Abstract
As expanded understanding of molecular tumor characteristics, which drive renal cancer growth and progression gives a promising future for renal carcinoma therapy. The objective of the present study was designed to examine the effect of β-sitosterol on a rat model of experimental renal carcinogenesis. Renal carcinogenesis was induced in rats treated with N-diethylnitrosamine (DEN; 200 mg/kg bw single i.p., injection) and ferric nitrilotriacetate (Fe-NTA; 9 mg Fe/kg bw i.p., twice a week for 16 weeks). β-sitosterol pretreatment (20 mg/kg bw in 0.1 % carboxymethyl cellulose (CMC) p.o., thrice a week for 24 weeks) was started 2 weeks before the exposure to carcinogens. Expression of angiogenesis marker (VEGF), proliferative markers (cyclin D1, PCNA) and apoptotic markers (Bcl-2, Bax, caspase-3 and caspase-9) were analyzed to assess the anti-cancer potential of β-sitosterol in renal carcinogenesis model. mRNA and protein expression changes were determined by qRT-PCR, Western blotting, ELISA technique and immunohistochemistry. Our results showed that oral administration of β-sitosterol pretreatment significantly (P < 0.05) reversed the expression of all the above mentioned markers and histological features which have been modified by renal carcinogen. It is concluded that, the protective effects of β-sitosterol against renal cancer is associated with the induction of apoptosis and the inhibition of cellular proliferation.
Keywords: Renal carcinogenesis, Fe-NTA, Angiogenesis, Proliferation, Apoptosis, β-Sitosterol
Introduction
There is a sharp increase in the incidence of genitourinary cancers, especially renal cell carcinoma (RCC) round the world. It has a higher mortality rate and more than 1/3rd of patients with RCC will die from the disease [1]. RCC is associated with numerous risk factors, including cigarette smoking, obesity, hypertension, inherited syndrome, long term dialysis, chronic kidney disease, genetic susceptibility and their interaction with environmental exposure [2]. RCC has been previously reported that, one of the most therapy resistant cancers [3]. A high degree of interest has been shown in the new therapeutic agents that modulate angiogenesis, signal transduction and immune response for patients with RCC. Hence, a very urgent need to explore powerful, non-toxic and less expensive chemopreventive agent that inhibit cancer growth and progression.
In the present study, rat model was chosen for ferric nitrilotriacetate (Fe-NTA) induced renal carcinogenesis due to the reason that strongest similarity pattern of renal cell carcinomas occurs between rat and human. Moreover, the Fenton reaction occurs faster in male rats when compared to female rats, which have lower whole-kidney and single nephron glomerular filtration rate [4]. Fe-NTA, a well established renal toxicant, its repeated administration leads to renal adenocarcinoma in experimental rats [5]. The kidney is reported to be the main target of Fe-NTA involving oxidative damage. It starts from the plasma compartment, where Fe-NTA finds the ideal environment to react with oxidisable lipids. The mechanism by which Fe-NTA causes its effects seems mainly due to its ability to interact with the membranes and to act as a powerful catalyst of lipid peroxidation [6]. In addition, Fe-NTA depletes the antioxidant battery and induces oxidative stress that cause ROS generation by iron catalyzed Fenton reaction plays a pivotal role in renal tumorigenesis [7]. ROS can modulate signal transduction cascade is mainly due to their ability to cause DNA damage. The oxidative attack and the modification of molecules like proteins and lipids can increase the risk of mutagenesis. The persistent tissue damage and cell proliferation could contribute to cancer-prone microenvironment [8].
Chemopreventive agents can exert anticarcinogenic effect by scavenging ROS, influencing apoptosis and inhibiting cell proliferation [9]. For these reasons, compounds derived from natural sources are gaining increasing interest as potential cancer therapeutics. Numerous studies have indicated that β-sitosterol inhibits cancer cell proliferation and also exhibits various pharmacological properties which includes anti-hypercholesterolemic [10], anti-inflammatory and anti-angiogenic properties [11, 12]. Profound studies have indicated that β-sitosterol activates the sphingomyelin cycle by 50 % increase in ceramide production that were associated with the induction of apoptosis in various cancer cells, including HT-29 human colon cancer cells [13], LNCaP human prostate cancer cells [14], Human leukemic U937 cells [15], SGC-7901 human stomach cancer cells [16] and MDA-MB-231 human breast cancer cells [17].
Therefore, in the light of above inferences we use renal carcinogenesis model to explore the anti-proliferative and apoptotic potential of β-sitosterol and interpret them according to the recent advancements to fight against cancer.
Materials and Methods
Drugs and Chemicals
β-sitosterol, DEN (N-diethylnitrosamine), nitrilotriacetic disodium salt, Trizol reagent and primer sequences for GAPDH and VEGF were purchased from Sigma-Aldrich Chemical Pvt. Ltd (St. Louis, MO, USA). Roche LightCycler Quantification Kit was obtained from Roche, USA. Antibodies for β-actin, Cyclin D1, PCNA, Bax, Bcl-2, cleaved caspase-3 and cleaved caspase-9 were purchased from Santa Cruz Biotechnology, USA. Enzyme-linked Immunosorbent Assay (ELISA) Kit was obtained from Biovision Research Products, USA. All other chemicals used were of analytical grade and purchased from Hi-media Laboratories Pvt. Ltd, Mumbai, India.
Preparation of Fe-NTA
0.16 mM ferric nitrate solution was mixed with a four-fold molar excess of 0.64 mM disodium salt of NTA and the pH was adjusted to 7.4 with a sodium bicarbonate solution [18].
Animals
Healthy adult male albino rats (8–10 weeks old; weighing 120–180 g) of Wistar strain, were purchased from the National Institute of Nutrition, Hyderabad, India, and were maintained in the Central Animal House, Department of Experimental Medicine, Rajah Muthaiah Medical College and Hospital, Annamalai University, Annamalainagar, India. The animals were housed in polypropylene cages and were provided with a standard pellet diet (Amrut laboratory Animal Feed Mysore Feeds Limited, Bangalore, Karnataka, India) and water ad libitum. The animals were maintained under controlled conditions of temperature (23 ± 2 °C) and humidity (65–70 %) with a 12 h light/dark cycle.
Experimental Design
A total number of 24 male albino Wistar rats were randomized into 4 groups (control and experimental) of 6 rats each. The Group 1 rats served as the Vehicle Control [0.1 % Carboxymethyl cellulose (CMC)]. The Group 2 and 3 rats were treated with N-diethylnitrosamine (200 mg/kg bw single i.p., injection) and ferric nitrilotriacetate (9 mg Fe/kg bw i.p., twice a week for 16 weeks). Group 2 rats received no other treatment. Group 3 rats were orally administered with β-sitosterol (20 mg/kg bw in 0.1 % CMC p.o., thrice a week for 24 weeks) starting 2 weeks before the exposure to the carcinogens. Based on the previous work of Baskar et al. [19], 20 mg/kg bw of β-sitosterol treatment is effective in colon cancer study and our previous published data shown that this dosage was effective against DEN and Fe-NTA [20]. Group 4 rats were orally administered with β-sitosterol alone throughout the experimental period. At the end of 24 weeks, all the animals were sacrificed by cervical dislocation. Four technical replicates were used for each molecular parameters in kidney tissues.
Western Blotting
Homogenates containing equal amounts of protein were resolved by 8–12 % SDS-PAGE and processed for Western Blotting and electrotransferred onto poly vinylidene fluoride membranes. The membranes were then incubated overnight at 4 °C with antibodies specific to Cyclin D1, PCNA, Bax, Bcl-2, cleaved caspase-3 and cleaved caspase-9 (Santa Cruz Biotechnology, USA). The membranes were washed with TBST [blocking buffer (containing 5 % skimmed milk powder in 0.5 M Tris-buffered saline, pH 7.5 containing 0.1 % Tween-20)] and incubated with respective secondary antibody for 2 h at room temperature. Protein band detection was performed by enhanced chemiluminescence assay. Blots were subsequently stripped, reprobed and processed for visualizing β-actin. The band density was normalized to that of β-actin. Quantitative comparisons of protein expression between various groups were performed using Image J software (from the US National Institutes of Health).
qRT-PCR
Total cellular RNA was extracted from renal tissue using Trizol reagent. The concentration and purity of RNA preparation were checked by using a Nano spectrophotometer (Implen, Germany) measuring the absorbed at 260 and 280 nm. Total RNA (2.0 µg) was reverse transcribed to cDNA in a reaction mixture containing 1 µl of oligo (dT) primer (0.2 µg/ml), 1 µl of RNase inhibitor (10 U/ml), 1 µl of 0.1 M DTT, 4 µl of 5× reaction buffer, 2.0 µl of 30 mM dNTP mix (7.5 mM each), 0.5 µl of M-MuLV reverse transcriptase (50 U/µl) and made up to 20 µl with DEPC water and kept at 37 °C for 1 h and then heated at 95 °C for 2 min. The primer sequences for VEGF and GAPDH were as follows: 5′GTGGACATCTTCCAGGAGGAGTA3′ as forward and 5′CTCTGAACAAGGCTCACAGT3′ as the reverse primer (Annealing temperature −58 °C; Size −270 bp) and 5′ACTCCCATTCCTCCACCTTT3′ as forward and 5′TTACTCCTTGGAGGCCATGT3′ as the reverse primer (Annealing temperature −60 °C; Size −516 bp). PCR amplification was performed with the Roche LightCycler Quantification Kit. The PCR conditions were as follows: 95 °C for 5 min, 40 cycles of 30 s at 95 °C, 30 s at 52 to 60 °C (based on the target), and 60 s at 72 °C. The reactions were run in triplicate for each sample. Relative quantitative folds change compared to control was calculated using the comparative Ct method using LightCycler® Relative Quantification Software.
ELISA Technique
Caspase-3 and caspase-9 activities in kidney tissues were measured using Enzyme-linked Immunosorbent Assay Kit (Biovision Research Products, USA). The caspase-3 and caspase-9 assays were based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labelled substrate DEVD-pNA and LEHD-pNA respectively at 405 nm in a microtiter plate reader.
Histopathological Studies
Histopathological investigations were performed on kidney and liver tissues of the control and experimental animals in each group. Tissues were fixed at 10 % buffered formalin and routinely processed and embedded in paraffin; 2–3 µm sections were cut on a rotary microtome, fixed on glass slides, and stained with hematoxylin and eosin.
Immunohistochemistry
Kidney tissues from control and experimental animals in each group were immediately fixed in buffered formalin (10 %), embedded in paraffin, cut Sects. (2–3 µm) using a rotary microtome, placed on polylysine coated clean glass slides, dried at 37 °C and used for immunohistochemical studies. Paraffin embedded tissue sections were dewaxed and rehydrated through graded ethanol diluted with distilled water. Endogenous peroxidase was blocked by incubation with hydrogen peroxide (3 %) in methanol for 10 min. The antigen retrieval was achieved by microwave in citrate buffer solution (pH 6.0) for 10 min, followed by washing step with Tris-buffered saline (pH 7.6). The tissue sections were then incubated with power Block™ reagent (BioGenex, San Ramon, CA, USA), universal proteinaceous blocking reagent, for 15 min at room temperature to block non-specific binding. These tissue sections were then incubated with specific primary antibody (PCNA and Bcl-2) over night at 4 °C. The bound primary antibody was incubated with the secondary antibody conjugated with horseradish peroxidase for 30 min at room temperature. After rinsing with Tris-buffered saline, the antigen–antibody complex was detected using 3, 3′-diaminobenzidine (Sigma, USA), the substrate of horseradish peroxidase. When acceptable colour intensity was reached, the slides were washed, counter stained with hematoxylin and covered with a mounting medium.
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test (DMRT) using SPSS version 17.0 for Windows (SPSS, Tokyo, Japan). Values are represented as mean ± SD and P < 0.05 were considered statistically significant.
Results
Effect of β-Sitosterol on Cell Proliferative Markers (Cyclin D1 and PCNA)
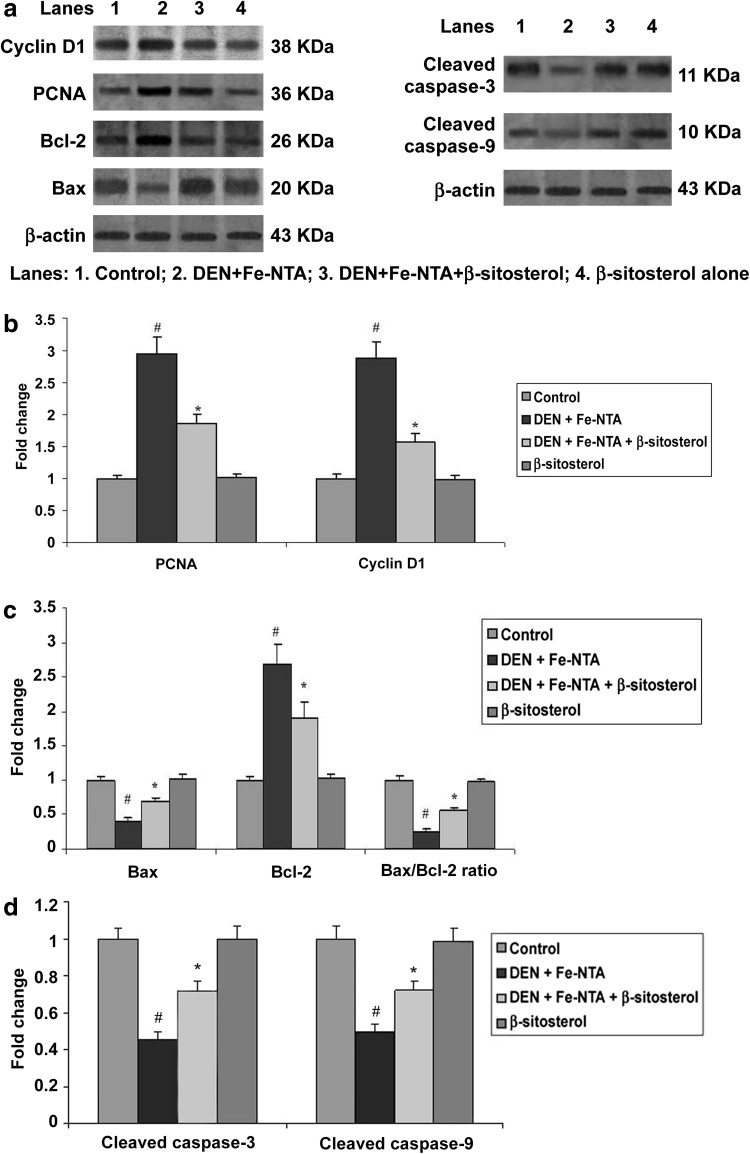
Expression of Cyclin D1 and PCNA were assessed by western blotting analysis (Fig. 1a, b). The expression of cell cycle regulatory protein Cyclin D1 and PCNA were induced in rats treated with DEN and Fe-NTA alone when compared to control rats (P < 0.05). However, β-sitosterol (20 mg/kg body weight) pretreated rats (Group 3) showed significant alteration of these protein expressions in kidney tissues compared to Group II (P < 0.05). There were no significant difference between the control (Group 1) and β-sitosterol alone (Group 4) rats.
Fig. 1.
Effect of β-sitosterol on Bax, Bcl-2, cleaved caspase-3, cleaved caspase-9, Cyclin D1 and PCNA protein expressions in the kidney tissue of control and experimental rats. For quantitative assessment, the band intensity was measured with a densitometer and normalized to β-actin expression and expressed as fold change with respect to control. The changes relative to control are represented in the bar diagram. Data shown are mean ± SD of four independent experiments. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT)
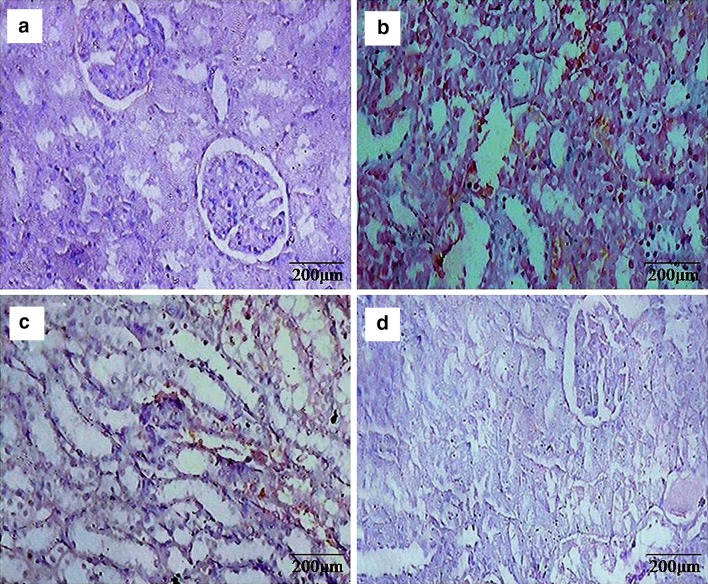
Anti-hyperproliferative effect of β-sitosterol was further confirmed through the immunohistochemical analysis of PCNA (Fig. 2). Intense expression of PCNA was observed in DEN and Fe-NTA treated rats when compared to control rats. Oral administration of β-sitosterol pretreated rats showed considerably lesser expression of PCNA when compared to Group 2 rats, which suggested that β-sitosterol has attenuated renal cell proliferation. No obvious difference between the control (Group 1) and β-sitosterol alone (Group 4) rats.
Fig. 2.
Representative photomicrographs of PCNA determined by immunohistochemistry (20×). a & d Normal expression was observed in control rats and β-sitosterol alone rats, b DEN + Fe-NTA administration increased the number of PCNA positive cells in renal sections of rats, c β-sitosterol + DEN + Fe-NTA treated rats showed a lesser number of PCNA positive cells as compared to group b as is evident from the figure
Effect of β-Sitosterol on Intrinsic Apoptotic Markers (Bax, Bcl-2, Caspase-3 and Caspase-9)
Induced expression of Bcl-2, declined expression of Bax, cleaved caspase-3, cleaved caspase-9 and also decreased the Bax/Bcl-2 ratio in rats treated with DEN and Fe-NTA alone when compared to control rats (P < 0.05; Fig. 1a, c, d). However, β-sitosterol (20 mg/kg body weight) pretreated rats (Group 3) significantly decreased Bcl-2 expression, followed by increased Bax, caspase-3, caspase-9 and Bax/Bcl-2 ratio compared to Group 2 (P < 0.05). There were no significant difference between the control (Group 1) and β-sitosterol alone (Group 4) rats.
Immunohistochemical evaluation showed very intense positive staining of Bcl-2 protein (Fig. 3) in rats treated with only DEN and Fe-NTA compared to control. However, β-sitosterol (20 mg/kg body weight) pretreated rats considerably downregulated the expression of Bcl-2 protein in renal tissues when compared to Group 2. No obvious difference between the control (Group 1) and β-sitosterol alone (Group 4) rats.
Fig. 3.
Representative photomicrographs of Bcl-2 determined by immunohistochemistry (20×). a & d Normal expression of Bcl-2 was observed in control rats and β-sitosterol alone rats, b DEN + Fe-NTA administration increased the expression of Bcl-2 in renal tubular sections of rats, c β-sitosterol + DEN + Fe-NTA treated rats showed decreased expression of Bcl-2 in renal tubular sections of rats as compared to group b as is evident from the figure
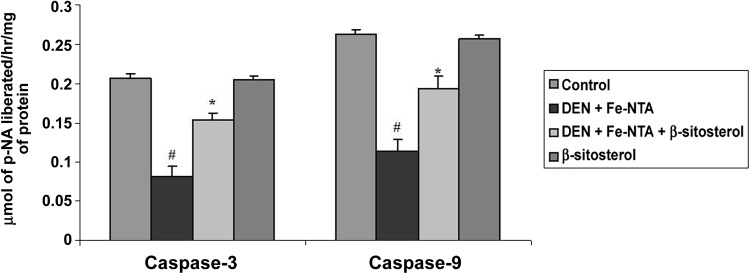
Levels of caspase-3 and caspase-9 in control and experimental rats were also evaluated by ELISA method (Fig. 4). DEN and Fe-NTA treated rats showed significantly decreased in the levels of caspase-3 and caspase-9 compared to control rats (P < 0.05). Pretreatment with β-sitosterol in Group 3 significantly (P < 0.05) increased the levels of caspase-3 and caspase-9. There is no significant difference between the control (Group 1) and β-sitosterol alone (Group 4) rats.
Fig. 4.
Effect of β-sitosterol on caspase-9 and caspase-3 activity in the kidney tissue of control and experimental rats. Data shown are mean ± SD of four independent experiments. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT)
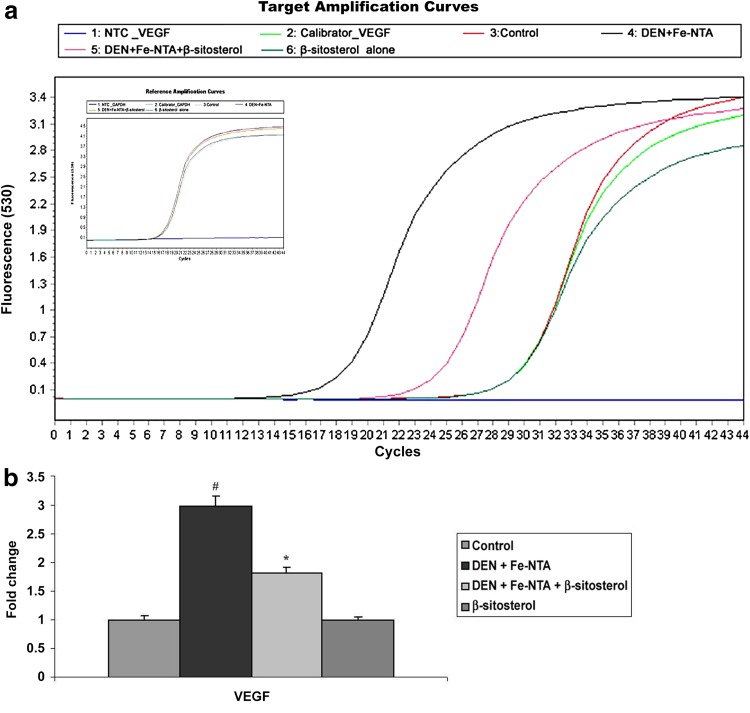
Effect of β-Sitosterol on the Expression of VEGF (Angiogenesis Marker)
VEGF mRNA expression patterns in renal tissues of control and experimental rats were depicted in Fig. 5a, b. DEN and Fe-NTA induced significant increase in the mRNA expression of VEGF in Group 2 rats when compared to control rats (P < 0.05). β-sitosterol pretreatment down regulated the VEGF mRNA expression in Group 3 when compared to Group 2 (P < 0.05). Similar patterns of VEGF mRNA expression was observed in control rats (Group 1) and rats treated with β-sitosterol alone (Group 4).
Fig. 5.
Effect of β-sitosterol on VEGF mRNA expression in the kidney tissue of control and experimental rats. VEGF mRNA expression was normalized to the expression level of the GAPDH mRNA expression. The changes relative to control are represented in the bar diagram. Data shown are mean ± SD of four independent experiments. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT)
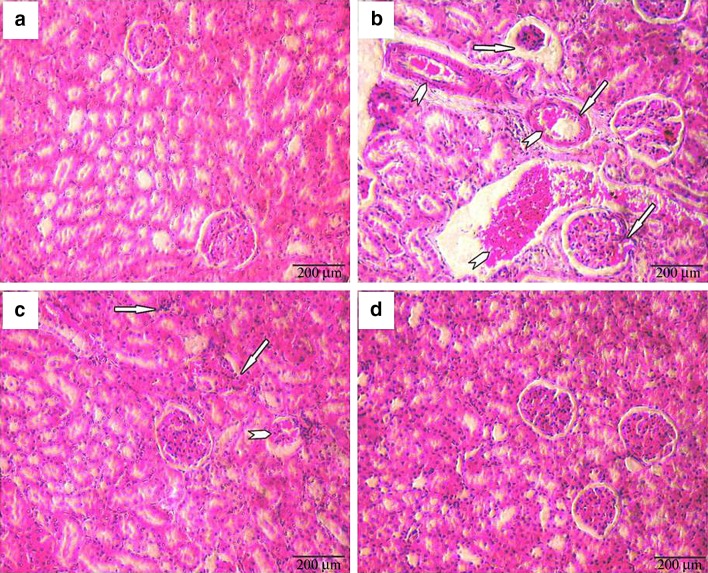
Effect of β-Sitosterol on Histopathological Observations
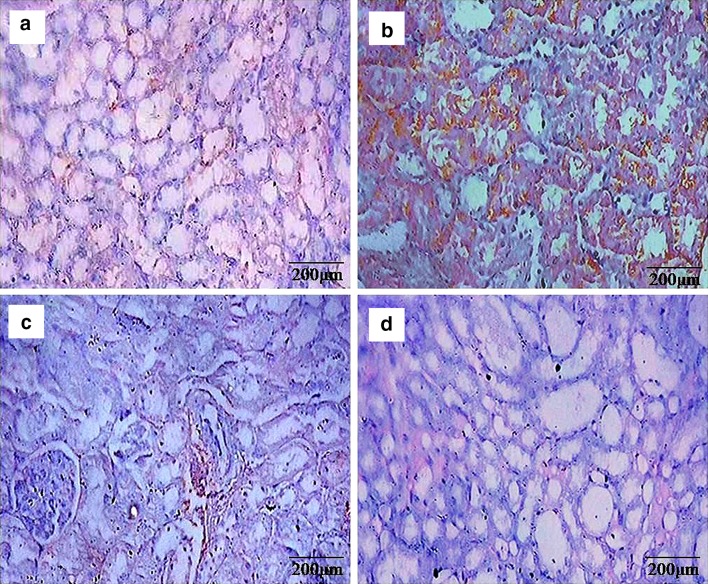
Histopathological manifestations in kidney tissues were depicted in Fig. 6. Normal histology of kidney tissue was observed in control and β-sitosterol alone treated animals. However, renal carcinogenesis was confirmed by evaluating the abnormal pathological symptoms includes glomerular congestion, tubular congestion, atrophy, blood sinusoids, interstitial hemorrhages and hyperchromatic deposits in DEN and Fe-NTA alone treated animals. Furthermore, β-sitosterol pretreatment to DEN and Fe-NTA treated animals considerably showed protective changes in the renal tissues. Mild inflammatory cell invasion and mild tubular degeneration were observed in β-sitosterol pretreatment of carcinogenic induced rats.
Fig. 6.
Histopathological evaluations in kidney tissues (20×). a & d Control and β-sitosterol alone treated animals, respectively, showing normal histology of kidney, b DEN + Fe-NTA alone treated animals showing glomerular congestion, tubular congestion, blood sinusoids, interstitial hemorrhages, hyperchromatic deposits and atrophy (arrow), c β-sitosterol + DEN + Fe-NTA treated animals showing mild tubular degeneration and mild inflammatory cell invasion (arrow)
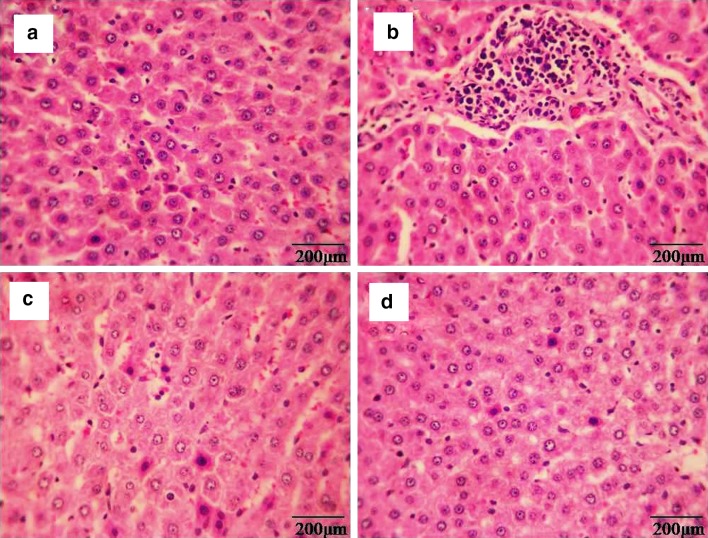
Histopathological manifestations in liver tissues were depicted in Fig. 7. Normal histology of liver tissue was observed in control (Group 1) and β-sitosterol alone (Group 4) treated animals. DEN and Fe-NTA alone treated animals (Group 2) showed disruption of the normal liver architecture, which was confirmed by evaluating the abnormal pathological symptoms, that includes pronounced dilatation of sinusoids, increased necrotic hepatocytes, lobular architecture and complete destruction of the hepatocytes. However, prior treatment of β-sitosterol to DEN and Fe-NTA treated animals noticeably showed protective changes in liver tissues. Pretreatment with β-sitosterol to DEN and Fe-NTA treated rat revealed reduction in necrotic hepatocytes and dilatation of sinusoids (Group 3) respectively.
Fig. 7.
Histopathological evaluations in liver tissues (20×). a & d Control and β-sitosterol alone treated animals, respectively, showing normal histology of liver, b DEN + Fe-NTA alone treated animals showing increased necrotic hepatocytes, pronounced dilatation of sinusoids and complete destruction of the hepatocytes, c β-sitosterol + DEN + Fe-NTA treated animals showing a reduction in necrotic hepatocytes and dilatation of sinusoids respectively
Discussion
The kidney is a dynamic organ and is an important control system that maintains body homeostasis. It is affected by many drugs and chemicals. Renal cell carcinoma (RCC) is a heterogeneous collection of kidney cancers, each driven by different genes and pathways which exhibited different clinical and pathological features, with distinct patterns of origin within the nephron, local invasion, and distant metastases. Worldwide morbidity and mortality rate from infectious diseases is being replaced by non-infectious diseases, including cancer, obesity, diabetes, cardiovascular diseases, neurodegenerative disease and aging [21]. Today nutrition research is focusing exclusively on chronic disease prevention. One of the main focuses is the search of potential chemopreventive agents without any adverse side effects. In the National cholesterol education program, adult treatment panel III has recognized the use of phytosterols as a key element of dietary therapy [11]. β-sitosterol is a major phytosterol that exhibit various pharmacological properties. In the present study, we tried to unravel the mechanisms of anti-proliferative and apoptotic potential of β-sitosterol in Fe-NTA model of renal cancer.
Cell proliferation is thought to play an important role in various steps of the carcinogenic process. Proliferating-cell nuclear antigen is identified as a cofactor of DNA polymerase-δ and its expression occurs in the late G1 and S phase of the cell cycle [22]. PCNA interacts with multiple protein partners; therefore it plays an important role in DNA replication and repair of DNA damage, chromatin structure maintenance, chromosome segregation and cell-cycle progression [23]. Overexpression of PCNA has been reported in many types of cancers including renal cancer. Elevated expression of PCNA indicates the hyper proliferative activity of tumor cells. Reducing the cellular proliferation was one of the hallmarks of controlling the carcinogenic process because PCNA plays an important role in nucleic acid metabolism as a component of replication [24]. Deregulation of G1 to S phase progression of the cell cycle is a frequent target in carcinogenesis. Cyclin D1, a key regulatory protein at the G1/S checkpoint of the cell cycle, act as a growth sensor and over expressed in many cancers. It provides links between mitogenic stimuli and the cell cycle. A previous study has reported that Fe-NTA is known to cause tumor promotion by inducing cellular proliferation [25]. Our results corroborated with previous literature that the elevated expression of PCNA in the kidney of DEN and Fe-NTA administered animals indicates the hyper proliferative activity of tumor cells. Vundru et al. [17] have been reported that β-sitosterol induced G0/G1 cell cycle arrest in human breast cancer MDA-MB-231 cells via the modulation of cell cycle regulators Cyclin D1. Modulatory expression of PCNA and Cyclin D1 by β-sitosterol in carcinogenesis induced rats in the present study explained its anti-proliferative and growth inhibitory effect.
One of the major mechanisms for the targeted chemotherapy of various cancers is apoptosis. Many genes have been acting as either inducers or repressors of apoptosis. In that, caspases are responsible for the cleavage of intracellular proteins and their activation is regulated by antiapoptotic Bcl-2 and proapoptotic Bax. During intrinsic pathway, the release of cytochrome c from mitochondria that activates caspase-9, which in turn activates caspase-3, an effector caspases [16]. Bcl-2 and its regulated protein control the cytochrome c and modulate the activity of caspases. In particular, Bcl-2 has been reported to directly inhibit caspases-3 and -9 activities. Vundru et al. [17] have been reported that β-sitosterol induce apoptosis via mitochondrial membrane depolarization and increase the Bax/Bcl-2 ratio in breast cancer cells. One of the consistent clues provided by the cell line study has shown that β-sitosterol induces apoptosis mediated through the activation of the sphingomyelin cycle [13]. β-sitosterol stimulates the de novo synthesis of ceramide in differentiated Caco2 cells [26]. Ceramide act as a second messenger in cells and increase in its intracellular concentration which is associated with the inhibition of cell proliferation and stimulation of apoptosis in many kinds of cancer cells [27, 28]. Previous study has been reported that β-sitosterol induced apoptosis in prostate cancer cells and human leukemic U937 cells by stimulation of caspase-3 activity and downregulation of Bcl-2 [15]. The Bax/Bcl-2 ratio is an important indicator to the cell, how it will respond to an apoptotic signal. Caspase enzymes are involved in apoptosis and their activities can be enhanced by internal stimuli through altered Bax/Bcl-2 ratio [17]. In the present study, β-sitosterol pretreatment to Fe-NTA induced rats increased the expression of Bax and Bax/Bcl-2 ratio and depleted the expression level of Bcl-2 which may contribute the induction of apoptosis via the mitochondrial apoptosis pathway mediated through caspases. Harada-Shiba et al. [29] have been shown that cholesterol can inhibit sphingomyelin degradation and thereby inhibit the ceramide pathway of apoptosis. Cholesterol lowering effect of β-sitosterol has been confirmed by Ikeda et al. [10]. These two research evidences suggested that the cholesterol lowering effect of β-sitosterol was favourable for apoptosis. These scientific evidences very strongly suggested that β-sitosterol administration increased the activity of caspases that could be due to the incorporation of β-sitosterol into lipid rafts of cancer cells, altering their structure, thereby result in beneficial alterations in signal transduction.
Increased angiogenesis is the hallmark of advanced RCC with increased VEGF expression which is an important factor underlying dysregulated angiogenesis during cancer progression [30]. Angiogenesis is the process in which more nascent blood vessels are developed to facilitate oxygen and nutrient supply to the center of the tumor, in turn facilitating tumor growth. ROS, particularly hydrogen peroxide, can act as second messengers in cell signaling. Increased ROS levels in tumor microenvironment have been directly proposed to augment the proliferation rate [31]. Increased ROS at moderate level mediates drug resistance and allow tumor cells to survive during treatment, resulting in cancer initiating capabilities. VEGF overexpression causes angiogenesis, vascular hyper permeability and stimulation of tumor development. In the present study, we found that overexpression of VEGF in DEN and Fe-NTA induced renal carcinogenic rats. However, β-sitosterol pretreatment suppress the VEGF overexpression by the deprivation of nutrition and hypoxia to cancer cells.
Histopathological examination of the kidneys of animals treated for 16 weeks with Fe-NTA revealed massive inflammatory response, glomerular congestion, tubular congestion, atrophy, blood sinusoids, interstitial hemorrhages and hyperchromatic deposits. However, kidney and liver from β-sitosterol pretreated rats suppressed the toxic effects of DEN and Fe-NTA which was confirmed through representative photomicrographs showing mild changes. Protective effect of β-sitosterol on renal tissue was evaluated as renal function markers like urea, uric acid and creatinine in our previous nephroprotective study are in lines with the present results [20]. Thus, the treatment with β-sitosterol 20 mg/kg body weight ameliorated the toxic manifestations of DEN and Fe-NTA in the kidney and liver which correlated with molecular endpoints.
Conclusion
Finally, we conclude from this study that the prior treatment of β-sitosterol to carcinogen exposed rats resulted in a marked decline in protein expression of cyclin-D1, PCNA, Bcl-2 and VEGF and considerable enhancement of caspases and Bax. These are the classical markers of cell proliferation, tumor promotion and apoptosis. Thus, in the present study, the anti-tumor activity of β-sitosterol against renal carcinogens was confirmed in experimental carcinogenesis. Based on our reports, we can strongly suggest that β-sitosterol can be utilised as a lead molecule to prepare anti-cancer drug for treating human renal cancer.
Acknowledgments
The author(s) sincerely thank Indian Council of Medical Research, India for providing financial support for this research project, in the form of Senior Research Fellowship (ICMR-SRF-IRIS ID: 2013-17190), to Ms. R. Sharmila.
Funding
This study was funded by Indian Council of Medical Research, India for providing financial support for this research project, in the form of Senior Research Fellowship (ICMR-SRF-IRIS ID: 2013-17190), to Ms. R. Sharmila.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
The animal treatment and protocol employed was approved by the Institutional Animal Ethics Committee (Registration Number 160/1999/CPCSEA; Proposal No. 1041: dated 06.08.2013), Annamalai University. The animals were kept in compliance with the “Guide for the care and use of laboratory animals” and committee for the purpose of control and supervision on experimental animals.
References
- 1.Cairns P. Renal Cell Carcinoma. Cancer Biomark. 2011;9:461–473. doi: 10.3233/CBM-2011-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqi A, Hasan SK, Nafees S, Rashid S, Saidullahb B, Sultana S. Chemopreventive efficacy of hesperidin against chemically induced nephrotoxicity and renal carcinogenesis via amelioration of oxidative stress and modulation of multiple molecular pathways. Exp Mol Pathol. 2015;99:641–653. doi: 10.1016/j.yexmp.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Akatsuka S, Yamashita Y, Ohara H, Liu YT, Izumia M, Abe K, et al. Fenton reaction induced cancer in wild type rats recapitulates genomic alterations observed in human cancer. PLoS One. 2012;7:e43403. doi: 10.1371/journal.pone.0043403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rashid S, Ali N, Nafees S, Hasan SK, Sultana S. Amelioration of renal carcinogenesis by bee propolis: a chemo preventive approach. Toxicol Int. 2013;20:227–234. doi: 10.4103/0971-6580.121676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehman MU, Tahir M, Ali F, Qamar W, Khan R, Khan AQ, et al. Chemopreventive effect of Quercus infectoria against chemically induced renal toxicity and carcinogenesis. Int J Drug Dev Res. 2012;4:336–351. [Google Scholar]
- 7.Kaur G, Athar M, Alam MS. Dietary supplementation of silymarin protects against chemically induced nephrotoxicity, inflammation and renal tumor promotion response. Invest New Drugs. 2010;28:703–713. doi: 10.1007/s10637-009-9289-6. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Woyengo TA, Ramprasath VR, Jones PJH. Anticancer effects of phytosterols. Eur J Clin Nutr. 2009;63:813–820. doi: 10.1038/ejcn.2009.29. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda I, Tanaka K, Sugano M, Vahouny GV, Gallo LL. Inhibition of cholesterol absorption in rats by plant sterols. J Lipid Res. 1988;29:1573–1582. [PubMed] [Google Scholar]
- 11.Loizou S, Lekakis I, Chrousos GP, Moutsatsou P. β-Sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol Nutr Food Res. 2010;54:551–558. doi: 10.1002/mnfr.200900012. [DOI] [PubMed] [Google Scholar]
- 12.Gupta MB, Nath R, Srivastava N, Shanker K, Kishor K, Bhargava KP. Anti-inflammatory and antipyretic activities of beta-sitosterol. Planta Med. 1980;39:157–163. doi: 10.1055/s-2008-1074919. [DOI] [PubMed] [Google Scholar]
- 13.Awad AB, von Holtz RL, Cone JP, Fink CS, Chen YC. β-Sitosterol inhibits the growth of HT-29 human colon cancer cells by activating the sphingomyelin cycle. Anticancer Res. 1998;18:471–473. [PubMed] [Google Scholar]
- 14.von Holtz RL, Fink CS, Awad AB. β-Sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr Cancer. 1998;32:8–12. doi: 10.1080/01635589809514709. [DOI] [PubMed] [Google Scholar]
- 15.Park C, Moon DO, Rhu CH, Choi BT, Lee WH, Kim GY, et al. β-Sitosterol induces anti-proliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biol Pharm Bull. 2007;30:1317–1323. doi: 10.1248/bpb.30.1317. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Chang SK, Qu G, Li T, Cui H. Beta-sitosterol inhibits cell growth and induces apoptosis in SGC-7901 human stomach cancer cells. J Agric Food Chem. 2009;57:5211–5218. doi: 10.1021/jf803878n. [DOI] [PubMed] [Google Scholar]
- 17.Vundru SS, Kale RK, Singh RP. β-Sitosterol induces G1 arrest and causes depolarization of mitochondrial membrane potential in breast carcinoma MDA-MB-231 cells. BMC Complement Altern Med. 2013;13:280–288. doi: 10.1186/1472-6882-13-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Athar M, Iqbal M. Ferric nitrilotriacetate promotes N-diethylnitrosamine-induced renal tumorigenesis in the rat: implications for the involvement of oxidative stress. Carcinogenesis. 1998;19:1133–1139. doi: 10.1093/carcin/19.6.1133. [DOI] [PubMed] [Google Scholar]
- 19.Baskar AA, Ignacimuthu S, Paulraj GM, Numair KSA. Chemopreventive potential of β-Sitosterol in experimental colon cancer model—an in vitro and in vivo study. BMC Complement Altern Med. 2010;10:24. doi: 10.1186/1472-6882-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharmila R, Sindhu G, Arockianathan PM. Nephroprotective effect of β-sitosterol on N-diethylnitrosamine initiated and ferric nitrilotriacetate promoted acute nephrotoxicity in Wistar rats. J Basic Clin Physiol Pharmacol. 2016 doi: 10.1515/jbcpp-2015-0085. [DOI] [PubMed] [Google Scholar]
- 21.Santangelo C, Vari R, Scazzocchio B, Benedetto RD, Filesi C, Masella R. Polyphenols, intracellular signaling and inflammation. Ann Ist Super Sanita. 2007;43:394–405. [PubMed] [Google Scholar]
- 22.Wang X, Hickey RJ, Malkas LH, Koch MO, Li L, Zhang S, et al. Elevated expression of cancer-associated proliferating cell nuclear antigen in high-grade prostatic intraepithelial neoplasia and prostate cancer. Prostate. 2011;71:748–754. doi: 10.1002/pros.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoimenov I, Helleday T. PCNA on the crossroad of cancer. Biochem Soc Trans. 2009;37:605–613. doi: 10.1042/BST0370605. [DOI] [PubMed] [Google Scholar]
- 24.Jagan S, Ramakrishnan G, Anandakumar P, Kamaraj S, Devaki T. Antiproliferative potential of gallic acid against diethylnitrosamine-induced rat hepatocellular carcinoma. Mol Cell Biochem. 2008;319:51–59. doi: 10.1007/s11010-008-9876-4. [DOI] [PubMed] [Google Scholar]
- 25.Rehman MU, Tahir M, Khan AQ, Khan R, Lateef A, Hamiza OO, et al. Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-κB. Toxicol Lett. 2013;216:146–158. doi: 10.1016/j.toxlet.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Awad AB, Fink CS, Trautwein EA, Ntanios FY. β-Sitosterol stimulates ceramide metabolism in differentiated Caco2 cells. J Nutr Biochem. 2005;16:650–655. doi: 10.1016/j.jnutbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- 28.Witty JP, Bridgham JT, Johnson A. Induction of apoptotic cell death in hen granulose cells by ceramide. Endocrinology. 1996;137:5269–5277. doi: 10.1210/endo.137.12.8940345. [DOI] [PubMed] [Google Scholar]
- 29.Harada-Shiba M, Kinoshita M, Kamido H, Shimokado K. Oxidized low density lipoprotein induces apoptosis in cultured human umbilical vein endothelial cells by common and unique mechanisms. J Biol Chem. 1998;273:9681–9687. doi: 10.1074/jbc.273.16.9681. [DOI] [PubMed] [Google Scholar]
- 30.Hudes GR, Carducci MA, Choueiri TK, Esper P, Jonasch E, Kumar R, et al. NCCN task force report: optimizing treatment of advanced renal cell carcinoma with molecular targeted therapy. J Natl Compr Canc Netw. 2011;9:1–29. doi: 10.6004/jnccn.2011.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]