Abstract
The mechanical properties of extracellular matrix (ECM) and connective tissues is largely dependent on the collagen and elastin structure. Lysyl oxidase (LOX) plays a critical role in the formation and repair of the ECM by oxidizing lysine residues in elastin and collagen, thereby initiating the formation of covalent cross linkages which stabilize these fibrous proteins. Due to its multiple functions both extracellularly and intracellularly, lysyl oxidase is involved in several processes in the tumorigenic pathway, in many different cancer types and stages. Alteration in LOX activity is implicated in many diseases and disorders including inflammation and inflammatory diseases, fibrosis of distinct organs and fibrotic disorders, cancer promotion and progression. There are only sparse reports of mutations or epigenetic alterations in the LOX gene. This review provides the recent clinical developments in the molecular mechanisms and pathologic process, pointing out LOX as a potential therapeutic target in translational medicine.
Keywords: Extracellular matrix, Lysyl oxidase, Tumorigenic pathway, Translational medicine
Introduction
The mechanical integrity of the tissues and structures such as skin, blood vessels, bones and tendons is based on a highly organized molecular structure of Collagen, in which three polypeptide chains are wound into a triple helix. Individual collagen molecules are organized in a head-to-tail quarter-staggered arrangement to make microfibrils and larger collagen fibrils [1]. These fibrils are strengthened by covalent cross-links formed enzymically during assembly between the collagen molecules [2].
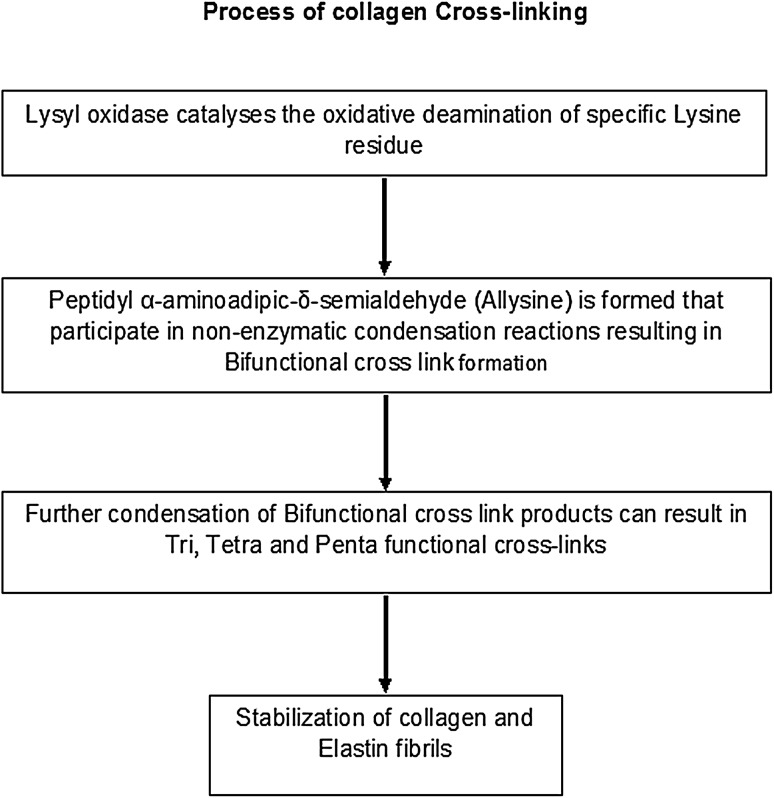
Lysyl oxidase (LOX) is an extracellular enzyme responsible for initiating covalent cross-link formation in collagen fibrils by oxidatively deaminating specific lysine and hydroxylysine residues to form allysines in the telopeptide domains of the collagen molecule [3]. Copper in lysyl oxidase appears to be involved in the transfer of electrons to and from oxygen to facilitate the oxidative deamination of targeted peptidyl lysyl groups in tropocollagen or tropoelastin and to internally catalyse quinone cofactor formation. Oxidation of peptidyl lysine results in the formation of peptidyl aminoadipic-δ- semialdehyde [4]. These aldehydes are highly reactive, and undergo spontaneous chemical reactions with other lysyl oxidase-derived aldehyde residues, or with unmodified lysine residues (Fig. 1). This results in cross-linking collagen and elastin, which is essential for stabilization of collagen fibrils and for the integrity and elasticity of mature elastin [5].
Fig. 1.
Process of collagen cross-linking
As LOX is necessary for the assembly and tensile strength, it is highly expressed in tissues containing fibrillar collagen and elastic fibres i.e. Skin, lung, cartilage, the cardiovascular system and the fibrous laminia propria, in the small intestine, liver, kidney, stomach, retina, and brain [6].
LOX has been classically characterized as an extracellular matrix enzyme. However, less is known about intracellular LOX. The LOX protein has been localized within the nuclei of various cells and tissues, and retains its catalytic activity inhibited by Beta-amino propionitrile (BAPN). Nuclear LOX has been shown to originate from extracellular LOX that enters the cytosol and concentrates within the nucleus. Histones have been reported as substrates for lysyl oxidase, and transfection of LOX yielded less tightly packed chromatin [7–9].
LOX Family Structure
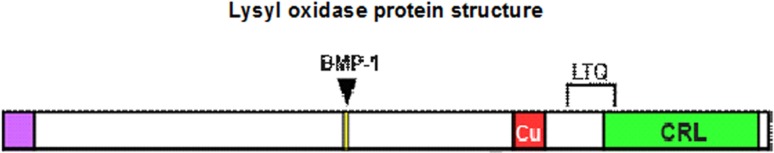
Besides the gene encoding LOX, new LOX-like genes have been identified and cloned, suggesting the existence of a family consisting of LOX and four LOX-like proteins (LOXL-1, -2, -3, and -4) [10, 11] having a complex tissue specific expression pattern and variation in mRNA levels. All members of the lysyl oxidase family of proteins share two highly conserved domains: a unique copper-binding (Cu) domain containing four histidines, shaded in red; and a cytokine-receptor like (CRL) domain similar to type I cytokine receptors, shaded in green. The predicted signal sequence is shaded in purple. The BMP-1 cleavage site, shaded in yellow, is noted by the arrow (Fig. 2). This region contains all of the elements required for enzymatic activity and is highly conserved. There is a great degree of diversity in the N terminal sequence of LOX family members [12]. LOX is secreted from fibrogenic cells as a 50-kDa proenzyme that appears to have little or no enzymatic activity and is processed in the extracellular environment to produce the 30-kDa catalytically active enzyme and a 18-kDa propeptide. This processing is mainly accomplished by the Zn-dependent Pro Collagen C Proteinase i.e. PCP (a member of the astacin family of enzymes, is a product of the Bmp1 gene i.e. bone morphogenic protein-1) and to a lesser degree by the mammalian Tolloid-like-1 protein and aminopeptidase B. Recent data suggest that the ECM glycoprotein fibronectin facilitates LOX processing by bringing PCP into close proximity or by altering the conformation of LOX proenzyme to make the cleavage site more accessible [13].
Fig. 2.
Lysyl oxidase protein structure
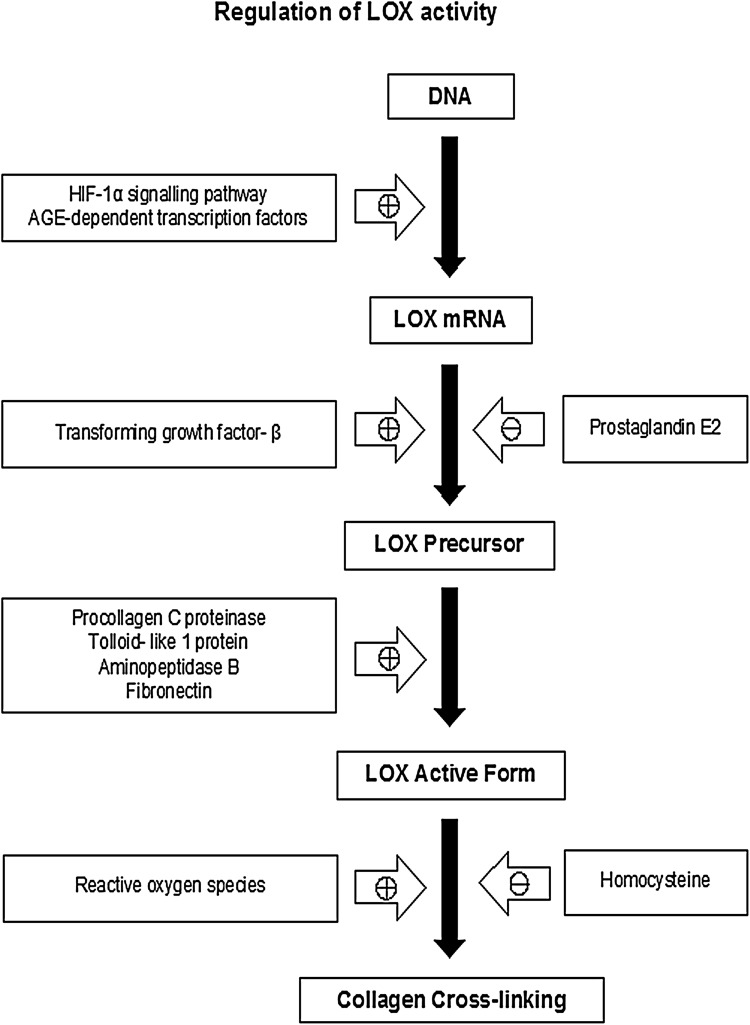
After these posttranslational modifications of Proenzyme in the endoplasmic reticulum and Golgi apparatus, it is secreted to the extracellular space where it is processed to form the mature active enzyme. Therefore, LOX can be regulated at three levels: synthesis of LOX precursor, extracellular conversion of the precursor into the mature enzyme and direct stimulation of the activity of the enzyme (Fig. 3). Hypoxia inducible factor-1 and the activation of hypoxia inducible factor-1 signalling regulate the expression of the LOX gene [14]. Advanced glycation end products (AGEs) induce the binding of transcription factors, such as nuclear factor κ-β and activator protein-1 on the LOX promoter, suggesting a possible involvement of AGEs in LOX gene regulation. LOX mRNA expression can also be regulated at the posttranscriptional level by humoral factors i.e. Transforming growth factor-β (TGF-β). On the contrary, the prostanoid prostaglandin E2 decreases the basal level of LOX mRNA and prevents the LOX precursor increase induced by TGF-β in fibroblasts [15, 16]. Homocysteine, a metabolic by-product of methyl transfer from s-adenosyl homocysteine, is able to directly inhibit LOX activity through a direct covalent interaction with the enzyme carbonyl cofactor lysine tyrosylquinone [17].
Fig. 3.
Regulation of LOX activity
LOX activity is required for the mechanical integrity of the collagen. So the consequences of LOX inhibition in producing lathyrism are well documented. Beta-aminopropionitrile (BAPN) treatment in tendon like construct (prepared from human tenocytes) showed structurally abnormal collagen fibril with irregular profiles resembling those seen in Ehlers Danlos Syndrome (EDS) phenotypes [18]. The Collagen type-V, Decorin, Fibromodulin and Tenascin –X were unaffected by Cross-link inhibition, suggesting that LOX regulates Fibrillogenesis independent of these Proteins [19].
Myocardial fibrosis directly contributes to adverse myocardial remodeling and the resulting alterations of left ventricular (LV) anatomy and function present in the major types of cardiac diseases [20]. Clinical as well as experimental studies show that LOX upregulation and/or over activity could underlie myocardial fibrosis and altered LV mechanics and contribute to the compromise of LV function in cardiac diseases [21]. Dysregulated matrix cross-linking and stability act as a pathological hallmark of pulmonary arterial hypertension.
LOX/LOXL family members contribute to the integrity and stabilization of a healthy vessel wall. Co-treatment with Angiotensin-II (ANG-II) and β-aminopropionitrile (β-APN) caused a rise in incidence of abdominal aortic aneurysm (AAA) and increased atherosclerotic lesion formation [22]. Therapeutic strategies to overexpress LOX/LOXL enzymes or to support the crosslinking of soluble matrix proteins in a polymeric scaffold are a promising opportunity to achieve stabilization of abdominal aortic aneurysm (AAA).
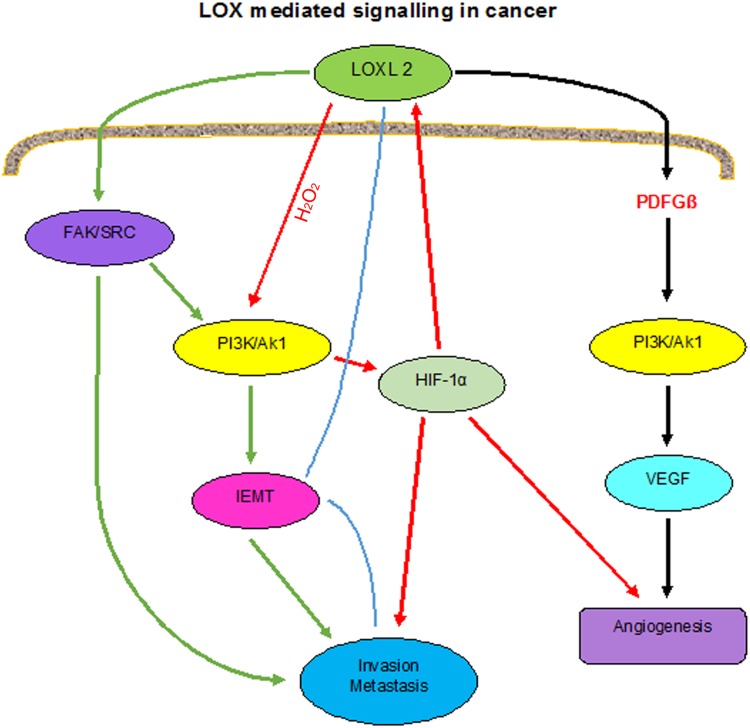
Using multiple models of colorectal cancer, it was found that lysyl oxidase (LOX) is essential for stimulating endothelial cells in vitro, and angiogenesis in vivo. LOX activates Akt (also known as Protein kinase B i.e. PKB), through platelet derived growth factor receptor β (PDGFRβ) stimulation, resulting in increased vascular endothelial growth factor (VEGF) expression. So LOX-driven angiogenesis can be abrogated through targeting LOX directly, or using inhibitors of PDGFRβ, Akt and VEGF signalling. LOX is clinically correlated with VEGF expression and blood vessel formation in 515 colorectal cancer patient samples [23].
Using DNA microarray analysis, it was found that the lysyl oxidase (LOX) gene was upregulated in tumour epithelial cell (TECs) compared with its expression in normal endothelial cells (NECs). LOX is an enzyme that enhances invasion and metastasis of tumour cells. Studies speculated that VEGF induced by hypoxia may be one possibility because LOX expression is regulated by hypoxia-inducible factors (HIFs) and VEGF in tumour cells [24]. LOX was upregulated even in NECs when they were exposed to hypoxic conditions or VEGF. As the primary function of LOX is to promote the covalent cross-linking of collagens and/or elastin in the extracellular matrix (ECM), which mediates tumour malignant transformation and the formation of premetastatic niches, TEC-derived LOX might be involved in pre-metastatic niche formation [25]. This explains that LOX is critical for pre-metastatic niche formation and its inhibition could prevent metastatic tumour growth through decreased recruitment of bone marrow-derived cells. Tumour cell entry into the blood may be prevented by suppressing tumour angiogenesis through LOX inhibition.
Many studies raised the importance of understanding the intracellular and the extracellular role of LOX. LOX inhibition by genetic or chemical means prevent in vitro invasion of breast, melanoma and pancreatic cancer cells. Role of LOX in enhanced invasiveness of hypoxic tumour cells provided a mechanism of how oxygen deprivation has a role in enhancing in vitro invasion of cancer cells. In Breast, head and neck, cervical, pancreatic, renal and lung cancer, hypoxia induced invasion can be prevented by treatment with LOX anti sense oligonucleotides and Short Hairpin(sh)RNA expression [26]. So hypoxic LOX expression results in increased actin polymerization, focal adhesion formation, cell–matrix adhesion, focal adhesion kinase (FAK) activation, cell movement and cell migration all these events essential for invasive migration of cancer cells (Fig. 4).
Fig. 4.
LOX mediated signalling in cancer
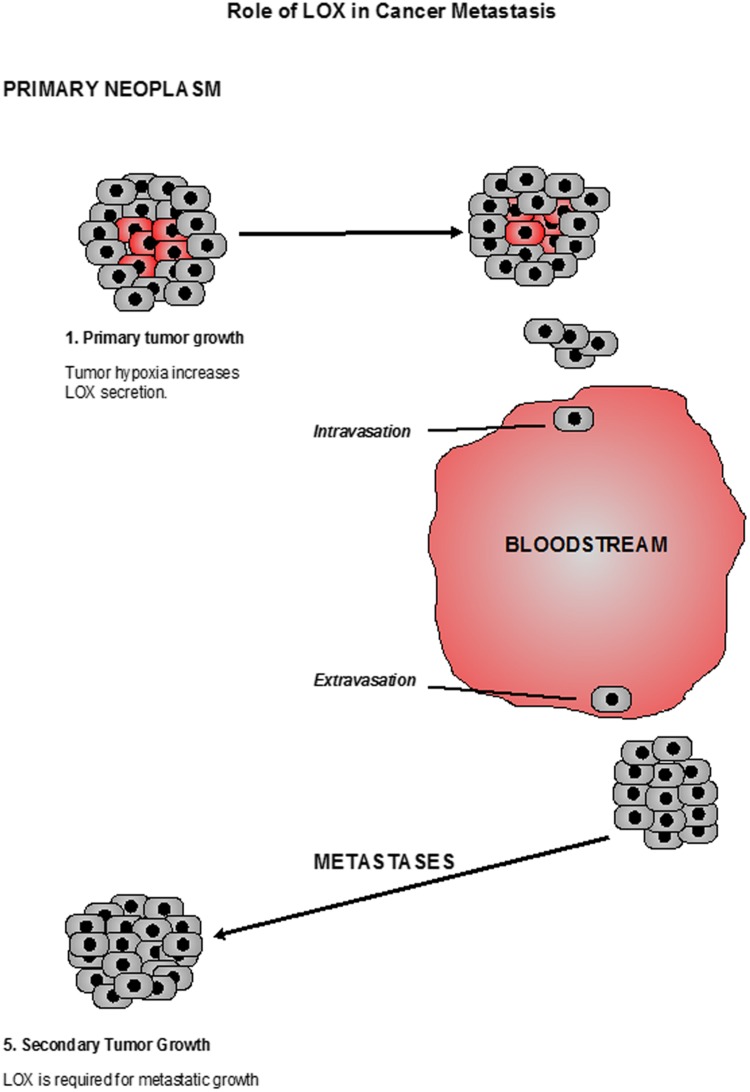
Role of LOX in metastasis could be explained by the fact that Hypoxia increases the amount of enzymatically active secreted LOX that act on collagen fibres outside of the cell increasing Integrin activity resulting in cell movement, leaving behind remodelled matrix tracks that create a route through which other cells may travel providing a “Highway to metastasis”. In the advanced stage of metastasis, the cells adhere to vessel wall, extravasate and migrate to colonize secondary organs (Fig. 5). LOX is required for the formation of mature extracellular Matrix at the secondary site, allowing cell proliferation and metastatic growth [27, 28]. So targeting secreted LOX could be an attractive mechanism to control all stages of metastasis.
Fig. 5.
Role of LOX in cancer metastasis
Latest and emerging research work showed that in models of breast cancer metastasis, targeting LOX, or its downstream effects, significantly inhibited premetastatic niche formation and the resulting metastatic burden, offering preclinical validation of this enzyme as a therapeutic target for metastatic breast cancer, supporting the fact that targeting of LOX could prevent the progression of multiple solid tumour types [29].
Hypoxia-inducible factor-1α (HIF-1α) play as an important contributing factor to radioresistance in tumor cells. Inhibition of LOX resulted in the reduction of the ability to repair double-stranded breaks (DSBs), promotion of apoptosis, relief of G2/M cycle arrest, and eventually reduction of hypoxia-induced radioresistance in the hypoxic A549 cells [30]. This suggests that LOX may play an important role in hypoxia-induced radioresistance.
Studies based on analysis of the effect of lysyl oxidases on drug distribution and efficacy in 3 Dimentional in vitro assay systems documented that elevated Lysyl oxidase (LOX) activity was responsible for reduced drug diffusion under hypoxic conditions and consequently impaired cytotoxicity of various chemotherapeutics. This effect was only observed in 3D settings but not in 2D-cell culture, confirming that lysyl oxidases affect drug efficacy by modification of the ECM and do not confer a direct desensitizing effect. Both drug diffusion and efficacy were strongly enhanced by inhibition of lysyl oxidases [28, 31].
It has been identified that lysyl oxidase (LOX) is associated with bone-tropism and relapse. Increased expression of LOX in primary breast tumours or systemic delivery of LOX leads to osteolytic lesion formation whereas silencing of LOX activity abrogates tumour-driven osteolytic lesion formation [32]. LOX acts as a novel regulator of osteoclast genesis contributing towards the formation of focal pre-metastatic lesions that could provide a platform for circulating tumour cells to colonize and form bone metastases.
Lysyl oxidase pro-peptide (LOX-PP) has the potential to promote bone cell differentiation, while inhibiting cancer cell effects in bone. Data showed that prostate cancer cell conditioned media inhibited osteoblast differentiation in bone marrow-derived cells, which was reversed by rLOX-PP treatment which could be explained as Prostate cancer conditioned media stimulated osteoclast differentiation which was further enhanced by rLOX-PP treatment [33]. rLOX-PP enhances both osteoclast and osteoblast differentiation. So rLOX-PP may serve to enhance coupling interactions between osteoclasts and osteoblasts helping to maintain a normal bone turnover in health, while contributing to bone abnormalities in disease [34].
LOX is the main isoenzyme expressed in human adipose tissue and its expression is upregulated in obese patients referred to bariatric surgery. Treatment with beta-aminopropionitrile (BAPN), a specific inhibitor of LOX, attenuated the increase in body weight and Fat mass. BAPN also ameliorated the increase in collagen content in adipose tissue of obese animals. It prevented the down regulation of adiponectin and Glucose transporter 4(GLUT-4) [35] pointing towards the fact that LOX plays a pathologically relevant role in metabolic dysfunction induced by obesity.
Hypoxia of obstructive sleep apnoea (OSA) increases hepatic production of lysyl oxidase (LOX). A recent study registered that the apnoea-hypopnea index as well as serum LOX was higher in patients with hepatic fibrosis than in those without fibrosis as was serum LOX [36]. In the sleep clinic sample, patients with severe OSA had higher baseline LOX than healthy controls and serum LOX decreased in patients with OSA on continuous positive airway pressure (CPAP) but not in untreated patients suggesting the fact that the hypoxic stress of obstructive sleep apnoea may increase circulating lysyl oxidase (LOX) levels, so LOX may serve as a biomarker of liver fibrosis in patients with severe obesity and non- alcoholic fatty liver disease [37].
Many studies were conducted to reveal the usefulness of serum lysyl oxidase in assessing liver fibrogenesis. Serum lysyl oxidase activity was increased in chronic persistent hepatitis, chronic active hepatitis and in cirrhosis, indicating an increase in concert with the development of liver fibrosis [38, 39]. In hepatocellular carcinoma, the serum activity, although significantly increased, was lower than that in cirrhosis. The magnitude of the increase and the abnormal percentage of serum lysyl oxidase activity were larger than those for serum prolyl hydroxylase and laminin P1. These results suggest that serum lysyl oxidase activity is a more sensitive indicator of liver fibrosis than serum prolyl hydroxylase and laminin P1 [40, 41].
In an experimental model of choroidal neovascularization (CNV), levels of LOX and LOXL2 in the posterior eye cups were found increased in a recent study [42]. Both antibodies i.e. against LOX and LOXL2 significantly inhibited fibrosis, angiogenesis and inflammation. Transcript levels of α-1 type I collagen (COL1A1) in the posterior eye cups were significantly decreased in lasered mice treated with antibodies. Vascular endothelial growth factor expression was also reduced suggesting that LOX and LOXL2 may play an important role in the pathogenesis of Age related Macular Degeneration [43].
Lysyl oxidase (LOX) and LOX like enzymes (LOXL1-4) contribute to cellular senescence under oxidative stress (OS).Recently a study showed that LOX gene expression was higher in fetal membranes from preterm prelabor rupture of membranes (pPROM)compared to preterm birth with intact membranes (PTB)and term. LOX and LOXL1, 2 and 4 were localized to both amniotic and chorionic cells, whereas LOXL3 was limited to chorion. Increase of LOX expression in pPROM, an Oxidative Stress related disease, and the apparent inhibition of LOX activity by water-soluble cigarette smoke extract (CSE), an OS inducer, restored by antioxidant treatment suggest that reactive oxygen species might influence LOX-mediated tissue remodeling in fetal membranes. Balanced antioxidant supplementation during pregnancy may reduce the risk of pPROM by increasing LOX activity [44].
In order to analyse the hypothesis of advanced glycation end products (AGE) signalling pathway interaction with LOX gene activity in polycystic ovarian (PCO) tissue, a study was conducted and distribution of LOX, collagen type IV and advanced glycation end products (AGE) molecules in the PCO tissue was found more as compared to control. Binding of AGE-induced transcription factors, NF-B and activator protein-1 (AP-1) on LOX promoter, indicated a possible involvement of AGEs in LOX gene regulation, which may account for the documented increase in LOX mRNA and protein levels. These findings suggest that deposition of excess collagen in PCO tissue may be due to AGE-mediated stimulation of LOX activity [45].
Recent study showed that LOX is elevated in the megakaryocytic lineage of mouse models of myeloproliferative neoplasms (MPNs). Transgenic mice expressing LOX in wild-type megakaryocytes and platelets were generated to gain insight into the role of LOX in thrombosis and platelet function. The wild type platelets adhere better to collagen and have greater aggregation response, demonstrating that LOX enhances platelet activation and thrombosis [46].
Conclusion
Extracellular Matrix remodelling is a common feature of diverse pathologic processes. Lysyl oxidase (LOX) act as an integral molecule in covalent cross-linking of collagens and elastin determining the mechanical properties of extracellular matrix (ECM) and connective tissues. Therefore, dysregulation of LOX could underlie the onset and progression of multiple pathologies affecting connective tissue, such as fibrotic processes, tumor progression and metastasis and neurodegenerative and cardiovascular diseases. This review provides an insight regarding the molecular basis as well as the recent clinical and experimental evidence that supports the role for LOX in health and Diseases.
Compliance with Ethical Standards
Conflict of interest
Authors Dr. Suchitra Kumari, Dr. Tarun Kumar Panda, Dr Tapaswini Pradhan have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Human and Animal Right Statement
For this type of study formal consent is not required. This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Wang R, Brewster LP, Gleason RL., Jr In situ characterization of the uncramping process of arterial collagen fibres using two photon confocal microscopy and digital image correlation. J Biomech. 2013;46:2726–2729. doi: 10.1016/j.jbiomech.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young RD, Knupp C, Pinali C, Png KM, Ralphs JR, Bushby AJ, et al. Three dimensional aspects of matrix assembly by cells in the developing cornea. Proc Natl Acad Sci USA. 2014;111:687–692. doi: 10.1073/pnas.1313561110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox TR, Erler JT. Remodelling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rucker Robert B, Kosonen Taru, Clegg Michael S, Mitchell Alyson E, Rucker Brian R, Uriu Janet Y, et al. Copper, lysyl oxidase and extracellular matrix protein cross-linking. Am J Clin Nutr. 1998;67(suppl):996S–1002S. doi: 10.1093/ajcn/67.5.996S. [DOI] [PubMed] [Google Scholar]
- 5.Baker AM, Bird D, Lang G, Cox TR, Ehler JT. Lysyl oxidase enzyme function increase the stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32:1863–1868. doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 6.Fogelgren Ben, Polgár Noémi, Szauter Kornélia Molnárné, Újfaludi Zsuzsanna, Laczkó Rozália, et al. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J Biol Chem. 2005;280:24690–24697. doi: 10.1074/jbc.M412979200. [DOI] [PubMed] [Google Scholar]
- 7.Turecek C, Fratzl-Zelman N, Rumpler M, Buchinger B, Spitzer S, Zoehrer R, et al. Collagen cross-linking influences osteoblastic differentiation. Calcif Tissue Int. 2008;82(5):392–400. doi: 10.1007/s00223-008-9136-3. [DOI] [PubMed] [Google Scholar]
- 8.Aydin S, Signorelli S, Lechleitner T, Joannidis M, Pleban C, Perco P, et al. Influence of microvascular endothelial cells on transcriptional regulation of proximal tubular epithelial cells. Am J Physiol Cell Physiol. 2008;294(2):C543–C554. doi: 10.1152/ajpcell.00307.2007. [DOI] [PubMed] [Google Scholar]
- 9.Buchinger B, Spitzer S, Karlic H, Klaushofer K, Varga F. Lysyl oxidase (LOX) mRNA expression and genes of the differentiated osteoblastic phenotype are upregulated in human osteosarcoma cells by suramin. Cancer Lett. 2008;265(1):45–54. doi: 10.1016/j.canlet.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Stewart GD, Nanda J, Brown DJ, Riddick AC, Ross JA, Habib FK. NO-sulindac inhibits the hypoxia response of PC-3 prostate cancer cells via the Akt signalling pathway. Int J Cancer. 2009;124(1):223–232. doi: 10.1002/ijc.23934. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez HM, Vaysberg M, MikelsA McCauley S, Velayo AC, Garcia C, et al. Modulation of Lysyl oxidase like 2 enzymatic activity by an allosteric antibody inhibitor. J Biol Chem. 2010;285:20964–20974. doi: 10.1074/jbc.M109.094136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Go EP, Finney J, Moon H, Lantz M, Rebicchi K, et al. Post translational modification of recombinant human lysyl oxidaselike -2 secreted from Drosophilla S2 cells. J Biol Chem. 2013;288:5357–5363. doi: 10.1074/jbc.C112.421768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimsby JL, Lucero HA, Trackman PC, Ravid K, Kagan HM. Role of lysyl oxidase pro-peptide in secretion and enzyme activity. J Cell Biochem. 2010;111:1231–1243. doi: 10.1002/jcb.22845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halberg N, Khan T, TrujilloME Wernstedt AI, Attie AD, Sherwani S, et al. Hypoxia –inducible factor 1 alphainduces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mello ML, Alvarenga EM, Vidal Bde C, Di Donato A. Chromatic supra organization and mitotic abnormalities, proliferation in cells with increased or down regulated LOX expression. Micron. 2011;42:8–16. doi: 10.1016/j.micron.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Lopez B, Querezeta R, Gonzalez A, Beaumont J, Larman M, Diez J, et al. Impact of treatment on Myocardial LOX expression and collagen cross linking in patients with heart failure. Hypertension. 2009;53:236–242. doi: 10.1161/HYPERTENSIONAHA.108.125278. [DOI] [PubMed] [Google Scholar]
- 17.Lucero HA, Kagan HM. Lysis oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63(19–20):2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox TR, Bird D, Baker AM, Baker HE, Ho MW, Lang G, et al. Lox mediated collagen cross linking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73:1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herchenhan A, Uhlenbrock F, Eliason P, Weis M, Eyre D, Karl E, et al. Lysyl oxidase activity is required for ordered collagen fibrillogenesis by tendon cells. J Biol Chem. 2015;290(26):16440–16450. doi: 10.1074/jbc.M115.641670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spurney CF, Knoblach S, Pistilli EE, Nagaraju K, Martin GR, Hoffman EP. Dystrophin-deficient cardiomyopathy in mouse: expression of Nox4 and Lox are associated with fibrosis and altered functional parameters in the heart. Neuromuscul Disord. 2008;18(5):371–381. doi: 10.1016/j.nmd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogaard Harm J, Mizuno Shiro, Guignabert Christophe, Aysar A, Hussaini Al, Farkas Daniela, et al. Copper dependence of angio -proliferation in pulmonary arterial hypertension in rats and humans. Am J Respir Cell Mol Biol. 2012;46(5):582–591. doi: 10.1165/rcmb.2011-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker Ann-Marie, Bird Demelza, Welti Jonathan C, Gourlaouen Morgane, Lang Georgina. Lysyl oxidase plays a critical role in endothelial cell stimulation to drive tumour angiogenesis. Cancer Res. 2013;73(2):583–594. doi: 10.1158/0008-5472.CAN-12-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucero HA, Ravid K, Grimsby JL, Rich CB, Di Camillo SJ, Maki JM, et al. Lysyl oxidase oxidizes cell membrane proteins and enhances the chemotactic response of vascular smooth muscle cells. J Biol Chem. 2008;283(35):24103–24117. doi: 10.1074/jbc.M709897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kothapalli CR, Ramamurthi A. Benefits of concurrent delivery of hyaluronan and IGF-1 cues to regeneration of crosslinked elastin matrices by adult rat vascular cells. J Tissue Eng Regen Med. 2008;2(2–3):106–116. doi: 10.1002/term.70. [DOI] [PubMed] [Google Scholar]
- 25.Osawa T, Ohga N, Akiyama K, Hida Y, Kitayama K, Kawamoto T, et al. Lysyl oxidase secreted by tumour endothelial cells promotes angiogenesis and metastasis. Br J Cancer. 2013;26:2237–2247. doi: 10.1038/bjc.2013.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linghong WU, Zhu Zing. The function and mechanism of action of LOXL2 in cancer. Int J Mol Med. 2015;36:1200–1204. doi: 10.3892/ijmm.2015.2337. [DOI] [PubMed] [Google Scholar]
- 27.Erler JT, Giaccia AJ. Lysyl oxidase mediates hypoxic control of metastasis. Cancer Res. 2006;66(21):10238–10241. doi: 10.1158/0008-5472.CAN-06-3197. [DOI] [PubMed] [Google Scholar]
- 28.Miller Bryan W, Morton Jennifer P, Pinese Mark, Saturno Grazia, Jamieson Nigel B, McGhee Ewan, et al. Targeting the LOX/Hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol Med. 2015;7(8):1063–1076. doi: 10.15252/emmm.201404827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox TR, Gartland A, Erler JT. Lysyl oxidase, a targetable secreted molecule involved in cancer metastasis. Cancer Res. 2016;76(2):188–192. doi: 10.1158/0008-5472.CAN-15-2306. [DOI] [PubMed] [Google Scholar]
- 30.Gu R, Gong C, Jin H, Sun Y, Li Z, Chen J, Wu G. Lysyl oxidase mediates hypoxia-induced radioresistance in non-small cell lung cancer A549 cells. Exp Biol Med. 2015 doi: 10.1177/1535370215609694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schütze F, Röhrig F, Vorlová S, Gätzner S, Kuhn A, Ergün S, et al. Inhibition of lysyl oxidases improves drug diffusion and increases efficacy of cytotoxic treatment in 3D tumor models. Sci Rep. 2015;5:17576. doi: 10.1038/srep17576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox TR, Rumney RM, Schoof EM, Perryman L, Høye AM, Agrawal A, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Bais MV, Ozdener GB, Sonenshein GE, Trackman PC. Effects of tumor-suppressor lysyl oxidase pro peptide on prostate cancer xenograft growth and its direct interactions with DNA repair pathways. Oncogene. 2015;34(15):1928–1937. doi: 10.1038/onc.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsulaiman M, Bais MV, Trackman PC. Lysyl oxidase pro peptide stimulates osteoblast and osteoclast differentiation and enhances PC3 and DU145 prostate cancer cell effects on bone in vivo. J Cell Commun Signal. 2015;12:1–15. doi: 10.1007/s12079-015-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miana M, Galen M, Ernesto MM, Sarey V, Raquel JL, Belen BM, et al. Lysyl oxidase inhibitor, beta-amino propio nitrile, reduces body weight gain and improves the metabolic profile in diet induced obesity. Dis Model Mech. 2015;4:124–128. doi: 10.1242/dmm.020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nobili V, Cutrera R, Liccardo D, Pavone M, Devito R, Giorgio V, et al. Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am J Respir Crit Care Med. 2014;189(1):66–76. doi: 10.1164/rccm.201307-1339OC. [DOI] [PubMed] [Google Scholar]
- 37.Mesarwi OA, Shin MK, Drager LF, Bevans-Fonti S, Jun JC, Putcha N, et al. Lysyl oxidase as a serum biomarker of liver fibrosis in patients with severe obesity and obstructive sleep Apnea. Sleep. 2015;38(10):1583–1591. doi: 10.5665/sleep.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;29(6):G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 39.He J, Tang HJ, Wang YY, Xiong MH, Zhou F, Shao K, et al. Expression of lysyl oxidase gene in upper digestive tract carcinomas and its clinical significance. Int J Cancer. 2002;21(6):671–674. [PubMed] [Google Scholar]
- 40.Murawaki Y, Kusakabe Y, Hirayama C. Serum lysyl oxidase activity in chronic liver disease in comparison with serum levels of prolyl hydroxylase and laminin. Hepatology. 1991;14(6):1167–1173. doi: 10.1002/hep.1840140635. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto M, Murawaki Y, Hirayama C. Serum lysyl oxidase activity in patients with various liver diseases. Gastroenterol Jpn. 1987;22(6):730–736. doi: 10.1007/BF02776746. [DOI] [PubMed] [Google Scholar]
- 42.Vadasz Z, Kessler O, Akiri G, Gengrinovitch S, Kagan HM, Baruch Y, et al. Abnormal deposition of collagen around hepatocytes in Wilson’s disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. J Hepatol. 2005;43(3):499–507. doi: 10.1016/j.jhep.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 43.Van Bergen T, Spangler R, Marshall D, Hollanders K, Van de Veire S, Vandewalle E, et al. The role of LOX and LOXL2 in the pathogenesis of an experimental model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2015;56(9):5280–5289. doi: 10.1167/iovs.14-15513. [DOI] [PubMed] [Google Scholar]
- 44.Polettini J, Silva MG, Kacerovsky M, Syed TA, Saade GR, Menon R. Screening of lysyl oxidase (LOX) and lysyl oxidase like (LOXL) enzyme expression and activity in preterm prelabor rupture of fetal membranes. J Perinat Med. 2016;44(1):99–109. doi: 10.1515/jpm-2014-0337. [DOI] [PubMed] [Google Scholar]
- 45.Papachroni Katerina K, Piperi Christina, Levidou Georgia, Korkolopoulou Penelope, Pawelczyk Leszek. Lysyl oxidase interacts with AGE signalling to modulate collagen synthesis in polycystic ovarian tissue. J Cell Mol Med. 2010;14(10):2460–2469. doi: 10.1111/j.1582-4934.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuura Shinobu, Mi Rongjuan, Koupenova Milka, Eliades Alexia, Patterson Shenia, Toselli Paul, et al. Lysyl oxidase is associated with increased thrombosis and platelet reactivity. Blood. 2016;127(11):1493–1501. doi: 10.1182/blood-2015-02-629667. [DOI] [PMC free article] [PubMed] [Google Scholar]