Abstract
Background and aims
Contingency management (CM) is one of the most effective behavioral interventions to promote drug abstinence, but availability of this treatment is limited. We evaluated the efficacy and acceptability of Internet-based CM relative to an Internet-based monitoring and goal setting control group in a nationwide sample of cigarette smokers.
Design
Randomized controlled trial with 3- and 6-month follow ups.
Setting
USA.
Participants
Smokers (n=94) from 26 states were enrolled (mean age 36, 56% female).
Intervention and comparator
Participants were randomized to earn financial incentives (up to $480 over 7 weeks) based on video-verified abstinence using breath carbon monoxide (CO) output (n=48; Abstinent Contingent Group, AC), or based on submitting CO samples (n=46, Submission Contingent, SC). Both groups also received the same CO-based goals. A $50 deposit was required in both groups that could be recouped from initial earnings.
Measures
The primary outcome was point prevalence at week 4. Secondary outcomes were point prevalence at the 3- and 6-month follow-ups, percentages of negative CO samples, adherence to the CO sampling protocol, and treatment acceptability ratings on a 0–100mm visual analog scale.
Findings
Abstinence rates differed at 4 weeks between the AC (39.6%) and SC (13.0%) groups (odds ratio=4.4, 95% CI=1.6–12.3), but not at the 3- (29.2% AC and 19.6% SC, odds ratio=1.7, 95% CI=.6–4.4), or 6- (22.9% AC and 13.0% SC, odds ratio=2.0, 95% CI=.7–5.9) month follow-ups. During the two main treatment phases, there were significant differences in negative COs (53.9% AC and 24.8% SC, odds ratio = 3.5, 95% CI=3.1–4.0; 43.4% AC and 24.6% SC, odds ratio = 2.3, 95% CI=1.6–3.4). Adherence to the CO submission protocol was equivalent (78% AC and 85% SC, difference = 7.0%, 95% CI = −10.3%–23.8%, x2=.75, p = .39). The lowest acceptability ratings were for the items assessing the deposit, whereas the highest ratings concerned the ease of the intervention, the graph of CO results, and earning money.
Conclusions
A contingency management/financial incentive program delivered via the Internet improved short-term abstinence rates compared with an internet program without the incentives.
Keywords: Cigarette smoking, contingency management, deposit contract, technology
Cigarette smoking continues to take an enormous toll on society. Smoking is associated with more than 6 million deaths worldwide (1). During the 50 years since the first Surgeon General’s report on the health consequences of smoking, more than 20 million Americans have died prematurely from tobacco-related diseases (2). The most recent Surgeon General’s report recommends several “end-game” tobacco control strategies such as reducing the levels of nicotine in cigarettes, increasing the use of smoke-free areas, restricting advertising, increasing the price of cigarettes, and increasing the prevalence of “barrier-free cessation support” (2).
Technology can play a key role in promoting barrier-free cessation support (3–5). In particular, technology can aid in the delivery of evidence-based behavioral treatments to promote smoking cessation. One such treatment is contingency management (CM; (6–8). Under CM procedures, desirable consequences such as financial incentives are provided based on objective evidence of abstinence. Typically, low levels of breath carbon monoxide (CO) indicate abstinence (e.g., < 4–6 parts per million), which can be determined quickly through a small, hand-held monitor. Due to the short half-life of breath CO (≈4 hr), samples must be collected at least twice per day to index abstinence (9). Because such frequent in-person meetings would pose a barrier to treatment, Dallery and colleagues developed an Internet-based method to collect CO and deliver CM (10–12). The procedure entails remote video capture of participants providing breath CO, and automated incentive delivery through a web-based platform called Mōtiv8. Internet-based CM has been shown to promote abstinence in heavy (11), adolescent (13), and rural smokers (14). Recent work has extended this method to mobile-phones (15,16), which has even greater potential for accessibility than computer-based access to the Internet.
Technology-based CM, however, has been restricted to relatively small geographical areas. For example, previous work by Dallery and colleagues enrolled participants from North Central Florida in the United States (17). Although several studies have delivered Internet-based CM to adults and adolescents living in rural Appalachia (14,18), these applications still represent a relatively small area in light of the potential reach of information technology. Even recent mobile-phone based CM studies enrolled participants who were local to the research site (15). Thus, the first purpose of the current study was to expand the reach of CM to qualified smokers in the contiguous United States. To our knowledge, this study represents the first remote, nationwide application of CM to cigarette smoking.
The second purpose of the study was to incorporate and evaluate the acceptability of a deposit contract procedure (19–22). To enroll in the study, participants were asked to deposit $50 into a PayPal account. Depending on group assignment, these funds could be recouped based on evidence of abstinence or based on submitting CO samples. The deposit contract was designed to deter non-smokers from enrolling in an incentive-based intervention, to motivate smokers to quit by avoiding losses in addition to receiving monetary gains (23), and to offset the costs associated with CM. After the deposit was recouped, participants in both groups could earn additional incentives based on CO abstinence or CO submissions.
The aims of this randomized trial were to (1) evaluate the efficacy of technology-based CM compared to technology monitoring and goal setting in a nationwide sample of smokers, and (2) access the acceptability of the intervention based on adherence rates to the CO sampling protocol and on acceptability ratings about treatment components (e.g., using a CO monitor, providing a deposit).
Method
Study design
Participants were randomized (1:1) to an Abstinence Contingent (AC) or a Submission Contingent (SC) group. Participants were asked to provide twice daily (for 4 weeks) and then twice weekly (for 3 weeks) video-recorded CO results to the Mōtiv8 website. Participants in the AC group received financial incentives based on abstinence (i.e., breath CO ≤ 4ppm), and participants in the SC group received financial incentives based on submitting CO samples. Participants in both groups were provided with the same CO-based goals. The only difference between groups was the target behavior to receive incentives: the AC group had to meet video-verified CO cutpoints and the SC group had to submit videos. Because baseline drug use has been inversely associated with responsiveness to contingency management (23,24), we stratified on the number of cigarettes smoked per day (≥30 cigarettes per day, Yes or No). Participants were also stratified based on gender (25). Randomization was automated by an Excel macro that coded each participant based on smoking severity and gender, and then assigned the participant to the group with a lower number of participants with that combined smoking severity and sex code. If smoking severity and gender distributions were equal, then the participant was assigned randomly to a group. The first participant enrolled on 28 August 2010 and the database was complete on 20 May 2014. Analysis was by intention to treat.
Interested persons were initially screened online, and then further screening was completed over the phone if they met the initial online screening criteria. A $50 deposit was required from all participants. Deposits were made to PayPal via debit, credit card, or direct bank transfer. The first $50 earned went toward reimbursement for the initial deposit incentive. All earnings were deposited to participants’ PayPal accounts.
Participants were loaned a CO monitor (Bedfont piCO+ Smokerlyzer, Kent, England) and a web-camera if needed (Logitech QuickCam or Creative Live! Cam Optia AF, Creative Labs 1.3 MP Webcam, Model VF0415). CO monitors were calibrated by the manufacturer and then at least every 6 months thereafter (CoVita™, Haddonfield, NJ).
Equipment was sent to participants via FedEx, and we exchanged CO monitors with participants via FedEx for the 3- and 6- month follow-ups. Participants also completed questionnaires assessing demographics, smoking history, prior drug use and abuse, general health, medication use, and the Fagerström Test for Nicotine Dependence (FTND) (26). Scores can range from 0–10, with a score of 0 representing no dependence and higher scores indicating greater dependence.
A Mōtiv8 webpage provided links to smoking cessation support websites, including smokefree.gov, QuitNet.com (archived at http://www.webcitation.org/6jUXwHxCm), and a link to the National Cancer Center’s booklet, Clearing the Air (27), which is a self-help guide to quitting.
Study sample
Participants were healthy smokers recruited from the contiguous United States. Table 1 lists participants’ demographic and smoking characteristics. Recruitment methods included Craigslist, Google Adwords, Facebook advertisements, newspapers, and ClinicalTrials.gov. Individuals interested in participating were routed to an online screening to determine eligibility. Applicants were excluded if they had a history of or current severe or unstable medical or psychiatric illness that would interfere with the study, or if they had unavoidable exposure to high levels of ambient air CO (e.g., occupational exposure). Participants were invited to participate if they were 18 years of age or older, scored 8 or higher on a 10-point Likert scale asking current desire to quit smoking (1 = not at all, 10 = very much), self-reported smoking at least 10 cigarettes per day, self-reported last cigarette within the previous 24 hours, had at least a 2 year history of daily smoking, provided permission to contact applicant by phone, self-reported having access to a computer, and had Internet access available at their residence. The Institutional Review Boards from the University of Florida and the National Development and Research Institute approved all study procedures.
Table 1.
Demographic and smoking characteristics of participants
| Characteristic | Abstinence Contingent (n=48) | Submission Contingent (n=46) |
|---|---|---|
| Age | 36.7 ± 11.20 | 34.9 ± 11.1 |
| Female (%) | 56 | 54 |
| Race/Ethnicity (%) | ||
| European American | 77 | 71 |
| Hispanic American | 4 | 7 |
| African American | 15 | 7 |
| Native American | 0 | 4 |
| Asian | 4 | 9 |
| More than one race | 0 | 2 |
| Yearly Income (%) | ||
| $0–$25,000 | 34 | 26 |
| $25,000–$50,000 | 29 | 39 |
| $50,000–$100,000 | 27 | 28 |
| Above $100,000 | 8 | 7 |
| Not reported | 2 | 0 |
| Education (%) | ||
| Less than high school degree | 2 | 2 |
| High school degree, no college | 8 | 0 |
| Some college | 44 | 48 |
| Associate/Professional Degree | 13 | 13 |
| Bachelor’s degree or higher | 33 | 37 |
| Cigarettes per day | 17.8 ± 7.0 | 18.3 ± 7.0 |
| Years smoking | 17.9 ± 11.0 | 16.5 ± 11.2 |
| FTND | 4.8 ± 2.0 | 4.8 ± 2.3 |
Note: Table values are means ± S.D. unless otherwise indicated.
Study procedures
Participants logged into the Mōtiv8 website using a unique username and password and completed the CO video sample collection by following simple on-screen and printed instructions. Each participant’s homepage consisted of a graph of CO sample results, voucher earnings history, a “Post Video” button, and a display showing their previous sample’s date/time, and the earliest date/time at which they could provide their next sample. The graph of CO sample results included horizontal lines indicating the CO cutpoint goals during the tapering, abstinence induction, and thinning phases (phases are described below). The exact CO target was also written in text above the “Post Video” button. An eight-hour interval had to elapse between CO samples, and the post video button was disabled for eight hours after a video was submitted. All samples were submitted between 5:00 AM and 4:00AM eastern standard time.
Baseline (3 days)
Participants experienced a three-day baseline phase with twice daily CO assessments, which served to familiarize them with the sampling procedure. No financial earnings were provided.
Tapering (4 days)
For participants in the AC group, the average CO during baseline was calculated and every pre-determined reduction from this value resulted in $3.00. The reductions were calculated for each participant such that the last day of this phase would serve as their quit day, with a target CO ≤ 4 ppm. The target of 4 ppm was chosen to be consistent with previous Internet-based CM research (17), and with recommendations for target CO levels in CM interventions (28). All samples ≤ 4 ppm were considered negative. For participants in the SC group, incentives were based on CO submissions only.
Abstinence induction (21 days)
For participants in the AC group, CO samples were judged as either positive or negative (≤ 4 ppm). Consistent with the escalating schedule developed by Higgins and colleagues (29) to promote continuous periods of abstinence, a $3.00 incentive was awarded for the first negative sample, and increased by $0.25 for each consecutive negative sample. In addition, every third consecutive negative CO resulted in a $5.00 bonus (11,17). For participants in the SC group, the same schedule of earnings was scheduled based on CO submissions alone.
Thinning (21 days)
A three-week thinning phase followed the treatment phase. Monitoring and the possibility of reinforcement was decreased, or thinned, from twice daily to twice per week (one sample between 5:00 AM and 4:00AM EST on Mondays and Thursdays). The incentive values were a continuation of the escalating pay schedule from the previous phase. The rationale for this phase stems from research suggesting that thinning, instead of abruptly eliminating, the schedule of reinforcement can maintain behavior change for longer periods (30,31). For participants in the SC group, the same schedule of reinforcement applied to CO submissions only.
Outcome measures and data analysis
Point prevalence (PP) of abstinence was defined by CO ≤ 4 ppm, and a self-reported answer of “No” to whether a participant had smoked cigarettes, even a puff, during the past seven days (Y/N). The primary outcome was PP at week 4. Secondary outcomes included PP at the 3- and 6-month follow-ups; and the twice-daily CO samples during baseline, tapering, and abstinence induction, and twice weekly CO samples during the thinning phase.
Two other secondary measures included a Treatment Acceptability Questionnaire (TAQ), which assessed the acceptability of components of the treatment and the deposit using a 0–100 visual analog scale (32). The TAQ was administered at week 4. Questions assessed the ease, helpfulness, fairness, effectiveness, convenience of the intervention overall, and of distinct elements of the intervention (e.g., seeing graphed CO results, using the CO meter). The TAQ included three specific questions related to the deposit (liking, effectiveness, and fairness of the deposit). The second measure, a Behavioral Change Inventory, included questions about factors that might have impacted the study results (e.g., use or introduction of new strategies for quitting, marijuana smoking, smoking or using other medications, lifestyle changes, adverse events). Participants received $10 or $75 for completing measures (the compensation was increased for both groups after year 2 in the clinical trial to improve adherence). All assessments occurred remotely using the Mōtiv8 platform.
Sample Size
The sample size was based on power calculations derived from a randomized controlled study conducted by Higgins and colleagues (33). The study evaluated the efficacy of CM to promote smoking cessation in pregnant and postpartum women. Although the study population differed from the current study, the procedures used in Higgins et al. were at the time the most similar to the procedures used in the current study (e.g., escalating schedule of voucher earnings for continued abstinence, a control group that also received financial earnings but independent of smoking status). Higgins and colleagues reported that point prevalence measures of abstinence in the contingent versus control groups were 37% versus 9%, 33% versus 0%, and 27% versus 0% at the end of treatment, and 3 and 6-month follow-up, respectively. The observed effect size in that study was .70. Based on this referred study and our power calculation results, we projected 32 subjects per group would be needed to detect an effect size of .70 with an estimated power of .80 using an alpha of .05. However, given the difference in study populations we proposed to randomly assign 50 subjects per group (n=100 total).
Analyses were conducted using SPSS version 22 and MPlus version 7.3. PP measures were analyzed using chi-square tests to examine the intervention effects on smoking cessation outcomes at each time point. Participants lost to follow-up were considered positive for smoking. We also used chi-square tests for binary CO data outcomes (CO≤4 ppm for abstinent versus smoking positive) across the 62 CO assessments to test the effect of intervention conditions within each treatment phase. For outcomes with significant group differences shown in chi-square tests, logistic regression models were applied while adjusting for baseline characteristics (age, sex, annual income, years of daily smoking, and the number of daily cigarettes smoked).
Results
Characteristics of the participants
Table 1 shows participant characteristics for each group. The mean age was 36 and 56% were female. Participants smoked an average of 17 cigarettes per day, and reported an average 17-year history of daily smoking. The majority of participants were European American (73%), and a majority earned between $0–$25,000 (30%) and $25,000–$50,000 (34%) per year. A majority also reported either some college (46%) or receiving a bachelor’s degree or higher (35%). Participants were enrolled from 26 states. The majority were from the South (58%; 29% resided in Florida), others resided in the West (11%), Midwest (17%), and Northeast (14%).
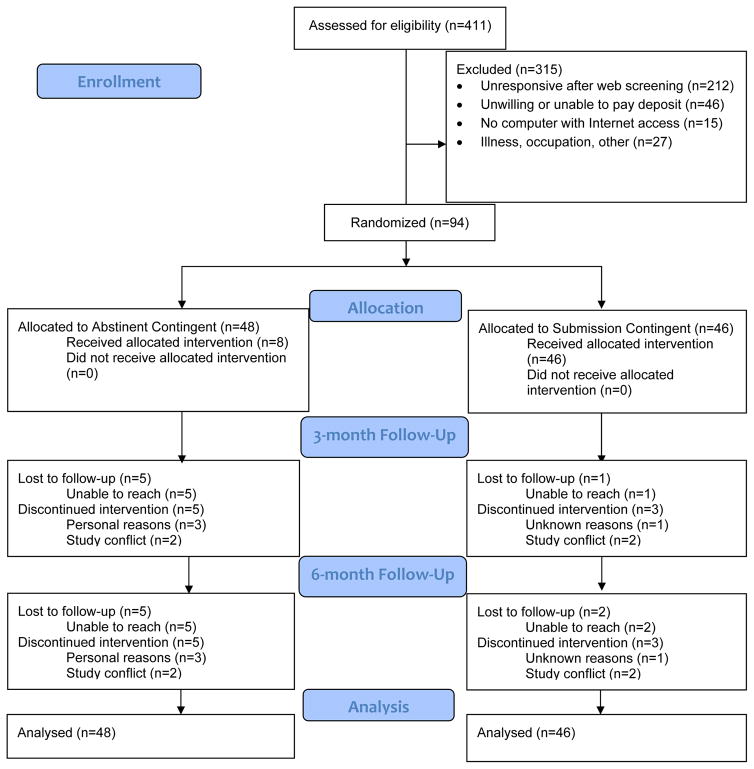
Figure 1 shows the flow of participants through the trial. 411 applicants visited the Mōtiv8 website, completed initial screening information, and received information about the study. 315 were excluded, and the majority (212) of these applicants were unresponsive to attempts to contact them via phone and/or email. Of those contacted, 46 were unwilling to pay the deposit, 15 did not have a home computer with Internet access, and 27 were excluded for other reasons (e.g., illness, occupational exposure to CO). We did not collect data systematically about reasons for being unwilling to pay the deposit, but many applicants shared that they did not have the financial ability, whereas others reported concerns about whether the study was a “scam” and concerns with transferring funds from a financial institution to PayPal. 94 applicants were randomized to the intervention groups. In the AC group, we lost contact with 5 participants and 5 withdrew from the study, and in the SC group we lost contact with 2 participants and 3 withdrew from the study. All missing data were counted as positive for smoking.
Figure 1.
CONSORT flow diagram.
Outcomes
Table 2 presents the PP rates and odds ratios at 4 weeks (primary outcomes), and at the 3- and 6-month follow-ups. At 4 weeks, 39.6% (n=19) and 13.0% (n=6) were negative in the AC and SC groups respectively, χ2(1,94)=8.5, p=.004, r=.30. A logistic regression model indicated that the AC group resulted in a 2.1 (SE=.65) increase in the logit variable of smoking abstinence at 4 weeks compared to the SC group (odds ratio=8.4, 95% CI=2.3–29.9). That is, the AC group, compared to the SC group, had an 8.4 times greater likelihood of abstinence at 4 weeks, which corresponded to the end of the Abstinence Induction phase. Differences in smoking abstinence between the AC and SC groups at the 3- (29% and 19%) and 6-month (23% and 13%) follow-ups were not statistically significant, Fs<2 with small effect sizes r = .11 and r = .13, respectively. Table 2 also presents the percentages of COs that were negative during each study phase. The daily CO data confirmed that during the entire intervention period (i.e., excluding 3- and 6-month follow-ups), abstinence rates were 43.9% in the AC group and 20.8% in the SC group, χ2(1, 5828)=353.29, p < .01 r=.25.
Table 2.
Smoking cessation outcome
| Outcome Measure | Abstinence Contingent | Submission Contingent | Odds ratio (95% CI) | Unadjusted χ2, p |
|---|---|---|---|---|
| ITT sample | N=48 | N=46 | ||
| Primary Outcome (PP 4 wks), % (n) | 39.6 (19) | 13.0 (6) | 4.4(1.5–12.3) | 8.5, .004 |
| Secondary Outcome (PP 3 mo), % (n) | 29.2 (14) | 19.6 (9) | 1.7 (0.6–4.4) | 1.2, .279 |
| Secondary Outcome (PP 6 mo), % (n) | 22.9 (11) | 13 (6) | 2.0 (0.7–5.9) | 1.5, .214 |
| Negative CO | ||||
| Secondary Outcome, Total, % (n) | 43.9 (1307/2976) | 20.8 (594/2852) | 3.0 (2.6–3.3) | 353.3, < .001 |
| Secondary Outcome, Baseline, % (n) | 6.6 (19/288) | 3.6 (10/276) | 1.9 (0.9–4.1) | 2.6, .110 |
| Secondary Outcome, Tapering, % (n) | 19.8 (76/384) | 9.8 (36/368) | 2.3 (1.5–3.5) | 14.8, < .001 |
| Secondary Outcome, Abstinence Induct. % (n) | 53.9 (1087/2016) | 24.8 (480/1932) | 3.5 (3.1–4.0) | 348.4, <.001 |
| Secondary Outcome, Thinning, % (n) | 43.4 (125/288) | 24.6 (68/276) | 2.3 (1.6–3.4) | 22.0, < .001 |
Adherence to the video submission protocol was equivalent across groups: 85% of all submissions in SC versus 78% in the AC group (difference = 7.0%, 95% CI=−10.3%–23.8%, F<1, p=.39).
The average amount distributed in the AC group was $218 (SD ± $196) and $366 (SD ± $153) in the SC groups, respectively. After deducting the deposits, the average earnings were $168 and $316 per participant in the AC and SC groups, respectively.
Results of the Treatment Acceptability Questionnaire are presented in Table 3. The AC group rated several items significantly lower than the SC group (items assessing fairness of the intervention, liking of the graph, earning money, and providing the deposit). The lowest ratings in both groups were for the items assessing the deposit. The highest ratings overall concerned the ease of the intervention, the graph of CO results, and earning money.
Table 3.
Mean (±SD) Treatment Acceptability Ratings based on a 100mm visual analog scale (0 = not favorable, 100 = very favorable)
| AC | SC | T-test (df=80) | |
|---|---|---|---|
| Easy | 86.6 (21.2) | 85.7 (19.4) | .20, p = 0.84 |
| Helpful | 66.7 (32.3) | 58.0 (28.2) | 1.30, p = 0.20 |
| Like CO meter | 70.3 (27.8) | 79.1 (16.5) | −1.72, p = 0.09 |
| Like Graph | 81.7 (21.4) | 90.7 (11.0) | −2.36, p = 0.02* |
| Earning money | 81.7 (22.5) | 92.9 (14.1) | −2.69, p = 0.009* |
| Convenient | 76.8 (21.5) | 78.2 (20.5) | −0.30, p = 0.76 |
| Effective | 67.4 (33.2) | 58.6 (29.3) | 1.27, p = 0.21 |
| Fair | 72.4 (30.3) | 90.6 (14.0) | −3.42, p = 0.001* |
| Like providing deposit | 54.5 (32.0) | 70.7 (26.4) | −2.49, p = 0.015* |
| Effective deposit to quit | 50.6 (33.6) | 53.7 (31.1) | −0.43, p = 0.67 |
| Fair deposit | 71.0 (28.9) | 79.6 (23.7) | −1.47, p = 0.15 |
Results from the Behavioral Change Inventory revealed that the groups did not differ meaningfully during treatment in the use of pharmacological interventions (2 participants in both groups), the use of educational or smoking cessation websites (14 in the AC and 18 in the SC group), smoking marijuana (1 in the AC and 3 in the SC group). The use of other nicotine products such as chewing tobacco was not reported in either group. No adverse events were reported in either group.
Discussion
The present study represents the first contingency management (CM) intervention that was accessible to any qualified smoker in the United States. We enrolled smokers from 26 states. Aside from phone contact, all procedures occurred remotely via the Internet. At week 4, point prevalence rates were 39.6% and 13.0% in the AC and SC groups, respectively. During the two treatment phases when negative COs were the goals, there were also significant differences in negative COs (53.9% AC and 24.8% SC during the Abstinence Induction phase, and 43.4% AC and 24.6% SC during the Thinning phase). Although there was no significant difference in abstinence at the 3- (29% AC and 19% SC) and 6-month (23% AC and 13% SC) follow-ups, the failure to detect the trend as a significant effect may be due in part to insufficient power. The relatively high rates of success in the SC group may be due to enrollment of motivated smokers or to frequent self-monitoring of smoking; these features may have reduced sensitivity to detect intervention effects. Adherence to the CO submission protocol was equivalent: 78% in AC and the 85% in the SC group. Thus, differences in smoking levels in the two groups can be attributed to the differences in the behavior targeted by the contingency: CO levels versus CO submissions. These results are consistent with previous technology-based interventions that involved in-person contacts for counseling and other study-related procedures (11,17). Overall, these results suggest that it is feasible and effective to extend the reach of CM interventions via technology.
The deposit contract procedure produced mixed findings. On the one hand, the deposit yielded some cost offsets. For the AC treatment group, the average amount earned per participant was $218, but after deducting the deposit the average decreased to $168. In addition, it is possible the deposit deterred non-smokers from enrolling in the study to gain access to incentives. All participants provided a positive CO at intake, and all except for one participant provided at least one positive CO during the intervention. Thus, we have biochemical evidence that the vast majority of these participants smoked cigarettes. On the other hand, participants rated aspects of the deposit contract as among the least acceptable (see Table 3). After learning about the study during the online screening process, 13.5% of applicants cited the deposit as a reason for not participating. Applicants cited the lack of available money as one reason for declining, but several others cited the possibility of the study being a “scam” and security issues about transferring funds. Security concerns raise caveats on the use of Internet-based CM procedures but do not preclude their utility. It is also possible that the deposit was an issue for a subset of the other 62% that were unresponsive to phone contacts after screening. Similarly, in a large clinical trial assessing various deposit and incentive arrangements, Halpern and colleagues (22) found that only 13.7% of applicants accepted assignment to a deposit contract group. Thus, one limitation of the current study is that the generality of our findings are restricted to those willing to pay a deposit.
Despite these mixed findings, deposits may be an important element of remote, technology-based interventions involving the provision of additional monetary incentives (19). The main rationale would be to deter faking of smoking to gain access to incentives. Even if additional incentives are not involved, deposits alone may represent a feasible CM delivery model (34,35). For example, web-based behavioral change programs such as StickK.com and Dietbet.com – both of which incorporate deposits - report high rates of access. As of 2016 both StickK.com and DietBet.com each reported over 300,000 end-users. Halpern and colleagues (36) outlined several design features of deposit contracts that warrant further investigation such as the ratio of the deposited amount to additional incentives, or whether the deposit is fixed or can be tailored by the individual. Indeed, more research is needed to enhance and evaluate the acceptability, effectiveness, and cost effectiveness of deposit contracts to promote behavior change.
Overall, participants found that the current intervention was acceptable. The highest rated items on the Treatment Acceptability Questionnaire in both groups concerned the ease of the intervention, the ability to view graphed CO progress, and earning money (all were rated > 80 on a 0–100 scale). These results are consistent with previous assessments of treatment acceptability of technology-based CM (32). Raiff and colleagues found that a number of stakeholders, including smokers, health-care professionals, and non-smokers, found that technology-based CM was acceptable. Of the health-care providers, 81% (n = 113) reported that they would be very likely to recommend the intervention to patients. One limitation of the present findings is that we did not assess concerns with confidentiality associated with technology-based methods.
One potential limitation of the study is that the sample’s demographic characteristics are not representative of the population of cigarette smokers in the United States (37). The requirement to own an Internet-connected computer at home may have yielded higher levels of education and income in the current study than the smoking population at large. To our knowledge, however, there is no evidence that education or income affects the efficacy of CM targeting smoking or substance use more generally (38).
Future iterations of technology-based CM will involve new digital technologies, and new applications of behavioral technologies (39). In terms of digital technologies, one study found that mobile phone CM (mCM) promoted higher rates of abstinence in smokers with post-traumatic stress disorder compared to a control group (15). In addition, lower cost CO monitors will make the intervention more economically attractive (40). Indeed, lower-cost monitors integrated with mobile phones have significant promise to deliver mCM. New applications of behavioral technologies might include group contingencies in which small teams of smokers must meet CO goals for incentives (41,42), or self-tailored deposits in which the end-user selects the amounts and timings of his or her deposits (43). Deposit contracts could be used as part of a lapse responsive method. That is, a smoker could re-initiate the intervention after a lapse or relapse by depositing a self-tailored amount of money.
The present results suggest that it is effective and acceptable to extend the reach of CM interventions via technology. Future work should continue to assess economically feasible and sustainable treatment delivery models.
Acknowledgments
Research and preparation of this paper was supported by Grants R01DA023469 (PI: J. Dallery) and P30DA029926 (PI: L. Marsch) from the National Institute on Drug Abuse.
We thank Alana Rojewski, Patrick Kurdila, Donna Paniry, Aaron Dumas, Hypatia Bolivar, Allison Kurti, Sarah Martner, and Yoko Fisher.
Footnotes
Clinical trial registration
ClinicalTrials.gov. Identifier: NCT00926939.
Declarations of interest
None.
Contributor Information
Jesse Dallery, University of Florida, Gainesville, FL, National Development and Research Institutes, New York, NY.
Bethany R. Raiff, Rowan University, Glassboro, NJ
Sunny Jung Kim, The Geisel School of Medicine at Dartmouth, Hanover, NH.
Lisa A. Marsch, The Geisel School of Medicine at Dartmouth, Hanover, NH
Maxine Stitzer, The Johns Hopkins University School of Medicine, Baltimore, MD.
Michael J. Grabinski, Red5hift LLC, Hanover, NH
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. U S Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 3.McClernon FJ, Roy Choudhury R. I Am Your Smartphone and I Know You Are About to Smoke: The Application of Mobile Sensing and Computing Approaches to Smoking Research and Treatment. Nicotine Tob Res. 2013 May 23; doi: 10.1093/ntr/ntt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strecher VJ, McClure J, Alexander G, Chakraborty B, Nair V, Konkel J, et al. The role of engagement in a tailored web-based smoking cessation program: randomized controlled trial. J Med Internet Res. 2008 Nov 4;10(5):e36. doi: 10.2196/jmir.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bricker J, Wyszynski C, Comstock B, Heffner JL. Pilot randomized controlled trial of web-based acceptance and commitment therapy for smoking cessation. Nicotine Tob Res. 2013 Oct;15(10):1756–1764. doi: 10.1093/ntr/ntt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stitzer M, Bigelow G, Lawrence C, Cohen J, D’Lugoff B, Hawthorne J. Medication take-home as a reinforcer in a methadone maintenance program. Addict Behav. 1977;2(1):9–14. doi: 10.1016/0306-4603(77)90003-x. [DOI] [PubMed] [Google Scholar]
- 7.Higgins ST, Silverman K, Heil SH. Contingency management in substance abuse treatment. New York, NY US: Guilford Press; 2008. [Google Scholar]
- 8.Petry NM. Contingency management treatments. Br J Psychiatry. 2006 Aug;189:97–98. doi: 10.1192/bjp.bp.106.022293. [DOI] [PubMed] [Google Scholar]
- 9.Sigmon SC, Lamb RJ, Dallery J. Tobacco. In: Higgins ST, Silverman K, Heil SH, editors. Contingency management in substance abuse treatment. New York, NY US: Guilford Press; 2008. pp. 99–119. [Google Scholar]
- 10.Dallery J, Glenn IM. Effects of an Internet-based voucher reinforcement program for smoking abstinence: a feasibility study. J Appl Behav Anal. 2005 Fall;38(3):349–357. doi: 10.1901/jaba.2005.150-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallery J, Glenn IM, Raiff BR. An Internet-based abstinence reinforcement treatment for cigarette smoking. Drug Alcohol Depend. 2007;86(2):230–238. doi: 10.1016/j.drugalcdep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Dallery J, Raiff BR. Contingency management in the 21st century: Technological innovations to promote smoking cessation. Subst Use Misuse. 2011;46(1):10–22. doi: 10.3109/10826084.2011.521067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds B, Dallery J, Shroff P, Patak M, Leraas K. A web-based contingency management program with adolescent smokers. J Appl Behav Anal. 2008;41(4):597–601. doi: 10.1901/jaba.2008.41-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoops WW, Dallery J, Fields NM, Nuzzo PA, Schoenberg NE, Martin CA, et al. An internet-based abstinence reinforcement smoking cessation intervention in rural smokers. Drug Alcohol Depend. 2009;105(1–2):56–62. doi: 10.1016/j.drugalcdep.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hertzberg JS, Carpenter VL, Kirby AC, Calhoun PS, Moore SD, Dennis MF, et al. Mobile Contingency Management as an Adjunctive Smoking Cessation Treatment for Smokers With Posttraumatic Stress Disorder. Nicotine Tobacco Res. 2013;15(11):1934–1938. doi: 10.1093/ntr/ntt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dan M, Grabinski MJ, Raiff BR. Smartphone-based contingency management for smoking cessation with smokers diagnosed with attention-deficit/hyperactivity disorder. Translational Issues in Psychological Science. 2016;2(2):116–127. [Google Scholar]
- 17.Dallery J, Raiff BR, Grabinski MJ. Internet-based contingency management to promote smoking cessation: A randomized controlled study. J Appl Behav Anal. 2013;46(4):750–764. doi: 10.1002/jaba.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds B, Harris M, Slone SA, Shelton BJ, Dallery J, Stoops W, et al. A feasibility study of home-based contingency management with adolescent smokers of rural Appalachia. Exp Clin Psychopharmacol. 2015 Dec;23(6):486–493. doi: 10.1037/pha0000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dallery J, Meredith S, Glenn IM. A deposit contract method to deliver abstinence reinforcement for cigarette smoking. J Appl Behav Anal. 2008;41(4):609–615. doi: 10.1901/jaba.2008.41-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paxton R. Prolonging the effects of deposit contracts with smokers. Behav Res Ther. 1983;21(4):425–433. doi: 10.1016/0005-7967(83)90012-8. [DOI] [PubMed] [Google Scholar]
- 21.Winett RA. Parameters of deposite contracts in the modification of smoking. The Psychological Record. 1973;23(1):49–60. [Google Scholar]
- 22.Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, et al. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372:2108–2117. doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. J Consult Clin Psychol. 1998;66(5):811–824. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
- 24.Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, et al. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996 May;53(5):409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- 25.Borrelli B, Spring B, Niaura R, Hitsman B, Papandonatos G. Influences of gender and weight gain on short-term relapse to smoking in a cessation trial. J Consult Clin Psychol. 2001 Jun;69(3):511–515. doi: 10.1037//0022-006x.69.3.511. [DOI] [PubMed] [Google Scholar]
- 26.Fagerstrom K, Schneider NG. Measuring nicotine dependence: A review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12(2):159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute. Clearing the Air: Quit Smoking Today. Bethesda, MD: U.S. Dept. of Health and Human Services, National Institutes of Health, National Cancer Institute; 2008. [Google Scholar]
- 28.Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. (100) 2005;(2):159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- 29.Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994 Jul;51(7):568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- 30.Chudzynski J, Roll JM, McPherson S, Cameron JM, Howell DN. Reinforcement Schedule Effects on Long-Term Behavior Change. Psychol Rec. 2015 Jun 1;65(2):347–353. doi: 10.1007/s40732-014-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St Peter Pipkin C, Vollmer TR. Applied implications of reinforcement history effects. J Appl Behav Anal. 2009 Spring;42(1):83–103. doi: 10.1901/jaba.2009.42-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raiff BR, Jarvis BP, Turturici M, Dallery J. Acceptability of an Internet-based contingency management intervention for smoking cessation: views of smokers, nonsmokers, and healthcare professionals. Exp Clin Psychopharmacol. 2013;21(3):204. doi: 10.1037/a0032451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res. 2004 Dec;6(6):1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- 34.Leahey T, Rosen J. DietBet: A Web-Based Program that Uses Social Gaming and Financial Incentives to Promote Weight Loss. JMIR Serious Games. 2014 Feb 7;2(1):e2. doi: 10.2196/games.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel MS, Asch DA, Rosin R, Small DS, Bellamy SL, Heuer J, et al. Framing Financial Incentives to Increase Physical Activity Among Overweight and Obese Adults: A Randomized, Controlled Trial. Ann Intern Med. 2016 Feb 16; doi: 10.7326/M15-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halpern SD, Asch DA, Volpp KG. Commitment contracts as a way to health. BMJ. 2012 Jan 30;344:e522. doi: 10.1136/bmj.e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamal A, Homa DM, O’Connor E, Babb SD, Caraballo RS, Singh T, et al. Current cigarette smoking among adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015 Nov 13;64(44):1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 38.Rash CJ, Olmstead TA, Petry NM. Income does not affect response to contingency management treatments among community substance abuse treatment-seekers. Drug Alcohol Depend. 2009 Oct 1;104(3):249–253. doi: 10.1016/j.drugalcdep.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dallery J, Kurti A, Erb P. A New Frontier: Integrating Behavioral and Digital Technology to Promote Health Behavior. The Behavior Analyst 2015. 2014;38(1):19–49. doi: 10.1007/s40614-014-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meredith SE, Robinson A, Erb P, Spieler CA, Klugman N, Dutta P, et al. A mobile-phone-based breath carbon monoxide meter to detect cigarette smoking. Nicotine Tob Res. 2014 Jun;16(6):766–773. doi: 10.1093/ntr/ntt275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meredith SE, Grabinski MJ, Dallery J. Internet-based group contingency management to promote abstinence from cigarette smoking: A feasibility study. Drug Alcohol Depend. 2011 Oct 1;118(1):23–30. doi: 10.1016/j.drugalcdep.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meredith SE, Dallery J. Investigating group contingencies to promote brief abstinence from cigarette smoking. Exp Clin Psychopharmacol. 2013;21(2):144–154. doi: 10.1037/a0031707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarvis BP, Dallery J. Internet-based self-tailored deposit contracts to promote smoking reduction and abstinence. J Appl Behav Anal. doi: 10.1002/jaba.377. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]