Abstract
Background
Gastrointestinal anastomosis remains associated with a considerable burden of morbidity and, in some cases, mortality. Functional end-to-end anastomosis, whilst extremely efficient, is vulnerable to increased intestinal pressure in the immediate postoperative period, which may predispose to development of anastomotic leakage or bleeding. Therefore, there is a requirement for new techniques that facilitate safe and efficacious anastomotic procedures.
Materials and methods
This study examined the clinical application of functional end-to-end anastomosis with a stapler that automatically applies a bioabsorbable polyglycolic acid sheet (Endo GIA™ Reinforced Reload with Tri-Staple™ Technology). A porcine model was used to examine functional end-to-end anastomosis with and without application of a bioabsorbable polyglycolic acid sheet. As the crotch of the anastomosis is considered the weakest point, a probe was used to test the integrity of these anastomoses. Furthermore, we performed functional end-to-end anastomosis using the Endo GIA™ Reinforced stapler in a clinical series of 20 patients undergoing gastrointestinal tract resection. In all cases, functional end-to-end anastomosis was performed without suture reinforcement.
Results
Small intestine anastomoses in the animal study exhibited no weakness at the crotch of the anastomosis, as tested with a probe, suggesting an increased resiliency to conventional complications of functional end-to-end anastomosis. In the clinical population, no postoperative complications were noted. No adhesive intestinal obstruction was noted.
Conclusion
Sutureless functional end-to-end anastomosis using the Endo GIA™ Reinforced appears to be safe, efficacious, and straightforward. Reinforcement of the crotch site with a bioabsorbable polyglycolic acid sheet appears to mitigate conventional problems with crotch-site vulnerability.
Keywords: Functional end-to-end anastomosis, Sutureless anastomosis, Polyglycolic acid felt, Linear stapler
Highlights
-
•
Functional end-to-end anastomosis is safe with the Endo GIA™ Reinforced stapler.
-
•
Conventional functional end-to-end anastomosis requires suture reinforcement.
-
•
The Endo GIA™ Reinforced allows sutureless anastomosis by creating staple planes.
1. Introduction
After gastrointestinal anastomosis, postoperative complications, such as anastomotic leakage, bleeding, and stricture, may occur [1], [2], [3], [4], [5], [6], [7], [8], [9]. Functional end-to-end anastomosis (FEEA) is a type of gastrointestinal anastomosis using an automatic suture device that was first reported in 1968 [10]. Compared to hand-sewn anastomoses, FEEA reduces the time for anastomosis, increases the anastomotic diameter, and permits anastomosis of ducts with different diameters, among other advantages. However, FEEA is vulnerable to increased intestinal pressure due to mucosal defects in the early postoperative period, as the staples partially intersect each other when the primary surgical incision (for insertion of the automatic suture device) is closed. Furthermore, in the crotch of side-to-side anastomoses, small gaps are created between staples, which are characteristically arrayed in parallel by an automatic suture device. Sutures are needed to reinforce such areas, given their propensity to result in leakage.
The Endo GIA™ Reinforced Reload with Tri-Staple™ Technology (GIA Reinforced™, Medtronic, Minneapolis, MN) is an automatic suture device using integrated bioabsorbable polyglycolic acid (PGA) felt, which is equipped with a cartridge on which Neoveil™ (Gunze, Kyoto), a bioabsorbable suture reinforcement sheet, is attached. This device was produced to reinforce staple lines on fragile tissue, prevent anastomotic leakage due to tissue laceration or increased tissue pressure, improve hemostatic effects, and provide 2-dimensional reinforcement using the bioabsorbable sheet.
In this study, the safety of sutureless FEEA for gastrointestinal anastomosis performed with the GIA Reinforced™ was assessed.
2. Material and methods
2.1. Patients
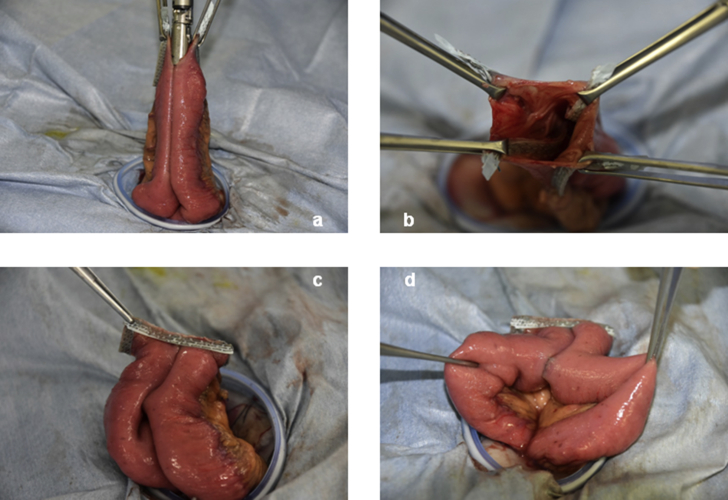
This study included 20 patients who underwent gastrointestinal tract resection followed by FEEA at the Department of Surgery, School of Medicine, Kitasato University during or after June 2016 (Table 1). FEEA was performed using the closed method. After treatment of the mesentery, the oral and anal sides of the intestine were sutured and resected to be aligned in parallel to the mesentery using the GIA Reinforced™ equipped with a purple 60-mm cartridge. A small incision was made in the occluded intestine contralateral to the mesentery with an electric scalpel. From the small incision, the GIA Reinforced™ was inserted into the intestine, and was then oriented to the planned anastomosis line contralateral to the mesentery. Whilst avoiding adipose and other surrounding tissue, the automatic suture device was used to make a side-to-side anastomosis. Subsequently, the GIA Reinforced™ was moved to keep anastomosis lines from overlapping, and the intestine was sutured and closed. Neither the suture-closured site nor the crotch of the side-to-side anastomosis was reinforced with suturing (Fig. 1).
Table 1.
Patient characteristics. ASA: American Society of Anesthesiologists.
| n = 20 | |
|---|---|
| Mean age (years±SD) | 65.3 ± 12.1 |
| Gender | |
| Male/Female | 7/13 |
| BMI | 21.5 ± 2.16 |
| ASA indicates | |
| I/II/III | 7/12/1 |
| Procedure | |
| Partial resection of small intestine | 2 |
| Partial resection of colon | 4 |
| Right hemicolectomy | 9 |
| Stoma close | 5 |
| Median follow-up times (days, range) | 130.9 (40–233) |
Fig. 1.
Sutureless functional end-to-end anastomosis with the Endo GIA™ Reinforced Reload with Tri-Staple™ Technology. a: Insertion of the linear stapler, b: After side-to-side anastomosis, c: After closure of the entry hole, d: Crotch.
All cases were given full explanation, and consents were received in accordance with the ethics committee based on the Declaration of Helsinki.
2.2. Experiment
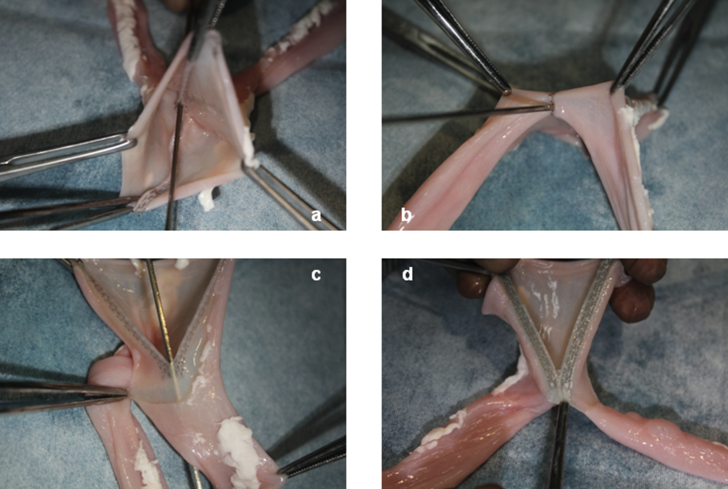
On the small intestine of crossbred three pigs originating from Duroc, Large Yorkshire, Yorkshire, and Landrace breeds, FEEA was performed with the GIA Reinforced™ and Endo GIA™ Tri-Staple™ Technology (Endo GIA™, Medtronic, Minneapolis, MN). After anastomosis, we attempted to insert a probe into the crotch of the side-to-side anastomosis (Fig. 2).
Fig. 2.
Experiment on the small intestine of a pig. a–c: When the Endo GIA™ Tri-Staple™ Technology was used, a probe could be inserted into the crotch. d: When the Endo GIA™ Reinforced Reload with Tri-Staple™ Technology was used, PGA felt turned staple lines into a plane.
2.3. Analysis
Postoperative complications were classified according to the Clavien-Dindo classification.
3. Results
3.1. Experiment using small intestine of pig
When the Endo GIA™ was used, a probe could be inserted into the crotch of the side-to-side anastomosis with staples aligned in parallel. Conversely, when the GIA Reinforced™ was used, the probe could not be inserted, as the gaps between staples were closed 2-dimensionally with bioabsorbable suture reinforcement sheets made of PGA (Fig. 2). Anastomosis and insertion of probe were performed three times each, with the same results obtained for those three times.
3.2. Operative and postoperative results
Anastomoses were created between 2 parts of the small intestine in 7 patients, between the small intestine and colon in 9 patients, and between 2 parts of the colon in 4 patients. The median postoperative follow-up period was 130.9 days (range: 40–233). Given results from the animal study, in which the probe could not be inserted into the crotch of the side-to-side anastomosis, suture reinforcement was not performed. No complications, such as anastomotic leakage, bleeding from the anastomotic site, or adhesive intestinal obstruction, were observed in follow-up periods (Table 2).
Table 2.
Operation results. Morbidity: Clavian-Dindo classification.
| n = 20 | |
|---|---|
| Operation time (min) | 134.7 ± 41.3 |
| Blood loss (ml) | 7.75 ± 15.9 |
| Intestine using anastomosis | |
| Colon - colon | 4 |
| Ileum - colon | 9 |
| Ileum - ileum | 5 |
| Jejunum - jejunum | 2 |
| Morbidity rates (%) | 0 |
4. Discussion
In gastrointestinal anastomoses, anastomotic leakage and bleeding from the anastomotic site are life-threatening critical complications, with a reported incidence of approximately 10% [1], [2], [3], [4], [5], [6], [7], [8], [9]. Since the use of an automatic suture device was reported in 1968, various automatic suture devices have been developed and evaluated to reduce the incidence of complications [10], [11], [12], [13], [14], [15].
Although a number of articles describe reinforcement materials for staple lines applied with automatic suture devices that are designed to reduce anastomotic complications, there are sporadic studies examining efficacious reinforcement materials for pneumonectomy and gastrointestinal anastomosis [16], [17], [18], [19], [20], [21], [22], [23]. The GIA Reinforced™ is an automatic suture device that applies a sheet of Neoveil™, a tissue reinforcement PGA felt for automatic suture devices, over 3 staple lines. The PGA felt is as thin as 0.15 mm, and is completely absorbed in 15 weeks. However, the tensile strength of the PGA is maintained at approximately 90% at 1 week, 30% at 2 weeks, and 4% at 3 weeks. Thus, the felt appears to be associated with no risk of causing tissue damage during intestinal anastomosis, and is sufficiently resistant to pressure. In addition, the felt reduces anastomotic complications by reducing blood flow disorders by preventing anastomotic bleeding. Using suture reinforcement sheets effectively converts staple lines into a plane, rather than numerous lines of separate points. In this study, we performed FEEA with the GIA Reinforced™ for gastrointestinal anastomosis, and analyzed the safety of a new FEEA technique without applying suture reinforcement to the crotch of the side-to-side anastomosis and other anastomotic sites.
In the 20 patients receiving anastomosis using this technique, no complications, such as anastomotic leakage or adhesive intestinal obstruction, were observed, suggesting that the technique is safe. There was no case in which overlapping sheets of PGA felt caused a particular technical difficulty.
It is possible that the Neoveil™ is sufficient to mitigate the need for suture reinforcement at weak points of FEEA when using the GIA Reinforced™. Because the sheets turn staple lines into a plane, and close gaps in the crotch of the side-to-side anastomosis, leakage of gastrointestinal contents can be prevented without suture reinforcement. Moreover, adhesion due to Neoveil™ has not been reported. Therefore, we conclude that this technique, which is not requisite of sutures, is extremely straightforward to implement clinically.
Since this study is a retrospective study in a single center, and the number of cases is small, there is a limit to describe efficacy and safety. However, sutureless FEEA is a simple and useful procedure, and is expected that prospective randomized control trial will be conducted to demonstrate effectiveness and safety.
Our study has demonstrated that sutureless FEEA with the GIA Reinforced™ is safe and efficacious. The appropriate use of this device not only reduces complications of gastrointestinal anastomosis, but also simplifies surgical techniques, thereby improving patient and surgical outcomes.
Ethical approval
None required.
Sources of funding
None received.
Author contribution
Masanori Naito: designed the study, performed procedures, collected data, composed the manuscript.
Masahiko Watanabe: reviewed the manuscript.
Hirohisa Miura, Takatoshi Nakamura, Takeo Sato, Takahiro Yamanashi and Atsuko Tsutsui: performed procedures.
Conflicts of interest
None to declare.
Guarantor
Masanori Naito, First Author.
Masahiko Watanabe, Senior Author.
Research registration unique identifying number (UIN)
N/A.
Trial registry number – ISRCTN
N/A.
References
- 1.Haverkamp L., Sluis P.C., Verhage R.J. Endo-to endocervical esophagogastric anastomoses are associated with a higher number of strictures compared with endo-to-side anastomoses. J. Gastrointest. Surg. 2013;17:872–876. doi: 10.1007/s11605-013-2159-8. [DOI] [PubMed] [Google Scholar]
- 2.He X., Chen Z., Huang J. Staoled side-to-side anastomosis might be better than handsewn end-to-end anastomosis in ileocolic resection for Crohn's disease: a meta-analysis. Dig. Dis. Sci. 2014;59:1544–1551. doi: 10.1007/s10620-014-3039-0. [DOI] [PubMed] [Google Scholar]
- 3.Kyzer S., Gordon P.H. The stapled functional end-to-end anastomosis following colonic resection. Int. J. Color. Dis. 1992;7:125–131. doi: 10.1007/BF00360351. [DOI] [PubMed] [Google Scholar]
- 4.Kracht M., Hay J.M., Fagniez P.L. Ileocolic anastomosis after right hemicolectomy for carcinoma: stapled or hand-sewn? A prospective, multicenter, randomized trial. Int. J. Color. Dis. 1993;8:29–33. doi: 10.1007/BF00341273. [DOI] [PubMed] [Google Scholar]
- 5.Platell C., Barwood N., Dorfmann G. The incidence of anastmotic leaks in patients undergoing colorectal surgery. Colorectal. Dis. 2007;9:71–79. doi: 10.1111/j.1463-1318.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 6.Byrn J.C., Schlager A., Divino C.M. The management of 38 anastomotic leaks after 1684 intestinal resection. Dis. Colon. Rectum. 2006;49:1346–1353. doi: 10.1007/s10350-006-0653-8. [DOI] [PubMed] [Google Scholar]
- 7.Hyman N., Manchester T.L., Osler T. Anastomotic leaks after intestinal anastomosis: it's later than you think. Ann. Surg. 2007;245:254–258. doi: 10.1097/01.sla.0000225083.27182.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin M., Akhtar S., Sasapu K. Clinical and subclinical leaks after low colorectal anastomosis: a clinical and radiologic study. Dis. Colon. Rectum. 2006;49:1611–1619. doi: 10.1007/s10350-006-0663-6. [DOI] [PubMed] [Google Scholar]
- 9.Phitayakorn R., Delaney C.P., Reymolds H.L. Standardized algorithms for management of anastomotic leaks and related abdominal and pelvic abscesses after colorectal surgery. World J. Surg. 2008;14:1238–1241. doi: 10.1007/s00268-008-9468-1. [DOI] [PubMed] [Google Scholar]
- 10.Felicien M., Steichen M.D. The use of staplers in anatomical side-to-side and functional end-to-end enteroanastomoses. Surgery. 1968;64:948–953. [PubMed] [Google Scholar]
- 11.Adam L. 1908. Sebeszeti Osztalyon Vegzett Gyomorresectiokrol, Magy Sebeszet Munk; p. 632. [Google Scholar]
- 12.Stechen F.M. The use of staplers in anastomical side-to-side and functional end-to-end enteroanastomoses. Surgery. 1968;64:948–953. [PubMed] [Google Scholar]
- 13.Simpler S.C., Erzinger J.M., Smith S.C. Comparison of laparoscopic linear staplers in clinical practice. Surg. Obes. Relat. Dis. 2007;3:446–451. doi: 10.1016/j.soard.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Baker R.S., Foote J., Kemmeter P. The science of stapling and leaks. Obes. Surg. 2004;14:1290–1298. doi: 10.1381/0960892042583888. [DOI] [PubMed] [Google Scholar]
- 15.Mery C.M., Shafi B.M., Binyamin G. Profiling surgical staplers: effect of staple height, buttress, and overlap on staple line failure. Surg. Obes. Relat. Dis. 2008;4:416–422. doi: 10.1016/j.soard.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Morris E.F., Jr., Keenan E.B., Paul P.A. Safety and efficacy of the use of bioabsorbable seamguard in colorectal surgery at the Texas endosurgery institute. Surg. Laparosc. Endosc. Percutan. Tech. 2005;15:9–13. doi: 10.1097/01/sle0000154019.83584.2e. [DOI] [PubMed] [Google Scholar]
- 17.Robertson L.D., Netherland D.E., Dhillon R. Air leaks after surgical stapling in lung resection: a comparison between stapling alone and stapling with staple line reinforcement materials in canine model. J. Thorac. Cardiovasc. Surg. 1998;116:353–354. doi: 10.1016/s0022-5223(98)70138-2. [DOI] [PubMed] [Google Scholar]
- 18.Vaughn C.C., Vaughn P.L., Vaughn C.C., III Tissue response to biomaterials used for staple-line reinforcement in lung resection. A comparison between expanded polytetrafluoroethylene and bovine pericardium. Eur. J. Cardiothorac. Surg. 1998;13:159–165. doi: 10.1016/s1010-7940(98)00012-8. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura M., Kase K., Sawafuji M. Staple-line reinforcement with a new type of polyglycolic acid felt. Surg. Laprosc. Endosc. Percutan. Tech. 2001;11:43–46. [PubMed] [Google Scholar]
- 20.Saito Y., Omiya H., Shomura Y. A new bioabsorbable sleeve for staple-line reinforcement: report of a clinical experience. Surg. Today. 2002;32:297–299. doi: 10.1007/s005950200041. [DOI] [PubMed] [Google Scholar]
- 21.De la Torre R.A., Scott J.S. Laparoscopic Roux-en-Y gastric bypass: a totally intra-abdominal approach. Obes. Surg. 1999;9:492–498. doi: 10.1381/096089299765552800. [DOI] [PubMed] [Google Scholar]
- 22.Naito M., Yamanashi T., Nakamura T. Safety and efficacy of a novel linear staple device with bioabsorbable polyglicolic acid felt in laparoscopic colorectal surgery. Asian J. Endosc. Surg. 2017;10:35–39. doi: 10.1111/ases.12314. [DOI] [PubMed] [Google Scholar]
- 23.Kimura M., Terashita Y. Use of bioabsorbable staple reinforcement material in side-to-side anastomoses: suture line reinforcement of the weak point of anastomosis. Ann. Med. Surg. 2016;6:50–55. doi: 10.1016/j.amsu.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]