Abstract
This study aimed to investigate the immediate impacts of Botulinum Toxin A (BoNT-A) injections on the inherent electrical properties of spastic muscles using a newly developed electrical impedance myography (EIM) technique. Impedance measures were performed before and after a BoNT-A injection in biceps brachii muscles of 14 subjects with spasticity. Three major impedance variables, resistance (R), reactance (X) and phase angle (θ) were obtained from three different configurations, and were evaluated using the conventional EIM frequency at 50 kHz as well as across multiple frequencies. Statistical analysis demonstrated a significant decrease of resistance in the injected muscles (Multiple-frequency: Rpre= 25.17±1.94 Ohm, Rpost= 23.65±1.63 Ohm, p<0.05; 50 kHz: Rpre= 29.06±2.16 Ohm, Rpost= 27.7±1.89 Ohm, p<0.05). Despite this decrease, there were no substantial changes in the reactance, phase angle, or anisotropy features after a BoNT-A injection. The significant changes of muscle resistance were most likely associated with the liquid injection of the BoNT-A-saline solution rather than the immediate toxin effects on the muscle. This study demonstrated high sensitivity of the EIM technique in the detection of alterations to muscle composition.
Introduction
Since the 1980s, Botulinum toxin type A (BoNT-A) has been widely used in the management of spasticity caused by stroke, spinal cord injury, or cerebral palsy [1–5]. The underlying mechanisms of BoNT-A is that it can effectively block the release of acetylcholine from the nerve endings, producing a progressive paralysis of the muscle [1, 6]. Subsequent to the intramuscular BoNT-A injection, alterations in the physiological and mechanical properties of muscle have been reported in various histological, mechanical, and medical imaging studies [7–11]. These modifications included decreases of muscle mass or muscle fiber cross-sectional area, a selective loss of fast-twitch muscle fibers, and changes in muscle fiber composition, sarcomere structure, and contractile proteins [10, 12, 13]. In electrophysiological studies following a BoNT-A injection, indications of progressive muscle denervation and reinnervation in the treated spastic muscles were shown through changes in compound muscle action potentials and increased muscle fiber jitter and density [3, 14]. It remains a question whether BoNT-A injection has an impact on the inherent electrical properties of the spastic muscle given that there is very limited information in the literature on the evaluation of muscular impedance changes in spastic muscles.
The development of electrical impedance myography (EIM) provides a novel and convenient technique for assessing muscle architecture and composition via measurement of electrical impedance from local muscles [15, 16]. The technique evolved from bioelectrical impedance analysis (BIA) which has been used for non-invasive bedside nutrition assessments for decades [15, 17, 18]. Conventional BIA studies usually focus on the estimation of the whole body volume and mass, fat-free mass or other body composition compartments based on prediction equations or population-specific models. Therefore, the technique is potentially influenced by a number of factors including hydration, fat fraction, and geometrical boundary conditions [15, 19]. In contrast, EIM measures the impedance of local muscle tissues to electrical current, i.e. resistivity and capacitance of skeletal muscles with relatively simple geometry, and thus minimizes the interference from skin and subcutaneous fat [16, 20].
This study aimed to understand the immediate impact of BoNT-A on these inherent electrical properties through the examination of changes to the resistance, reactance and phase angle before and after injection. The anisotropy of the muscle, which represents the directional dependence of the impedance to electrical current, was also investigated. Evaluation of all muscle impedance variables was performed using the conventional analysis at 50 kHz as well as using multi-frequency analysis.
Methods
Human sample
This study examined fourteen subjects (3 Females, 11 Males, and aged 37–73 years) who had spasticity in various muscles of the upper limbs after a neurological injury (13 stroke and 1 spinal cord injury). All subjects were hemiplegic except that one stroke subject who was bilaterally affected. The spasticity of the affected muscles were examined using the Modified Ashworth Scale (MAS) and their score range varied from 1 to 3 (Table 1). The duration of injury ranged from 6 months to 26 years. Experiments were conducted under institutional policies and procedures approved by the Institutional Review Board of University of Texas Health Science Center and the TIRR Memorial Herman (Houston, USA). The informed consent was obtained from all subjects prior to the experiments.
Table 1.
Subject information, dose of the Botox® and impedance changes
| Subject ID | MAS | Affected side | Duration (years) | Dose (U) | ΔR (Ohm) | ΔX (Ohm) | Δθ (°) |
|---|---|---|---|---|---|---|---|
| 1 | 1+ | Right | 3.5 | 75 | 1.33 | 0.31 | 0.02 |
| 2 | 3 | Left | 7.7 | 50 | 5.57 | 0.04 | 1.84 |
| 3 | 1 | Left | 10.7 | 50 | 2.55 | 0.16 | 1.68 |
| 3 | Right | 100 | 1.64 | 0.00 | 0.64 | ||
| 4 | 2 | Right | 4.2 | 50 | 1.70 | 0.66 | 1.47 |
| 5 | 3 | Right | 5.3 | 50 | 2.50 | 0.16 | 0.64 |
| 6 | 2 | Right | 7.0 | 50 | 0.35 | 0.37 | 1.02 |
| 7 | 3 | Right | 1.9 | 100 | 1.67 | 0.55 | 1.97 |
| 8 | 1 | Left | 1.2 | 50 | 1.00 | 0.85 | 1.81 |
| 9 | 1+ | Right | 0.5 | 50 | 4.80 | 1.27 | 3.87 |
| 10 | 2 | Left | 16.0 | 50 | 3.22 | 0.85 | 0.08 |
| 11 | 3 | Right | 4.0 | 75 | 1.55 | 0.48 | 1.28 |
| 12 | 3 | Left | 2.8 | 100 | 0.27 | 1.39 | 2.78 |
| 13 | 2 | Left | 1.9 | 100 | 3.85 | 0.49 | 0.83 |
| 14 | 3 | Right | 26.0 | 75 | 3.01 | 0.07 | 0.84 |
MAS: Modified Ashworth Scale
ΔR, ΔX, Δθ: absolute changes of impedance before and after injections averaged over multiple frequencies
BoNT-A injection
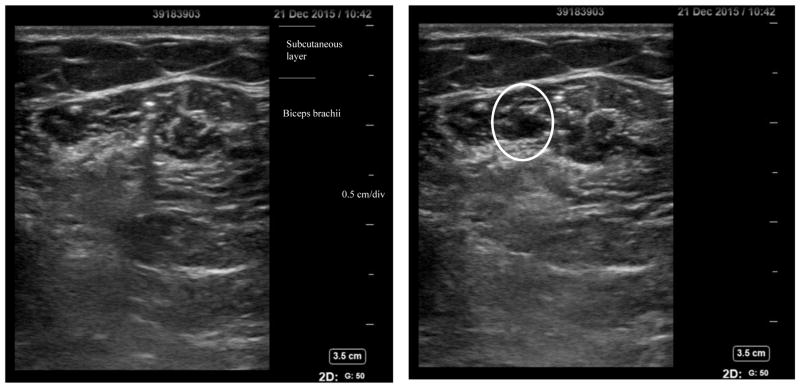
Impedance measurement took place on the day that subjects were scheduled a BoNT-A (Botox®) injection in the spastic biceps brachii muscle. The toxin doses and injection sites were determined by the physicians depending on each individual’s muscle condition. A fixed dilution of 100U of Botox® in 2 mL of normal saline was usually suggested for big muscles such as the biceps brachii. The ultrasound images in Figure 1 illustrate changes before and after injection of Botox® in the biceps brachii muscle. Impedance tests were performed on the injected muscle 20 minutes before and after the injection.
Figure 1.
Ultrasound images of biceps brachii pre- (left) and post-(right) Botox® injection. The oval part highlights injection volume.
EIM test
All EIM measurements were made using the mView® EIM system (Myolex Inc, Boston, MA). During the test, subjects were seated upright in a chair or bedside with the shoulder slightly abducted and flexed with neutral internal rotation. The elbow was held by the examiner at 90-degrees flexion. Subjects remained relaxed throughout the experiments while the EIM device delivered low-intensity alternating current to the muscle. Routine preparation of the muscle involved wiping the skin over the biceps muscle with a saline pad before the handheld electrode array (P/N: 20-00045) was gently pressed on the muscle. The position of the handheld sensor was carefully selected to cover the bulk area of the muscle and the injection site. The same position was used for EIM measures before and after the injection. In each test, the biceps were measured at least three times and the most consistent three trials were saved in the system.
The mView® handheld EIM sensor contains an array of current electrode pairs in three different configurations. Electrode pairs in Configuration 1 and 2 are aligned parallel to the muscle direction with the inter-electrode distance set as 68 mm and 43 mm respectively. The current electrode pair in Configuration 3 is arranged perpendicular to the direction of the other two pairs with an inter-electrode distance of 43 mm. All impedance parameters are automatically collected across a range of frequencies from 1000 Hz to 10 MHz.
Data analysis
Offline analysis of the impedance variables was performed using Matlab® (MathWorks, Natick, MA). Three channels of muscular impedance variables, resistance (R), reactance (X), and phase angle (θ=arctan(X/R)) were obtained from each of the three current-electrode configurations. Our analysis of the basic parameters was primarily based on data from Configuration 1, as suggested by a previous study which showed that impedance measured from larger distances yielded more reliable and representative signals than those from narrower distances [21].
Impedance analysis involved averaging the variables of resistance, reactance and phase angle over three trials at a single frequency of 50 kHz (referred to as the conventional analysis) or averaging across the range of multiple frequencies. In addition, the anisotropy ratio (AR) of each impedance variable was calculated using impedance data from Configurations 2 and 3. The AR of a variable is defined as: , where VCon2 and VCon3 represent the impedance variables (R, X or θ) from Configuration 2 and 3, respectively. The ARs of the impedance variables were analyzed at 50 kHz and across multiple frequencies as well.
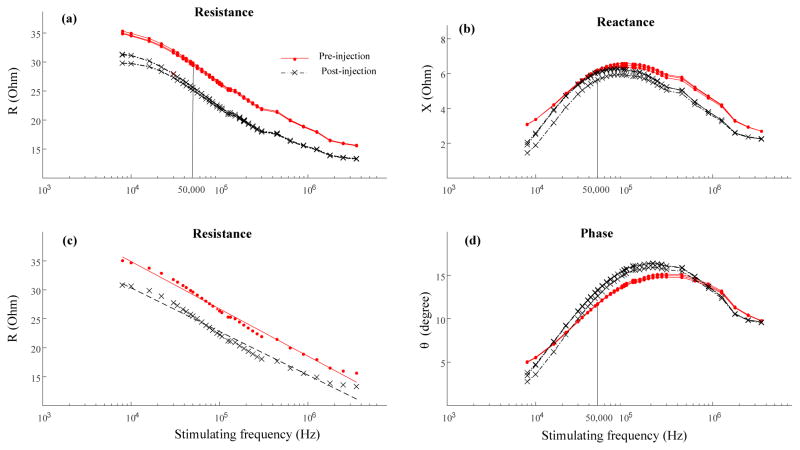
For the multi-frequency analysis, occasional negative impedance values were observed at low frequencies, the range of which also varied from trial to trial. To guarantee positive impedance data for all analyses, an all-positive frequency range was selected for each subject. Note that for the same muscle, the same frequency range was used for the data collected both before and after the BoNT-A injection. Determination of the lower boundary of the frequency range involved two steps: 1) finding the threshold frequencies for each trial; and 2) selecting the largest threshold. The threshold frequency corresponded to the second positive value in reactance following the negative values of lower frequencies from each trial. The largest value of the threshold frequencies was selected from across all trials of the same muscle as the lower boundary of the frequency range. The upper boundary of the frequency range was 3.55 MHz for all subjects. Examples of the impedance variables obtained from Configuration 1 in a selected frequency range are presented in Figure 2.
Figure 2.
Semi-log plot of impedance variables across multiple frequencies from a representative stroke subject pre- and post- BoNT-A injection. Each test involved three trials with the ‘Pre-injection’ test marked in red solid lines (or dots) and the ‘Post-injection’ test marked in black dashed lines (or ‘x’). (a) Resistance (R). (b) Reactance (X). (c) Linear relation of resistance and logarithm of frequency (see text for details). (d) Phase angle (θ).
Once the frequency range was determined, mean impedance variables were calculated by averaging the values within the range. Additional multi-frequency analysis involved regression analysis of the linear relationship between muscle resistance and the stimulating frequency. For confirmed linear relationships, the slopes of the resistance-frequency regressions were compared between the pre- and post- injection tests.
Statistical analysis
The paired t-test was used to assess differences of impedance variables before and after the BoNT-A injection, which included the 50k Hz and multi-frequency averaged R, X, θ, AR of R, AR of X and AR of θ, and the slope of resistance with frequency. In addition, regression analysis was applied to examine whether pre-injection impedance variables (pre_R, pre_X and pre_θ) were associated with the time course of the neurological injury. Spearman ρ coefficient was also computed to examine the correlation between the MAS and pre-injection impedance variables.
Results
As described previously, the inherent electrical properties of muscles were examined at a single frequency of 50 kHz or averaged over a selected multi-frequency range. In addition, most of the impedance parameters used for analysis was obtained from Configuration 1 except the anisotropy ratios, which were calculated from Configurations 2 and 3.
Impedance variables across multiple frequencies
Impedance variables of resistance, reactance and phase angles flrom a representative subject’s biceps brachii collected before and after the BoNT-A injection are displayed in Figure 2. Solid lines indicate 50 kHz to highlight the impedance values at that frequency (50 kHz: Rpre=29.62 Ohm, Rpost=25.49 Ohm; Xpre=6.14 Ohm, Xpost=5.92 Ohm; θpre=11.71°, θpost=13.07°). The mean impedance variables for this subject were averaged from 8 kHz to 3.55 MHz (mean value: Rpre=25.89 Ohm, Rpost=22.04 Ohm; Xpre=5.49 Ohm, Xpost=4.99 Ohm; θpre=12.24°, θpost=13.07°). In addition, the resistance decreased linearly with frequency in both pre-injection and post-injection tests (Rpre=67.64–8.18*log10 (freq), r2=0.988, p<0.0001; Rpost=60.48–7.54*log10 (freq), r2=0.973, p<0.0001, ‘freq’ represents the frequency).
Changes of impedance variables with BoNT-A injection
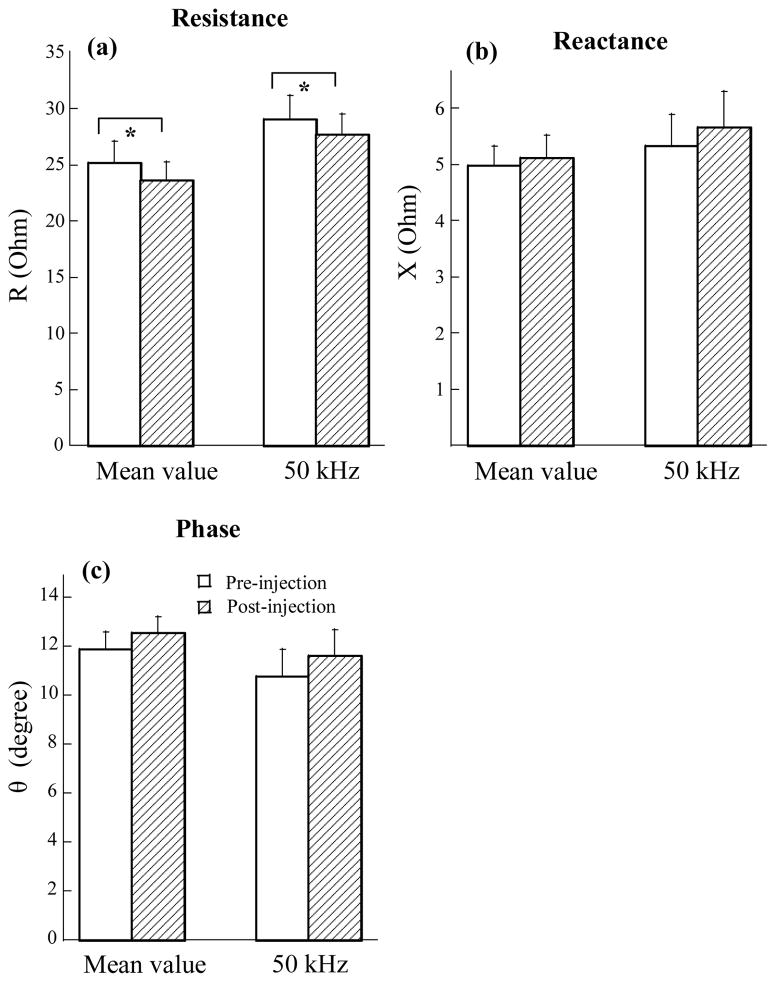
Comparisons of impedance changes before and after BoNT-A injection were made on the average of 15 muscles from 14 subjects (one subject received bilateral BoNT-A injections in the biceps brachii muscle). Despite the fact that female subjects showed larger impedance values in both the pre- and post-injection tests, a comparison of the pre/post changes demonstrated similar tendencies for both men and women. In addition, examination of the normality of impedance changes (ΔR, ΔX, and Δθ in Table 1) indicated a normal distribution in the three variables (p>0.17 for all). Therefore, gender differences might be negligible in the current analysis. Averaged impedance values of R, X and θ at 50 kHz and over the multi-frequency range are presented in Figure 3. Results of a paired t-test indicated a significant decrement of resistance in the biceps muscle after the BoNT-A injection (Fig 3a: Multi-freq: Rpre= 25.17±1.94 Ohm, Rpost= 23.65±1.63 Ohm, p<0.05; 50 kHz: Rpre= 29.06±2.16 Ohm, Rpost= 27.7±1.89 Ohm, p<0.05). Examination of the other two parameters, reactance and phase angle, however, did not present any significant differences before and after the BoNT-A injection in the two conditions (Fig. 3b: Multi-freq: Xpre= 4.97±0.35 Ohm, Xpost= 5.11±0.41 Ohm, p=0.43; 50 kHz: Xpre= 5.33±0.57 Ohm, Xpost= 5.65±0.65 Ohm, p=0.38) (Fig 3c: Multi-freq: θpre= 11.86±0.74°, θpre= 12.54±0.65°, p=0.12; 50 kHz: θpre= 10.75±1.14°, θpre= 11.58±1.1°, p=0.23).
Figure 3.
Comparison of impedance variable changes between pre- and post- BoNT-A injections. (a) Averaged resistance (R) across multiple frequencies and at 50 kHz. (b) Averaged reactance (X) across multiple frequencies and at 50 kHz. (c) Averaged phase angle (θ) across multiple frequencies and at 50 kHz.
All muscles demonstrated that the muscular resistance decreased linearly with an increase in stimulating frequency (Pre-injection: r2>0.9, p<0.0001; Post-injection: r2>0.93, p<0.0001). The averaged slope of the resistance-frequency was similar before and after the injection (Pre-injection: -7.76±0.54, post-injection: -7.81±0.56, p=0.83).
In addition, regression analysis did not confirm any significant relationship between the pre-injection impedance variables at 50 kHz or over multiple frequencies (pre_R, pre_X, pre_θ) and the duration of the injury (p>0.2 for all). Similarly, no correlatins were observed between the pre-injection impedance and the MAS score (p>0.2 for all). Resultant doses of Botox® injection varied from 50U to 100U in the current study. Injection dose and the corresponding changes of impedance variables of individual muscles are presented in Table 1.
Comparisons of the anisotropy ratios of R and X did not disclose any significant differences before and after the BoNT-A injection (Mean value of AR of R: AR_Rpre= 0.97±0.02, AR_Rpost=0.96±0.02, p=0.44; 50 kHz: AR_Rpre= 0.98±0.02, AR_Rpost=0.95±0.02, p=0.25. Mean value of AR of X: AR_Xpre= 1.05±0.04, AR_Xpost=0.99±0.05, p=0.17; 50 kHz: AR_Xpre= 1.11±0.07, AR_Xpost=1.03±0.06, p=0.26. Mean value of AR of θ: AR_θpre= 1.07±0.05, AR_θpre= 1.02±0.04, p=0.19; 50 kHz: AR_θpre= 1.13±0.06, AR_θpre= 1.03±0.06, p=0.19).
Discussion
This study examined the immediate impact of BoNT-A injection on the inherent electrical properties of spastic muscles. Despite a significant decrease of resistance in the treated muscle, there were no substantial changes in the other impedance variables including reactance, phase angles, or the anisotropy of each of the parameters (i.e resistance, reactance, and phase angle) after toxin injection.
Effect of BoNT-A injection on impedance of spastic muscle
The EIM technique has been used to assess changes of local muscle impedance in neuromuscular disease and for tracking disease progress in amyotrophic lateral sclerosis (ALS) [16, 22, 23]. For example, patients with ALS demonstrated reduced reactance and phase angle in the biceps brachii muscles and changes of phase angle correlated well with severity of the disease [23, 24]. Thus, the EIM has been used as a new non-invasive technique for assessment of muscle structural alterations in ALS. In the current impedance analysis there were no significant changes in the reactance or phase angle after a BoNT-A injection.
In contrast, the study disclosed a significant reduction of resistance in the treated spastic muscle which is different from previous findings of elevated resistance in neuromuscular disease [16]. According to the literature, an increase of resistance or decrease of reactance and phase angle observed in the affected muscle may reflect muscle fiber atrophy or changes to muscular composition including increased fat infiltration or connective tissue [24]. In particular, phase angle is a very sensitive biomarker in tracking neuromuscular disease-related muscle structural modifications such as ALS or other myopathies [22–24]. Current observation of decreased muscle resistance and no changes in other impedance parameters (reactance, phase angle, AR of resistance/reactance/phase angle) tends to suggest that the changes are not attributable to the toxin effects of the muscle. The effect of BoNT-A therapy usually starts 2–3 days after injection. Rather, the decreased resistance is likely to be caused by the injection of the liquid botox-saline solution itself. As showed in the ultrasound image (Fig 1), the injected saline solution is not likely to be absorbed and dissipated in the 20 minutes after injection. Decreased muscle resistance thus reflects local fluid accumulation due to BoNT-A injection according to an inverse correlation between the extracellular water and resistance [25]. A previous study examining muscle impedance changes after injury reported similar findings that local fluid accumulation secondary to muscle injury can cause reduction in resistance [26]. On the other hand, it is unlikely that injection of BoNT-A will produce any immediate modifications to cell membrane mass or function, which may explain why no significant changes of muscle reactance were observed in the injected muscle. Significantly reduced reactance was reported in the severely injured muscles as a result of membrane disruption [27]. Given the injection of small volume of saline (largest dose in this study, 100 units = 2 mL) to a large muscle like biceps brachii, this study identified the fluid accumulation as detected by resistance changes, which indicates that EIM is a very sensitive measurement of muscle impedance. Previous studies reported significant gender- and age-related differences in electrical impedance measurements with substantial reduction of impedance values in the lower extremity muscles in men [28]. The influence of gender on upper limb muscles such as the biceps brachii, however, was limited as there were no significant differences in the impedance observed between male and female subjects [28]. In this study, we focused on the relative changes of impedance variables before and after the BoNT-A injection rather than the global impedance variables. We also examined the normality of changes of impedance variables in terms of ΔR, ΔX, and Δθ and found a normal distribution in all three variables. This implies that the effect of gender can be negligible in the current analysis on relative impedance changes before and after the injection.
Since doses were chosen by the physician in practical amounts for the clinic, only three different doses (50U, 75U and 100U) of BoNT-A were delivered to the spastic muscle in this study. This made it impractical to use regression analysis for evaluation of whether changes of resistance were associated with the injection dose. In a previous electromyography (EMG) study, force generation and mass in the injected muscle confirmed effects of dose and injection volume on neuromuscular blockade in the mouse gastrocnemius [29]. By tracking changes of compound muscle action potentials (CMAPs), researchers found a detectable ‘blocking’ effect took place 24–48 hours after the BoNT-A injection followed by a progressive decrease of CMAP amplitude with peak reduction around 6–7 days and a slow recovery process in 30 days [3]. The time course of muscle structural changes varied from two weeks to four months with observations of peak post-injection atrophy of 30% to 60% of the muscle mass and an incomplete recovery of 90% of the initial mass in the treated muscles [11, 12]. Such muscular alterations may presumably influence the inherent electrical properties of the injected muscle and the long-term effect of toxin injection on muscle impedance has not been fully explored in the literature. A previous study measuring muscle impedance in patients with cervical dystonia demonstrated reduced assymmetry of muscle ressitance after a BoNT-A treatment [30]. Future studies may involve tracking subsequent changes of muscle impedance in the injected spastic muscle given the advantage of high sensitivity and convenience of the EIM technique.
Multiple frequency analysis in EIM
The present study involved both conventional analysis at 50 kHz and multi-frequency analysis of muscular impedance. Similar findings in impedance changes with BoNT-A injection were observed in both the conventional and multi-frequency analysis. Compared with the conventional method using impedance data from a single frequency (50 kHz), multi-frequency analysis yielded smaller variations in impedance variables and similar sensitivity to muscle architectural changes. Multi-frequency analysis has also demonstrated new applications in characterizing the structural reorganization of muscle with neuromuscular disease [31, 32]. For example, across multiple frequencies, ALS patients showed elevated an anisotropy ratio of phase angle and distorted anisotropy patterns whereas myopathic patients had a normal or reduced anisotropy ratio of phase angle and regular patterns [31]. In the current study, linear regression was used for quantifying the pattern of changes of resistance with frequency. Although we did not find any significant differences in the changes of the slope of the resistance-frequency, this analysis may provide a new means for exploring the changes of impedance patterns in disease-related impedance analysis in a future study with larger sample size.
Conclusion
This study applied both conventional single frequency analysis and multiple frequency analysis in the examination of the immediate impact of a BoNT-A injection on muscle impedance. Both methods yielded consistent findings of muscle resistence reduction with local injection, which provided evidence of the high sensitivity of the EIM techique in the detection of alterations to muscle composition. Future studies may focus on understanding the long-term changes in muscle structure post BoNT-A injection by integrating the EIM and other electrophysiological or imaging techniques to achive a more comprehensive analysis.
Highlights.
The manuscript aims to investigate the immediate impact of Botulinum Toxin A (BoNT-A) injection on the inherent electrical properties of spastic muscle using the newly developed electrical impedance myography (EIM) technique.
Muscle impedance and anisotropy features were examined in spastic muscles before and after BoNT-A injection.
There is a significant change of muscle resistance in the injected muscle which could be related to the injection of BoNT-A-saline solution indicating high sensitivity of the EIM technique in detection of muscle composition alterations.
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health of the U.S. Department of Health and Human Services under Grant R01NS080839, and in part by the Memorial Hermann Foundation.
Footnotes
Competing interests: None declared.
Ethical approval: The study was approved by Institutional Review Board of University of Texas Health Science Center and the TIRR Memorial Herman (Reference number: HSC-MS-16-0403).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208–10. doi: 10.1136/pgmj.65.762.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba MDY, Osborne MDM, Wszolek MDZ, Kwolek A, Druzbicki Ph DM. Treatment of spasticity with botulinum toxin. Ortop Traumatol Rehabil. 2004;6:665–72. [PubMed] [Google Scholar]
- 3.Eleopra R, Tugnoli V, De Grandis D. The variability in the clinical effect induced by botulinum toxin type A: the role of muscle activity in humans. Movement disorders: official journal of the Movement Disorder Society. 1997;12:89–94. doi: 10.1002/mds.870120115. [DOI] [PubMed] [Google Scholar]
- 4.Hastings-Ison T, Blackburn C, Rawicki B, Fahey M, Simpson P, Baker R, et al. Injection frequency of botulinum toxin A for spastic equinus: a randomized clinical trial. Dev Med Child Neurol. 2015 doi: 10.1111/dmcn.12962. [DOI] [PubMed] [Google Scholar]
- 5.Hesse S, Brandi-Hesse B, Bardeleben A, Werner C, Funk M. Botulinum toxin A treatment of adult upper and lower limb spasticity. Drugs Aging. 2001;18:255–62. doi: 10.2165/00002512-200118040-00003. [DOI] [PubMed] [Google Scholar]
- 6.Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87:1044–9. doi: 10.1016/s0161-6420(80)35127-0. [DOI] [PubMed] [Google Scholar]
- 7.Fortuna R, Vaz MA, Youssef AR, Longino D, Herzog W. Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox) J Biomech. 2011;44:39–44. doi: 10.1016/j.jbiomech.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Legerlotz K, Matthews KG, McMahon CD, Smith HK. Botulinum toxin-induced paralysis leads to slower myosin heavy chain isoform composition and reduced titin content in juvenile rat gastrocnemius muscle. Muscle Nerve. 2009;39:472–9. doi: 10.1002/mus.21247. [DOI] [PubMed] [Google Scholar]
- 9.Picelli A, Bonetti P, Fontana C, Barausse M, Dambruoso F, Gajofatto F, et al. Is spastic muscle echo intensity related to the response to botulinum toxin type A in patients with stroke? A cohort study. Arch Phys Med Rehabil. 2012;93:1253–8. doi: 10.1016/j.apmr.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Thacker BE, Tomiya A, Hulst JB, Suzuki KP, Bremner SN, Gastwirt RF, et al. Passive mechanical properties and related proteins change with botulinum neurotoxin A injection of normal skeletal muscle. J Orthop Res. 2012;30:497–502. doi: 10.1002/jor.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathevon L, Michel F, Decavel P, Fernandez B, Parratte B, Calmels P. Muscle structure and stiffness assessment after botulinum toxin type A injection. A systematic review. Ann Phys Rehabil Med. 2015 doi: 10.1016/j.rehab.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Tsai FC, Hsieh MS, Chou CM. Comparison between neurectomy and botulinum toxin A injection for denervated skeletal muscle. J Neurotrauma. 2010;27:1509–16. doi: 10.1089/neu.2010.1320. [DOI] [PubMed] [Google Scholar]
- 13.Dubowitz WS. Muscle biopsy: a practical approach. Philadelphia: Saunders Elsevier; 2007. [Google Scholar]
- 14.Bogucki A. Serial SFEMG studies of orbicularis oculi muscle after the first administration of botulinum toxin. Eur J Neurol. 1999;6:461–7. doi: 10.1046/j.1468-1331.1999.640461.x. [DOI] [PubMed] [Google Scholar]
- 15.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gomez JM, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23:1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Rutkove SB, Aaron R, Shiffman CA. Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve. 2002;25:390–7. doi: 10.1002/mus.10048. [DOI] [PubMed] [Google Scholar]
- 17.Lukaski HC. Evolution of bioimpedance: a circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur J Clin Nutr. 2013;67(Suppl 1):S2–9. doi: 10.1038/ejcn.2012.149. [DOI] [PubMed] [Google Scholar]
- 18.Buffa R, Saragat B, Cabras S, Rinaldi AC, Marini E. Accuracy of specific BIVA for the assessment of body composition in the United States population. PLoS One. 2013;8:e58533. doi: 10.1371/journal.pone.0058533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulasi U, Kuchnia AJ, Cole AJ, Earthman CP. Bioimpedance at the bedside: current applications, limitations, and opportunities. Nutr Clin Pract. 2015;30:180–93. doi: 10.1177/0884533614568155. [DOI] [PubMed] [Google Scholar]
- 20.Tarulli A, Esper GJ, Lee KS, Aaron R, Shiffman CA, Rutkove SB. Electrical impedance myography in the bedside assessment of inflammatory myopathy. Neurology. 2005;65:451–2. doi: 10.1212/01.wnl.0000172338.95064.cb. [DOI] [PubMed] [Google Scholar]
- 21.Sung M, Spieker AJ, Narayanaswami P, Rutkove SB. The effect of subcutaneous fat on electrical impedance myography when using a handheld electrode array: the case for measuring reactance. Clin Neurophysiol. 2013;124:400–4. doi: 10.1016/j.clinph.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography correlates with standard measures of ALS severity. Muscle Nerve. 2014;49:441–3. doi: 10.1002/mus.24128. [DOI] [PubMed] [Google Scholar]
- 23.Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotroph Lateral Scler. 2012;13:439–45. doi: 10.3109/17482968.2012.688837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutkove SB. Electrical impedance myography: Background, current state, and future directions. Muscle Nerve. 2009;40:936–46. doi: 10.1002/mus.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukaski HC, Moore M. Bioelectrical impedance assessment of wound healing. J Diabetes Sci Technol. 2012;6:209–12. doi: 10.1177/193229681200600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nescolarde L, Yanguas J, Lukaski H, Alomar X, Rosell-Ferrer J, Rodas G. Localized bioimpedance to assess muscle injury. Physiol Meas. 2013;34:237–45. doi: 10.1088/0967-3334/34/2/237. [DOI] [PubMed] [Google Scholar]
- 27.Nescolarde L, Yanguas J, Lukaski H, Alomar X, Rosell-Ferrer J, Rodas G. Effects of muscle injury severity on localized bioimpedance measurements. Physiol Meas. 2015;36:27–42. doi: 10.1088/0967-3334/36/1/27. [DOI] [PubMed] [Google Scholar]
- 28.Kortman HG, Wilder SC, Geisbush TR, Narayanaswami P, Rutkove SB. Age- and gender-associated differences in electrical impedance values of skeletal muscle. Physiol Meas. 2013;34:1611–22. doi: 10.1088/0967-3334/34/12/1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone AV, Ma J, Callahan MF, Smith BP, Garrett JP, Smith TL, et al. Dose- and volume dependent-response to intramuscular injection of botulinum neurotoxin-A optimizes muscle force decrement in mice. J Orthop Res. 2011;29:1764–70. doi: 10.1002/jor.21434. [DOI] [PubMed] [Google Scholar]
- 30.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]
- 31.Esper GJ, Shiffman CA, Aaron R, Lee KS, Rutkove SB. Assessing neuromuscular disease with multifrequency electrical impedance myography. Muscle Nerve. 2006;34:595–602. doi: 10.1002/mus.20626. [DOI] [PubMed] [Google Scholar]
- 32.Rutkove SB, Shefner JM, Gregas M, Butler H, Caracciolo J, Lin C, et al. Characterizing spinal muscular atrophy with electrical impedance myography. Muscle Nerve. 2010;42:915–21. doi: 10.1002/mus.21784. [DOI] [PubMed] [Google Scholar]