respectively (Figure 4B–E), confirming that cGAS is the main DNA sensor involved in IFN-β induction in response to WT bacteria.
In the same conditions, the ΔcdnP* mutant induced 1.6 to 2 times more IFN-β in WT and cGAS−/− THP-1 cells compared with WT GBS strains (Figure 4B). The decreased IFN induction between WT and cGAS−/− THP-1 cells in response to the ΔcdnP* mutant (around 64%) is similar to the one observed with the WT bacteria controls (Figure 4B). Similar results were obtained by quantifying the production of the related IP-10 cytokine by ELISA (Figure 4C). These results indicate that the relative contribution of DNA sensing by cGAS is not affected by CdnP inactivation.
In contrast, we observed a similar low level of IFN-β induction and IP-10 production by WT bacteria and the ΔcdnP* mutant when using STING−/− THP-1 cells (Figures 4C and 4D). The absence of a difference between WT and mutated bacteria in STING−/− THP-1 cells demonstrates that the increased IFN-β induction is cGAS-independent and STING-dependent, suggesting a role for c-di-AMP in increased IFN-β induction by ΔcdnP*. Indeed, cell transfection with c-di-AMP results in IFN-β induction and IP-10 production in WT and cGAS−/− THP-1 cells (Figures 4B and 4C) but not in STING−/− cells (Figures 4D and 4E). Similarly, incubation of digitonin-permeabilized THP-1 cells with a nucleotide fraction of GBS supernatants results in IFN-β induction only with ΔcdnP* supernatants (Figure S2C). Addition of rCdnP to the purified ΔcdnP* supernatant or inactivation of STING abolished IFN-β induction (Figure S2C). These results demonstrate that THP-1 cells induce more IFN-β in response to infection with the ΔcdnP* mutant, and that this increase response is dependent on STING and on the increased amount of c-di-AMP present outside the mutated bacteria.
The same experiments were repeated in BMDMs to confirm the conserved role of c-di-AMP in IFN-β induction. As observed with THP-1 cells, the ΔcdnP* mutant induced IFN-β by a cGAS and STING-dependent pathway similar to the WT GBS but, in addition, activated a cGAS-independent and STING-dependent pathway that accounts for the increased IFN-β induction of the mutated strain (Figure 4F and 4G). In both cell lines, no difference in phagocytosis, intracellular killing, and TNF-α induction was observed for the three GBS strains in WT and mutated cell lines (Figure S4). Overall, we conclude that the ΔcdnP* mutant induces a stronger IFN-β response due to the cumulative effect of DNA recognition by cGAS, and c-di-AMP by a STING-dependent pathway. The DNA-cGAS pathway is similarly activated by the WT and the ΔcdnP* mutant, while the c-di-AMP-STING pathway is activated only by the ΔcdnP* mutant.
CdnP inactivation induces higher IFN-β levels in vivo and reduces GBS virulence
Type I IFN is important for controlling GBS infection in mice (Mancuso et al., 2007) and our results showed that the GBS ΔcdnP* mutant induced higher IFN-β levels in THP-1 and BMDMs. To test the effect of CdnP inactivation in vivo, we infected WT, cGAS−/− and STING−/− mice with GBS WT, WTbk, and the ΔcdnP* mutant. Consistent with our in vitro results, the ΔcdnP* mutant induced higher IFN-β production in WT and cGAS−/− mice, but not in STING−/− mice, when compared to WT and WTbk bacteria (Figure 5A). The difference in IFN-β induction observed with the ΔcdnP* mutant appears specific since TNF-α induction is not affected by CdnP inactivation in the three mice backgrounds (Figure 5B). Interestingly, inactivation of cGAS or STING decreased the absolute level of IFN-β induction for the three GBS strains, demonstrating the important role of the cGAS-STING pathway during in vivo infection (Figure 5A).
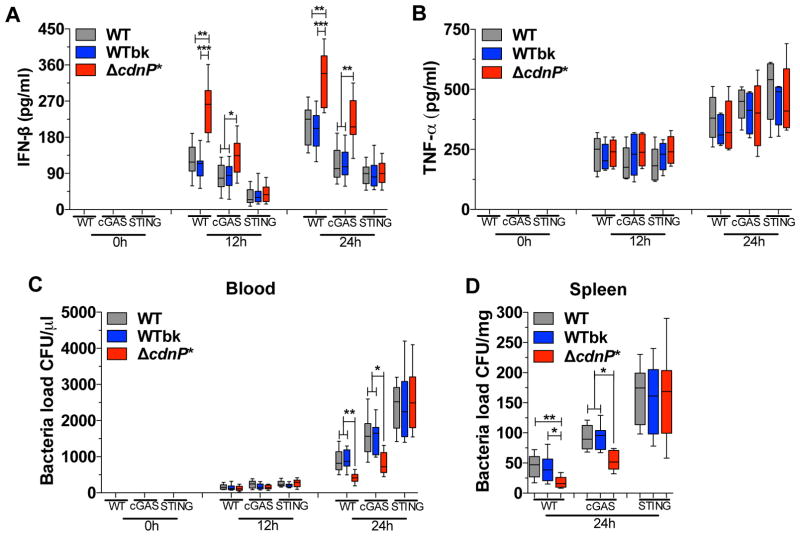
Figure 5. GBS ΔcdnP* mutant induces higher type I interferon response in vivo in both WT and cGAS−/−, but not in STING−/− mice.
(A–C) WT, cGAS−/−, and STING−/− mice (n = 10 for each genotype) were infected by intravenous injection with 1.5 × 107 CFUs of GBS WT, WTbk, or ΔcdnP* mutant. Mice were bled 12 and 24 post-infection. Serum IFN-β (A) and TNF-α (B) levels were measured by ELISA, and blood bacteremia was evaluated by plating serial dilutions (C).
(D) Same experimental condition as in (A–C) evaluating spleen bacteremia after 24 of infection (n = 8 for each time point).
Data are presented as mean ± SD of one of the two independent experiments that yielded similar results. Asterisks indicate that differences are statistically significant (*,p < 0.05; **, p < 0.01 and ***, p < 0.001).
To confirm the link between IFN-β level and the control of GBS infection, we quantified the number of bacteria in the blood and spleen. The WT and cGAS−/− mice, but not the STING−/− mice, infected by the ΔcdnP* mutant showed decreased bacteremia in both blood (Figure 5C) and spleen (Figure 5D) at 24 hours post-infection. Moreover cGAS or STING inactivation increased bacteremia, demonstrating their role to restrict bacterial infection by GBS (Figure 5C and D). In conclusion, CdnP inactivation results in higher IFN-β induction in mice and allows a more efficient control of the infection by a STING-dependent pathway. It is therefore likely that GBS expresses CdnP at its surface to degrade its own released c-di-AMP and to promote its virulence.
DISCUSSION
Type I IFNs are better known for their role in antiviral host defense, but they are likewise important in the host response to bacterial infection with favorable or detrimental outcomes to the host depending on the pathogen (McNab et al., 2015; Monroe et al., 2010). Both viral and bacterial intracellular pathogens have been reported to induce type I IFNs by the cGAS/STING axis (Barber, 2014; Cai et al., 2014). Given the conserved and ancient origin of this signaling pathway (Kranzusch et al., 2015), it is likely that microbes have developed mechanisms to manipulate this innate immune sensing pathway. Consistently, viruses express specific proteins that increase infectiveness by interfering with cGAS or STING activation (Paludan, 2015; Wu et al., 2015; Ma and Damania, 2016).
In this study, we demonstrated that type I IFN induction by the extracellular pathogen GBS is also dependent on the cGAS/STING axis. We identified cGAS as the main upstream sensor leading to IFN-β production in response to WT GBS, in agreement with our previous study showing that GBS DNA is the main agonist of this response (Charrel-Dennis et al., 2008). However, we identified that WT GBS expresses a CdnP ectonucleotidase that degrades c-di-AMP outside the bacteria. Inactivation of CdnP leads to c-di-AMP accumulation outside GBS, higher levels of IFN-β in vitro and in vivo, and reduced virulence, with lower bacterial burden in infected organs. The overproduction of IFN-β is STING-dependent but cGAS-independent, showing that bacterial cdN can activate STING either directly or with the help of additional cdN receptors such as DDX41 (Danilchanka and Mekalanos, 2013; Parvatiyar et al., 2012). By degrading its own c-di-AMP, GBS avoids the overactivation of STING, limits IFN-β induction, and promotes its virulence.
To the best of our knowledge, a cdN catabolic activity at the bacterial surface has never been reported, or tested, in any species. Interestingly, CdnP is an enzyme belonging to the widespread ectonucleotidase family usually involved in nucleotide catabolism at the cell surface of prokaryotic and eukaryotic cells (Matange et al., 2015; Zimmermann et al., 2012). This family of enzymes is structurally unrelated to the currently known bacterial cdN phosphodiesterases, which are all involved in the regulation of the intracellular concentration of cdN to control bacterial physiology (Corrigan and Grundling, 2013; Romling et al., 2013). The current functional annotation in databases of CdnP suggests an activity on 2′,3′ cyclic nucleotides. Although enzymes with this predicted activity are widespread, their biological functions are unknown (Matange et al., 2015; Wilson et al., 2012) probably because the predicted 2′,3′ cNMP substrates have never been detected in vivo and are unstable intermediates that do not require a specific enzyme for their degradation (Rao et al., 2010). The CdnP activity on 3′,5′ cdN is therefore more likely to be a relevant physiological function instead of the activity on 2′,3′ cNMPs. CdnP is active on specific cdNs, being more potent on c-di-AMP, the only cdN synthesized by GBS, and unable to cleave the eukaryotic 2′3′ cGAMP. Following this original CdnP enzymatic characterization, it is expected that other annotated 2′,3′ cNMP-degrading enzymes are in fact phosphodiesterases acting on specific cdNs.
Prior to this study, CdnP homologues in bacteria were identified but their biological functions have been elusive. The best-characterized homologue to date is the Vibrio cholera CdpB enzyme necessary for bacterial growth when extracellular DNA is the only phosphate source (McDonough et al., 2015), but the nutritional function of extracellular cdN is unlikely. The secretion or release of cdNs by bacteria is common and might be a way to regulate the intracellular pool of cdN in response to environmental stresses. However, cdNs have no known function outside bacteria. No effect on GBS growth was observed by inactivating CdnP, suggesting that the extracellular accumulation of c-di-AMP has no apparent physiological role in the tested conditions. In contrast, intracellular c-di-AMP synthesis is essential for bacterial growth in almost all conditions (Corrigan and Grundling, 2013; Whiteley et al., 2015) and we were unable to inactivate the only c-di-AMP synthase gene in GBS with our conventional methods (data not shown), suggesting that c-di-AMP synthesis is indeed essential for growth in standard condition.
The biological significance of having a dedicated c-di-AMP phosphodiesterase at the GBS surface becomes relevant when considering the host-pathogen relationship (Danilchanka and Mekalanos, 2013). We found that IFN-β induction by GBS-infected human and murine cells is dependent on cGAS, in agreement with GBS DNA as the main inducer of this TLR-independent response (Charrel-Dennis et al., 2008). This result is also consistent with cGAS activation by DNA as predominant over bacterial cdN activation of STING, as observed recently with M. tuberculosis (Collins et al., 2015; Wassermann et al., 2015; Watson et al., 2015), L. monocytogenes (Hansen et al., 2014), and C. trachomatis (Zhang et al., 2014). However, by inactivating CdnP in GBS, IFN-β induction is increased and results from the additive effect on STING of the cGAS-synthesized 2′3′ cGAMP in response to DNA and of the secreted bacterial c-di-AMP (Figure 6). The increased amount of cdN outside bacteria has been previously linked to an increase in STING-dependent IFN-β induction (Barker et al., 2013; Dey et al., 2015; Schwartz et al., 2012; Woodward et al., 2010). Interestingly, we did not detect a CdnP homologue in L. monocytogenes. This is not surprising since IFN-β production has mainly negative effects on the host during infections by intracellular bacterial pathogens. It might even be possible that this pathogen class positively regulates the secretion of cdN to increase IFN-β production. In contrast, IFN-β is necessary for host protection against GBS and related pathogens. It is therefore advantageous for GBS to diminish STING activation by degrading c-di-AMP to promote invasion and organ colonization.
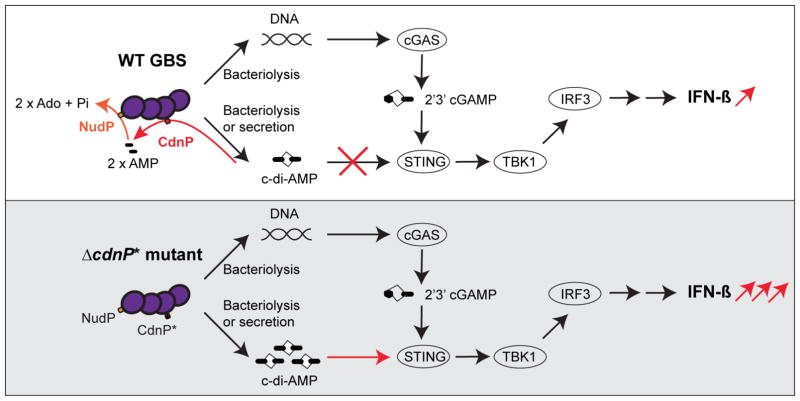
Figure 6. Model of cGAS/STING activation by WT GBS and ΔcdnP* mutant.
Top panel: WT GBS induces type I IFN by a cGAS-dependent pathway following bacterial DNA sensing, synthesis of 2′3′ cGAMP, and activation of the STING/TBK1/IRF3 axis. The c-di-AMP released by GBS is degraded by the CdnP cell wall-anchored ectonucleotidase into AMP, which is further degraded by the second ectonucleotidase NudP into adenosine (Ado).
Bottom panel: in the absence of the CdnP ectonucleotidase (GBS ΔcdnP* mutant), c-di-AMP accumulation outside the bacteria activates STING independently of cGAS, and, together with GBS DNA sensing by cGAS, leads to type I IFN over-production.
In conclusion, we extend the role of the cGAS-STING axis to the extracellular bacterial pathogen S. agalactiae and identified cGAS as the main sensor leading to IFN-β production by infected macrophages. We also uncovered that wild-type GBS dampens IFN-β production by degrading c-di-AMP due to the CdnP activity, thus avoiding the direct activation of STING. Our discovery shows the importance of c-di-AMP in the host immune response to GBS infection, and suggests that the development of CdnP inhibitors could be envisioned as a treatment strategy for infectious diseases.
EXPERIMENTAL PROCEDURES
Ethics Statement
All experiments involving animals were performed in accordance with guidelines set forth by the American Association for Laboratory Animal Science (AALAS) and were approved by the Institutional Animal Care and Use Committee (IACUC A-1332) at the University of Massachusetts Medical School (UMMS).
Bacterial strains and growth conditions
GBS strains used in this study are derivatives of NEM316, a ST-23 and serotype III clinical isolate (Glaser et al., 2002). The ΔcylE non-hemolytic and the ΔnudP deletion mutant were described previously (Firon et al., 2014; Firon et al., 2013). The ΔcdnP* mutant was constructed, as described in the supplemental experimental procedures, by mutating the His199-encoding codon to an Ala-encoding codon. The marker-less substitution and the isogenic WTbk control were confirmed by sequencing. Liquid cultures were maintained at 37°C in TH broth (TH, Difco-BD) or in a chemically defined medium (CDM).
Cells, cultures, and infections
WT, cGAS−/−, STING−/− and IFI16−/− THP-1 cells generated with CRISPR-Cas9 (Supplemental procedures and Figure S1) were grown in RPMI1640 supplemented with 4 mM glutamine and 10% FBS. Primary BMDMs from C57BL/6 mice were generated as described previously (Charrel-Dennis et al., 2008) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL) supplemented with 4 mM glutamine and 10% FBS. cGAS−/− and STING−/− mice were obtained from G. Barber (University of Miami). Cells were infected with live GBS cultures as described (Charrel-Dennis et al., 2008) at an MOI of 6, unless otherwise stated. Cells were harvested 6 to 18 hrs post-infection for RNA preparation and supernatant analysis.
Quantitative RT-PCR and ELISA
Total RNA was extracted from infected cells using Trizol (Invitrogen) and one microgram of total RNAs was used for cDNA synthesis and quantitative PCR (Bio-Rad). Levels of human and mouse IFN-β and TNF-α were normalized against their respective GAPDH housekeeping genes. Quantification by ELISA was performed on cell supernatants after the indicated time of infection as described for mouse IFN-β (Roberts et al., 2007) and according to the manufacturer’s instructions (R&D Research) for human CXCL10/IP-10 and mouse and human TNF-α.
Quantification of GBS intracellular cdN by LC-MS/MS
Quantification of cdNs was performed by LC-MS/MS by BioLog Life Science GmbH using internal labeled standards. Extraction of cdNs on 5 ml of overnight GBS cultures was performed with Acetonitrile/Methanol/Water (2/2/1, v/v/v) buffer as recommended. Quantities of cdNs were normalized against the quantity of total protein extract.
Quantification of cdN and nucleotides by RR-HPLC
Rapid Resolution High Performance Liquid Chromatography (RR-HPLC) was used to determine kinetics of extracellular c-di-AMP degradation on whole GBS cells, extracellular c-di-AMP quantification, and for CdnP enzymatic characterization as described in supplemental experimental procedures. Reagents and standards were purchased from BioLog Life Sciences GmbH (c-di-AMP, c-di-GMP, cGAMP, 2′3′ cGAMP, 2′,3′ and 3′,5′ cyclic NMPs) or from Sigma Aldrich (NMP, NDP, nucleotides, nucleosides).
Extracellular c-di-AMP degradation by GBS
GBS in early stationary phase were incubated with 0.1 mM c-di-AMP in 50 mM Tris and 5 mM MnCl2, at 37°C with agitation. Aliquots were taken at different times, centrifuged two times to eliminate bacteria, and the kinetics of c-di-AMP degradation and product formation was analyzed by RR-HPLC.
Quantification of extracellular c-di-AMP in GBS supernatants
Enrichment of c-di-AMP was done by affinity purification on 40 ml of GBS supernatants with the c-di-AMP binding protein CabP (Bai et al., 2014), as detailed in the supplemental experimental procedure. Relative quantification of extracellular c-di-AMP was performed by RR-HPLC with an external standard (40 ml of PBS containing 1.25 μM c-di-AMP) treated in the same condition to normalize samples.
Characterization of rCdnP enzymatic activities
Recombinant CdnP (residues 29-768) was produced in E. coli and analyzed by ultracentrifugation (An60Ti rotor, Beckman Coulter) as described in the supplemental experimental procedures. The rCdnP metallo-phosphatase activities were first assayed on 2′,3′ cyclic nucleotides by measuring the release of inorganic phosphate (Pi) with Malachite Green reagent (BIOMOL GREEN™, Enzo Life Sciences). Inorganic phosphate (Pi) was quantified (absorbance at 620 nm) against a standard Pi curve. Kinetics with cdNs were done at 37°C in 50 mM Tris, pH 7.5, containing 5 mM MnCl2, 100–200 μM of substrates and 3 nM purified rCdnP enzyme. Substrate degradation and product formation were followed every 7 min by RR-HPLC.
In vivo infection and bacterial burden
6-week-old female C57BL/6 mice (Charles River) were injected intravenously with 1.5 × 107 CFUs of bacteria harvested during the exponential growth phase, washed in PBS, and resuspended in 100 μl. At 12 and 24 hrs after infection, mice were bled to evaluate serum IFN-β levels. Bacterial counts in blood and spleen homogenates were determined by plating serial dilutions on blood agar plates.
Statistical Analysis
All in vitro data were analyzed using an unpaired, two-tailed Student’s t test with a 95% confidence interval. In vivo data were analyzed using a non-parametric ANOVA (Kruskal-Wallis) (Prism; GraphPad Software, Inc.). Data are represented as means ± SD.
Supplementary Material
Highlights.
Induction of type I IFN by Group B Streptococcus (GBS) depends on the cGAS/STING axis
GBS expresses an ectonucleotidase (CndP) that degrades its own secreted c-di-AMP
A GBS CndP mutant secrets increased amounts of c-di-AMP and induces higher IFN-β levels
Mice infected with a GBS CndP mutant produce more IFN-β and exhibit lower bacteremia
Acknowledgments
We thank Dr. S. Pochet for the RR-HPLC and B. Raynal from the PFBMI for analytical ultracentrifugation experiment. This work was supported by NIH grant (to D.T.G.), the Institut Pasteur and CNRS (to P.T.C.), French Government’s Investissement d’Avenir program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases” Grant ANR-10-LABX-62-IBEID (to P.T.C.), and Fondation pour la Recherche Médicale Grant DEQ20130326538 (to P.T.C.). W.A.A. was supported by a fellowship from CNPq (Brazil). The authors declare no competing financial interests.
Footnotes
Author contributions
W.A.A., A.F., P.T.C, D.T.G. and P.A.K. conceived and designed experiments. W.A.A., A.F., T.S., and P.A.K performed experiments. V.H., and K.A.F provided reagents. W.A.A., A.F., E.K.J., P.T.C, D.T.G. and P.A.K. analyzed data. W.A.A., A.F., P.T.C, D.T.G. and P.A.K. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, Hopfner KP, Ludwig J, Hornung V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anraku Y. A New Cyclic Phosphodiesterase Having a 3′-Nucleotidase Activity from Escherichia Coli B. II. Further Studies on Substrate Specificity and Mode of Action of the Enzyme. J Biol Chem. 1964;239:3420–3424. [PubMed] [Google Scholar]
- Archer KA, Durack J, Portnoy DA. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS pathogens. 2014;10:e1003861. doi: 10.1371/journal.ppat.1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Yang J, Zarrella TM, Zhang Y, Metzger DW, Bai G. Cyclic di-AMP impairs potassium uptake mediated by a cyclic di-AMP binding protein in Streptococcus pneumoniae. Journal of bacteriology. 2014;196:614–623. doi: 10.1128/JB.01041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends in immunology. 2014;35:88–93. doi: 10.1016/j.it.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Barker JR, Koestler BJ, Carpenter VK, Burdette DL, Waters CM, Vance RE, Valdivia RH. STING-dependent recognition of cyclic di-AMP mediates type I interferon responses during Chlamydia trachomatis infection. mBio. 2013;4:e00018–00013. doi: 10.1128/mBio.00018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell. 2014;54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- Charrel-Dennis M, Latz E, Halmen KA, Trieu-Cuot P, Fitzgerald KA, Kasper DL, Golenbock DT. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host Microbe. 2008;4:543–554. doi: 10.1016/j.chom.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Cai H, Li T, Franco LH, Li XD, Nair VR, Scharn CR, Stamm CE, Levine B, Chen ZJ, et al. Cyclic GMP-AMP Synthase Is an Innate Immune DNA Sensor for Mycobacterium tuberculosis. Cell Host Microbe. 2015;17:820–828. doi: 10.1016/j.chom.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan RM, Grundling A. Cyclic di-AMP: another second messenger enters the fray. Nat Rev Microbiol. 2013;11:513–524. doi: 10.1038/nrmicro3069. [DOI] [PubMed] [Google Scholar]
- Danilchanka O, Mekalanos JJ. Cyclic dinucleotides and the innate immune response. Cell. 2013;154:962–970. doi: 10.1016/j.cell.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BW, Bogard RW, Young TS, Mekalanos JJ. Coordinated Regulation of Accessory Genetic Elements Produces Cyclic Di-Nucleotides for V. cholerae Virulence. Cell. 2012;149:358–370. doi: 10.1016/j.cell.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B, Dey RJ, Cheung LS, Pokkali S, Guo H, Lee JH, Bishai WR. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med. 2015;21:401–406. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firon A, Dinis M, Raynal B, Poyart C, Trieu-Cuot P, Kaminski PA. Extracellular nucleotide catabolism by the Group B Streptococcus ectonucleotidase NudP increases bacterial survival in blood. J Biol Chem. 2014;289:5479–5489. doi: 10.1074/jbc.M113.545632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firon A, Tazi A, Da Cunha V, Brinster S, Sauvage E, Dramsi S, Golenbock DT, Glaser P, Poyart C, Trieu-Cuot P. The Abi-domain protein Abx1 interacts with the CovS histidine kinase to control virulence gene expression in group B Streptococcus. PLoS Pathog. 2013;9:e1003179. doi: 10.1371/journal.ppat.1003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, Serganov AA, Liu Y, Jones RA, Hartmann G, et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013a;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Ascano M, Zillinger T, Wang W, Dai P, Serganov AA, Gaffney BL, Shuman S, Jones RA, Deng L, et al. Structure-function analysis of STING activation by c[G(2′,5′)pA(3′,5′)p] and targeting by antiviral DMXAA. Cell. 2013b;154:748–762. doi: 10.1016/j.cell.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P, Rusniok C, Buchrieser C, Chevalier F, Frangeul L, Msadek T, Zouine M, Couve E, Lalioui L, Poyart C, et al. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol Microbiol. 2002;45:1499–1513. doi: 10.1046/j.1365-2958.2002.03126.x. [DOI] [PubMed] [Google Scholar]
- Gratz N, Hartweger H, Matt U, Kratochvill F, Janos M, Sigel S, Drobits B, Li XD, Knapp S, Kovarik P. Type I interferon production induced by Streptococcus pyogenes-derived nucleic acids is required for host protection. PLoS Pathog. 2011;7:e1001345. doi: 10.1371/journal.ppat.1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K, Prabakaran T, Laustsen A, Jorgensen SE, Rahbaek SH, Jensen SB, Nielsen R, Leber JH, Decker T, Horan KA, et al. Listeria monocytogenes induces IFN expression through an IFI16-, cGAS- and STING-dependent pathway. The EMBO Journal. 2014;33:1654–1666. doi: 10.15252/embj.201488029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubrel C, Tazi A, Six A, Dmytruk N, Touak G, Bidet P, Raymond J, Trieu-Cuot P, Fouet A, Kerneis S, et al. Group B Streptococcus neonatal invasive infections, France 2007–2012. Clin Microbiol Infect. 2015;21:910–916. doi: 10.1016/j.cmi.2015.05.039. [DOI] [PubMed] [Google Scholar]
- Kranzusch PJ, Wilson SC, Lee AS, Berger JM, Doudna JA, Vance RE. Ancient Origin of cGAS-STING Reveals Mechanism of Universal 2′,3′ cGAMP Signaling. Molecular Cell. 2015;59:891–903. doi: 10.1016/j.molcel.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Damania B. The cGAS-STING Defense Pathway and Its Counteraction by Viruses. Cell Host Microbe. 2016;19:150–158. doi: 10.1016/j.chom.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso G, Midiri A, Biondo C, Beninati C, Zummo S, Galbo R, Tomasello F, Gambuzza M, Macri G, Ruggeri A, et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J Immunol. 2007;178:3126–3133. doi: 10.4049/jimmunol.178.5.3126. [DOI] [PubMed] [Google Scholar]
- Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. Mycobacterium Tuberculosis Activates the DNA-Dependent Cytosolic Surveillance Pathway within Macrophages. Cell Host Microbe. 2012;11:469–480. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matange N, Podobnik M, Visweswariah SS. Metallophosphoesterases: structural fidelity with functional promiscuity. Biochem J. 2015;467:201–216. doi: 10.1042/BJ20150028. [DOI] [PubMed] [Google Scholar]
- McDonough E, Kamp H, Camilli A. Vibrio cholerae phosphatases required for the utilization of nucleotides and extracellular DNA as phosphate sources. Mol Microbiol. 2015 doi: 10.1111/mmi.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M, Joncker NT, Ishii KJ, Akira S, Colonna M, Chen ZJ, et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med. 2009;206:1899–1911. doi: 10.1084/jem.20082874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe KM, McWhirter SM, Vance RE. Induction of type I interferons by bacteria. Cell Microbiol. 2010;12:881–890. doi: 10.1111/j.1462-5822.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L, Madureira P, Andrade EB, Bouaboud A, Morello E, Ferreira P, Poyart C, Trieu-Cuot P, Dramsi S. Group B streptococcus GAPDH is released upon cell lysis, associates with bacterial surface, and induces apoptosis in murine macrophages. PLoS One. 2012;7:e29963. doi: 10.1371/journal.pone.0029963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan SR. Activation and regulation of DNA-driven immune responses. Microbiol Mol Biol Rev. 2015;79:225–241. doi: 10.1128/MMBR.00061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D, Planet PJ, Soong G, Narechania A, Prince A. Induction of type I interferon signaling determines the relative pathogenicity of Staphylococcus aureus strains. PLoS Pathog. 2014;10:e1003951. doi: 10.1371/journal.ppat.1003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13:1155–1161. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao F, Qi Y, Murugan E, Pasunooti S, Ji Q. 2′,3′-cAMP hydrolysis by metal-dependent phosphodiesterases containing DHH, EAL, and HD domains is non-specific: Implications for PDE screening. Biochem Biophys Res Commun. 2010;398:500–505. doi: 10.1016/j.bbrc.2010.06.107. [DOI] [PubMed] [Google Scholar]
- Roberts ZJ, Goutagny N, Perera PY, Kato H, Kumar H, Kawai T, Akira S, Savan R, van Echo D, Fitzgerald KA, et al. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204:1559–1569. doi: 10.1084/jem.20061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the First 25 Years of a Universal Bacterial Second Messenger. Microbiol Mol Biol Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz KT, Carleton JD, Quillin SJ, Rollins SD, Portnoy DA, Leber JH. Hyperinduction of host beta interferon by a Listeria monocytogenes strain naturally overexpressing the multidrug efflux pump MdrT. Infect Immun. 2012;80:1537–1545. doi: 10.1128/IAI.06286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann R, Gulen MF, Sala C, Perin SG, Lou Y, Rybniker J, Schmid-Burgk JL, Schmidt T, Hornung V, Cole ST, et al. Mycobacterium tuberculosis Differentially Activates cGAS- and Inflammasome-Dependent Intracellular Immune Responses through ESX-1. Cell Host Microbe. 2015;17:799–810. doi: 10.1016/j.chom.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, Cox JS. The Cytosolic Sensor cGAS Detects Mycobacterium tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell Host Microbe. 2015;17:811–819. doi: 10.1016/j.chom.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley AT, Pollock AJ, Portnoy DA. The PAMP c-di-AMP Is Essential for Listeria Growth in Macrophages and Rich but Not Minimal Media due to a Toxic Increase in (p)ppGpp. Cell Host Microbe. 2015;17:788–798. doi: 10.1016/j.chom.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Schoggins JW, Zang T, Kutluay SB, Jouvenet N, Alim MA, Bitzegeio J, Rice CM, Bieniasz PD. Inhibition of HIV-1 particle assembly by 2′,3′-cyclic-nucleotide 3′-phosphodiesterase. Cell Host Microbe. 2012;12:585–597. doi: 10.1016/j.chom.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Li W, Shao Y, Avey D, Fu B, Gillen J, Hand T, Ma S, Liu X, Miley W, et al. Inhibition of cGAS DNA Sensing by a Herpesvirus Virion Protein. Cell Host Microbe. 2015;18:333–344. doi: 10.1016/j.chom.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Hara H, Tsuchiya K, Sakai S, Fang R, Matsuura M, Nomura T, Sato F, Mitsuyama M, Kawamura I. Listeria monocytogenes strain-specific impairment of the TetR regulator underlies the drastic increase in cyclic di-AMP secretion and beta interferon-inducing ability. Infect Immun. 2012;80:2323–2332. doi: 10.1128/IAI.06162-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yeruva L, Marinov A, Prantner D, Wyrick PB, Lupashin V, Nagarajan UM. The DNA sensor, cyclic GMP-AMP synthase, is essential for induction of IFN-beta during Chlamydia trachomatis infection. J Immunol. 2014;193:2394–2404. doi: 10.4049/jimmunol.1302718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.