Abstract
Background
Diarrhea, a common complication after solid organ transplant (SOT), is associated with allograft failure and death. No evidence-based guidelines exist for the evaluation of diarrhea in SOT recipients. We performed a cost-analysis to derive a testing algorithm for the diagnosis of community-onset diarrhea that minimizes costs without compromising diagnostic yields.
Design
A cost-analysis was performed on a retrospective cohort of 422 SOT admissions for community-onset diarrhea over an 18-month period. A stepwise testing model was applied on a population level to assess test costs relative to diagnostic yields.
Results
Over an 18-month period, 1564 diagnostic tests were performed and 127 (8.1%) returned positive. Diagnostic testing accounted for $95 625 of hospital costs. The tests with the lowest cost per decrease in the false-omission rate (FOR) were stool Clostridium difficile polymerase chain reaction (PCR) ($156), serum cytomegalovirus quantitative PCR ($1529), stool norovirus (NV) PCR ($4673), and stool culture ($6804). A time-to-event analysis found no significant difference in the length of hospital stay between patients with and without NV testing (P=.520).
Conclusions
A stepwise testing strategy can reduce costs without compromising diagnostic yields. In the first-stage testing, we recommend assessment for C. difficile, cytomegalovirus, and food-borne bacterial pathogens. For persistent diarrheal episodes, second-stage evaluation should include stool NV PCR, Giardia/Cryptosporidium enzyme immunoassay, stool ova and parasite, reductions in immunosuppressive therapy, and possibly endoscopy. Although NV testing had a relatively low cost per FOR, we recommend NV testing during second-stage evaluation, as an NV diagnosis may not lead to changes in clinical management or further reductions in length of hospital stay.
Keywords: Clostridium difficile, cost-analysis, cytomegalovirus, diarrhea, norovirus, solid organ transplant
1 INTRODUCTION
Diarrhea is a frequent complication after solid organ transplant (SOT) and can lead to alterations in immunosuppressive therapy, hospitalizations, allograft failure, and mortality.1–3 In community-onset diarrhea, most episodes are from non-infectious etiologies, including immunosuppressive therapy, and only 20%–40% are related to infections.3–5 Clostridium difficile, norovirus (NV), and cytomegalovirus (CMV) are the most common causes of infectious diarrhea, while opportunistic and parasitic infections occur infrequently.3–6 Most episodes of post-transplant diarrhea, regardless of the cause, ultimately resolve. 3–6
No evidence-based guidelines are available for the evaluation of diarrhea in SOT recipients. Expert recommendations include stepwise3,4 and simultaneous7–10 testing approaches, but it is unclear how these strategies compare in terms of accuracy, timing, and costs. Many testing approaches recommend diagnostics directed at the most common causes of infectious diarrhea including NV.3,6,10 It is unknown if NV testing is a worthwhile use of resources, as no specific treatment exists beyond providing supportive care and reducing immunosuppressive therapy. However, confirming an NV diagnosis may lead to indirect cost savings by informing infection control measures, reducing length of hospital stay, and preventing additional diagnostic evaluation including endoscopy.
We performed a cost-analysis on a previously described retrospective cohort to derive a testing algorithm for the evaluation of community-onset diarrhea in SOT recipients.5 We hypothesized a stepwise testing strategy would reduce costs without compromising diagnostic yields.
2 MATERIALS AND METHODS
A cost analysis was performed on a retrospective cohort of SOT recipients admitted with community-onset diarrhea from March 1, 2012 through September 30, 2013 to Northwestern Memorial Hospital, Chicago, IL USA.5 Patient identification methods and inclusion and exclusion criteria were previously described.5 Diagnostic evaluation was at the discretion of the treating physician. Hospital SOT protocol recommends stool C. difficile toxin B polymerase chain reaction (PCR), plasma CMV DNA quantitative PCR (qPCR), stool NV PCR, stool culture, and stool Giardia/Cryptosporidium enzyme immunoassay (EIA). Other routinely performed tests included blood culture and stool ova and parasite examination (O&P). For tests yielding positive results, we identified the number performed and their diagnostic yields.5 Rarely performed tests, each accounting for <1% of evaluations, were omitted. Testing costs were obtained from the hospital cost center database.
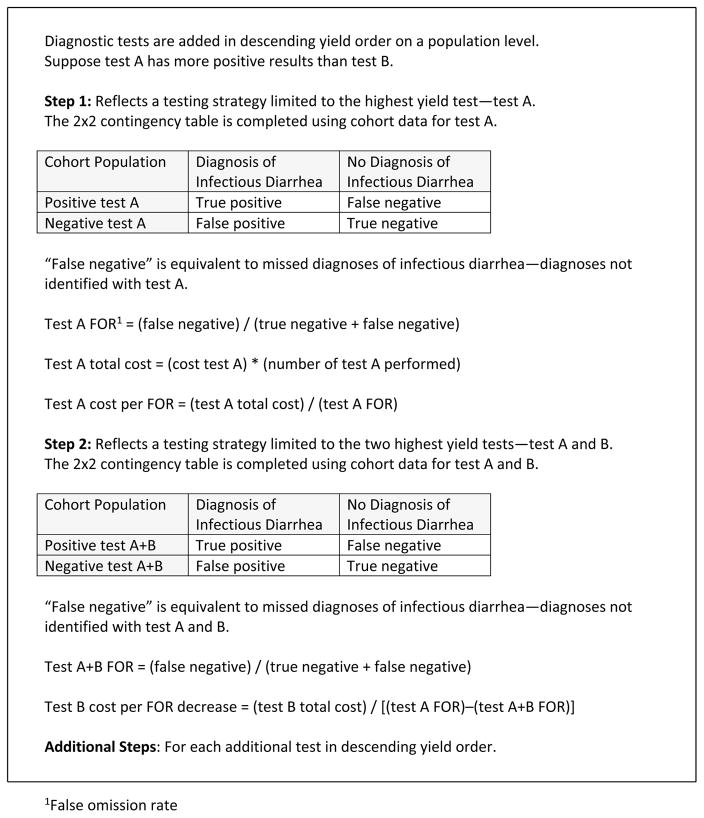
A stepwise testing model was applied to assess test cost relative to diagnostic yield (Figure 1). The model assumed diagnostic tests were added stepwise in descending yield order on a population level. The first step of the model reflected a testing strategy limited to the highest yield test. In the second step, the testing strategy included the two highest yield tests. A test was added to each step of the model until the final step included all tests.
Figure 1.
Stepwise Testing Model for Cost-Analysis
A binary classification analysis was performed for each step of the model. In the two-by-two contingency tables, disease outcomes were “diagnosis of infectious diarrhea” and “no diagnosis of infectious diarrhea.” Test outcomes included the results for all diagnostic tests performed during the step. The analyses were performed using testing rates and diagnostic yields from the original cohort.
For each step in the model, the false-omission rate (FOR) was calculated to assess the contribution of each test to the diagnostic yield. The FOR was defined as “false-negative tests” divided by the sum of “false-negative tests” and “true-negative tests.” False-negative tests were equivalent to missed diagnoses of infectious diarrhea—diagnoses not identified by the tests in each step. By definition, the FOR would decrease as tests were added to each step of the model. We compared tests by calculating test cost per decrease in FOR.
A secondary time-to-event analysis was conducted to assess if NV testing led to indirect cost-savings by decreasing the length of hospital stay. Patients who had testing for CMV, C. difficile, and/or NV were included (n=389) in the analysis. Time to discharge was compared between those who had stool NV PCR testing to those who did not. The non-parametric Wilcoxon test of equality of the survival functions was used with an alpha of 0.05 to reject the null hypothesis of both groups being equal.
3 RESULTS
A total of 422 admissions for community-onset diarrhea (314 unique patients) were identified. Demographic and clinical characteristics were previously described.5 Onset of diarrhea occurred a median 1028 days after transplant (interquartile range 240–2372 days). Transplant types included kidney (42.7%), liver (24.6%), heart (11.1%), pancreas (2.6%), bowel (0.7%), pancreas-kidney (9.0%), and liver-kidney (5.2%). No infectious cause was identified for the majority of admissions (62.2%). A total of 1564 diagnostic tests were performed and 127 (8.1%) returned positive (Table 1).
TABLE 1.
Diagnostic testing costs and false-omission rates
| Diagnostic test | Number of tests performed (%)a | Positive test results (%)b | Disease prevalence (%) | Cumulative prevalence (%) | Test cost ($) | Total cost ($) | Cumulative cost ($) |
|---|---|---|---|---|---|---|---|
| Stool Clostridium difficile PCR | 324 (76.8) | 60 (18.5) | 14.2 | 14.2 | 36 | 11 664 | 11 664 |
| Serum CMV qPCR | 309 (73.2) | 31 (10.0) | 7.4 | 21.6 | 93 | 28 737 | 40 401 |
| Stool norovirus PCR | 127 (30.1) | 28 (22.1) | 6.6 | 28.2 | 195 | 24 765 | 65 166 |
| Stool culture | 243 (57.6) | 5 (2.1) | 1.2 | 29.4 | 28 | 6804 | 71 970 |
| Blood culture | 276 (65.4) | 1 (0.4) | 0.2 | 29.6 | 44 | 12 144 | 84 114 |
| Stool Giardia/Cryptosporidium EIA | 138 (32.7) | 1 (0.7) | 0.2 | 29.8 | 44 | 6072 | 90 186 |
| Stool O&P | 147 (34.8) | 1 (0.7) | 0.2 | 30.0 | 37 | 5439 | 95 625 |
| Total | 1,564 | 127 (8.1) | 30.0 | 95 625 |
The denominator for percentage is the total number of admissions for community-acquired diarrhea (n=422).
The denominator for percentage is the total number of tests performed (presented in the second column).
PCR, polymerase chain reaction, CMV, cytomegalovirus; qPCR, quantiative PCR; EIA, enzyme immunoassay; O&P, ova and parasites test.
In a testing strategy limited to the highest yield test, stool C. difficile PCR, the FOR was 25.4%. Inclusion of the second highest yield test, plasma CMV qPCR, decreased the FOR to 6.6%. Each additional test decreased the FOR as follows: stool NV PCR 1.3%, stool culture 0.3%, blood culture 0.2%, Giardia/Cryptosporidium EIA 0.08%, and stool O&P 0%.
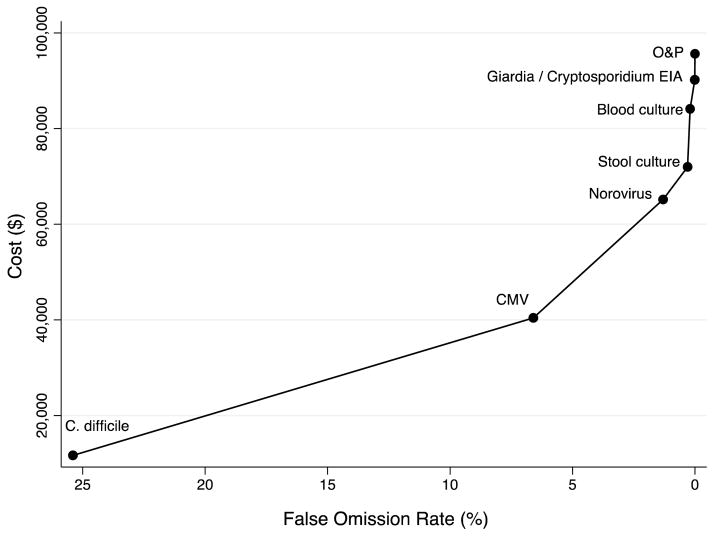
During the study period, diagnostic testing accounted for $95 625 of hospital costs. As stool C. difficile PCR accounted for $11 664 of testing costs, it achieved an FOR of 25.4% at a cost per FOR decrease of $156. With the additional $28 737 spent on serum CMV qPCR, the FOR decreased from 25.4% to 6.6% at a cost per FOR decrease of $1529. The cost per FOR decrease for each additional test was as follows: stool NV PCR $4673, stool culture $6804, blood culture $121 440, stool Giardia/Cryptosporidium $50 600, and stool O&P $67 988 (Figure 2).
Figure 2. Diagnostic Testing Cumulative Cost per Decrease in False Omission Rate for Solid Organ Transplant Recipients Admitted with Community-Onset Diarrhea.
The slope represents test cumulative cost increase driven by the corresponding decrease in the false omission rate: C. difficile $156, CMV $1,529, norovirus $4,673, stool culture $6,804, blood culture $121,440, Giardia/Cryptosporidium $50,600, and stool O&P $67,988.
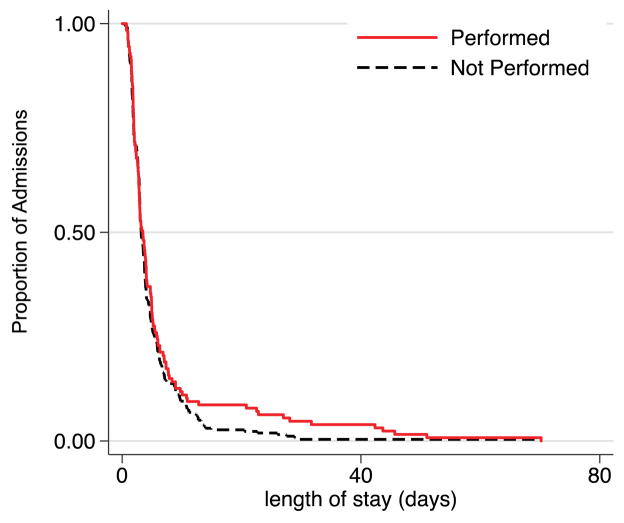
A total of 127 (30.1%) patients received NV testing. In the time-to-event analysis, no significant difference in the hospital length of stay (time to discharge) was seen between patients with and without stool NV PCR testing (χ2=0.41, P=.520) (Figure 3).
Figure 3. Time-to-Event Analysis for Stool Norovirus PCR and Length of Hospital Stay.
There was no significant difference between the length of hospital stay between patients with (n=127, 30.1%) and without (n=72.5%) norovirus testing (Wilcoxon χ2=0.41; p=0.520).
4 DISCUSSION
Diarrhea is a frequent occurrence in SOT recipients, but infections are responsible for only 20%–40% of episodes.3–6 The majority of infectious episodes can be attributed to a few clinically indistinguishable pathogens.3–6 Based on our findings, cost containment can be achieved with a stepwise testing strategy, where the initial evaluation is limited to the most common pathogens with further assessment reserved for persistent episodes. Our cost-analysis suggests the initial evaluation should include stool C. difficile PCR, serum CMV qPCR, stool NV PCR, and stool culture—the four tests with the lowest cost per FOR decrease. This approach accepts initially missing diagnoses identified with blood culture, stool O&P, and Giardia/Cryptosporidium EIA—studies that can be reserved for second-stage testing if necessary. In our study population, omitting these studies would lead to three (2.4%) missed diagnoses on initial testing: Listeria by blood culture, Giardia by EIA, and Blastocystis by stool O&P. Parasites are a rare cause of post-transplant diarrhea in our region; therefore, our findings may not be generalizable to centers with higher incidences of parasitic infections.3–6 For persistent diarrheal episodes, second-stage evaluation may include Giardia/Cryptosporidium EIA, stool O&P, reductions in immunosuppressive therapy, and possibly endoscopy.
This testing strategy is based on comparing the costs per FOR decrease for each test. While the cost-analysis helps define the relative cost of NV testing, its ultimate value remains unclear. Although NV is a common cause of post-transplant diarrhea, stool NV PCR was the most expensive test, while providing results with minimal impact on clinical management. No specific treatment exists for NV, and management includes supportive therapy and reduction in immunosuppression, measures most patients already receive empirically on admission. Delayed diagnosis of NV may lead to minimal or no changes in initial clinical management. Nonetheless, it is unclear if confirming a diagnosis leads to indirect cost-savings at the patient- and center-level by informing infection control measures, preventing additional diagnostic testing (including endoscopy), and facilitating hospital discharge. Our time-to-event analysis showed performing NV testing did not significantly reduce the length of hospital stay. Until further studies assess the cost-benefit of NV testing in SOT recipients, we recommend reserving stool NV PCR for second-stage evaluation, to achieve additional reductions in testing costs. Thus, if first-stage testing is limited to stool C. difficile PCR, serum CMV qPCR, and stool culture, we would achieve a cost-savings of $48 420 (51% of total cost) with an FOR of 4%—a reasonable FOR, as NV accounts for 90% of missed diagnoses.
A major limitation to our testing strategy is its reliance on molecular diagnostics. The testing strategy may result in over-diagnosis and, as a result, over-treatment of C. difficile, as it relies exclusively on molecular testing without considering toxin production and host immune response.11 Additional studies are needed to assess testing strategies in SOT recipients including both C. difficile PCR and toxin immunoassay. Furthermore, the possibility of missing diagnoses of CMV gastrointestinal disease exists, because plasma CMV qPCR has a sensitivity of 85%.12 In our cohort, two patients with CMV colitis had a negative qPCR on initial evaluation (2/26, 7.7%). A negative plasma CMV qPCR should not exclude CMV gastrointestinal disease and we recommend endoscopy in the second-stage evaluation of persistent episodes.
Multiplex PCR assays for gastrointestinal pathogens have several advantages including high sensitivity, rapid turnaround time, and potentially lower costs with consolidated laboratory testing and workflow.13 However, exclusive reliance on molecular diagnostics without considering the clinical presentation and immune response may lead to over-diagnosis and over-treatment.6,11 Furthermore, results have been inconsistent across seven commercially available multiplex PCR assays in kidney transplant recipients.6 Our testing strategy may reduce the risk of over-diagnosis and over-treatment, as the first-stage testing is limited to the most common causes of post-transplant diarrhea. Prospective studies are needed to assess how multiplex PCR assays impact clinical management, outcomes, and healthcare costs in SOT recipients.
Our study has several limitations. The cost-analysis may not be generalizable to hospital-onset diarrhea, chronic diarrhea, and other centers with differences in epidemiology, organ transplant types, and testing costs. The stepwise model assumes tests were performed incrementally on the population at a single period of time without considering how results impacted decisions for further testing on the individual level. Additional studies are needed to better define the optimal testing strategy for community-onset diarrhea. The care of transplant patients may be improved with an evidence-based and cost-conscious synthesis of classical and molecular diagnostic techniques.
Practice guidelines indicate that patient-specific epidemiologic history should provide the foremost guidance for the evaluation of diarrhea.14 We feel that our proposed strategy may serve as a general starting point for the evaluation of community-onset diarrhea in SOT recipients where no clear etiology is suggested by the epidemiological history. Thus, whereas a specific history and epidemiologic exposure should prompt consideration for individualized testing, a history of SOT does not necessarily denote a need for extensive first-line testing beyond that proposed in this study.
5 CONCLUSIONS
In SOT recipients admitted with community-onset diarrhea, a stepwise testing strategy reduces costs without compromising diagnostic yields. The cost-analysis suggests initial evaluation should be directed to the most frequently encountered infectious etiologies: C. difficile, CMV, NV, and food-borne bacterial pathogens. This testing strategy captures the vast majority of infectious episodes and rarely misses a diagnosis. However, we recommend a strategy that reserves stool NV testing to second-stage evaluation, as this approach may further reduce costs without impacting initial supportive care and the length of stay. Thus, we recommend first-stage testing with stool C. difficile PCR, serum CMV qPCR, and stool culture. If clinically necessary, second-stage management should include stool NV PCR, stool Giardia/Cryptosporidium EIA, stool O&P, consideration for further reductions in immunosuppressive therapy, and possibly endoscopy. In light of the increasing prevalence of molecular diagnostics, prospective studies are necessary to assess the direct and indirect costs of different testing strategies.
Acknowledgments
Oluwatoyosi Akinlade performed data retrieval for the time-to-event analysis. Nathaniel Rhodes conceived the idea of a stepwise testing model for the cost-analysis. This study utilized data derived from the Northwestern Medicine Enterprise Data Warehouse Pilot Data Program, supported by the National Center for Research Resources (Grant 5UL1RR025741), now at the National Center for Advancing Translational Sciences (Grant 8UL1TR000150).
Funding Support:
None
Footnotes
Author contributions:
Concept/design: S.A.T., I.A.E., S.P., and M.P.A.; Data collection: I.A.E. and S.A.T.; Data analysis/interpretation: S.A.T., I.A.E., S.P., and M.A.; Statistics: S.A.T. and S.P.; Drafting article: S.A.T. and I.A.E.; Critical revision of article: S.A.T., I.A.E., S.P., and M.P.A.; Approval of article: S.A.T., I.A.E., S.P., and M.P.A..
Disclosures:
The authors declare no conflicts of interest.
References
- 1.Bunnapradist S, Neri L, Wong W, et al. Incidence and risk factors for diarrhea following kidney transplantation and association with graft loss and mortality. Am J Kidney Dis. 2008;51:478–486. doi: 10.1053/j.ajkd.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Altiparmak MR, Trablus S, Pamuk ON, et al. Diarrhoea following renal transplantation. Clin Transplant. 2002;16:212–216. doi: 10.1034/j.1399-0012.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- 3.Roos-Weil D, Ambert-Balay K, Lanternier F, et al. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation. 2011;92:61–69. doi: 10.1097/TP.0b013e31821c9392. [DOI] [PubMed] [Google Scholar]
- 4.Maes B, Hadaya K, de Moor B, et al. Severe diarrhea in renal transplant patients: Results of the DIDACT study. Am J Transplant. 2006;6:1466–1472. doi: 10.1111/j.1600-6143.2006.01320.x. [DOI] [PubMed] [Google Scholar]
- 5.Echenique IA, Penugonda S, Stosor V, Ison MG, Angarone MP. Diagnostic yields in solid organ transplant recipients admitted with diarrhea. Clin Infect Dis. 2015;60:729–737. doi: 10.1093/cid/ciu880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coste JF, Vuiblet V, Moustapha B, et al. Microbiological diagnosis of severe diarrhea in kidney transplant recipients by use of multiplex PCR assays. J Clin Microbiol. 2013;5:1841–1849. doi: 10.1128/JCM.03366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginsburg PM, Thuluvath PJ. Diarrhea in liver transplant recipients: Etiology and management. Liver Transplant. 2005;11:881–890. doi: 10.1002/lt.20500. [DOI] [PubMed] [Google Scholar]
- 8.Weclawiak H, Ould-Mohamed A, Bournet B, et al. Duodenal villous atrophy: A cause of chronic diarrhea after solid-organ transplantation. Am J Transplant. 2011;11:575–582. doi: 10.1111/j.1600-6143.2010.03423.x. [DOI] [PubMed] [Google Scholar]
- 9.Rice JP, Spier BJ, Cornett DD, Walker AJ, Richie K, Pfau PR. Utility of colonoscopy in the evaluation of diarrhea in solid organ transplant recipients. Transplantation. 2009;88:374–379. doi: 10.1097/TP.0b013e3181ae98ab. [DOI] [PubMed] [Google Scholar]
- 10.Aulagnon F, Scemla A, DeWolf S, Legendre C, Zuber J. Diarrhea after kidney transplantation: A new look at a frequent symptom. Transplantation. 2014;98:806–816. doi: 10.1097/TP.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 11.Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile Infection in the Molecular Test Era. JAMA Intern Med. 2015;175:1792–1801. doi: 10.1001/jamainternmed.2015.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durand CM, Marr KA, Arnold CA, et al. Detection of cytomegalovirus DNA in plasma as an adjunct diagnostic for gastrointestinal tract disease in kidney and liver transplant recipients. Clin Infect Dis. 2013;57:1550–1559. doi: 10.1093/cid/cit521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenberg SD, Bacelar M, Brazier P, Bisnauthsing K, Edgeworth JD. A cost benefit analysis of the Luminex xTAG Gastrointestinal Pathogen Panel for detection of infectious gastroenteritis in hospitalised patients. J Infect. 2015;70:504–511. doi: 10.1016/j.jinf.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Guerrant RL, Van Gilder T, Steiner TS, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–351. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]