Abstract
Trichomes, or leaf hairs, are epidermal extensions that take a variety of forms and perform many functions in plants, including herbivore defense. In this study, I document genetically determined variation, within-generation plasticity, and a direct role of trichomes in herbivore defense for Mimulus guttatus. After establishing the relationship between trichomes and herbivory, I test for transgenerational effects of wounding on trichome density and herbivore resistance. Patterns of inter-annual variation in herbivore density and the high cost of plant defense makes plant-herbivore interactions a system in which transgenerational phenotypic plasticity (TPP) is apt to evolve. Here, I demonstrate that parental damage alters offspring trichome density and herbivore resistance in nature. Moreover, this response varies between populations. This is among the first studies to demonstrate that TPP contributes to variation in nature, and also suggests that selection can modify TPP in response to local conditions.
Keywords: Trichomes, Phenotypic Plasticity, Transgenerational Plasticity, Mimulus, Herbivory
Introduction
By exposing plants to a variety of environments, Clausen, Keck, and Heisey developed the common garden experimental design to distinguish between genetically determined, environmentally dictated, and genetic × environmental effects on growth and fitness (Clausen et al., 1948). This ability for an individual to react to an environmental stimulus and alter its growth, development, or state is termed “phenotypic plasticity” (Bradshaw, 1965, West-Eberhard, 1989, Via et al., 1995). Phenotypic plasticity comes in many forms; often classified as being either active or passive, adaptive or non-adaptive, and continuous or discrete (West-Eberhard, 2003). When an environmental cue is a reliable predictor of future ecological conditions, and a plastic response increases fitness in that condition, the given plasticity is advantageous and expected to evolve (West-Eberhard, 1989, Agrawal, 2001a, Herman et al., 2013, Kuijper & Hoyle, 2015). In this way, the current environment acts as a source of information from which an individual can use to modify future development (English et al., 2015).
Following the hypotheses regarding within-generation plasticity, if current environmental conditions are a good predictor of the conditions experienced by the next generation (positive inter-generational autocorrelation), then the transmission of altered developmental trajectories between generations (transgenerational phenotypic plasticity, TPP, Figure 1c and 1d) should also be adaptive (Herman & Sultan, 2011, Herman et al., 2013, Jablonka, 2013, Leimar & McNamara, 2015). For example, if annual herbivore patterns exhibit positive autocorrelations across two generations, selection should favor genotypes of plants that, when exposed herbivory, produce offspring transgenerationally primed for herbivore defense (Hoyle & Ezard, 2012, Prizak et al., 2014). However, if the variability does not exhibit any autocorrelation over the inter-generational time scale, TPP is expected to evolve to a zero or slightly negative level (Kuijper & Hoyle, 2015). With this conceptual framework, this manuscript addresses TPP to wounding in M. guttatus, and considers the role of parental environment and local adaptation on offspring trichome density and herbivore resistance in the field.
Figure 1.
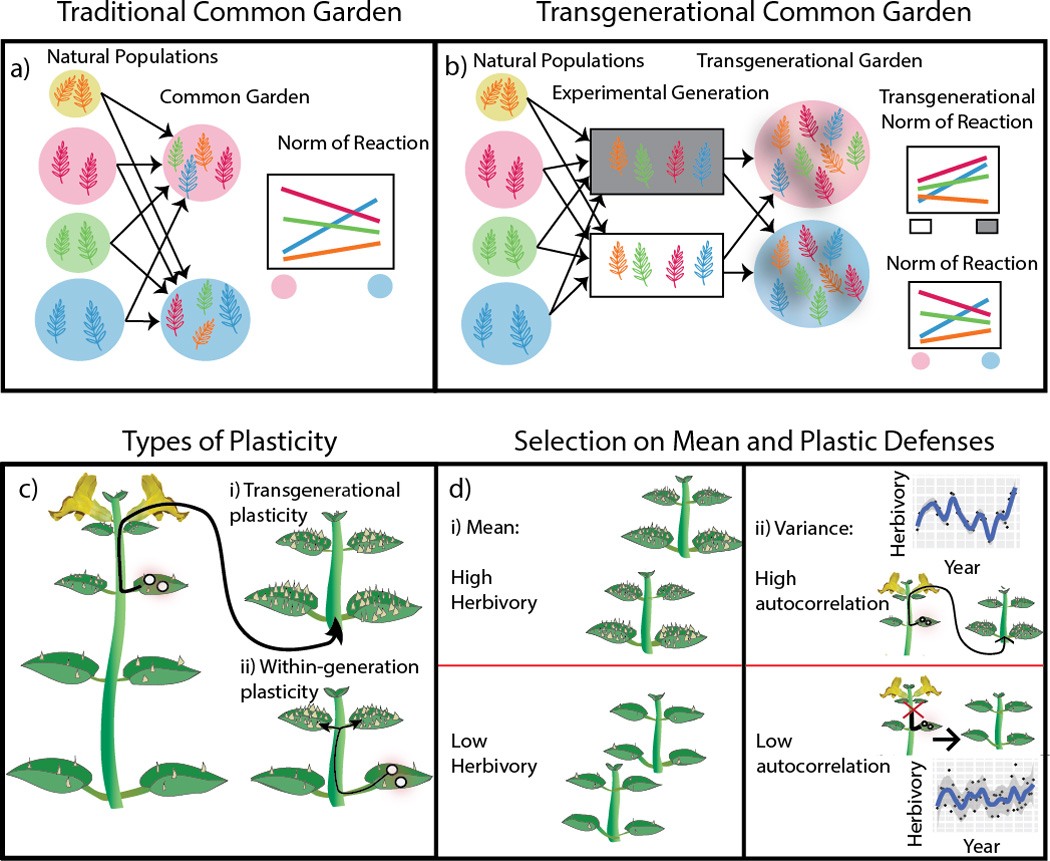
Diagram of a traditional common garden experiment (a) compared to the transgenerationally extended common garden experiment (b) utilized in this study. Both experimental designs allow genetic, environmental, and genetic × environmental effects to be parsed out through norms of reaction and associated analyses. The extended transgenerational approach utilizing an experimental generation (b), allows for transgenerational effects to be considered within this this framework. This framework allows transgenerational plasticity (c.i) to be considered alongside traditional within generation plasticity (c.ii) to study damage induced trichome production in M. guttatus. While constitutive trichome densities are expected to evolve in accordance with mean herbivore abundance at a given site (d.i), it is the presence or absence of inter-annual autocorrelations in herbivore abundance that are expected to select for or against transgenerational plasticity of trichome induction (d.ii).
Since the demonstration of transgenerationally induced defenses in wild radish (Agrawal et al., 1999), plant-herbivore interactions have become a model system for studying TPP. Agrawal et al. (1999) demonstrated that the progeny of plants exposed to caterpillar herbivory were themselves more resistant to caterpillars (and that Daphnia exposed to predators produced better defended offspring), accelerating the study of TPP on biotic interactions (Agrawal et al., 1999). Numerous studies across taxa have since demonstrated that the offspring of wounded plants produce more chemical and physical defenses than the offspring of control plants (Agrawal, 2001b, Agrawal, 2002, Holeski, 2007, Holeski et al., 2012, Rasmann et al., 2012, Ballhorn et al., 2016). This framework is conceptually tied to the optimal defense theory (McKey, 1974), which predicts that the costs/benefits and probability of attack provide information to the plant which selects for plants to defend various organs at levels that maximize fitness under local conditions. In this context, parental wounding provides an additional source of information that a plant can utilize to produce appropriate levels of defenses given the cost/benefit tradeoff. However, the optimal defense theory also predicts a tradeoff in constitutive vs. induced defenses (McKey, 1974, Fritz & Simms, 1992, Zangerl & Rutledge, 1996), which is not necessarily predicted through this information theory framework. Instead, the mean levels of herbivory over time would be expected to select for baseline constitutive levels of defense, while the patterns of variation in herbivore frequencies would select for or against within-generation plasticity or TPP. In other words, constitutive and inducible defenses respectively, reflect the elevation and slope of the norm of reaction of defense on variation in herbivory.
Concurrent with theoretical advances of when TPP should be advantageous (Herman et al., 2014, Kuijper & Hoyle, 2015), recent studies of the transcriptional basis of TPP (Colicchio et al., 2015b), its epigenetic origin (Boyko et al., 2010, Lang-Mladek et al., 2010, Verhoeven et al., 2010, Calarco et al., 2012, Rasmann et al., 2012, Herrera & Bazaga, 2013), and taxonomic prevalence (Holeski et al., 2012) have confirmed the complex and widespread role of TPP in plant defense. In Mimulus guttatus (yellow monkeyflower), simulated herbivory (mechanical leaf wounding) to parental plants leads to offspring with more trichomes—defensive hair-like epidermal structures—than offspring of undamaged parental plants (Holeski, 2007). The offspring of wounded plants differ from the offspring of control plants via a broad, multifaceted transcriptional response (Colicchio et al., 2015b). Molecular epigeneticists have recently demonstrated the presence of an intricate three part system through which histone modifications (Greenberg et al., 2013), DNA methylation (Matzke & Mosher, 2014), and small-RNAs (Sunkar et al., 2007, McCue et al., 2012) are responsive to environmental conditions (Dowen et al., 2012), alter gene expression (Eichten et al., 2014), and persist into the following generations(Verhoeven & van Gurp, 2012). These epigenetic mechanisms appear likely to represent the underlying basis of transgenerational plasticity (Rasmann et al., 2012), although maternal nutrient provisioning and seed coat modifications have also been implicated in TPP (Galloway, 2001, Luzuriaga et al., 2006). While this paper focuses on TPP’s role in plant-herbivore interactions, the role of TPP in evolution extends to many environmental variables (Bossdorf et al., 2008), and across a wide diversity of life, including a significant breadth of the animal kingdom (Lancaster et al., 2007, Donelson et al., 2012, Gapp et al., 2014, Woestmann & Saastamoinen, 2016).
While the molecular underpinnings, and the theoretical ramifications of TPP have become increasingly understood, field studies addressing the role of transgenerational effects in nature are still relatively sparse. In Campanulastrum americanum, maternal light conditions impact the life history strategy (annual vs. biennial) of their offspring in a way that increases the fitness of the next generation if it too is grown in the same light condition (Galloway & Etterson, 2007, Galloway & Etterson, 2009). In Phaseolus lunatus (wild lima bean) a recent study utilized a similar common garden approach to the one applied here to study transgenerational chemical defense induction and the role of this induction on plant survival in nature (Ballhorn et al., 2016). However, inter-population variation in TPP was not considered in either of these studies. Work outside of the plant-herbivore defense literature has demonstrated that genetic differences exist that alter the role of parent environment on offspring phenotype (Schmitt et al., 1992, Sultan, 1996, Vu et al., 2015), but these studies have not tested the effects of transgenerational effects in field conditions or in regard to insect herbivory.
Previous studies in the M. guttatus system have demonstrated the presence of transgenerational trichome induction to wounding, and variation in this response across recombinant inbred lines and their progenitor inbred lines(Holeski, 2007, Holeski et al., 2010, Scoville et al., 2011, Colicchio et al., 2015b). M. guttatus is fed on by a wide-range of Lepidoptera, including both generalists and specialists (Rotter and Holeski, In Review), and produces a diverse suite of PPGs (Keefover-Ring et al., 2014) that can provide additional levels of herbivore defense or attraction (Holeski et al., 2013). While the parental stress imposed in those experiments and the one used here, mechanical damage though hole-punching, does not perfectly mimic herbivory (Reymond et al., 2000), at least in Arabidospsis, the transcriptomic responses to mechanical damage and herbivore damage do overlap significantly (Reymond et al., 2000).
Over the past decade, the genetic (Holeski, 2007, Holeski et al., 2010) and transcriptional (Scoville et al., 2011, Colicchio et al., 2015b) basis of TPP in M. guttatus has become better understood, but until this point no one has tested for a role of trichomes or TPP in herbivore defense within the M. guttatus system. Here, I utilize an extended common garden approach (Figure 1b) to gain insight into the function of M. guttatus trichomes, the role of TPP on plant-herbivore interactions in nature, and population level differences in transgenerational plasticity.
Methods
Natural Population Phenotyping, Herbivory assays, and Collection
During the summer of 2014 I identified 16 natural populations of annual Mimulus guttatus within a 150 by 50-mile area in Central Oregon. These sites ranged in elevation from 89 to 1,481 meters (Table S1). When over 50% of plants at a given site began setting seed (between June 7th and August 5th), 12–20 plants were collected per site and brought to the Plant Biology lab at HJ Andrews Experimental Forest. Here, I assayed herbivory on every leaf of the primary axis on a 0 to 5 ranking (0: no leaf damage, 1: 1–10% leaf area removed, 2: 11–25%, 3: >26%) using a visual estimation of leaf damage that has been found accurate (Johnson et al., 2015). I also measured plant height and width, and counted the number of flowers produced by each plant. In addition, I counted trichomes from three leaves per plant (one of which was always of the second leaf pair, and two of which were later leaves) as described previously (Colicchio et al., 2015b). At the end of the growing season (August 10th–September 2nd) I revisited these sites and randomly selected and collected seed from 10 plants per site.
Experimental Greenhouse Generation
In the fall of 2014, I grew seed from 6 maternal lines for eight of the sixteen populations sampled in the field (chosen to represent a wide-range of ecological conditions) at the University of Kansas Greenhouse. Seeds were germinated individually in 1-inch cells, before being transplanted to 2-inch containers for continued growth. I phenotyped one third of the plants for second leaf trichome density at the third leaf pair expanded stage. Of the remaining plants, half were subject to damage through the hole-punch method from the third through the sixth leaf pair which was previously shown to induce increases in trichome production in inbred M. guttatus lines (Holeski, 2007, Colicchio et al., 2015b). Within each population, I randomly selected one damaged and one control plant from each maternal line to use as parent plants for the next generation, while the remainders were phenotyped for 2nd and 7th leaf pair trichomes. Plants derived from the location “Trailbridge Road” (TBR) did not continue leaf development to the 7th leaf pair, and were therefore excluded from the analysis of within-generation trichome induction. I performed crosses using a circular crossing design for both damaged and control breeding individuals. Briefly, a wounded plant from maternal line one was used as the pollen donor for a cross with a wounded plant from maternal line two. This same wounded plant from maternal line two was then used as a pollen donor for a cross with a wounded plant from maternal line three, and so forth, until a wounded plant from maternal line six was used as the pollen donor for the original wounded plant from maternal line one. This same exact pattern was repeated with control plants, and done for each of the eight populations. From this I generated six paired lines for each of the eight populations, deriving from either the offspring of two control or two damaged plants (96 lines total).
Field Common Garden Design
During the summer of 2015, I germinated seeds from each of these populations at the University of Oregon greenhouse in 1” flats and transplanted one to two week old seedlings into two common garden sites in the Cascade mountain region of Central Oregon. At both sites, individuals were planted in a randomized design across the site. One common garden, HJ Andrews Experimental Forest (HJA), was located >2 mi away from any native M. guttatus population and differed from typical M. guttatus habitat in that it contained a greater composition of soil organic matter and received fewer hours per day of direct sunlight (Personal Observations). The other site, Browder Ridge (BR), features a large native population and has been the site of numerous prior common garden experiments (Mojica et al., 2012, Monnahan & Kelly, 2015).
At HJA, 1,232 seeds from 44 lines (4 were excluded due to insufficient seed set), each replicated with either damaged or control parents, were planted on April 18th, 799 (64.8%) germinated and were planted into the field on May 12th, with 224 of these plants (28.0%) eventually flowering. 1,056 seeds from these same lines were planted for the BR garden on May 4th, 573 (54.3%) germinated and were transplanted into this field site on May 25th, of these 94 reached flowering (16.4%). During the growing season, rapid dry down due to the drought conditions in the Cascades during the summer of 2015 necessitated the addition of supplemental water at both sites at the rate of 5 gallons per common garden site every other day for 3 weeks during the growing season. Of the surviving 318 plants, 153 were the offspring of damaged parent plants, while 165 were the offspring of control plants. Trichome counts were completed for 271 of 318 plants.
I surveyed plants every other day, and on the day that a plant produced its first flower, the following traits were scored: largest leaf length and width, number of leaves, plant height, node of flower, peduncle length. I assayed herbivory by the same method as described above, and one second node leaf was collected for trichome phenotyping.
Greenhouse Common Gardens
To test whether the observed transgenerational effects persisted in greenhouse as well as field conditions, the same offspring of damaged and control plants were grown at the University of Kansas greenhouse during the fall of 2015 and spring of 2016. Seeds were germinated and grown in 1-inch cells, and when plants reached the third leaf pair expanded stage, a second node leaf was collected and phenotyped for trichome density as described above.
Statistical Analysis
Natural Field Survey
Population Level Analysis
Incidence of herbivory (percent of leaves at a site with any herbivore damage), elevation, aridity (Zomer et al., 2008), latitude and longitude were all considered as possible explanatory variables in a least squares regression of population mean second leaf pair trichome density in JMP v10 (SAS Institute Inc., Cary, NC). Significance in this model was determined through t-tests. The linear relationship between population incidence of herbivory and trichome density was carried forward using linear regression. The same approach was used with incidence of herbivory as the response variable, and elevation, aridity, latitude, and longitude as explanatory variables. Correlations between both population mean field trichome density, and greenhouse trichome density, with population incidence of herbivory were calculated in JMP.
Leaf level Analysis
Mixed models were constructed using the (R:lmee:glmer) package followed by ANOVAs comparing models to test if trichome densities affected severity or likelihood of damage in this model(Bates et al., 2014). Within this model population and individual leaf trichome densities were treated as fixed effects, with plant nested within population treated as a random effect. The response variable was either a binary variable of whether or not the plant received and wounding (binomial family model), or an ordinal variable representing the severity of wounding (Poisson family model). All leaves (654) were considered for the binary herbivory or no herbivory model, while only leaves with at least minor herbivory (320) were considered to test for an effect of trichomes on limiting herbivore severity.
Trichome/Fitness Relationship
Least squares regression was used to determine the relationship between population, trichome density (averaged across the three leaves per plant), a “population × trichome” interaction term, and stem width on plant flower production. Stem width was included in this model as a covariate to help partition out variation due to general plant vigor, and get more directly at the relationship between trichome density and flower production for a plant of a given size. Effect significance was determined by F-ratio tests based on factor sum of squares.
Within Generation Plasticity
For analyses, with trichome density as the response variable, I transformed the densities as log (trichome density + 1). Trichome counts were right skewed with a large number of 0 values. To detect signatures of phenotypic plasticity, log (7th leaf trichome density +1) was treated as the response variable with population, family nested within population, damage treatment, and a “population × damage” interaction term explanatory variables. Using a GLM framework in JMP v10 (SAS Institute Inc., Cary, NC) log-ratio chi-square tests were performed between models to determine significance. The residuals in this model were approximately normally distributed, and were not correlated with predicted trichome density. Subsequently, the data was split according to population and GLMs were fit with the above terms to test if individual populations did in fact exhibit significant within-generation plasticity.
Transgenerational Plasticity
Population of origin, line nested within population, growth environment (field or greenhouse), parental damage, and all possible two interaction effects between population, parental damage, and growth environment, as well as their three-way interaction were considered in a GLM model with log (2nd leaf trichome density +1) as the response variable. Using JMP v10 (SAS Institute Inc., Cary, NC) log-ratio chi-square tests were performed between models to determine the significance of the various terms. The residuals in this model were approximately normally distributed, and were not correlated with predicted trichome density (Figure S1). Subsequently, the data was split according to population and GLMs were fit with the above terms to test if specific populations exhibit significant transgenerational plasticity.
Considering all leaves, I created a model to test which, if any, factors limit the likelihood that a plant experiences herbivore damage. I used a stepwise model selection method in JMP v10 (SAS Institute Inc., Cary, NC), with herbivore wounding coded as binary response variable, and possible explanatory variables to include were population, site, parental treatment, days to flower, leaf width, plant height, trichomes, treatment × population, site × treatment, site × trichomes, and site × population as possible explanatory factors. Considering leaves from which there was at least some sign of insect damage I created a model to test which, if any, factors limit insect herbivore damage. Using a stepwise model selection method in JMP v10 (SAS Institute Inc., Cary, NC), with severity of damage coded as minor, moderate, or high as the response variable, and the same possible explanatory variables. For both models I then selected a model using both a minimum BiC and AiCc criterion.
Environmental Autocorrelations in the Oregon Cascades
Mean annual temperature and precipitation data from the northern, southern, western, and eastern most populations within this study system between 1895–2014 were downloaded at 4 sq. km. resolution using the data explorer tool from the PRISM climate group (PRISM Climate Group, Oregon State University, http://prism.oregonstate.edu, 12 Feb 2016). Autocorrelations were calculated using R:acf (Team, 2013) at a lag of one.
Results
Natural Population Surveys
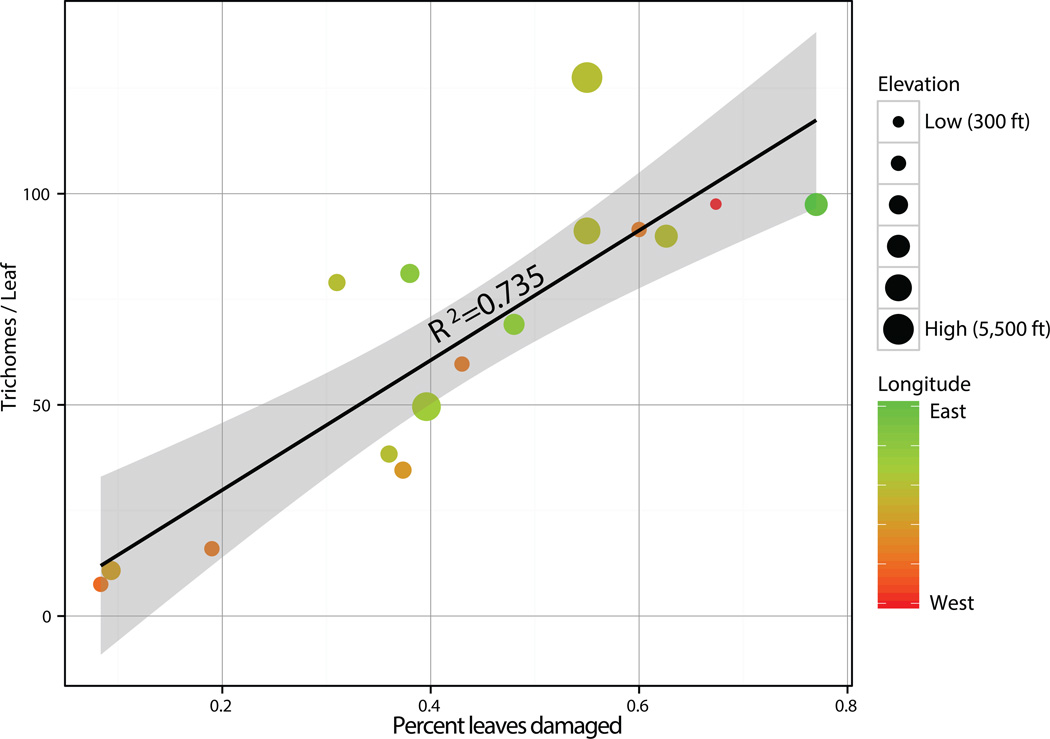
Central Oregon M. guttatus exhibit vast natural variation in trichome density (7.5 trichomes/cross section: 127.5 trichomes/cross section, Table S1) and experience quite different magnitudes of leaf herbivory (8% of leaves experiencing herbivory : 67% of leaves, Table S1). Incidence of herbivory at a site was the only factor that significantly covaried with population mean trichome density in a least-squares regression (t1=3.96, p=0.0027, Table S2) when considered alongside latitude, longitude, aridity, and elevation. Additionally, incidence of herbivory was not significantly correlated with any of the preceding environmental variables (Table S3). The strong positive correlation between population incidence of herbivory and trichome density among plants at native field sites (R2 = 0.735, Figure 2), and in the greenhouse (R2=0.82) suggests that herbivory driven natural selection plays a role in driving population trichome density variation.
Figure 2.
Scatterplot and regression of population mean trichome density to the percent leaves damaged at a given site. Point size is coded to represent the elevation of the site and color to the longitude, both of which were not found to be significantly correlated with leaf trichome density.
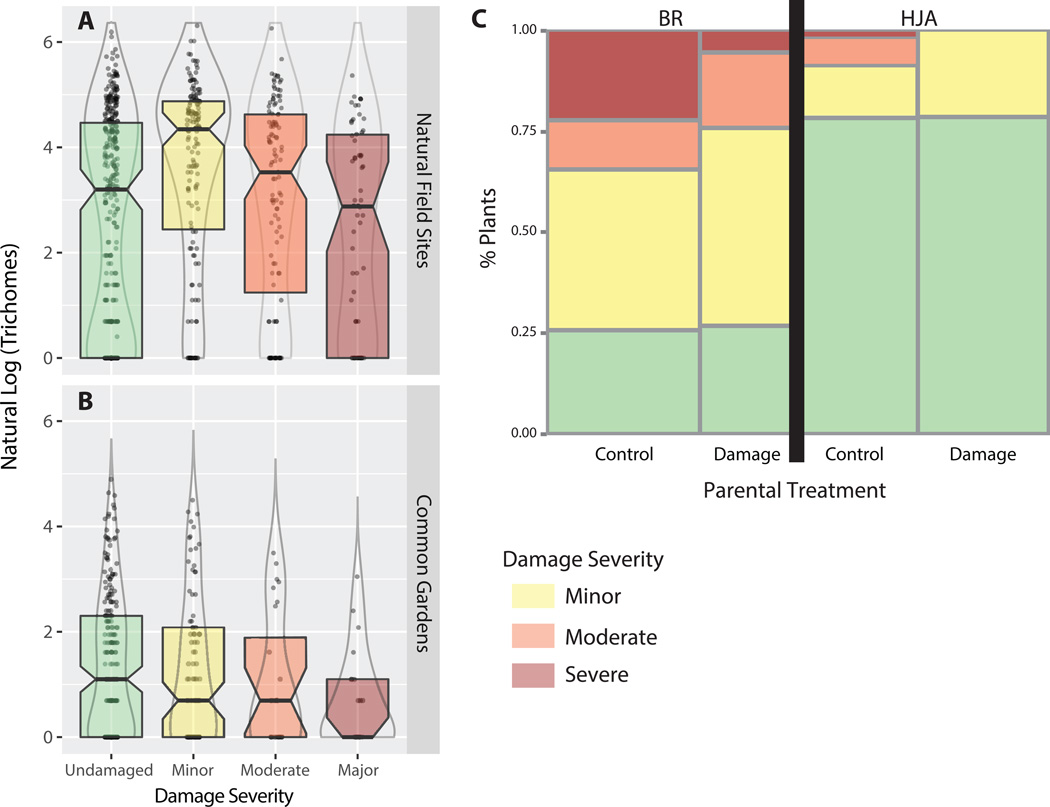
While plants in the two different common garden locations did significantly differ in the probability that a leaf would receive herbivore damage (Mixed-model logistic regression: x152=100.85, P<9×10−15, Figure 3a), trichomes did not alter the likelihood that a leaf would receive herbivore damage (x12=0.23, P=0.63). On the other hand, trichomes did significantly reduce the severity of herbivory on leaves that showed at least minimal signs of damage (x12=7.74, P=0.0054, Figure 3a), but there was not a significant effect of common garden on severity of damage (Mixed-model Poisson regression x152=8.5, P=0.90. Leaves receiving minor damage had an average of 90 trichomes (n=147, SE=7.7), while those receiving moderate damage had an average of 63 trichomes (n=111, SE= 7.6), and those receiving severe damage had an average of 44 trichomes (n=53, SE=7.04). The results shown in Figure 2 suggest that trichome density evolves in response to the prevalence of herbivory at a given location, while the results in Figure 3 suggest that trichomes may not reduce the likelihood but rather the severity of herbivory (Figure 3a and 3b).
Figure 3.
The role of trichomes (a,b) and transgenerational plasticity to damage (c) in plant herbivore resistance. In both natural field sites (a) and field common gardens (b) plants experiencing minor damage tended to have more trichomes than those receiving moderate or severe damage. (c) At both HJA and BR field common garden sites, the progeny of damaged plants experienced less severe herbivore damage than the progeny of control plants.
There was not a significant relationship between plant trichome density and flower production in the least-squares regression (SS=1.36, F1,197=0.025, P=0.872, Table S4), but a “trichome × population” interaction term did significantly affect flower production (SS=1765, F14,184=2.3, P=0.0067, Table S4). This implies that there was variation across sites regarding the effect of trichomes on plant fitness. Of the populations carried forward for common garden experiments: trichomes were positively associated with fitness in WC, CSR, IM, and TBR, and negatively associated with fitness in BR, HOL, LPD, and MWL.
Within Generation Plasticity
Log-transformed trichome density of 7th leaf pairs in the greenhouse was plastic with regard to wounding of earlier leaves (GLM: x12=7.2, P=0.0074), population (x62=171.9, P < 0.0001), and family nested within population (x322=53.5, P=0.01, Table 1). Wounded plant produced on average 18.5 (SE=1.46) trichomes per cross section on 7th leaves compared to 14.5 (SE=1.27) by control plants. Within the populations considered there was not a significant “population × damage” effect on trichome density (x62=8.3, P=0.22, Table 1, Figure S2). This suggests that although wounded plants generally produced a greater number of trichomes than control plants, there was no evidence for inter-population variation in within-generation trichome plasticity. Individual population tests found wounded plants produced significantly more trichomes in WC (x12 =4.46, P=0.035), TBR (x12 =4.94, P=0.026), and CSR (x12 =4.01, P=0.043), but not in the other populations (Figure S2).
Table 1.
Generalized linear model of trichome density accounting for within generation plasticity to leaf wounding
| Source | DF | X2 | P-value |
|---|---|---|---|
| Population | 6 | 171.9 | < 0.0001 |
| Wounding | 1 | 7.2 | 0.0074 |
| Wounding × Population | 6 | 8.3 | 0.2163 |
| Family[population] | 32 | 53.5 | 0.0101 |
Transgenerational Phenotypic Plasticity
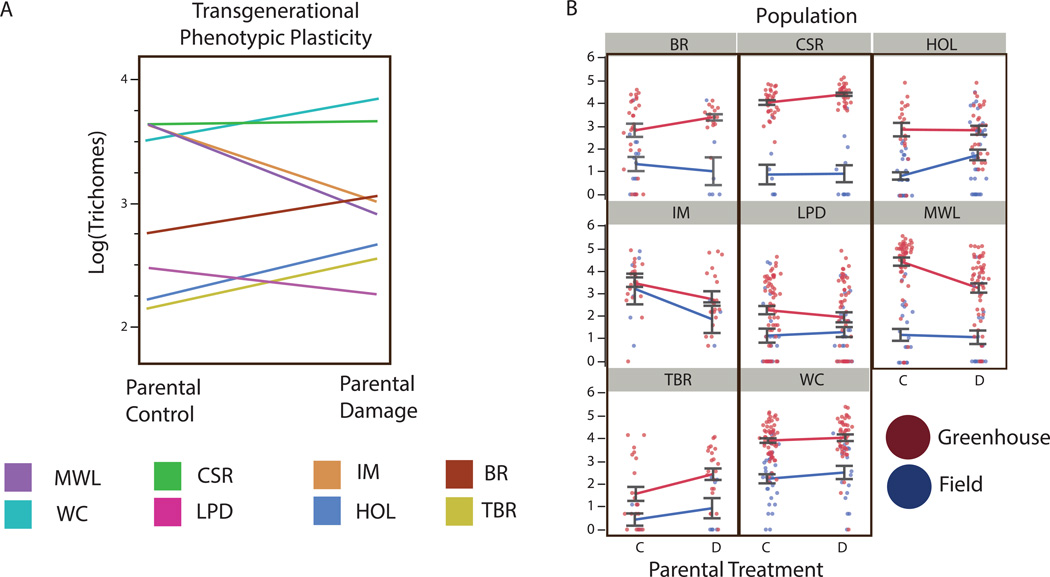
In both field and common garden environments parental wounding had a significant population dependent effect on log transformed offspring second leaf trichome density (GLM: x72=21.96, P=0.0026, Table 2). The offspring of damaged plants from WC, HOL, TBR, CSR, and BR had increased trichome density, while trichome production declined in the offspring of damaged plants from MWL, LPD, and IM (Figure 4a). Individual population tests find that transgenerational trichome increases were statistically significant in HOL (x12=6.92, P=0.0085) and CSR (x12=8.95, P=0.0028), but not in BR (x12=3.35, P=0.067), WC (x12=0.25, P=0.62), or TBR (x12=2.6, P=0.11) (Figure 4a and 4b). Transgenerational wounding induced trichome reductions were significant for IM (x12=6.56, P=0.010) and MWL (x12=13.8, P=0.0002), but not LPD (x12=2.2, P=0.14) (Figure 4a and 4b).
Table 2.
Generalized linear model of trichome density accounting for transgenerational phenotypic plasticity
| Source | DF | X2 | P-value |
|---|---|---|---|
| Environment1 | 1 | 184.7 | < 0.0001 |
| Parental wounding | 1 | 0.33 | 0.5683 |
| Environment × Parental wounding | 1 | 0.075 | 0.7849 |
| Population | 7 | 107.1 | < 0.0001 |
| Environment × Population | 7 | 48.6 | < 0.0001 |
| Population × Parental wounding | 7 | 21.9 | 0.0026 |
| Environment × Population × Parental wounding | 7 | 16.2 | 0.0237 |
| Family[population] | 35 | 135.9 | < 0.0001 |
Environment: Field vs. Greenhouse
Figure 4.
Transgenerational norm of reaction for trichome density in the offspring of control and damaged parent plants across the study populations. Positive slopes represent increased trichome production in the offspring of wounded plants, negative slopes represent decreases in trichome production. (a) Standard norm of reactions demonstrating mean population responses to parental damage. (b) Expanded norm of reactions showing population responses to parental damage, and variability of this effect across different offspring environments.
Along with parental wounding, field vs. greenhouse conditions also had a large effect on trichome production (x12=184.73, P < 0.0001, Table 2, Figure 4b), with plants grown in the greenhouse producing many more trichomes than their siblings transplanted to the field. Different populations varied in the scale to which they produced more trichomes in the greenhouse (offspring environment × population, x72= 48.61, P < 0.0001, Table 2), but plants from all populations produced more trichomes in greenhouse compared to field environments. There was not a significant environment × parental wounding effect on trichome density (x12 = 0.074, P=0.785), but was a significant environment × parental wounding × population effect (x72= 16.16, P=0.024). This three-way interaction term can be conceptualized as population level differences in the different phenotypic outcomes of parental wounding in field vs. greenhouse offspring environments, and visualized as the differences between transgenerational reaction norms in field vs. greenhouse environments across populations (Figure 4b).
Trichomes and Transgenerational Herbivore Resistance
At field common garden sites, plants with a greater number of 2nd leaf pair trichomes experienced less severe herbivory than those with fewer trichomes (Poisson Regression: n=135, x12=5.14, P=0.023, Figure 3b). At common garden sites, leaves that experienced no herbivory produced an average of 9.75 trichomes (n=217, SE=1.25), leaves that experienced minor damage produced an average of 9.39 (n=88, SE=1.9), moderate damage 5.80 (n=25, SE=3.5), and severe damage 2.27 (n=22, SE=3.7). There was not a significant difference in trichome density between undamaged and minor damaged leaves, or moderate and severely damaged leaves, but leaves experiencing minor or no damage produced significantly more trichomes than those experiencing moderate or severe damage (n=352, F=5.46, P=0.02, Figure 3b).
To further analyze the factors effecting plant herbivore damage in the field, models were constructed via both minimum BiC and AiCc criteria to select factors impacting plant herbivore damage. Both minimum BiC and AiCc constructed models identified only common garden site as having a significant effect on whether or not a plant received herbivore damage (n=319, BiC=345, AiCc= 338, x12=82.8, P < 0.0001, Figure 3c). While 73% of plants at BR received at least minor herbivore damage, only 21% of plants at HJA experienced any herbivory.
On the other hand, our models constructed to compare the severity of herbivore damage varied greatly between minimum BiC and AiCc constructed models. Using a minimum BiC criterion a model, only common garden site, parental damage, and their interaction term were selected as explanatory factors explaining herbivore severity (n=115, BiC=190.84, AiCc= 177.66, x32=25.29, P < 0.0001, Table 3a, Figure 3c). The offspring of damaged plants experienced less severe herbivore damage than the offspring of control plants (x12=14.8, P < 0.0001, Table 3a, Figure 3c), and plants at the BR common garden had more severe herbivory than those at HJA (x12= 14.6, P < 0.0001). Of the 25 offspring of damaged plants grown at HJA that experienced herbivory, all of them experienced only minor leaf damage (less than 10% leaf area removal), while 6/19 offspring of undamaged plants at this same site experienced moderate herbivory (between 10%–30% leaf area removal, Figure 3c). At BR, the most striking difference was found in the severe herbivory category; only 2/27 (7.5%) offspring of damaged plants experienced severe damage (>30% leaf area removal), while 13/43 (30%) offspring of control plants experienced this level of damage.
Table 3.
Minimum BiC (a) and AiCc (b) selected models of the factors explaining herbivore damage at two field common garden sites
| a) | |||
|---|---|---|---|
| Source | DF | Χ2 | P-value |
| Site | 1 | 14.6 | 0.0001 |
| Parental Wounding | 1 | 14.8 | 0.0001 |
| Site × Parental Wounding | 1 | 5.9 | 0.0154 |
| b) | |||
| Source | DF | Χ2 | P-value |
| Parental Wounding | 1 | 9.7 | 0.0018 |
| Pop3 × Parental Wounding | 1 | 9.4 | 0.0022 |
| Pop1 | 1 | 9.1 | 0.0026 |
| Site × Parental Wounding | 1 | 6.8 | 0.0091 |
| Site × Pop2 | 1 | 5.5 | 0.0186 |
| Site × Trichomes | 1 | 5.3 | 0.0216 |
| Site | 1 | 5.0 | 0.0251 |
| Pop3 | 1 | 4.5 | 0.0341 |
| Height | 1 | 2.8 | 0.0954 |
| Pop2 | 1 | 0.2 | 0.6435 |
| Trichomes | 1 | 0.2 | 0.6969 |
Pop1: LPD/WC/MWL/HOL/TBR vs. IM/BR/CSR
Pop2: LPD/WC/MWL vs. HOL/TBR
Pop3: LPD/WC vs. MWL
Under the minimum AiCc selected model, those same terms, along with eight additional terms were found to influence herbivory levels (n=93, BiC=163.3, AiCc=134.9, x112=50.9, P < 0.0001, Table 3b). This less conservative model included population, height, trichome density, parental damage × population, trichome × site, site × population, and site × trichome × population interaction terms (Table 3b). Of particular interest, while there is a general trend of increased herbivore resistance in the offspring of damaged plants across all populations, the magnitude and direction of this response varied across populations. The offspring of plants derived from IM, BR, and CSR generally received the least severe damage (x21=9.1, P=0.003, Table 3b). The offspring of damaged plants from WC showed the greatest increase in resistance, while the offspring of damaged plants from MWL and LPD were less resistant than the offspring of control plants (x21=9.4, P=0.002, Table 3b, Figure S3). Of the other populations, BR, CSR, and IM showed similar resistance in the offspring of control and damaged individuals, while HOL, LPD, and TBR showed increased resistance in the offspring of damaged plants (Figure S3).
Year-to-Year Autocorrelation in Temperature and Precipitation
Between 1895 and 2014 sites within the Oregon Cascades exhibited substantial year-to-year autocorrelations in mean annual temperature, and only minor inter-annual autocorrelations for precipitation. Of the four locations considered, LPD had the highest inter-annual autocorrelation in temperature (0.43) while WC had the lowest (0.22) (Figure S4), all significantly higher than expected by chance. A second peak in temperature autocorrelations at a lag time of 8 years (Figure S5) suggests that El Nino cycles may lead to lagged periodicity in temperature autocorrelations in the central cascades (Rasmusson & Wallace, 1983). Inter-annual autocorrelations in total precipitation in this region were negative in the cases of LPD (−0.15), CSR (−0.01), and MWL (−0.06), but positive at the western WC population (0.03); however, none of these autocorrelations were outside the 95% confidence interval.
Discussion
The experiments described above reiterate the role of plastic responses to the environment, genetic differentiation, and the inferred role of selection on phenotypic differentiation in plants using an extension of the common garden technique popularized by Clausen, Keck, and Heisey (Figure 1a, Clausen et al., 1948). The addition of an intermediate greenhouse generation, in which plants were exposed to mechanical wounding, extends the framework to consider transgenerational effects on phenotype and plant-herbivore interactions (Figure 1b).
In this study, both common garden and natural population surveys indicate that M. guttatus trichomes limit the severity of herbivory in the field (Figure 3a and 3b), parental wounding alters offspring herbivore resistance (Figure 3c) and defense phenotypes (Table 2), and that there is natural variation in these responses (Table 2 and Table 3b). While previous studies in M. guttatus have implicated their likely role in herbivore defense (Holeski, 2007, Holeski et al., 2010, Scoville et al., 2011), and found their induced production coincides with the differential expression of many genes involved in plant defense (Colicchio et al., 2015b), other studies in Mimulus (Hendrick et al., 2016) and elsewhere (Bickford, 2016) have also implicated trichomes in other processes. For this reason, the natural surveys of M. guttatus trichome variation, and tests of the relationship between trichomes and herbivore defense provided here deliver much needed support for future experimental work in this system.
Trichomes: Natural Variation and Role in Herbivore Defense
Population mean trichome density is strongly correlated with population level incidences of herbivory (R2=0.735, Figure 2), and at both common gardens and natural field sites plants with more trichomes experienced less severe herbivory (Figure 3a and b). The significant interaction between trichome density and population on flower number suggests that trichomes are related to plant fitness in a population dependent manner. There are a number of potential causes for this interaction, such as correlations between trichome density and other phenotypes (Lande & Arnold, 1983), a variable role of trichomes as specialist vs. generalist herbivore deterrents (Rotter and Holeski, In Review), or an interaction between nutrient availability, trichome density, and plant fitness (Wilkens et al., 1996). Additionally, flower number is limited as a proxy for fitness due to variation in flower size, seed set per flower, germination rates of seeds, and other offspring fitness components (Reznick & Travis, 1996). Still, taken together the population level positive correlation between trichome density and herbivore incidence, the individual level negative correlation between trichome density and herbivore damage severity, and the population dependent relationship between trichomes and flower number suggest that M. guttatus trichomes play a role in herbivore defense, and that there are costs associated with increased defenses (Mauricio & Rausher, 1997, Mauricio, 1998) that could lead to intermediate trichome densities dependent on local herbivory.
While our population level analyses suggest that trichome densities may evolve in response to local herbivore prevalence (Figure 2), the evidence here suggests that they may not function in preventing herbivory per se, but rather in limiting the scale of damage given that an herbivore does arrive. Trichomes did not have a significant effect on reducing the likelihood of leaves receiving herbivore damage in natural populations (P=0.63, Figure 3a), and at common garden sites undamaged leaves had similar trichome densities to those with minor damage (Figure 3b). However, in both natural populations and field common gardens trichome density was associated with a decrease in herbivore damage severity, given that a leaf received at least minor signs of herbivory (Figure 3a and 3b). Thus, it appears that while the frequency of herbivory selects for population trichome density levels (Figure 2), trichomes may function to reduce the severity of damage rather than reducing the incidence of herbivory.
Unlike the hypotheses put forth under the optimal defense theory (McKey, 1974), and supported in a number of cases (Zangerl & Berenbaum, 1990, Lewinsohn et al., 1991), I do not find any evidence for a tradeoff between constitutive and within-generationally (Figure S2) or transgenerationally (Figure 4) induced defenses in this study. This result reiterates findings from M. guttatus recombinant inbred lines (RILs) where a similar lack of correlation between induced and constitutive defenses was found (Holeski, 2007). In both cases, there is no pattern in which lines that produce constitutively higher levels of defense are any more or less plastic than their less defended relatives. Rather than herbivore abundance patterns imparting a single selective force on induced and constitutive defenses, the mean and variability of such herbivore incidences over time may select somewhat independently upon constitutive, plastic, and TPP induced defenses. The uncoupling of norm of reaction intercept and slope could allow organisms to adapt to their local conditions more precisely than if these two parameters were tightly linked.
Future studies that include herbivore observations will be necessary to determine if specialist and generalist herbivores are differentially impacted by transgenerational induction, as might be expected due to the largely different manner in which plant-generalist and specialist interactions are expected to evolve (van der Meijden, 1996, Ali & Agrawal, 2012). The prevalence of TPP within the Lepidopteran herbivores that feed on M. guttatus in nature (Woestmann & Saastamoinen, 2016) further complicates the role of TPP in natural environments, as does the potential that complex suites of traits (Lancaster et al., 2007) in M. guttatus are impacted by parental wounding, as suggested by the transcriptomic evidence (Colicchio et al., 2015b).
Transgenerational Effects on Trichomes
In both greenhouse and field experiments leaf trichome density was affected by wounding in the previous generation (Table 2). By considering TPP in multiple offspring environments, I hoped to mitigate biases due to a single beneficially or detrimentally saturated environment (Engqvist & Reinhold, 2016). While some populations showed increased trichome density in the offspring of damaged individuals, plants derived from damaged LPD, MWL, and IM parents produced fewer trichomes. This parental wounding × population interaction term is the most natural evidence for inter-population variation in TPP (Sultan, 1996). Similar to (Sultan, 1996) and (Schmitt et al., 1992) this study demonstrates that not only can the slope of transgenerational norms of reaction vary, but so to can the direction (Figure 4a). This suggests that transgenerational effects could evolve to match both positive environmental autocorrelations (through positive TPP) as well as negative environmental autocorrelations (negative TPP) (Kuijper et al., 2014, Kuijper & Hoyle, 2015).
Plants grown in greenhouse common gardens produced more trichomes than those that were transplanted to field conditions. Interestingly, plants collected from natural populations had a greater number of trichomes than either greenhouse or field common garden plants, a similar result to one previously described in Cajanus cajun (Romeis et al., 1999). This result demonstrates the need to take caution when using solely greenhouse experiments as a proxy for plant defenses in nature (Thaler et al., 1996).
The significant offspring environment (field vs greenhouse) × parental environment (wounded vs. control) × population effect on trichome density demonstrates that the current environment has a genotype dependent effect on TPP (Table 3, Figure 4b). Current environment × parental environment effects on phenotype have been observed previously (Schmitt et al., 1992), and while not surprising, here I find evidence that these effects vary across populations. This somewhat curious effect demonstrates that rather than transgenerational effects providing a discrete signal to direct phenotypic change in the next generation, it acts in conjunction with genetic regulatory elements and within-generation plasticity to further alter plant development. Recent work across a diversity of plants has demonstrated that these interactions between genotype, parental environment, and offspring environment are widespread and suggest that within-generation plasticity, transgenerational inheritance, and genetic variation all interact and contribute to local adaptation (Sultan, 2015). In Polygonum persicaria the three-way interaction between parental drought treatment, offspring germination demethylation treatment, and genetic line explained over 30% of the unexplained variance in seedling biomass(Herman & Sultan, 2016). In Arabidopsis, recent work has highlighted the complex interactions between multi-generation exposure to salt stress, and offspring environment on a number of plant phenotypes (Groot et al., 2016). Finally, the “priming” effect, in which the offspring of stressed plants are able to rapidly induce defenses in response to a similar stress represents another known system in which this interaction between current and parental environment impacts plant phenotype (Cayuela et al., 1996, Beckers & Conrath, 2007, Beckers et al., 2009, Freitak et al., 2009, Conrath, 2011). Future experiments aimed at discovering and analyzing transgenerational effects must therefore not only carefully consider genetic variation and transgenerational environments when designing experiments, but also the offspring environments. This three-way interaction highlights the complexity of TPP, and poses new questions regarding the interaction between within-generation and transgenerational plasticity.
Transgenerational Effects on Herbivory
Though relating parental environment with offspring phenotype has been the end goal of most transgenerational studies (Reymond et al., 2000, Holeski, 2007, Colicchio et al., 2015b), others have additionally considered the fitness effects of transgenerational plasticity (Galloway & Etterson, 2007, Herman et al., 2012). In the plant herbivory system (Ballhorn et al., 2016) recent results demonstrate that parental wounding can increase offspring resistance. Here I corroborate that result; the offspring of wounded plants experience significantly less severe herbivory than the progeny of control individuals (Figure 3c). Herbivory imposes strong selective pressures (Coley et al., 1985), suggesting that parental wounding plays a role in altering offspring development in a way that will directly affect their fitness.
While M. guttatus trichomes appear to evolve according to the probability that a plant will experience herbivory (Figure 2), and slightly reduce the probability that a plant will be damaged (Figure 3b), they also reduce the severity of damage after herbivore arrival (Figure 3a and 3b). Future studies that include herbivore monitoring will be necessary to elucidate the precise stage at which trichomes function to reduce or avoid herbivore damage.
This distinction between traits that allow plants to resist herbivore damage, avoid herbivore damage, and tolerate herbivore damage represent distinct but non-mutually exclusive mechanisms that allow plants to cope with herbivores (Strauss & Agrawal, 1999, Tiffin, 2000), and parallels the diversity of mechanisms (tolerance, avoidance, escape) that plants employ to deal with drought (Kooyers, 2015) or freezing (Sakai & Larcher, 2012). Considering adaptation more generally, (i) escaping stress before it limits fitness, (ii) minimizing initial stress to reduce concomitant fitness loss, and (iii) mitigating the long-term detrimental effects of stress, presents a chronological and intuitive method to parameterize how traits mediate adaptation in the face of stress. Here we model the possible role of trichomes or general parental wounding effects via mechanisms (i) and (ii), but future experiments utilizing herbivore manipulations will be necessary to test the role of herbivory tolerance (iii) in this system. Natural genetic variation in the fitness impacts of herbivory has been demonstrated (Strauss & Agrawal, 1999, Więski & Pennings, 2014), but to this point no one has tested the possibility that parental wounding may impact this relationship.
In much the same way that there was a population × parental wounding effect on trichome production, this same interaction term also significantly affected herbivore resistance (Table 3b, Figure S3). While the two components of this finding have been previously demonstrated, transgenerational effects alter plant herbivore resistance in field conditions (Ballhorn et al., 2016) and TPP varies across genotypes (Galloway & Etterson, 2009), this is the first evidence that TPP for herbivore resistance varies across populations. Considered alongside theory regarding the evolution of TPP in different environments (Kuijper & Hoyle, 2015, Uller et al., 2015), this result suggests that the natural variation in TPP exists upon which environmental patterns could act to increase or decrease the relative importance and direction of transgenerational plasticity.
While herbivores in other locations exhibit population dynamics that lead to inter-annual autocorrelations in their density (Turchin, 1990, Klapwijk et al., 2013), future studies will be necessary to test for inter-annual autocorrelations in herbivory and TPP responses in the same system. Alternatively, natural variation in TPP could be due variable spatial heterogeneity in herbivory, similar to its hypothesized role in life-history shifts due to parental shading (Galloway & Etterson, 2007). If the location of a parent plant within a population is correlated with the herbivore wounding severity the offspring are likely to receive, the evolution of TPP may also be expected (Herman et al., 2013).
One interesting dichotomy between spatial and temporal autocorrelation driven TPP is that in the case of spatial heterogeneity, maternal environment is likely to contain higher quality information than paternal information and thus lead to the evolution of maternal effects (Fox & Mousseau, 1998). However, if transgenerational plasticity evolves in response to patterns of temporal autocorrelation, both maternal and paternal environment will contain information of similar value, and thus the integration of paternal and maternal effects would likely prove adaptive (although explicit theory and modeling will be needed to consider both spatial and temporal autocorrelations in tandem). In M. guttatus, both paternal and maternal wounding is known to induce trichome production to a similar scale (Akkerman et al., 2016), but 5-azacytadine experiments demonstrate that the molecular mechanism is distinct between the maternally and paternally inherited signals. This suggests that maternal and paternal effects may evolve separately, and at least in a single recombinant inbred line, the nearly equal dual inheritance is indicative of the flavor of TPP that is expected to evolve in response temporal rather than spatial autocorrelations.
Natural Variation in TPP to Herbivory
Considering inter-population variation in TPP for trichome production (Figure 4a and 4b, Table 2) alongside variation for TPP in herbivore resistance (Table 3, Figure S3), there is substantial support for natural variation in transgenerational phenotypic plasticity within these populations of M. guttatus. In five of the eight populations there was support for TPP of induced herbivore resistance that is in part mediated through increased trichome production (Figure 3a). Within LPD there is evidence of induced herbivore resistance, but not trichome production, potentially implicating other forms of defense, such as leaf PPG synthesis (Holeski et al., 2013) in the transgenerational response. Seed herbivory in the developing fruit is extremely common at LPD (Personal Observations). As leaf trichomes have no clear effect on seed herbivory, it is possible that floral defense traits rather than vegetative traits, such as increased floral tissue thickness (Roubik, 1982) or calyx trichomes may be more relevant to local adaptation to herbivory at this site.
Peculiarly, in MWL there is evidence of negative transgenerational induction (reduced trichome production and herbivore resistance in the progeny of damaged plants, Figure 4b, Figure S2), with moderate support for negative induction in IM (reduced trichome density but no change in herbivore resistance, Figure 4b, Figure S2). Out of the eight populations considered, the two that showed signs of negative transgenerational induction, IM (Iron Mountain) and MWL (Mount Washington Lookout), were derived from the highest elevation and furthest north sites (Table S1). However, IM is located only 8 miles from BR, which, along with the next most northern population, CSR, both show signs of positive transgenerational plasticity. Additionally, IM and BR share a great deal of common genetic variation (average pairwise FST = 0.065) (Monnahan et al., 2015), suggesting that neutral divergence is unlikely to account for the observed differences in TPP. Still, it could be that IM and MWL share a common ancestry relative to the rest of the populations, and that drift processes rather than selection have led to negative TPP arising to high frequencies within those populations. Alternatively, being derived from high elevation and latitude, it could be that a certain aspect of one, or both, of these variables generates patterns of inter-annual herbivore abundance that favors negative TPP.
Potential Role of Climatic Autocorrelations in TPP Evolution
While long term herbivory data is not available at these locations, climate data from 1895–2014 in the central Cascades does exhibit significant patterns of year-to-year autocorrelation for annual mean temperature, but not precipitation. Both temperature and moisture availability impact herbivore activity (Bale et al., 2002), so these climatic variables should relate to patterns of inter-annual herbivore variation. At all four locations there were positive autocorrelations in mean annual temperature, with autocorrelation values varying from 0.22 to 0.43 (Figure S4). As mean annual temperature influences herbivore activity (Lemoine et al., 2014), the observed pattern of temperature autocorrelation may give rise to positive autocorrelations in herbivore abundance, in turn selecting for genotypes that transmit herbivore induced defenses between generations. Long-term studies of insect densities (Turchin, 1990) and more recently insect herbivore damage (Klapwijk et al., 2013) confirm that herbivore densities between consecutive years tend to be positively autocorrelated. While the direct relationship between climatic autocorrelations and biotic autocorrelations, such as herbivore abundance, is unclear (Jactel et al., 2012), and for some climatic parameters appear non-existent (Swanson, 1998), there is evidence that underlying abiotic autocorrelations can select for the evolution of transgenerational effects (Petchey, 2000, Dey et al., 2016).
While high temperatures during the growing season should increase herbivore activity in the short-term, it also reduces snowpack going into winter (Walker et al., 1999, Pederson et al., 2011). During the following growing season decreased snowpack will reduce moisture availability (Luus et al., 2013). Thus, while at high elevations inter-annual temperature autocorrelations exist, the relationship between temperature and snowpack may generate a negative autocorrelation in inter-annual herbivore activity. Low elevation sites are less arid (Table S1), and moisture availability is not as heavily reliant on snow-melt (Personal Observations), therefore this negative autocorrelation is not expected to be as prominent. This elevation dependent relationship between temperature and moisture availability, and the further complex relationship of drought conditions on herbivore activity (Jactel et al., 2012) may explain the presence of positive transgenerational trichome induction and herbivore resistance at low but not high elevation sites, however this prediction remains to be tested. Supporting the possibility that elevation, latitude, or growing season length may in some way drive differences in TPP to herbivory in M. guttatus, previous studies demonstrated that trichome induction is prevalent in individuals derived from low elevation Point Reyes coastal perennial plants, but not in high elevation derived Iron Mountain (IM) plants (Holeski, 2007).
Conclusions
The optimal allocation of resources towards defense traits will evolve to an equilibrium based on the costs and benefits of this defense (McKey, 1974, Caswell & Reed, 1976). This study provides evidence that along with natural variation in constitutive and inducible defenses (Lewinsohn et al., 1991, Moreira et al., 2014), natural variation in TPP can also impact plant defense in nature. This variation in plant abilities to integrate environmental information between individuals across space, through volatile compounds (Kessler & Baldwin, 2001), and time (shown here) begins to reveal the complexity of plant-plant communication. Inter-plant communication through TPP and volatile compound communication (Kessler & Baldwin, 2001) both serve to increase the information available to an individual, and in turn increase their ability to produce optimal phenotypes under a given environment. In this light, genetic regulation, plastic responses, and inter-individual communication act in the same vein to maximize the mutual-information between organism phenotype and selective environment (Frank, 2009, English et al., 2015).
While the contribution of transgenerational effects on fitness in nature has numerous ecological implications, the evolutionary significance of TPP depends upon genetic variation for transgenerational plasticity. This study demonstrates the presence of inter-population variation for TPP (Figure 4a and 4b), which, alongside evidence of within population variation for TPP (Galloway & Etterson, 2007), suggests that local environmental patterns of variation can favor genotypes with different capacity to transmit information between generations. M. guttatus trichome induction can be stable across two generations (grandmaternal effects) (Akkerman et al., 2016), bringing up the possibility that there is variation not only in the TPP signal across a single generation, but also in its persistence (Prizak et al., 2014). It could be that populations strongly affected by long-term climatic oscillations such as El Nino may select for the multi-generation stability of TPP. The persistence of M. guttatus trichome induction across at least two generations suggests that future studies considering inter-annual autocorrelations in herbivory and TPP along altitudinal, latitudinal, and climatic autocorrelation clines will be necessary to test the different possibilities presented above. In this study, the use of a replicated circular crossing design in the parental generation was used to limit the confounding effects of multi-generation TPP by ensuring that the progeny of damaged and control plants derived from the same grandparents, but grandparental effects could be responsible for some of the observed mean trichome density differences between populations.
One possible explanation for this system of inherited environmental information is that a portion of the environmentally induced epigenetic changes (such as DNA methylation or histone modifications) are not reset, but rather passed between generations (Verhoeven et al., 2010). Further work is necessary to determine the mechanism through which epigenetic effects are reiterated in the germ line, but evidence for the epigenetic basis of TPP is mounting. Methylation changes in response to environmental stress (Wang et al., 2010, Dowen et al., 2012), stably transmitted epigenetic markings (Rasmann et al., 2012, Slaughter et al., 2012, Schmitz et al., 2013, Li et al., 2014), and epigenetic effects on gene expression (Colicchio et al., 2015a) all point towards epigenetic inheritance as the source of TPP. Additionally, recent work has demonstrated that the erasure of DNA methylation markers reduces or completely erases TPP to wounding (Akkerman et al., 2016) and drought stress (Herman & Sultan, 2016). Additionally, maternal effects due to seed nutrient allocation, morphology, or coating (Galloway, 2001, Luzuriaga et al., 2006) could alter offspring defenses through currently unknown mechanisms.
Adaptive TPP in nature was first demonstrated to play a role in the transition between annual and biennial life history strategies in response to maternal light conditions in Campanulastrum americanum (Galloway & Etterson, 2007, Galloway & Etterson, 2009). Here, utilizing the system of plant herbivore defense, this work gets expanded upon by considering natural TPP variation across multiple populations, measures of offspring fitness and phenotype in field common gardens, and patterns of inter-annual autocorrelation of environmental factors. Future experiments considering the molecular variation in TPP across populations and species, direct measures of inter-annual herbivory variation, and plant secondary metabolite phenotypes in conjunction with trichome density will be necessary to further increase our understanding of how transgenerational plasticity alters biotic interactions in nature.
Supplementary Material
Acknowledgments
I thank J. Kelly, L. Hileman, B. Blackman, Jos. Colicchio, and P. Vogler for valuable comments and critiques. P. Monnahan, N. Kooyers, and N. Handloser for much needed and appreciated aid in the field; Cheryl Friesen and the National Forest Service for permission to perform common garden experiments at Browder Ridge, and Mark Schulze, Oregon State, and the HJ Andrews Experimental Forest for permission to construct common gardens, and live, at HJ Andrews. This study was primarily supported through the University of Kansas Botany endowment and National Institutes of Health grant R01 GM073990 (to J. Kelly). Finally, I would like to thank J. Vogler, R. Vogler, S. Colicchio, and Jo. Colicchio for inspiration, insight, laughs, and heritable material, both genetic and epigenetic.
Literature Cited
- Agrawal AA. Phenotypic Plasticity in the Interactions and Evolution of Species. Science. 2001a;294:321–326. doi: 10.1126/science.1060701. [DOI] [PubMed] [Google Scholar]
- Agrawal AA. Transgenerational consequences of plant responses to herbivory: an adaptive maternal effect? The American Naturalist. 2001b;157:555–569. doi: 10.1086/319932. [DOI] [PubMed] [Google Scholar]
- Agrawal AA. Herbivory and maternal effects: mechanisms and consequences of transgenerational induced plant resistance. Ecology. 2002;83:3408–3415. [Google Scholar]
- Agrawal AA, Laforsch C, Tollrian R. Transgenerational induction of defences in animals and plants. Nature. 1999;401:60–63. [Google Scholar]
- Akkerman KC, Sattarin A, Kelly JK, Scoville AG. Transgenerational plasticity is sex-dependent and persistent in yellow monkeyflower (Mimulus guttatus) Environmental Epigenetics. 2016;2 doi: 10.1093/eep/dvw003. dvw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali JG, Agrawal AA. Specialist versus generalist insect herbivores and plant defense. Trends in plant science. 2012;17:293–302. doi: 10.1016/j.tplants.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biology. 2002;8:1–16. [Google Scholar]
- Ballhorn DJ, Kautz S, Laumann JM. Herbivore damage induces a transgenerational increase of cyanogenesis in wild lima bean (Phaseolus lunatus) Chemoecology. 2016;26:1–5. [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823. 2014 [Google Scholar]
- Beckers GJ, Conrath U. Priming for stress resistance: from the lab to the field. Current opinion in plant biology. 2007;10:425–431. doi: 10.1016/j.pbi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Beckers GJ, Jaskiewicz M, Liu Y, Underwood WR, He SY, Zhang S, Conrath U. Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana . The Plant Cell. 2009;21:944–953. doi: 10.1105/tpc.108.062158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford CP. Ecophysiology of leaf trichomes. Functional Plant Biology. 2016 doi: 10.1071/FP16095. [DOI] [PubMed] [Google Scholar]
- Bossdorf O, Richards CL, Pigliucci M. Epigenetics for ecologists. Ecol Lett. 2008;11 doi: 10.1111/j.1461-0248.2007.01130.x. [DOI] [PubMed] [Google Scholar]
- Boyko A, Blevins T, Yao Y, Golubov A, Bilichak A, Ilnytskyy Y, Hollander J, Meins F, Jr, Kovalchuk I. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS One. 2010;5:e9514. doi: 10.1371/journal.pone.0009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in genetics. 1965;13:115–155. [Google Scholar]
- Calarco JP, Borges F, Donoghue MT, Van Ex F, Jullien PE, Lopes T, Gardner R, Berger F, Feijó JA, Becker JD. Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell. 2012;151:194–205. doi: 10.1016/j.cell.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell H, Reed FC. Plant-herbivore interactions. Oecologia. 1976;26:151–156. doi: 10.1007/BF00582893. [DOI] [PubMed] [Google Scholar]
- Cayuela E, Pérez-Alfocea F, Caro M, Bolarin M. Priming of seeds with NaCl induces physiological changes in tomato plants grown under salt stress. Physiologia Plantarum. 1996;96:231–236. [Google Scholar]
- Clausen JC, Keck DD, Hiesey WM. Experimental Studies on the Nature of Species: Environmental Responses of Climatic Races of Archillea. III. Carnegie Institution of Washington; 1948. [Google Scholar]
- Coley PD, Bryant JP, Chapin FS. Resource availability and plant antiherbivore defense. Science. 1985;230:895–899. doi: 10.1126/science.230.4728.895. [DOI] [PubMed] [Google Scholar]
- Colicchio JM, Miura F, Kelly JK, Ito T, Hileman LC. DNA methylation and gene expression in Mimulus guttatus . BMC genomics. 2015a;16:507. doi: 10.1186/s12864-015-1668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicchio JM, Monnahan PJ, Kelly JK, Hileman LC. Gene expression plasticity resulting from parental leaf damage in Mimulus guttatus . New Phytologist. 2015b;205:894–906. doi: 10.1111/nph.13081. [DOI] [PubMed] [Google Scholar]
- Conrath U. Molecular aspects of defence priming. Trends in plant science. 2011;16:524–531. doi: 10.1016/j.tplants.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Dey S, Proulx SR, Teotonio H. Adaptation to temporally fluctuating environments by the evolution of maternal effects. PLoS Biol. 2016;14:e1002388. doi: 10.1371/journal.pbio.1002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelson J, Munday P, McCormick M, Pitcher C. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nature Climate Change. 2012;2:30–32. [Google Scholar]
- Dowen RH, Pelizzola M, Schmitz RJ, Lister R, Dowen JM, Nery JR, Dixon JE, Ecker JR. Widespread dynamic DNA methylation in response to biotic stress. Proceedings of the National Academy of Sciences. 2012;109:E2183–E2191. doi: 10.1073/pnas.1209329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichten SR, Schmitz RJ, Springer NM. Epigenetics: Beyond Chromatin Modifications and Complex Genetic Regulation. Plant Physiol. 2014;165:933–947. doi: 10.1104/pp.113.234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English S, Pen I, Shea N, Uller T. The information value of non-genetic inheritance in plants and animals. PloS one. 2015;10:e0116996. doi: 10.1371/journal.pone.0116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist L, Reinhold K. Adaptive trans-generational phenotypic plasticity and the lack of an experimental control in reciprocal match/mismatch experiments. Methods in Ecology and Evolution. 2016 [Google Scholar]
- Fox CW, Mousseau TA. Maternal effects as adaptations for transgenerational phenotypic plasticity in insects. Maternal effects as adaptations. 1998;159 [Google Scholar]
- Frank SA. Natural selection maximizes Fisher information. Journal of Evolutionary Biology. 2009;22:231–244. doi: 10.1111/j.1420-9101.2008.01647.x. [DOI] [PubMed] [Google Scholar]
- Freitak D, Heckel DG, Vogel H. Dietary-dependent trans-generational immune priming in an insect herbivore. Proceedings of the Royal Society of London B: Biological Sciences. 2009;276:2617–2624. doi: 10.1098/rspb.2009.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz RS, Simms EL. Plant resistance to herbivores and pathogens: ecology, evolution, and genetics. University of Chicago Press; 1992. [Google Scholar]
- Galloway LF. The effect of maternal and paternal environments on seed characters in the herbaceous plant Campanula americana (Campanulaceae) American Journal of Botany. 2001;88:832–840. [PubMed] [Google Scholar]
- Galloway LF, Etterson JR. Transgenerational plasticity is adaptive in the wild. Science. 2007;318:1134–1136. doi: 10.1126/science.1148766. [DOI] [PubMed] [Google Scholar]
- Galloway LF, Etterson JR. Plasticity to canopy shade in a monocarpic herb: within-and between-generation effects. New Phytologist. 2009;182:1003–1012. doi: 10.1111/j.1469-8137.2009.02803.x. [DOI] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nature neuroscience. 2014;17:667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg MV, Deleris A, Hale CJ, Liu A, Feng S, Jacobsen SE. Interplay between active chromatin marks and RNA-directed DNA methylation in Arabidopsis thaliana . PLoS Genet. 2013;9:e1003946. doi: 10.1371/journal.pgen.1003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot MP, Kooke R, Knoben N, Vergeer P, Keurentjes JJ, Ouborg NJ, Verhoeven KJ. Effects of multi-generational stress exposure and offspring environment on the expression and persistence of transgenerational effects in Arabidopsis thaliana . PloS one. 2016;11:e0151566. doi: 10.1371/journal.pone.0151566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick MF, Finseth FR, Mathiasson ME, Palmer KA, Broder EM, Breigenzer P, Fishman L. The genetics of extreme microgeographic adaptation: an integrated approach identifies a major gene underlying leaf trichome divergence in Yellowstone Mimulus guttatus . Molecular Ecology. 2016 doi: 10.1111/mec.13753. [DOI] [PubMed] [Google Scholar]
- Herman JJ, Spencer HG, Donohue K, Sultan SE. How stable ‘should’ epigenetic modifications be? Insights from adaptive plasticity and bet hedging. Evolution. 2013 doi: 10.1111/evo.12324. [DOI] [PubMed] [Google Scholar]
- Herman JJ, Spencer HG, Donohue K, Sultan SE. How stable ‘should’epigenetic modifications be? Insights from adaptive plasticity and bet hedging. Evolution. 2014;68:632–643. doi: 10.1111/evo.12324. [DOI] [PubMed] [Google Scholar]
- Herman JJ, Sultan SE. Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Frontiers in plant science. 2011;2:102. doi: 10.3389/fpls.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JJ, Sultan SE. Proc. R. Soc. B. Vol. 283. The Royal Society; 2016. DNA methylation mediates genetic variation for adaptive transgenerational plasticity; p. 20160988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JJ, Sultan SE, Horgan-Kobelski T, Riggs C. Adaptive transgenerational plasticity in an annual plant: grandparental and parental drought stress enhance performance of seedlings in dry soil. Integrative and comparative biology. 2012;52:77–88. doi: 10.1093/icb/ics041. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Bazaga P. Epigenetic correlates of plant phenotypic plasticity: DNA methylation differs between prickly and nonprickly leaves in heterophyllous Ilex aquifolium (Aquifoliaceae) trees. Botanical Journal of the Linnean Society. 2013;171:441–452. [Google Scholar]
- Holeski L. Within and between generation phenotypic plasticity in trichome density of Mimulus guttatus . Journal of Evolutionary Biology. 2007;20:2092–2100. doi: 10.1111/j.1420-9101.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- Holeski L, Keefover-Ring K, Bowers MD, Harnenz Z, Lindroth R. Patterns of Phytochemical Variation in Mimulus guttatus (Yellow Monkeyflower) Journal of Chemical Ecology. 2013;39:525–536. doi: 10.1007/s10886-013-0270-7. [DOI] [PubMed] [Google Scholar]
- Holeski LM, Chase-Alone R, Kelly JK. The genetics of phenotypic plasticity in plant defense: trichome production in Mimulus guttatus . The American Naturalist. 2010;175:391–400. doi: 10.1086/651300. [DOI] [PubMed] [Google Scholar]
- Holeski LM, Jander G, Agrawal AA. Transgenerational defense induction and epigenetic inheritance in plants. Trends in ecology & evolution. 2012;27:618–626. doi: 10.1016/j.tree.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Hoyle RB, Ezard TH. The benefits of maternal effects in novel and in stable environments. Journal of The Royal Society Interface. 2012;9:2403–2413. doi: 10.1098/rsif.2012.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka E. Epigenetic inheritance and plasticity: the responsive germline. Progress in Biophysics and Molecular Biology. 2013;111:99–107. doi: 10.1016/j.pbiomolbio.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Jactel H, Petit J, Desprez-Loustau ML, Delzon S, Piou D, Battisti A, Koricheva J. Drought effects on damage by forest insects and pathogens: a meta-analysis. Global Change Biology. 2012;18:267–276. [Google Scholar]
- Johnson MTJ, Bertrand JA, Turcotte MM. Precision and accuracy in quantifying herbivory. Ecological Entomology. 2015;41:112–121. [Google Scholar]
- Keefover-Ring K, Holeski LM, Bowers MD, Clauss AD, Lindroth RL. Phenylpropanoid glycosides of Mimulus guttatus (yellow monkeyflower) Phytochemistry Letters. 2014;10:132–139. [Google Scholar]
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Klapwijk MJ, Csóka G, Hirka A, Björkman C. Forest insects and climate change: long-term trends in herbivore damage. Ecology and evolution. 2013;3:4183–4196. doi: 10.1002/ece3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooyers NJ. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Science. 2015;234:155–162. doi: 10.1016/j.plantsci.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Kuijper B, Hoyle RB. When to rely on maternal effects and when on phenotypic plasticity? Evolution. 2015;69:950–968. doi: 10.1111/evo.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper B, Johnstone RA, Townley S. The evolution of multivariate maternal effects. PLoS Comput Biol. 2014;10:e1003550. doi: 10.1371/journal.pcbi.1003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster LT, McAdam AG, Wingfield JC, Sinervo BR. Adaptive social and maternal induction of antipredator dorsal patterns in a lizard with alternative social strategies. Ecology Letters. 2007;10:798–808. doi: 10.1111/j.1461-0248.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Lang-Mladek C, Popova O, Kiok K, Berlinger M, Rakic B, Aufsatz W, Jonak C, Hauser M-T, Luschnig C. Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in Arabidopsis . Molecular plant. 2010;3:594–602. doi: 10.1093/mp/ssq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimar O, McNamara JM. The evolution of transgenerational integration of information in heterogeneous environments. The American Naturalist. 2015;185:E55–E69. doi: 10.1086/679575. [DOI] [PubMed] [Google Scholar]
- Lemoine NP, Burkepile DE, Parker JD. Variable effects of temperature on insect herbivory. PeerJ. 2014;2:e376. doi: 10.7717/peerj.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn E, Gijzen M, Croteau R. Defense Mechanisms of Conifers: Differences in Constitutive and Wound-Induced Monoterpene Biosynthesis Among Species. Plant Physiology. 1991;96:44–49. doi: 10.1104/pp.96.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Eichten SR, Hermanson PJ, Springer NM. Inheritance Patterns and Stability of DNA Methylation Variation in Maize Near-Isogenic Lines. Genetics. 2014;196:667–676. doi: 10.1534/genetics.113.158980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luus K, Gel Y, Lin J, Kelly R, Duguay C. Pan-Arctic linkages between snow accumulation and growing-season air temperature, soil moisture and vegetation. Biogeosciences. 2013;10:7575–7597. [Google Scholar]
- Luzuriaga A, Escudero A, Perez-Garcia F. Environmental maternal effects on seed morphology and germination in Sinapis arvensis (Cruciferae) Weed Research. 2006;46:163–174. [Google Scholar]
- Matzke MA, Mosher RA. RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nature Reviews Genetics. 2014;15:394–408. doi: 10.1038/nrg3683. [DOI] [PubMed] [Google Scholar]
- Mauricio R. Costs of resistance to natural enemies in field populations of the annual plant Arabidopsis thaliana . The American Naturalist. 1998;151:20–28. doi: 10.1086/286099. [DOI] [PubMed] [Google Scholar]
- Mauricio R, Rausher MD. Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution. 1997;51:1435–1444. doi: 10.1111/j.1558-5646.1997.tb01467.x. [DOI] [PubMed] [Google Scholar]
- McCue AD, Nuthikattu S, Reeder SH, Slotkin RK. Gene expression and stress response mediated by the epigenetic regulation of a transposable element small RNA. PLoS genetics. 2012;8:e1002474. doi: 10.1371/journal.pgen.1002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKey D. Adaptive patterns in alkaloid physiology. American Naturalist. 1974:305–320. [Google Scholar]
- Mojica JP, Lee YW, Willis JH, Kelly JK. Spatially and temporally varying selection on intrapopulation quantitative trait loci for a life history trade-off in Mimulus guttatus . Molecular ecology. 2012;21:3718–3728. doi: 10.1111/j.1365-294X.2012.05662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnahan PJ, Colicchio J, Kelly JK. A genomic selection component analysis characterizes migration-selection balance. Evolution. 2015;69:1713–1727. doi: 10.1111/evo.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnahan PJ, Kelly JK. Naturally segregating loci exhibit epistasis for fitness. Biology Letters. 2015;11:20150498. doi: 10.1098/rsbl.2015.0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira X, Mooney KA, Rasmann S, Petry WK, Carrillo-Gavilán A, Zas R, Sampedro L, Novotny V. Trade-offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecology Letters. 2014;17:537–546. doi: 10.1111/ele.12253. [DOI] [PubMed] [Google Scholar]
- Pederson GT, Gray ST, Woodhouse CA, Betancourt JL, Fagre DB, Littell JS, Watson E, Luckman BH, Graumlich LJ. The unusual nature of recent snowpack declines in the North American Cordillera. Science. 2011;333:332–335. doi: 10.1126/science.1201570. [DOI] [PubMed] [Google Scholar]
- Petchey OL. Environmental colour affects aspects of single-species population dynamics. Proceedings of the Royal Society of London B: Biological Sciences. 2000;267:747–754. doi: 10.1098/rspb.2000.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prizak R, Ezard TH, Hoyle RB. Fitness consequences of maternal and grandmaternal effects. Ecology and evolution. 2014;4:3139–3145. doi: 10.1002/ece3.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmann S, De Vos M, Casteel CL, Tian D, Halitschke R, Sun JY, Agrawal AA, Felton GW, Jander G. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant physiology. 2012;158:854–863. doi: 10.1104/pp.111.187831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson EM, Wallace JM. Meteorological aspects of the El Nino/southern oscillation. Science. 1983;222:1195–1202. doi: 10.1126/science.222.4629.1195. [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis . The Plant Cell. 2000;12:707–719. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick D, Travis J. Adaptation. San Diego, CA: Academic Press; 1996. The empirical study of adaptation in natural populations; pp. 243–289. [Google Scholar]
- Romeis J, Shanower T, Peter A. Trichomes on pigeonpea [Cajanus cajan (L.) Millsp.] and two wild Cajanus spp. Crop science. 1999;39:564–569. [Google Scholar]
- Roubik DW. The ecological impact of nectar-robbing bees and pollinating hummingbirds on a tropical shrub. Ecology. 1982;63:354–360. [Google Scholar]
- Sakai A, Larcher W. Frost survival of plants: responses and adaptation to freezing stress. Springer Science & Business Media; 2012. [Google Scholar]
- Schmitt J, Niles J, Wulff RD. Norms of reaction of seed traits to maternal environments in Plantago lanceolata . American Naturalist. 1992:451–466. [Google Scholar]
- Schmitz RJ, He Y, Valdes-Lopez O, Khan SM, Joshi T, Urich MA, Nery JR, Diers B, Xu D, Stacey G, Ecker JR. Epigenome-wide inheritance of cytosine methylation variants in a recombinant inbred population. Genome Res. 2013;23:1663–74. doi: 10.1101/gr.152538.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville AG, Barnett LL, Bodbyl-Roels S, Kelly JK, Hileman LC. Differential regulation of a MYB transcription factor is correlated with transgenerational epigenetic inheritance of trichome density in Mimulus guttatus . New Phytologist. 2011;191:251–263. doi: 10.1111/j.1469-8137.2011.03656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter A, Daniel X, Flors V, Luna E, Hohn B, Mauch-Mani B. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant physiology. 2012;158:835–843. doi: 10.1104/pp.111.191593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss SY, Agrawal AA. The ecology and evolution of plant tolerance to herbivory. Trends in Ecology & Evolution. 1999;14:179–185. doi: 10.1016/s0169-5347(98)01576-6. [DOI] [PubMed] [Google Scholar]
- Sultan S. Phenotypic plasticity for offspring traits in Polygonum persicaria . Ecology. 1996;77:1791–1807. [Google Scholar]
- Sultan SE. Organism and Environment: Ecological Development, Niche Construction, and Adaptation. USA: Oxford University Press; 2015. [Google Scholar]
- Sunkar R, Chinnusamy V, Zhu JH, Zhu JK. Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends in Plant Science. 2007;12:301–309. doi: 10.1016/j.tplants.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Swanson BJ. Autocorrelated rates of change in animal populations and their relationship to precipitation. Conservation Biology. 1998;12:801–808. [Google Scholar]
- Team RC. R: A language and environment for statistical computing. 2013 [Google Scholar]
- Thaler JS, Stout MJ, Karban R, Duffey SS. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. Journal of Chemical Ecology. 1996;22:1767–1781. doi: 10.1007/BF02028503. [DOI] [PubMed] [Google Scholar]
- Tiffin P. Are tolerance, avoidance, and antibiosis evolutionarily and ecologically equivalent responses of plants to herbivores? The American Naturalist. 2000;155:128–138. doi: 10.1086/303301. [DOI] [PubMed] [Google Scholar]
- Turchin P. Rarity of density dependence or population regulation with lags? Nature. 1990;344:660–663. [Google Scholar]
- Uller T, English S, Pen I. Proc. R. Soc. B. Vol. 282. The Royal Society; 2015. When is incomplete epigenetic resetting in germ cells favoured by natural selection? p. 20150682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meijden E. Proceedings of the 9th International Symposium on Insect-Plant Relationships. Springer; 1996. Plant defence, an evolutionary dilemma: contrasting effects of (specialist and generalist) herbivores and natural enemies; pp. 307–310. [Google Scholar]
- Verhoeven KJ, Jansen JJ, van Dijk PJ, Biere A. Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytologist. 2010;185:1108–1118. doi: 10.1111/j.1469-8137.2009.03121.x. [DOI] [PubMed] [Google Scholar]
- Verhoeven KJ, van Gurp TP. Transgenerational effects of stress exposure on offspring phenotypes in apomictic dandelion. PloS one. 2012;7:e38605–e38605. doi: 10.1371/journal.pone.0038605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via S, Gomulkiewicz R, De Jong G, Scheiner SM, Schlichting CD, Van Tienderen PH. Adaptive phenotypic plasticity: consensus and controversy. Trends in Ecology & Evolution. 1995;10:212–217. doi: 10.1016/s0169-5347(00)89061-8. [DOI] [PubMed] [Google Scholar]
- Vu WT, Chang PL, Moriuchi KS, Friesen ML. Genetic variation of transgenerational plasticity of offspring germination in response to salinity stress and the seed transcriptome of Medicago truncatula . BMC evolutionary biology. 2015;15:1. doi: 10.1186/s12862-015-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M, Walker D, Welker J, Arft A, Bardsley T, Brooks P, Fahnestock J, Jones M, Losleben M, Parsons A. Long-term experimental manipulation of winter snow regime and summer temperature in arctic and alpine tundra. Hydrological processes. 1999;13:2315–2330. [Google Scholar]
- Wang W-S, Pan Y-J, Zhao X-Q, Dwivedi D, Zhu L-H, Ali J, Fu B-Y, Li Z-K. Drought-induced site-specific DNA methylation and its association with drought tolerance in rice (Oryza sativa L.) Journal of experimental botany. 2010 doi: 10.1093/jxb/erq391. erq391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard MJ. Phenotypic Plasticity and the Origins of Diversity. Annual Review of Ecology and Systematics. 1989;20:249–278. [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. Oxford University Press; 2003. [Google Scholar]
- Więski K, Pennings S. Latitudinal variation in resistance and tolerance to herbivory of a salt marsh shrub. Ecography. 2014;37:763–769. [Google Scholar]
- Wilkens RT, Shea GO, Halbreich S, Stamp NE. Resource availability and the trichome defenses of tomato plants. Oecologia. 1996;106:181–191. doi: 10.1007/BF00328597. [DOI] [PubMed] [Google Scholar]
- Woestmann L, Saastamoinen M. The importance of trans-generational effects in Lepidoptera. Current Zoology. 2016 doi: 10.1093/cz/zow029. zow029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangerl AR, Berenbaum MR. Furanocoumarin Induction in Wild Parsnip: Genetics and Population Variation. Ecology. 1990;71:1933–1940. [Google Scholar]
- Zangerl AR, Rutledge CE. The probability of attack and patterns of constitutive and induced defense: a test of optimal defense theory. American Naturalist. 1996:599–608. [Google Scholar]
- Zomer RJ, Trabucco A, Bossio DA, Verchot LV. Climate change mitigation: A spatial analysis of global land suitability for clean development mechanism afforestation and reforestation. Agriculture, ecosystems & environment. 2008;126:67–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.