Abstract
Introduction
Brunner's gland hamartoma (BGH) is an infrequently encountered, benign, polypoid proliferation of Brunner's glands. Usually these lesions are asymptomatic, just only occasionally presenting with duodenal obstruction or bleeding signs and mimicking a tumoral lesion.
Case presentation
A 72-year-old male, referred for recurrent vomiting and epigastralgia, was investigated and all preoperative findings were suggestive of a tumour of the duodenum. During the scheduled pancreaticoduodenectomy a mass, resultant to a polyp, was palpatory felt inside the duodenum and then successfully and completely resected through a duodenotomy avoiding surgical overtreatment and connected postoperative morbidities. Histological analysis showed hyperplasia of Brunner's glands correspondent to a Brunner's gland hamartoma. BGH was undiagnosed before surgery, due to its particular sub-mucosal growth simulating an expanding process starting from the duodenum, and secondly due to unsuccessful biopsies performed during endoscopic procedure.
Conclusion
BGH is a rare lesion featuring, when symptomatic, obstructive or bleeding symptoms. Surgical treatment represents the gold standard approach in case of lesions that are technically impossible to remove endoscopically or in case of an undiagnosed lesion. Herein, we report a case of a patient presenting with a duodenal lesion mimicking, in all preoperative findings, a tumour of the duodenum. Duodenotomy and resection of the BGH provided a definitive cure avoiding surgical overtreatment. An intraoperative deep analysis of all surgical cases still remain crucial for a right therapeutic choice even in a new era for surgical technology. For similar intraoperative findings we recommend this technique.
Keywords: Brunner's gland hamartoma, Case report, EUS, Surgical overtreatment
Highlights
-
•
Brunneroma is an extremely uncommon benign tumour of the duodenum.
-
•
The case is completely mimicking a malignant tumour of the duodenum.
-
•
The report is good opportunity to take a stock of the situation from a clinical stand point.
1. Introduction
Brunner's gland hamartoma (BGH), also known as brunneroma or Brunner's gland adenoma, is an extremely uncommon benign tumour arising from alkaline-based mucin-secreting glands of the duodenum, with an estimated incidence in the population of around 0.008% [1]. These lesions are typically discovered incidentally, even though they can eventually lead, in case of increasing size, to obstructive or haemorrhagic symptoms such as: abdominal pain, nausea, dyspepsia, vomiting, intussusception, upper gastrointestinal bleeding and recurrent pancreatitis [2]. BGH therapeutic management is essentially based on endoscopic removal in case of peduncolated lesion or surgical resection for broad-based structures or in case of an unsuccessful endoscopic procedure. The current case report focuses on a Brunner's gland hamartoma causing intermittent duodenal obstruction, and reviews its clinical presentation, histopathological features and its therapy management.
1.1. Case presentation
A 72-year-old Caucasian male in moderately good general health conditions (medical history was positive for mild arterial hypertension, hepatitis C and previous laparoscopic appendectomy appendicitis and cholecystectomy, patient's BMI was 25) was referred to our department by his family physician due to a six-month history of strong epigastric discomfort unrelated with food intake, recurrent vomiting and epigastralgia non-responding to PPI administration. He denied medications, allergies or previous colonoscopy or esophagogastroduonenoscopy (EGDS). Physical examination revealed a soft diffusely tender abdomen, without palpable mass. Haematological examinations were regular with haemoglobin concentration of 14.3 gr/dl and a coagulation profile within normal limits, normal amylase, lipase, liver function studies and neoplastic markers (Ca19.9, CEA). Upon referral, EGDS was then performed to investigate eventual upper intestinal obstruction. On endoscopy he was found to have a broad-based lesion on the posterior-inferior wall of the second duodenal portion with no signs of active ulcer, particularly close to the pylorus. EGDS also revealed a normal oesophagus with hyperaemic gastric mucosa. The lesion, which was by-passable by the endoscopic instrument, deformed pylorus and duodenal bulb and the duodenum appeared normal until the passage D1-D2. Resection was not considered due to the broad-based nature of the lesion and secondly to its extension (judged around 35mm).
No signs of active bleeding were observed and the lesion was then unsuccessfully biopsied. In order to radiologically investigate the lesion, we performed a high-resolution contrast-enhanced computer tomography (CT) that confirmed the presence of a whorled 40 × 16 mm vegetant lesion characterized by inhomogeneous arterial contrast enhancement. This lesion was established to be very close to the pyloric area, occupying almost completely the first part of the duodenum without a real surgical cleavage plan of resection. No dilatation of common bile duct (CBD) or pancreatic duct was found. We considered mandatory a better characterization of the lesion, and, particularly, ascertain its depth of involvement within the duodenal layers, and for this reason, an endoscopic ultrasound (EUS) was completed. The EUS showed a sub-mucosal lesion of mixed echogenicity measuring around 4cm in diameter arising from D2-D3, with disomogeneous parenchymal distribution visible following contrast enhancing with Sonovue™. The EUS did not display, like the CT-scan previously, any clear planes between the mass and the head of the pancreas nor lymphnodes involvement. All the above preoperative findings led our group to a provisional diagnosis of duodenal tumour with a second-line diagnosis of tumour of the head of the pancreas (Fig. 1). For these reasons, 10 days after the referral, the patient was taken up for a laparotomy with a plan to perform Whipple's pancreaticoduodenectomy. After a midline laparotomy, the distal stomach and duodenum were carefully kocherized and a mass lesion was felt inside the duodenum, just after the pylorus on palpation. The lesion seemed to be moderately mobile at the duodenal bulb with hard parenchymal consistency. No adhesions suggestive of a chronic inflammation status were noticed during the entire operation. Due to these features Whipple's procedure was at the moment interrupted in order to deeply investigate intraoperatively the origin of the mass. Via a duodenotomy a large polyp occluding almost the entire duodenal lumen was observed (Fig. 2). The ampullar papilla was not involved. A very short and large stalk characterized the polyps' base, so we decided to excise it by using mechanical endo-GIA (Fig. 3). The duodenotomy was then closed via a double layer reconstruction and a close-suction drain was positioned close to it. There was no lymph node involvement. The procedure was carried out by an experienced senior surgeon in an academic high volume hospital/setting.
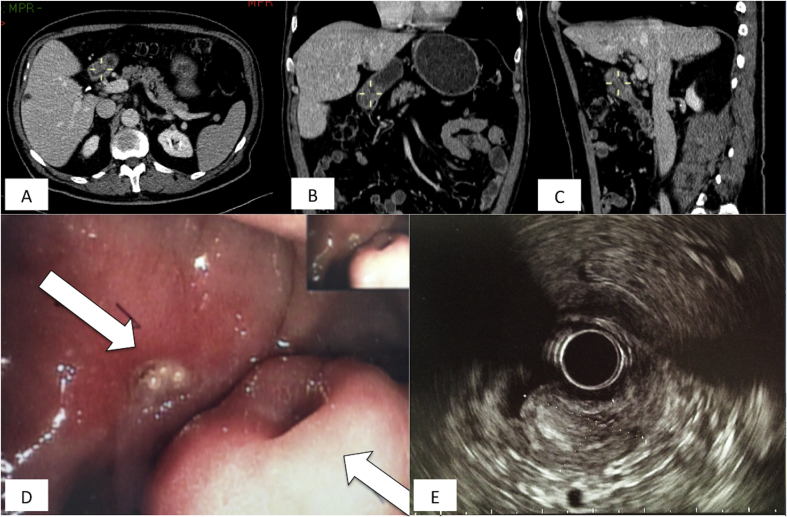
Fig. 1.
Contrast enhanced computer tomography scan axial image shows a lesion confined to the first and second part of the duodenum (D1 and D2) [Pict. A - white arrows] characterized by inhomogeneous arterial contrast enhancement. Size of the lesion was estimated around 40 × 16mm. Not clear images potentially attributable to lymphnodal structures were observed too. Coronal reconstruction displays the solid component of the mass [Pict. B] whereas sagittal cut [Pict. C] the longitudinal involvement of the duodenal wall. [Pict. D] Upper endoscopic view shows Brunner's gland hamartoma extremely attached to the posterior wall of the duodenum with no evidence of active bleeding and normal appearing mucosa. Endoscopic ultrasound of duodenum revealed a submucosal middle-echoic mass sizing 24.5 × 17.1 mm (lightly lower than measures obtained from CT-scan) with unclear margins [Pict. E]. Echoic characteristic of the mass permitted differential diagnosis between sub-mucosal lesions from pseudo-submucosal lesions.
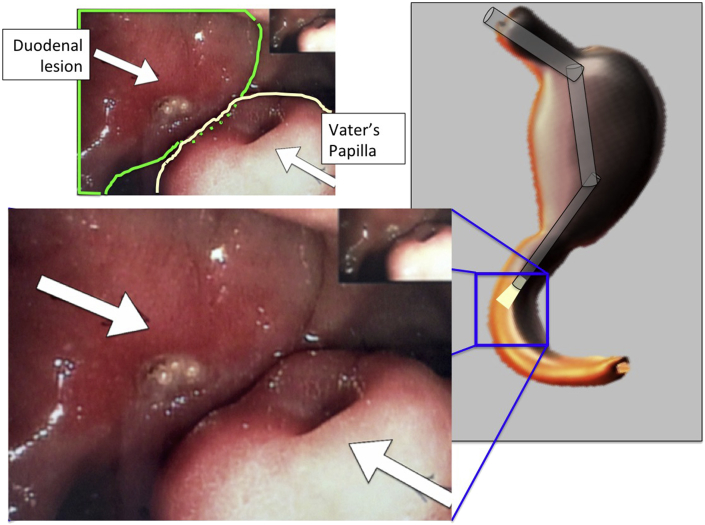
Fig. 2.
Endoscopic picture showing the Vater's papilla and the duodenal lesion.
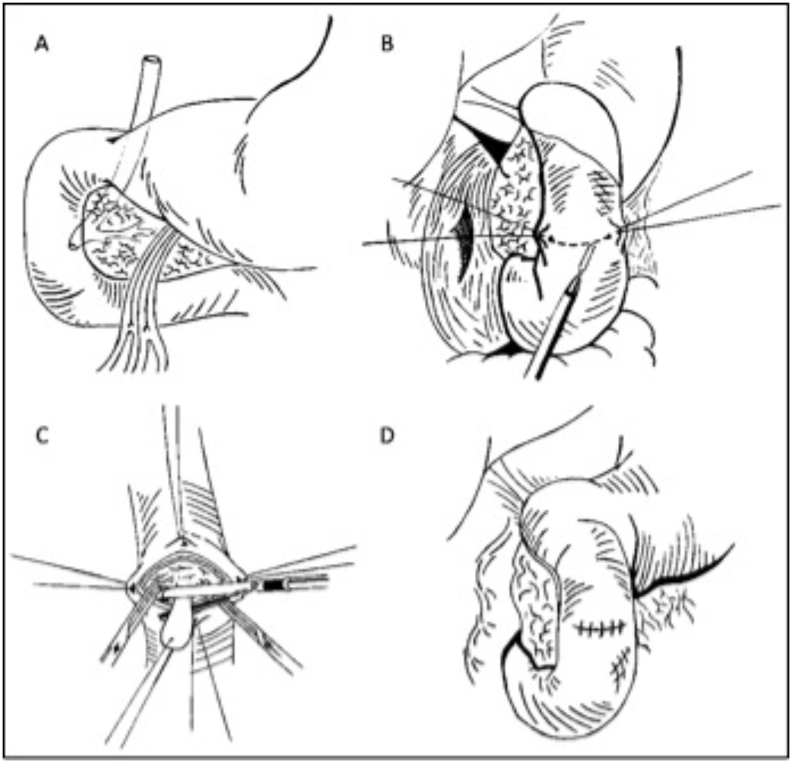
Fig. 3.
Anatomically duodenal exposure was mandatory in order to intraoperatively explore and investigate the cause of the compression [Pict. A]. Starting from the epiploic foramen, lateral parietal peritoneum was excised along descending duodenum to perform Kocher maneuver [Pict. B] permitting the careful examination of the posterior wall of the duodenum and of the retroduodenal portion of the pancreas. Longitudinal duodenotomy was performed assessing the presence of the polypoid formation that was transected by vascular mechanical stapler [Pict. C]. Duodenal opening was then close in layers [Pict. D] and rüsch drainage was positioned sub-liver.
The post-operative period was characterized in third POD by signs of intestinal perforation: fever, abdominal pain and mild leukocytosis (15.2 × 10/l). A CT scan showed a small collection near the duodenal suture. For this reason the patient was conducted in operation theatre but at the laparotomy no perforative sites or collections were observed (Clavien Dindo Grade IIIb). The residual postoperative period was uneventful. On POD 5 the NGT was removed and he tolerated diet, on POD 6 the patient reported bowel movements and on POD 7 he was discharged. Histopathological findings finally showed that the resected polyp (2,8 × 2,8 × 1,7 cm) was a Brunner gland hamartoma with hyperplastic glands without histopathological evidence of atypia. Immunohistochemical profile was positive for Cit7, Cit20, MUC1, MUC6 and MUC5AC. Brunner glands appeared mixed to adipose tissue and lymphoid infiltrate. Some gastric metaplasia areas were observed in the overlying mucosal layer (MUC1+ and MUC5AC+) (Fig. 4).
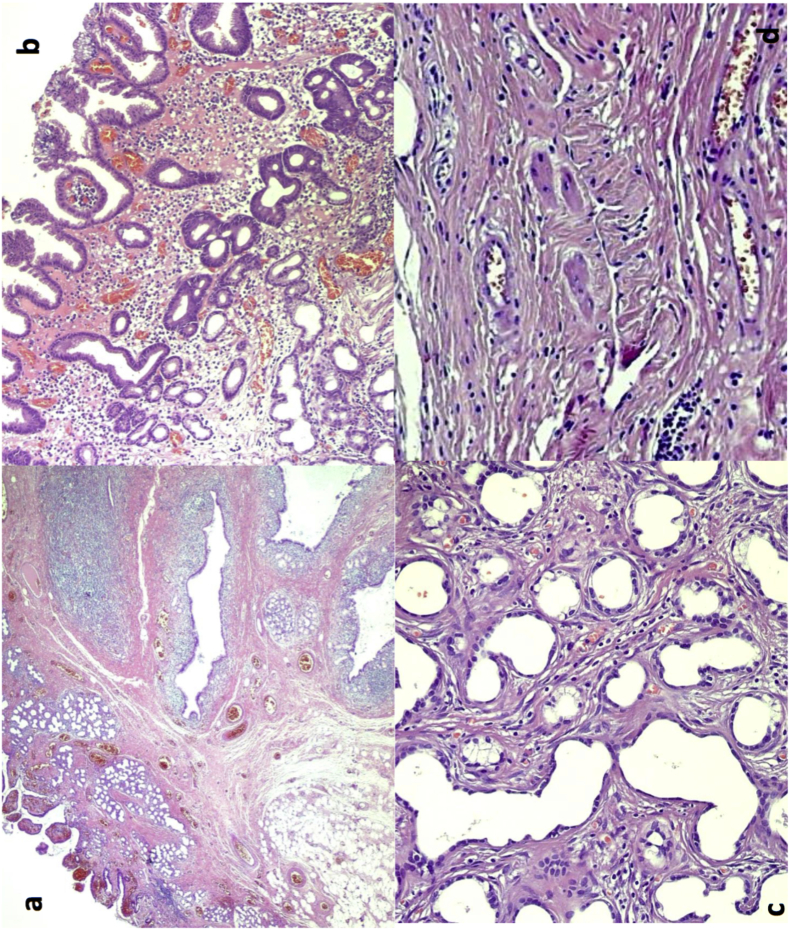
Fig. 4.
Histologic features of Brunner's gland hamartoma. a) At low power, Brunner gland hamartoma is composed of lobules of Brunner's glands, extending into the mucosa and admixed with dilated ducts, adipose tissue and lymphoid tissue. b) Gastric foveolar metaplasia of the surface epithelium can be observed. c) Dilated Brunner's glands with some structural irregularity and lined by cuboidal cells with scant cytoplasm are present in this sclerotic glandular focus, which, however, retained a lobular architecture. Note the bland cytology with absent mitotic activity. d) A cystically dilated duct surrounded by overt lymphoid tissue is shown.
2. Conclusion
Brunner glands are acinotubular glands located in the duodenal bulb, and the proximal and distal duodenum with a decreasing prevalence [3] (rarely Brunner glands are located into the jejunum [4]) and an anti-acid function related to the secretion of a specific mucus coating the duodenal epithelium. Described for the first time by Cruveilhier and Savioli, in 1835 [5] and 1876 [6] respectively, between 150 and 200 cases of this type of benign tumour counts have been documented in English literature [7], [8] and approximately 5% of these were duodenal masses [1].
In 1934, Feyter (cited by Goldman [9]) a classification of the abnormal proliferation of Brunner's glands into three different subsets has been proposed: type 1, diffuse nodular hyperplasia, type 2 circumscribed nodular hyperplasia, and type 3 glandular adenoma [10], [11]. Moreover, this classification of hyperplasia of Brunner glands seems to be potentially designed as hamartoma, principally due to its histological features such as lack of surfacing capsule, network combination of acini, smooth muscle, adipose tissue, Paneth cells and mucosal glands, and the lack of cellular atypia. Pathogenesis of BGH still needs to be completely explored but the three most quoted hypotheses that have been proposed are the hyperchlorhydria hypothesis, the pancreatitis-related hypothesis and the inflammation-based hypothesis.
Zangara et al. [11] suggested that the natural anti-acid function (and subsequent hyperchlorhydria) could promote hyperplasia of Brunner's glands. This theory has been also reported in 1985, with a relationship between chronic gastric erosions (due to hyperchlorhydria) and the presence of BGH [12] although, on the other hand, some studies do not endorse this theory [13].
In 2008 [10], in a study enrolling more than 19000 patients, Abbass R showed an association for 5 patients out of 7 between BGH and H. Pylori positivity, and the researchers considered the infection to be a potential trigger for BHG onset or growth. The chronic pancreatitis hypothesis on BGH pathogenesis has its roots in large case series evidence illustrated by Stole et al. [15]. That research speculates on a possible adaptive reaction resulting in Brunner's glands hyperplasia due to the exocrine inefficiency of the pancreas caused by chronic pancreatitis.
Finally, inflammation is indicated as an additional likely trigger for BHG. Inflammatory histological features, particularly the always-present lymphocytic infiltrate, suggests that hamartoma can be the result of a hyperplastic reaction to any inflammation stimuli. Most patients affected by Brunner gland hamartoma are clinically silent, leading to a possible classification that differentiates between non-symptomatic and symptomatic cases. These symptoms are anyway non-specific manifestations, including obstructive symptoms such as nausea or imprecise abdominal pain, or upper gastrointestinal bleeding symptoms resulting in melena.
The eventual bleeding is related to the lesion localization along the duodenum and BGH size. Lesions located in the second or third portion of the duodenum have a major propensity for bleeding, probably due to the major physical stress that they have to cope with or the weak vascular provision. Bleeding BGHs have usually a diameter of between 2.1 and 2.8 cm whereas BGH sized 1.6 cm are asymptomatic most of the time [3].
The different diagnoses of a duodenal mass include many entities such as adenomatous polyp, leiomyoma/leiomyosarcoma, gastro intestinal stromal tumour, ampullary neoplasm or pancreatic pseudocyst/tumour, and all require upper endoscopy or imaging studies. Upper endoscopy is the first-line study that is usually performed with this type of condition. Through direct visualization, the position of the lesion can be exactly located, which allows a hypothetical histological assessment. Unfortunately, due to the submucosal nature of BGH [14], biopsies are frequently negative or non-diagnostic, providing just a small advantage but ruling out malignancy. Our case report confirms this theory. It is also important to note that the high frequency of non-diagnostic endoscopic biopsies is the main reason that the real incidence of BGH is hard to exactly define. Endoscopic ultrasound (EUS) is specifically useful in evaluating the extension, the morphological characteristics and the vascularity of the BGH. In 2002, Hizawa K. and colleagues studied the endosonographic features of BGH [16] in depth, demonstrating that there was a “heterogeneous solid or cystic mass within the submucosal layer” and highlighting the resection as the only possible tool to have a histological definition. To our knowledge, EUS plays the pivotal role in assessing the layer of origin, typically the submucosal layer. The CT-scan is a second-line study that permits the physician to analyse the extent of BGH and its relationship with adjacent structures. In the literature, few reports or case reports correlate the CT-scan enhancement distribution to the nature of the lesion. In 2006, Patel et al. [17] recognized, in a series of 26 patients, a correlation between contrast-enhancement homogenous scattering and solid gland proliferation whereas, conversely, heterogeneous enhancement was suggested to be associated with fat and smooth muscle proliferation and to cystic dilatation of Brunner's gland. More recently, in 2010 Hur S. and colleagues [18] described essential CT-scan features of BGH for differential diagnoses with other entities, above all GIST, neuroendocrine tumours or ectopic pancreas. Discrimination between such diseases is essential for a more/less aggressive surgical/endoscopic approach. They concluded that CT-scan images of low-attenuating, contrast-enhanced duodenal masses with inner cystic components are strongly evocative for BGH, improving the diagnostic accuracy. Use of contrast-enhancement is mandatory due to the BGH iso-attenuation to the pancreatic parenchyma in a non-enhanced CT-scan.
Surgical resection is actually considered the best treatment to perform in the case of symptomatic BGH, and accurate pre-operative surgical planning must precede it. The morphological characteristics of BGH, along with its size are the most significant properties to discern between endoscopic or radical surgical resection (open or laparoscopic).
As we mentioned before, upper endoscopic biopsies usually fail, so only endoscopic imaging studies can drive decision such as the expertise of endoscopic team and health general condition of the patient. The Yamada classification [19] categorizes polyps into four different groups. Type I polyps are elevated with unspecified edges. Type II polyps are more protruding than type I, with a distinctive margin and no notch. Type III polyps are elevated lesions that are starting to have develop a distinguishable broad base. Finally Type IV polyps are typically peduncolated [20].
Endoscopic resection is indicated for the removal of stalk polyps (Type IV). Regarding sizing, it has been suggested that in the case of duodenal lesions measuring less than 1 cm (even with the involvement of the submucosal layer) or a maximum of 2 cm but confined to the mucosa, the endoscopic polypectomy approach still remains the best treatment [21]. It is suggested that lesions larger than 2 cm (+/− involvement of the submucosal) should be treated by surgical resection in order to guarantee complete excision. Transduodenal polypectomy is the suggested treatment for duodenal lesions occupying the first part of the duodenum (D1) whereas more aggressive treatment, even going as far as pancreaticoduodenectomy [22], [23] has been reported in the case of D2 or D3 BGH localization. There is no evidence of pancreaticoduodenectomy being the best treatment for BGH especially since 99% of the lesions is benign and no report has described effectively malignant transformation.
The majority of BGH (99%) is benign but some papers have reported its possible hypothetical evolution into dysplasia, which raises the question of a subsequent malignant transformation. To our knowledge, no report has described effectively such growth, suggesting conservative management.
Histologically BGH has been described as hyperplastic proliferation of Brunner's glands involving inconstant quantity of fibrous and adipose tissue, smooth muscle, ducts and inflammatory infiltration (lymphocytes) and, in their report, in 2014, Akaki et al. also suggested an immunohistochemical connection between BGH proliferative activity and gastric foveolar metaplasia [24].
In order to investigate the role of mucins for morphologic modifications that occur in BGH, mucin profile (MUC1, MUC2, MUC5 and MUC6) has been explored [25]. The loss of MUC5 and MUC6 seems to be linked both to sclerotic glands formation and mucosal and submucosal destroying. One last point to review is the potential malignancy of BGH. Several reports highlight the cancerous changes arising from BGH with different evolutions [26], [27], [28] even if Kim et al. [25] didn't support this hypothesis (based on p53 and Ki-67 stainings). Based on literature review on this focus BGH biological behaviour (cancerous/pre-cancerous lesion) still remains ambiguous [29].
Limitation to our preoperatory workup include not testing the patient for H. Pylori; the strength of our workup was performing a preoperative thorough EUS assesment that led us to a dubitative management and to question the scheduled procedure.
In our patient, we could not assess the lesion exact origin and nature and a pancreaticoduodenectomy was scheduled. Accurate intraoperative findings allowed us to palpatory appreciate the mass within the duodenal lumen even if it is confined to the submucosal. After duodenotomy a smooth dissection of the lesion's stalk was performed assessing the peduncolated nature of the mass (resulted broad-base during the endoscopy). This finding prompted us to use conservative management, avoiding the risk of over-treatment and saving the patient from an unnecessary Whipple's procedure.
The primary take-home lesson from this case report is to question the extent of the scheduled procedure, when the preoperative imaging is unclear (or even if preoperative imaging suggest a wide extension), assessing intraoperatively the extent of the lesion.
For similar intraoperative findings we recommend the conservative management.
In conclusion, BGH is a challenging diagnosis that needs multidisciplinary expertise (endoscopic, radiological and surgical) to be reached but even if extremely rare, might be considered in case of duodenal submucosal lesions. The present case report follows the SCARE guidelines [30].
3. Additional information
The patient gave his informed consent for the publication of case report and images, no conflict of interest declared. No sources of founding declared, no ethical committee approval required.
Conflicts of interest
All the Authors have no conflicts of interest.
Sources of funding
We did not have any sources of funding.
Ethical approval
The study did not need an ethical approval.
Author contribution
Peloso; Dominioni; Zonta and Bugada treated the patient and collected data. Vanoli and Viglio did the histopathology; Cobianchi and Calabrese wrote the manuscript; Dionigi and Maestri did the review of literature.
Registration of research studies
No registration of research study was needed.
Guarantor
The Guarantor is the one or more people who accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Lorenzo Cobianchi is the guarantor and has the full responsibility for the present work.
Competing interest section
All the authors declare that they have no competing interest.
Consent
We had the consent to publish the case from the patient.
Contributor Information
Andrea Peloso, Email: andreapeloso@hotmail.it.
Jacopo Viganò, Email: j.vigano@smatteo.pv.it.
Alessandro Vanoli, Email: a.vanoli@smatteo.pv.it.
Tommaso Dominioni, Email: t.dominioni@smatteo.pv.it.
Sandro Zonta, Email: s.zonta@smatteo.pv.it.
Dario Bugada, Email: dariobugada@gmail.com.
Carlo Maria Bianchi, Email: cm.bianchi@smatteo.pv.it.
Francesco Calabrese, Email: Francesco.calabrese01@ateneopavia.it.
Ilaria Benzoni, Email: ilaria.benzoni@hotmail.it.
Marcello Maestri, Email: mmaestri@smatteo.pv.it.
Paolo Dionigi, Email: p.dionigi@smatteo.pv.it.
Lorenzo Cobianchi, Email: lorenzo.cobianchi@unipv.it, l.cobianchi@smatteo.pv.it.
References
- 1.Botsford T.W., Crowe P., Croker D.W. Tumours of the small intestine. A review of experience with 115 cases including a report of a rare case of malignant hemangio-endothelioma. Am. J. Surg. 1962;103:358–365. doi: 10.1016/0002-9610(62)90226-x. [DOI] [PubMed] [Google Scholar]
- 2.Jansen J.M., Stuifberg W.N., Van Milligen A.V.V. Endoscopic resection of a large Brunner's gland adenoma. Neth J. Med. 2002;60(6):253–255. [PubMed] [Google Scholar]
- 3.Levine J.A., Burgart L.J., Batts K.P., Wang K.K. Brunner's gland hamartomas: clinical presentation and pathological features of 27 cases. Am. J. Gastroenterol. 1995;90(2):290–294. [PubMed] [Google Scholar]
- 4.Gao Y.P., Zhu J.S., Zheng W.J. Brunner's gland adenoma of duodenum: a case report and literature review. World J. Gastroenterol. 2004;10(17):2616–2617. doi: 10.3748/wjg.v10.i17.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruveilhier J. Harper and Bros; New York, NY: 1844. Anatomy of the Human Body. [Google Scholar]
- 6.Savioli G. Contribuzione allo studio degli adenomi. L'Osservatore Gazetta Medica Torino. 1876;12:481. [Google Scholar]
- 7.Chattopadhyay P., Kundu A.K., Bhattacharyya S., Bandyopadway A. Diffuse nodular hyperplasia of Brunner gland presenting as upper gastrointestinal haemorrhage. Singap. Med. J. 2008;49(1):81–83. [PubMed] [Google Scholar]
- 8.Rajeev S., Veena G., Nisha S., Nikita C., Sanjay K., Shivani M. Brunner gland hamartoma masquerading as malignancy: a rare case report. MEJoDD – Middle East J. Dig. Dis. 2014;6:237–239. [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman L. Hamartomatous polyp of Brunner's gland. Gastroenterology. 1963;44:57–62. [PubMed] [Google Scholar]
- 10.Abbass R., Al-Kawas F.H. Brunner gland hamartoma. Gastroenterol. Hepatol. 2008;4:473–475. [PMC free article] [PubMed] [Google Scholar]
- 11.Zangara J., Kushner H., Drachenberg C., daly B., Flowers J., Fantry G. Iron deficiency anemia due to a Brunner gland hamartoma. J. Clin. Gastroenterol. 1998;27(4):353–356. doi: 10.1097/00004836-199812000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Franzin G., Musola R., Ghidini O., Manfrini C., Fratton A. Nodular hyperplasia of Brunner's glands. Gastrointest. Endosc. 1985;31(6):374–378. doi: 10.1016/s0016-5107(85)72251-1. [DOI] [PubMed] [Google Scholar]
- 13.Spelberg M.A., Vucelic B. A case of Brunner gland hyperplasia with diarrhea responsive to cimetidine. Am. J. Gastroenterol. 1980;73(6):519–522. [PubMed] [Google Scholar]
- 14.Walden D.T., Marcon N.E. Endoscopic injection and polypectomy for bleeding Brunner gland hamartoma: case report and expanded literature review. Endoscopy. 1998;47:403–407. doi: 10.1016/s0016-5107(98)70228-7. [DOI] [PubMed] [Google Scholar]
- 15.Stolte M., Schwabe H., Prestele H. Relationship between diseases of the pancreas and hyperplasia of Brunner glands. Virchows Arch. A Pathol. Anat. Histol. 1981;394(1–2):75–87. doi: 10.1007/BF00431666. [DOI] [PubMed] [Google Scholar]
- 16.Hizawa K., Iwai K., Esaki M., Suekane H., Inuzuka S., Matsumoto T., Yao T., Iida M. Endosonographic features of Brunner's gland hamartomas which were subsequently resected endoscopically. Endoscopy. 2002;34(12):956–958. doi: 10.1055/s-2002-35849. [DOI] [PubMed] [Google Scholar]
- 17.Patel N.D., Levy A.D., Mehrotra A.K., Sobin L.H. Brunner's gland hyperplasia and hamartoma: imaging features with clinicopathological correlation. AJR Am. J. Roentgenol. 2006;187(3):715–722. doi: 10.2214/AJR.05.0564. [DOI] [PubMed] [Google Scholar]
- 18.Hur S., Han J.K., Kim M.A., Bae J.M., Choi B.I. Brunner's gland hamartoma: computed tomographic findings with histopathological correlation in 9 cases. J. Comput. Assist. Tomogr. 2010;34(4):543–547. doi: 10.1097/RCT.0b013e3181d472dc. [DOI] [PubMed] [Google Scholar]
- 19.Yamada T., Ichikawa H. X-ray diagnosis of elevated lesion of the stomach. Radiology. 1974;110(1):79–83. doi: 10.1148/110.1.79. [DOI] [PubMed] [Google Scholar]
- 20.Morais D.J., Yamanaka A., Zeitune J.M., Andreollo N.A. Gastric polyps: a retrospective analysis of 26000 digestive endoscopies. Arq. Gastroenterol. 2007;44(1):14–17. doi: 10.1590/s0004-28032007000100004. [DOI] [PubMed] [Google Scholar]
- 21.Perez A., Saltzman J.R., Carr-Locke D.L. Benign nonampullary duodenal neoplasm. J. Gastrointest. Surg. 2003;7(4):563–641. doi: 10.1016/S1091-255X(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 22.Lee W., Yang H., Lee Y., Jung S., Choi G., Go H. Brunner's gland hyperplasia: treatment of severe diffuse nodular hyperplasia mimicking a malignancy of the pancreatico-duodenal area. J. Korean Med. Sci. 2008;23(3):540–543. doi: 10.3346/jkms.2008.23.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iusco D., Roncoroni L., Violi V., Donadei E., Sarli L. Brunner's gland hamartomas: “over treatment” of a voluminous mass simulating a malignancy of the pancreatico-duodenal area. JOP. 2005;6(4):348–353. [PubMed] [Google Scholar]
- 24.Akaki M., Taniguchi S., Hatakeyama K., Kushima R., Kataoka H. Duodenal mucosal damage is associated with proliferative activity of Brunner's gland hamartoma: a case report. BMC Gastroenterol. 2014;14(14):14. doi: 10.1186/1471-230X-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim K., Jang S.J., Song H.J., Yu E. Clinicopathologic characteristics and mucin expression in Brunner's gland proliferating lesions. Dig. Dis. Sci. 2013;58(1):194–201. doi: 10.1007/s10620-012-2320-3. [DOI] [PubMed] [Google Scholar]
- 26.Itsuno M., Makiyama K., Omagari K., Tanaka T., Hara K., Tsuda N., Ajioka Y., Watanabe H. Carcinoma of duodenal bulb arising from the Brunner's gland. Gastroenterol. Jpn. 1993;28(1):118–125. doi: 10.1007/BF02775012. [DOI] [PubMed] [Google Scholar]
- 27.Ohta Y., Saitoh K., Akai T., Uesato M., Ochiai T., Matsubara H. Early primary duodenal carcinoma arising from Brunner's glands synchronously occurring with sigmoid colon carcinoma: report of a case. Surg. Today. 2008;38(8):756–760. doi: 10.1007/s00595-007-3707-1. [DOI] [PubMed] [Google Scholar]
- 28.Akino K., Kondo Y., Ueno A., Yamazaki K., Hosokawa M., Shimoji H. Carcinoma of duodenum arising from Brunner's gland. J. Gastroenterol. 2002;37(4):293–296. doi: 10.1007/s005350200038. [DOI] [PubMed] [Google Scholar]
- 29.Brookes M.J., Manjunatha S., Ca Allen, Cox M. Malignant potential in Brunner's gland hamartoma. Postgrad. Med. J. 2003;79(933):416–417. doi: 10.1136/pmj.79.933.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S. Orgill DP and the SCARE Group. The SCARE Statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]