Abstract
We evaluated the impact of revised Clinical and Laboratory Standards Institute (CLSI) breakpoints for broad-spectrum cephalosporins (BSCs) on the susceptibilities of 1,742 isolates of Enterobacter species, Serratia marcescens, Citrobacter freundii, and Morganella morganii. The 2011 CLSI criteria for cefotaxime and ceftazidime reduced the rates of susceptibility by 2.9% and 5.9%, respectively. The 2014 CLSI criteria for cefepime reduced the rate of susceptibility by 13.9%, and categorized 11.8% isolates as susceptible-dose dependent (SDD) for cefepime. Among 183 isolates with extended-spectrum ß-lactamase (ESBL) phenotype, implementation of the new criteria reduced the rates of susceptibility to cefotaxime, ceftazidime, and cefepime by 2.8%, 14.8%, and 53.6%, respectively. The proportion of ESBL phenotype among BSC-susceptible isolates was low (0.9% for cefotaxime, 3.0% for ceftazidime, and 3.3% for cefepime). In summary, implementation of new CLSI criteria led to little change in susceptibility to cefotaxime and ceftazidime but a substantial change in susceptibility to cefepime. The recognition of revised CLSI criteria for BSC and SDD will help clinicians to select the optimal antibiotic and dosing regimen.
Keywords: Cephalosporin, Susceptibility, Enterobacteriaceae
Broad-spectrum cephalosporin is a commonly utilized antimicrobial agent for empirical and directed therapy against infections involving Enterobacteriaceae. The Clinical and Laboratory Standards Institute (CLSI) recently revised the cephalosporin breakpoints against Enterobacteriaceae (Table 1). Prior to 2011, the CLSI susceptibility breakpoints for cefotaxime and ceftazidime were ≤8 μg/mL for Enterobacteriaceae, but they were reduced in January 2011 to ≤1 μg/mL for cefotaxime and ≤4 μg/mL for ceftazidime [1]. Prior to 2014, the CLSI susceptibility breakpoints for cefepime was ≤8 μg/mL for Enterobacteriaceae, but in January 2014, it was reduced to ≤2 μg/mL [2]. Along with this breakpoint revision for cefepime, the CLSI introduced the Enterobacteriaceae susceptible-dose dependent (SDD) category with a breakpoint of 4–8 μg/mL in order to encourage clinicians to use higher doses for organisms with higher minimum inhibitory concentrations (MICs) [2]. The background and rationale of this revision for susceptibility criteria is mainly based on the data for Escherichia coli and Klebsiella species [3].
Table 1. Comparison of the susceptibility rates for revised and previous clinical and laboratory standards institute (CLSI) for broad-spectrum cephalosporins for Enterobacteriaceae a .
| Drug | MIC Interpretive Criteria (µg/mL) | Zone Diameter Interpretive Criteria (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| S | SDD | I | R | S | SDD | I | R | |
| Cefotaxime | ||||||||
| Previous | ≤8 | - | 16–32 | ≥64 | ≥23 | - | 15–22 | ≤14 |
| Revised | ≤1 | - | 2 | ≥4 | ≥26 | - | 23–25 | ≤22 |
| Ceftazidime | ||||||||
| Previous | ≤8 | - | 16–32 | ≥64 | ≥18 | - | 15–17 | ≤14 |
| Revised | ≤4 | - | 8 | ≥16 | ≥21 | - | 18–20 | ≤17 |
| Cefepime | ||||||||
| Previous | ≤8 | - | 16 | ≥32 | ≥18 | - | 15–17 | ≤14 |
| Revised | ≤2 | 4–8 | - | ≥16 | ≥25 | 19–24 | - | ≤18 |
Enterobacter species, Serratia marcescens, Citrobacter freundii, and Morganella morganii have emerged as major causes of nosocomial infections. These organisms are characterized by inducible resistance that is mediated by the chromosomal AmpC β-lactamase [4]. Limited data exist on the impact of revised cephalosporin CLSI breakpoints on susceptibility in these organisms. Hence, the aim of the study was to evaluate the impact of new susceptibility criteria on the reported broad-spectrum cephalosporin susceptibility profiles for Enterobacteriaceae producing AmpC β-lactamase.
This study was performed using samples collected in our previous prospective cohort studies at the Asan Medical Center, Seoul, Republic of Korea [5,6]. This 2,700-bed university-affiliated teaching hospital provides both primary and tertiary care and has an average of approximately 124,000 annual patient discharges and 2,000,000 outpatient visits. Between January 2005 and December 2008, we collected clinical isolates of Enterobacter species, S. marcescens, C. freundii, and M. morganii.
Blood samples were cultured using the BACTEC 9240 system (Becton Dickinson, Sparks, MD, USA). Identification of the bacterial isolates was performed using the MicroScan system (Dade Behring, West Sacramento, CA, USA). Antimicrobial susceptibility tests for cefotaxime, ceftazidime, and cefepime were performed with Kirby-Bauer disk diffusion method as recommended in the CLSI guideline [1]. Extended-spectrum ß-lactamase (ESBL) production was tested using the double disk synergy test. Briefly, the inhibitory zone diameters of disks containing 30 μg of cefotaxime, ceftazidime, or cefepime, either alone or in combination with 10 μg of clavulanate, were compared [6,7,8,9]. An ESBL production phenotype was defined as a ≥5 mm increase in zone diameter for at least one of the combination disks relative to its corresponding single antibiotic disk [6,7,8,9]. The E. coli strain, ATCC 25922 (American Type Culture Collection, Georgetown, DC, USA) and the Klebsiella pneumoniae strain, ATCC 700603, were used as negative and positive controls for ESBL production, respectively.
During the study period, a total of 1,742 clinical isolates were collected. Of the 1,742 isolates, 1,056 (60.6%) were Enterobacter species (651 E. cloacae, 376 E. aerogenes, 19 E. agglomerans, 5 E. asburiae, 4 E. gergoviae, and 1 E. sakazakii), 318 (18.3%) were Citrobacter species (304 C. freundii, 10 C. koseri, and C. amalonaticus), 230 (13.2%) were S. marcescens, and 138 (7.9%) were M. morganii.
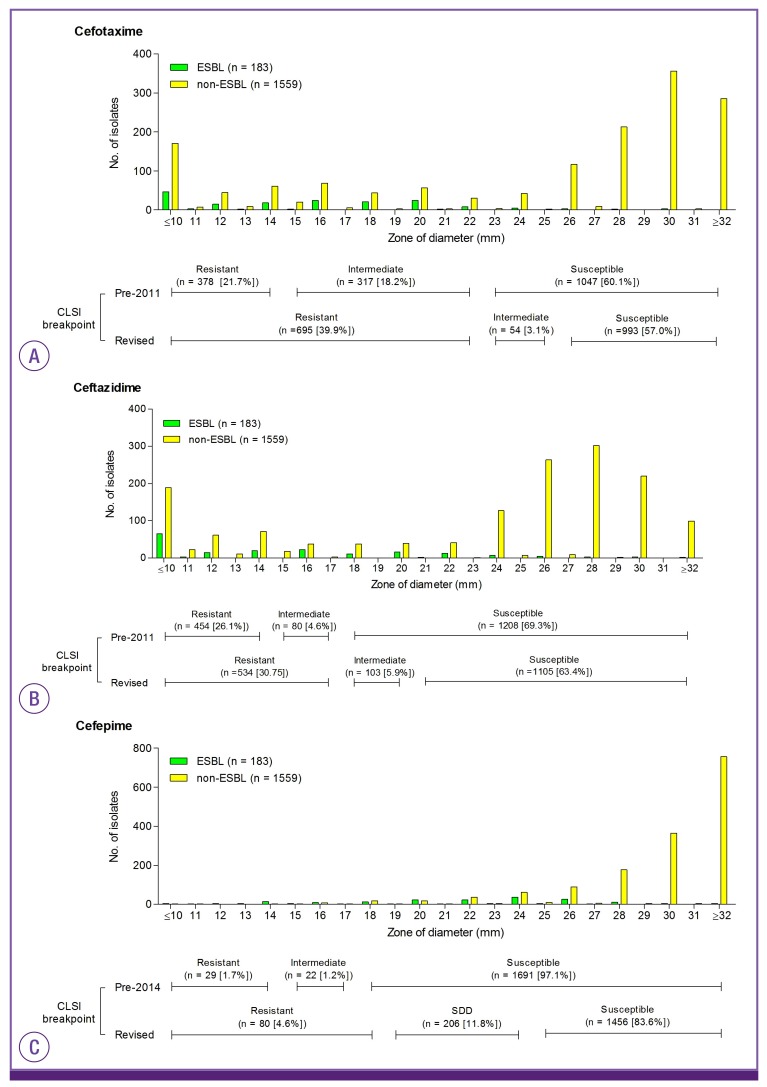
The results for 1,742 Enterobacteriaceae isolates were analyzed (Table 2). Implementation of the new interpretative criteria reduced the rates of susceptibility to cefotaxime, ceftazidime, and cefepime by 2.9%, 5.9%, and 13.6%, respectively (Table 2). The distribution of the zone diameters around the cefotaxime, ceftazidime, and cefepime disk is displayed in Fig. 1. The difference in the categorization between the old and new interpretative criteria can largely be attributed to a decrease in the number of isolates in the intermediate range and an increase in the number of isolates in the resistant range (Fig. 1). Of 1,742 isolates, 206 (11.8%) isolates were categorized as SDD for cefepime, according to the revised criteria. To assess how this revision might have affected therapeutic decisions, we further analyzed the categorization for the subset of isolates with ESBL phenotype. Among 183 isolates with ESBL phenotype, implementation of the new interpretative criteria reduced the rates of susceptibility to cefotaxime, ceftazidime, and cefepime by 2.8%, 14.8%, and 53.6%, respectively (Table 2). Eighty-six (47.0%) isolates were categorized as SDD for cefepime, according to the new criteria. The prevalence of ESBL phenotype for cefotaxime-, ceftazidime-, and cefepime-susceptible isolates was 0.9% (9/993), 3.0% (33/1,105), and 3.3% (48/1,456), respectively.
Table 2. Comparison of the susceptibility rates for isolates to broad-spectrum cephalosporin using the previous and new clinical and laboratory standards institute (CLSI) interpretative criteria.
| Agent, specimen source, and ESBL phenotype | Total, No. | % susceptibility to cefotaxime | Difference in % susceptible | % susceptibility to ceftazidime | Difference in % susceptible | % susceptibility to cefepime | Difference in % susceptible | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLSI 2010a | CLSI 2011a | CLSI 2010a | CLSI 2011a | CLSI 2013b | CLSI 2014b | |||||||||||||||||
| S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | SDD | R | |||||
| All cases | 1,742 | 60.1 | 18.2 | 21.7 | 57 | 3.1 | 39.9 | −3.1 | 69.3 | 4.6 | 26.1 | 63.4 | 5.9 | 30.7 | −5.9 | 97.1 | 1.2 | 1.7 | 83.6 | 11.8 | 4.6 | −13.5 |
| Bacterial groups | ||||||||||||||||||||||
| Enterobacter species | 1,056 | 56.7 | 18.8 | 24.5 | 53.5 | 3.2 | 43.3 | −3.2 | 64.6 | 5.0 | 30.4 | 59.1 | 5.5 | 35.4 | −5.5 | 96.9 | 1.3 | 1.8 | 80.4 | 14.4 | 5.2 | −16.5 |
| Citrobacter species | 318 | 55.0 | 17.9 | 27.0 | 51.6 | 3.5 | 45.0 | −3.4 | 61.9 | 5.7 | 32.4 | 55.7 | 6.3 | 38.1 | −6.2 | 96.5 | 1.9 | 1.6 | 85.5 | 9.1 | 5.4 | −11.0 |
| Serratia marcescens | 230 | 73.9 | 16.1 | 10.0 | 70.4 | 3.5 | 26.1 | −3.5 | 93.0 | 1.3 | 5.7 | 87.4 | 5.7 | 7.0 | −5.6 | 96.9 | 0.9 | 2.2 | 86.5 | 10.0 | 3.5 | −10.4 |
| Morganella morganii | 138 | 74.6 | 18.1 | 7.2 | 73.9 | 0.7 | 25.4 | −0.7 | 83.3 | 4.3 | 12.3 | 74.6 | 8.7 | 16.7 | −8.7 | 100 | 0 | 0 | 98.6 | 1.4 | 0 | −1.4 |
| Specimens types | ||||||||||||||||||||||
| Blood | 429 | 58.7 | 16.3 | 24.9 | 55.9 | 2.8 | 41.3 | −2.8 | 67.1 | 2.6 | 30.3 | 60.8 | 6.3 | 32.9 | −6.3 | 95.6 | 2.8 | 1.6 | 80.4 | 12.4 | 7.2 | −15.2 |
| Urine | 493 | 68.0 | 17.4 | 14.6 | 64.1 | 3.9 | 32.0 | −3.9 | 75.8 | 4.5 | 19.7 | 70.6 | 5.3 | 24.1 | −5.3 | 97.6 | 0.8 | 1.6 | 88.0 | 9.3 | 2.7 | −9.5 |
| Sputum | 312 | 64.7 | 19.9 | 15.4 | 60.9 | 3.8 | 35.3 | −3.8 | 76.6 | 4.2 | 19.2 | 70.5 | 6.1 | 23.4 | −6.1 | 98.7 | 0.3 | 1.0 | 88.0 | 9.3 | 2.6 | −10.7 |
| Wound discharge | 96 | 64.6 | 18.8 | 16.7 | 63.5 | 1.0 | 35.4 | −1.0 | 76.1 | 3.1 | 20.8 | 69.8 | 6.2 | 24.0 | −6.2 | 97.9 | 0 | 2.1 | 88.0 | 9.3 | 2.6 | −9.9 |
| Other | 412 | 47.6 | 19.7 | 32.8 | 45.1 | 2.4 | 52.4 | −2.4 | 56.8 | 7.5 | 35.7 | 50.7 | 6.1 | 43.2 | −6.1 | 96.6 | 1.2 | 2.2 | 88.0 | 9.3 | 2.7 | −8.6 |
| ESBL phenotype | ||||||||||||||||||||||
| ESBL positive | 183 | 7.7 | 45.9 | 46.4 | 4.9 | 2.7 | 92.3 | −2.8 | 32.8 | 12.0 | 55.2 | 18.0 | 14.8 | 67.2 | −14.8 | 79.8 | 7.1 | 13.1 | 26.2 | 47.0 | 26.8 | −53.6 |
| ESBL negative | 1,559 | 66.3 | 14.9 | 18.8 | 63.1 | 3.1 | 33.7 | −3.2 | 73.7 | 3.7 | 22.6 | 68.7 | 4.9 | 26.4 | −4.8 | 99.1 | 0.6 | 0.3 | 90.3 | 7.7 | 2.0 | −8.8 |
ESBL, extended-spectrum ß-lctamase; S, susceptible; I, intermediate; R, resistant; SDD, susceptible-dose dependent.
aAccording to the interpretative criteria published by the CLSI in January 2010 (M100-S20) and January 2011 (M100-S21).
bAccording to the interpretative criteria published by the CLSI in January 2013 (M100-S23) and January 2014 (M100-S24).
Figure 1. Distribution of zone of diameters around the cefotaxime (A), ceftazidime (B), and cefepime (C).
ESBL, extended-spectrum ß-lactamase; CLSI, clinical and laboratory standards institute; SDD, susceptible-dose dependent.
The revised CLSI document (M23) specifically outlines several reasons for the reassessment of susceptibility breakpoints that were applied for cephalosporins, including new resistance mechanisms, new pharmacokinetic/pharmacodynamics (PK-PD) data, and elimination of differences in breakpoints proposed by CLSI and those by other organizations (the European Committee on Antimicrobial Susceptibility Testing [EUCAST]) and the Food and Drug Administration [FDA]) [3]. Using ESBL-producing isolates in mouse models of infection, PK-PD relationships for efficacy of cephalosporins against ESBL- or non-ESBL-producing isolates were similar, irrespective of ESBL production [10]. In addition, new breakpoints would simplify testing and eliminate the need for additional tests to detect specific resistance mechanisms such as those associated with ESBL. Of 42 patients infected with ESBL-producing E. coli and Klebsiella species bacteremia, reduced clinical response was associated with increasing MIC, with a considerable decrease in efficacy for cephalosporin at MIC of >2 mg/L [10]. When using the revised breakpoints (MIC ≤1 for cefotaxime and ceftriaxone), routine testing for ESBLs (screen plus phenotypic confirmation test) is no longer required.
A recent study showed that when the revised CLSI breakpoints were applied to 264 isolates of E. coli and Klebsiella species, the rates of susceptibility to cefotaxime and ceftazidime were reduced by 8% and 3%, respectively [11]. Similarly, we found that the revised susceptibility breakpoint criteria led to little change in the rates of susceptibility of cefotaxime (0.9%) and ceftazidime (3.0%) to Enterobacteriaceae producing AmpC β-lactamase. Moreover, another study showed that the revised cefepime CLSI breakpoints resulted in only ≤ 2% reduction in cefepime susceptibility of E. coli and K. pneumoniae, with 1–2% in the SDD category [12]. In contrast, we found that applying the new cefepime CLSI criteria led to a substantial change in the susceptibility rates of cefepime against Enterobacteriaceae producing AmpC β-lactamase. This finding is in line with the recent results by Lee et al. [13]. They reported that 63.6% of 217 E. cloacae blood isolates were cefepime-susceptible and 19.8% were cefepime-SDD [13]. Hence, laboratories that choose to implement the new CLSI criteria for Enterobacteriaceae should clearly inform treating physicians regarding the SDD category, so that they can be alert to the possible need for higher doses of cefepime in treating infections caused by these organisms. In addition, clinicians should note that cefepime, even at the recommended higher doses, may be inefficient against Enterobacteriaceae isolates for which the MIC is >2 μg/mL. In patients with ESBL-producing Enterobacteriaceae infection who received cefepime, the crude mortality rate was higher for isolates with a MIC of 2–8 μg/mL than for those with a MIC of ≤1 μg/mL (68.8% versus 30.0%; P = 0.045] [14]. Of patients with E. cloacae bacteremia who were treated with definite cefepime therapy, 30-day mortality rate of those infected by cefepime-susceptible isolates was significantly lower than that of patients infected by cefepime-SDD isolates (16.1% [9/56] versus 62.5% [10/16]; P <0.001)[13].
No guidelines have been issued for ESBL detection in members of the Enterobacteriaceae with inducible chromosomal AmpC β-lactamase although these species may have emerged as major causes of nosocomial infections. The absence of such recommendations likely has two reasons. First, phenotypic detection of ESBLs in members of the Enterobacteriaceae producing an AmpC β-lactamase is complex, because AmpC expression may mask the synergy required for ESBL detection between broad-spectrum cephalosporins and clavulanic acid. Here, we tried to circumvent this problem by demonstrating synergy between clavulanic acid and cefepime, a fourth-generation cephalosporin hydrolyzed by ESBLs but generally not by AmpC β-lactamase [7,8]. Second, unlike E. coli and Klebsiella species, ESBL production was not associated with poorer outcomes in patients with Enterobacter species bacteremia [15,16]. In addition, our study showed that the prevalence of ESBL phenotype for cefotaxime-, ceftazidime-, and cefepime-susceptible isolates was low (0.9%, 3.0%, and 3.3%, respectively). The proportion of broad-spectrum cephalosporin-susceptible isolates among ESBL isolates was markedly decreased after application of revised susceptibility criteria. Taken together, our results support the CLSI recommendation that routine testing for ESBLs (screening plus phenotypic confirmation test) is no longer required for Enterobacteriaceae isolates.
In conclusion, implementation of the new CLSI susceptibility breakpoints leads to a small change in cefotaxime and ceftazidime susceptibility of Enterobacteriaceae producing AmpC β-lactamase. However, a substantial proportion of isolates were in the SDD category for cefepime, and clinicians should be alert to the possible need for higher doses in treating infections caused by these organisms. The rarity of ESBL phenotype among broad-spectrum cephalosporin-susceptible isolates based on the revised criteria eliminated the need for ESBL screening and phenotypical confirmation for broad-spectrum cephalosporin-susceptible isolates. Further studies should be performed to evaluate the impact of revised broad-spectrum cephalosporin CLSI susceptibility criteria on clinical management of infections caused by these organisms.
Footnotes
This work was supported by a grant from Korean Society for Chemotherapy in 2014.
Conflict of Interest: No conflicts of interest.
References
- 1.Clinical and Laboratory Standards Institue (CLSI) Performance standards for antimicrobial susceptibility resting: 21th infomational supplment CLSI document M100-S21. Wayne, PA: CLSI; 2011. [Google Scholar]
- 2.Clinical and Laboratory Standards Institue (CLSI) Performance standards for antimicrobial susceptibility resting: 24th infomational supplment CLSI document M100-S24. Wayne, PA: CLSI; 2014. [Google Scholar]
- 3.Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, Jones RN, Antimicrobial Susceptibility Testing Subcommittee of the Clinical and Laboratory Standards Institute Background and rationale for revised clinical and laboratory standards institute interpretive criteria (breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa: I. cephalosporins and aztreonam. Clin Infect Dis. 2013;56:1301–1309. doi: 10.1093/cid/cit017. [DOI] [PubMed] [Google Scholar]
- 4.Sanders WE, Jr, Sanders CC. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin Microbiol Rev. 1997;10:220–241. doi: 10.1128/cmr.10.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SH, Lee JE, Park SJ, Kim MN, Choo EJ, Kwak YG, Jeong JY, Woo JH, Kim NJ, Kim YS. Prevalence, microbiology, and clinical characteristics of extended-spectrum beta-lactamase-producing Enterobacter spp., Serratia marcescens, Citrobacter freundii, and Morganella morganii in Korea. Eur J Clin Microbiol Infect Dis. 2007;26:557–561. doi: 10.1007/s10096-007-0308-2. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Lee JE, Park SJ, Choi SH, Lee SO, Jeong JY, Kim MN, Woo JH, Kim YS. Emergence of antibiotic resistance during therapy for infections caused by Enterobacteriaceae producing AmpC beta-lactamase: implications for antibiotic use. Antimicrob Agents Chemother. 2008;52:995–1000. doi: 10.1128/AAC.01083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Towne TG, Lewis JS, 2nd, Herrera M, Wickes B, Jorgensen JH. Detection of SHV-type extended-spectrum beta-lactamase in Enterobacter isolates. J Clin Microbiol. 2010;48:298–299. doi: 10.1128/JCM.01875-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stürenburg E, Sobottka I, Noor D, Laufs R, Mack D. Evaluation of a new cefepime-clavulanate ESBL Etest to detect extended-spectrum beta-lactamases in an Enterobacteriaceae strain collection. J Antimicrob Chemother. 2004;54:134–138. doi: 10.1093/jac/dkh274. [DOI] [PubMed] [Google Scholar]
- 9.Jacoby GA, Han P. Detection of extended-spectrum beta-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli . J Clin Microbiol. 1996;34:908–911. doi: 10.1128/jcm.34.4.908-911.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andes D, Craig WA. Treatment of infections with ESBL-producing organisms: pharmacokinetic and pharmacodynamic considerations. Clin Microbiol Infect. 2005;11(Suppl 6):10–17. doi: 10.1111/j.1469-0691.2005.01265.x. [DOI] [PubMed] [Google Scholar]
- 11.Kohner PC, Robberts FJ, Cockerill FR, 3rd, Patel R. Cephalosporin MIC distribution of extended-spectrum-β-lactamase- and pAmpC-producing Escherichia coli and Klebsiella species. J Clin Microbiol. 2009;47:2419–2425. doi: 10.1128/JCM.00508-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamada Y, Sutherland CA, Nicolau DP. Impact of revised cefepime CLSI breakpoints on Escherichia coli and Klebsiella pneumoniae susceptibility and potential impact if applied to Pseudomonas aeruginosa . J Clin Microbiol. 2015;53:1712–1714. doi: 10.1128/JCM.03652-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee NY, Lee CC, Li CW, Li MC, Chen PL, Chang CM, Ko WC. Cefepime therapy for monomicrobial Enterobacter cloacae bacteremia: Unfavorable outcomes in patients infected by cefepime-susceptible dose-dependent isolates. Antimicrob Agents Chemother. 2015;59:7558–7563. doi: 10.1128/AAC.01477-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, Ko WC. Cefepime therapy for monomicrobial bacteremia caused by cefepime-susceptible extended-spectrum beta-lactamase-producing Enterobacteriaceae: MIC matters. Clin Infect Dis. 2013;56:488–495. doi: 10.1093/cid/cis916. [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, Park KH, Chung JW, Sung H, Choi SH, Choi SH. Prevalence and impact of extended-spectrum beta-lactamase production on clinical outcomes in cancer patients with Enterobacter species bacteremia. Korean J Intern Med. 2014;29:637–646. doi: 10.3904/kjim.2014.29.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheong HS, Ko KS, Kang CI, Chung DR, Peck KR, Song JH. Clinical significance of infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae blood isolates with inducible AmpC beta-lactamase. Microb Drug Resist. 2012;18:446–452. doi: 10.1089/mdr.2011.0126. [DOI] [PubMed] [Google Scholar]