Abstract
Serum thyroglobulin (Tg) and thyroid stimulating hormone (TSH) measurements have evolved as important analytes for monitoring the prognosis of patients with differentiated thyroid cancer, post-thyroidectomy. Individual analyte immunoassay is the current practice in clinical pathology, but the simultaneous assay for all relevant analytes for a given disease, can reduce assay costs, improve patient compliance and give the clinician more information for an unequivocal diagnosis. Microarray immunoassay (MI) can achieve this goal and, hence, we have developed and validated a immuno-radiometric MI for quantitation of serum TSH and Tg by using highly micro-porous polycarbonate (PC) track-etched membranes (TEM) to immobilize the monoclonal anti-TSH and polyclonal anti-Tg antibodies in ~1 mm diameter spots. Non-competitive immunoassays were performed using mixture of 125I labeled monoclonal anti-TSH and anti-Tg antibodies. Phosphorimager was used to quantify the bound radioactivity. TSH and Tg were detected with detection limit of 0.07 µIU/ml and 0.13 ng/ml respectively, which is lower than the clinically required cut-off level. The assay showed: acceptable intra-assay precision within 20 % and recovery in the range of 76–111.2 %. MI compared well with the established immunoradiometric assay (IRMA) with r = 0.98, p < 0.01 (n = 41). No cross-reactivity was seen between the immobilized antibodies. Although two hormones are addressed in this report, MI using PC TEM and isotopic/non-isotopic tracers has the potential for highly automated multiplexed analysis.
Keywords: Microarray immunoassay, Track-etched membranes, Antibody chip, Thyroid stimulating hormone, Thyroglobulin
Introduction
The profiling of analytes in serum for a given disease is often required for both clinical diagnosis and management of patients. The quantitation of analytes, present in serum in minute concentrations, e.g., hormones and tumour markers, is mostly done using immunoassays, which currently, are developed to assay one analyte at a time.
Since the original description by Ekins, [1, 2] and early advances by Huang [3], Schweitzer et al. [4], Tam et al. [5] and Knight et al. [6], MI (also referred to as multi-analyte immunoassay) has been demonstrated to be promising in area of clinical diagnosis [7–10] and several other areas such as environmental monitoring and food safety [11, 12]. Also, by creating disease-associated analyte panels, sensitivity and specificity of clinical diagnosis and/or prognosis is increased by the ‘weight’ of evidence derived from comparing associated analytes. Although several MI have been developed, but most of them are for targeting immunoglobulin or cytokines and MI for routine use in endocrinology is limited to that from Randox for thyroid hormones [13–15]. Technical and operational considerations have hindered implementation of MI in clinical settings [14].
One of the important considerations while developing MI, is a process for the immobilization of the antibodies at high density while maintaining its functionality. This is essential to achieve the required sensitivities along with dynamic ranges appropriate for biological relevance for each of the analytes. Moreover, immobilization procedure should provide low variability to achieve good reproducibility of the assay. Other considerations in developing MI include the elimination of assay cross-reactivity under highly multiplexed condition to achieve high sensitivity and specificity. This report describes the development of MI for simultaneous estimation of TSH and Tg useful in monitoring patients with differentiated thyroid cancer. PC-TEM, which is highly microporous (108 pores per cm2) and very thin (10 micron), was used as an immobilization support. TEMs were earlier described by us, for the first time, as an optimal support providing efficient immobilization of antibodies with low background [16–18]. Capture antibodies, at high density, for both the analytes were immobilized on PC-TEM as small spots (~1 mm diameter).
Detection, which is the key step in development of MI, was accomplished using 125I because of its easy availability at our Centre and detection. Using a mixture of 125I labeled detection antibodies against both TSH and Tg, a non-competitive immunoassay approach was used for development of MI.
MI for TSH and Tg, as an analytical technique, was validated with respect to sensitivity, accuracy, precision and reproducibility, which are important parameters for any bioanalysis [19]. The performance of MI was compared with individual IRMA using linear regression analysis. The developed MI has scope for multiplexing several assays.
Materials and Methods
Materials
Reagents
Bovine serum albumin (BSA) was purchased from Sigma, USA. Human recombinant TSH (thyrogen) was purchased from Genzyme Corporation, USA. Tg standards were obtained along with human Tg IRMA kit from M/s Izotop, Budapest, Hungary. All other chemicals and reagents required for this study were purchased locally and were of analytical or equivalent grade. Low conductivity deionised water was used wherever required.
TEM
PC TEMs having 25 mm diameter with pore size of 0.4 micron and pore density of 108/cm2 were procured from Millipore, USA.
Antibodies and Tracers
Monoclonal antibodies to TSH were purchased from Biodesign International, USA. Polyclonal antibodies against Tg were produced in camel at NRCC, Bikaner, and the Institutional Animal Ethics Committee approval was obtained for this purpose. The total Ig component of the antiserum was purified with 50 % ammonium sulphate and dialyzed in phosphate buffered saline (PBS). 125I labeled monoclonal anti-TSH antibodies and 125I labeled monoclonal anti-Tg antibodies were obtained along with human TSH IRMA kit from BRIT and human Tg IRMA kit from Izotop respectively.
Methods
Preparation of Antibody-chip
Surface Functionalization of TEM
Surface functionalization was carried out by immersing PC TEMs in 2.5 % glutaraldehyde in PBS (PBS: 0.025 M PO4, pH 7.4 and 1 % NaCl) for 2 h with gentle agitation to provide reactive aldehyde groups. The TEMs were washed thrice with PBS and air-dried.
Antibody Immobilization and Blocking
Monoclonal anti-TSH antibodies and polyclonal anti-Tg antibodies were diluted in PBS (0.025 M, pH-7.4) to contain antibodies at a concentration of 1 mg/ml. Antibody solution (0.5 µl) were spotted manually on glutaraldehyde functionalized PC TEM using micropipette at defined locations with two replicate spots for each sample or standard. Spotted TEMs were incubated for 2 h at room temperature and rinsed with PBS to remove excess unbound antibodies. To block nonspecific binding on functionalized TEMs, TEMs were incubated for 2 h at room temperature with blocking solution having 4 % bovine serum albumin (BSA) in PBS with gentle agitation and rinsed thoroughly with PBS.
Preparation of Standards and Tracer for MI
Standards for MI use were prepared by mixing human recombinant TSH at required concentrations in Tg standards from Tg-IRMA kits from M/s Izotop. Prepared standards were calibrated against reference TSH preparation IRP 80/558. Seven standards (S1–S7) having TSH at 0, 0.15, 0.5, 1.5, 5, 15 and 50 µIU/ml and Tg at 0, 0.3, 1, 4, 20, 100 and 250 ng/ml respectively were prepared. To perform MI, mixture of tracer was prepared containing optimized concentration of 125I-labeled anti-TSH monoclonal antibodies and 125I-labeled anti-Tg monoclonal antibodies at 100,000 cpm/100 µl each.
Non-competitive Immunoassays
The antibody spotted TEM were cut into 5 × 20 mm pieces and placed in a 12-well plate for performing the immunoassays. 50 µl of standard or serum sample and 200 µl of tracer cocktail was pipetted in each well of plate and incubated on rotary shaker. After the reaction, the TEMs were washed thrice with PBS containing 0.1 % Tween-20 to remove unbound reactants and air-dried.
Signal Measurement and Analysis
Digital autoradiography of TEMs was performed using Phosphorimager (Typhoon Trio+) from GE Healthcare Biosciences The TEMs were exposed to the storage phosphor screen for 2 h. The images were generated using Imagequant TL software provided by GE Healthcare Biosciences. Phosphorimager, which is based on use of BaFBr:Eu2+ as a detector, provides high resolution images and is more accurate in quantifying the amount of radioactivity as compared to conventional autoradiography since it has wide, linear dynamic range (~1000 times greater than x-ray film) and high sensitivity (10 times more than x-ray film). When the screen is exposed to ionizing radiation such as α, β, or γ radiation, or wavelengths of light shorter than 380 nm, the electrons from Eu+2 are excited and then trapped in an “F-center” of the BaFBr-complex; this results in the oxidation of Eu+2 to Eu+3, which forms the latent image on the screen. After exposure, the latent image is released by scanning the screen with a laser (633 nm). During scanning, Eu3+ reverts back to Eu+2, releasing a photon at 390 nm. The luminescence can then be collected and measured in relation to the position of the scanning laser beam. The result is a representation of the latent image on the storage phosphor imaging plates. The image can then be viewed on a video monitor and analyzed with the aid of appropriate software. This enables visualization and quantification of both strong and weak signal on the same exposure. Moreover, storage phosphor screen exposure takes 10 % of the time required for an equivalent exposure to conventional film.
Open source image analysis software, ImageJ (http://rsbweb.nih.gov/ij/) was used for quantification of spot intensity. Average spot intensity was determined by placing a circle of fixed diameter over each spot and the software provided the average signal intensity on a gray scale in arbitrary units within the selection. This is the sum of the gray values of all the pixels in the selection divided by the number of pixels. Local background signal was collected the similar manner from the surrounding pixels and was subtracted from the average spot intensities. Background subtracted mean spot intensity of the duplicate spots for each sample, was plotted against the analyte concentration and calibration curve was obtained. Gamma in vitro test (available with Stractec Gamma Counter) analysis software was used for standard curve plotting and intrapolating the concentration of analytes in the sample.
Determination of MI Performance
Spot Cross-talk
Cross-talk assessment was done by reacting the TEMs immobilized with both capture antibodies with standard S7, containing high concentration of both the analytes. The TEMs were then reacted with either 125I labeled TSH monoclonal antibodies or 125I labeled Tg monoclonal antibodies or a mixture of both.
Assay Sensitivity
Assay sensitivity can be defined as the lowest concentration of the analyte that can be consistently measured. The lowest analyte concentration that can be detected in the clinical samples (limit of detection [LOD]) is based on analyzing multiple replicate measurements of the ‘0’ standard. To obtain the LOD, we typically add 3 standard deviations to the average spot intensity of the ‘0’ standard. Values expressed as mean spot intensity are then interpolated from the standard curve to be expressed as LOD.
Assay Precision
Intra-assay variability is determined by assaying ten replicates of two samples in a single assay. Three serum samples were assayed over 10 days to calculate the inter-assay CV. Both determinations are expressed as a coefficient of variation (%CV, defined as variability/mean × 100).
Matrix Evaluation
One of the primary conditions in immunoassay is that the standard and the sample should be identical. Standards should be prepared in the same matrix as the analyzed samples. We have prepared standards in human hormone free serum. Recovery and linearity tests were done to find out the errors arising out of differences in the matrix between the standard and sample. Recovery was determined in two serum samples which were spiked with two standards, S4 and S5, in 1: 1 ratio and results were expressed as a percentage of the expected values. Linearity was determined in a serum sample containing high concentration of both TSH and Tg which was serially diluted in standard matrix.
Method Comparison
Serum samples were analyzed by commercial TSH IRMA kit from BRIT and Tg IRMA kit from Izotop according to manufacturer’s instructions. TSH and Tg concentration obtained by MI were compared to IRMA results.
Results
MI Performance
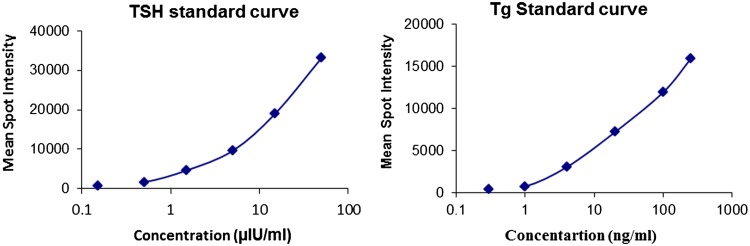
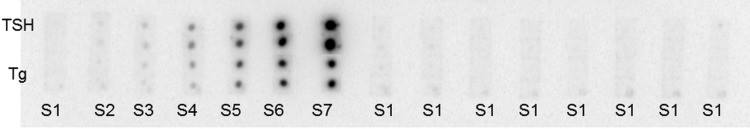
Using optimized conditions, MI was developed for TSH and Tg on glutaraldehyde activated PC TEM. Background subtracted, average spot intensity of the individual spot was determined. Quantification of the signal intensities were followed by estimation of analyte concentration using calibration curve, where mean intensity of duplicate spots spotted on TEM were plotted against analyte concentration. Standard curves generated for TSH and Tg using MI are shown in Fig. 1. The LOD values, calculated as mean + 3SDs, by averaging multiple replicates of zero standards were 0.07 µIU/ml and 0.13 ng/ml for TSH and Tg respectively. The upper limit of the working range of assay was 50 µIU/ml for TSH and 250 ng/ml for Tg as shown in Fig. 2.
Fig. 1.
Standard curve for TSH and Tg obtained using MI. The standard curves were generated by plotting the mean of spot intensity of the duplicate spots against each dilution of standards
Fig. 2.
Autoradiogram showing sensitivity of MI. The membranes are reacted with standard cocktail (S1–S7) or S1 (0 concentration for both analytes) along with mixture of 125I labeled detection antibodies against both the analytes
Spot Cross-talk
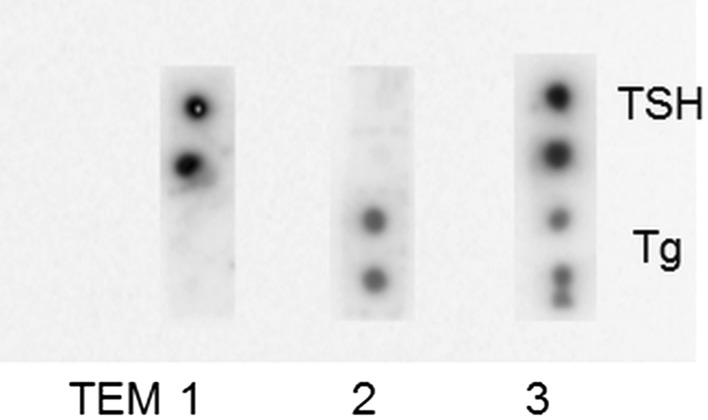
When reacted with 125I labeled TSH monoclonal antibodies, only the capture antibodies against TSH showed signal and no signal was observed with anti-Tg antibody spots. Similarly, vice versa was true when reacted with 125I labeled Tg monoclonal antibodies and no signal observed with anti-TSH antibody spots. This shows that MI was highly specific and cross-talk free. The results of cross-talk are summarized in Fig. 3.
Fig. 3.
Image showing the cross-talk of MI. Each membrane was reacted with standard cocktail containing high concentration of TSH and Tg. TEM1 was reacted with 125I-TSH detection antibodies. TEM 2 was reacted with 125I-Tg detection antibodies. TEM 3 was reacted with tracer cocktail of 125I-TSH and 125I-Tg detection antibodies
Assay Precision
The intra-assay imprecision (CVs), tested using two serum samples within an assay, varied from 9.1 to 11.6 % (Table 1). The inter-assay CVs for duplicate analyses for three serum samples across multiple assays over 10 days were 5–20 % (Table 1).
Table 1.
Intra- and inter-assay variation of MI
| Analyte | TSH | Tg | ||||
|---|---|---|---|---|---|---|
| Concentration (µIU/ml) | Mean ± SD | CV (%) | Concentration (ng/ml) | Mean ± SD | %CV | |
| Intra-assay variation (n = 10) | ||||||
| Sample A | 2 | 1.6 ± 0.15 | 9.1 | 20 | 21.1 ± 2.1 | 9.9 |
| Sample B | 16.8 | 17.2 ± 1.79 | 10.3 | 67 | 65.2 ± 7.6 | 11.6 |
| Inter-assay variation (n = 10) | ||||||
| Sample A | 1.8 | 1.64 ± 0.32 | 20 | 2 | 2.42 ± 0.43 | 18 |
| Sample B | 14 | 14.3 ± 1.75 | 12 | 56 | 58.4 ± 7.56 | 13 |
| Sample C | 4 | 4.02 ± 0.20 | 5 | 19.2 | 19.38 ± 3.84 | 20 |
Matrix Evaluation
The recoveries of TSH (1.5 and 5 µIU/mL) and Tg (4 and 20 ng/ml) added to two samples having known concentrations of TSH and Tg were 76–110.6 % and 78.8–111.2 % (Table 2). For linearity studies, a serum sample with high TSH and Tg concentrations was diluted in standard matrix. Measured concentrations were 69.3–116 % of expected concentrations (Table 3).
Table 2.
Recovery of serum sample spiked with calibrators
| Analyte | Standard concentration added to samples in 1:1 ratio | Recovery % | ||
|---|---|---|---|---|
| Sample A | Sample B | Mean | ||
| TSH | (IU/L) | |||
| 1.5 | 100.9 | 83.7 | 92.3 | |
| 5 | 110.6 | 76 | 93.3 | |
| Tg | ng/ml | |||
| 4 | 111.2 | 97.1 | 104.2 | |
| 20 | 78.8 | 82.9 | 80.8 | |
A and B are serum samples having TSH concentration of 19.5 and 0.09 µIU/ml and Tg concentration of 10.2 and 58.8 ng/ml. The serum samples were spiked with standards S4 and S5 in 1:1 ratio
Table 3.
Linearity of serum sample serially diluted in standard matrix
| Analyte | Dilution factor | Measured/Expected % |
|---|---|---|
| TSH | 2 | 82.6 |
| 4 | 106.8 | |
| 8 | 88.1 | |
| 16 | 79.2 | |
| 32 | 116 | |
| 64 | 73.3 | |
| Tg | 2 | 79.6 |
| 4 | 84.2 | |
| 8 | 69.3 | |
| 16 | 87.1 | |
| 32 | 100 | |
| 64 | 105.3 |
Sample having TSH = 100 µIU/ml and Tg = 50 ng/ml was serially diluted in standard matrix
Method Comparison
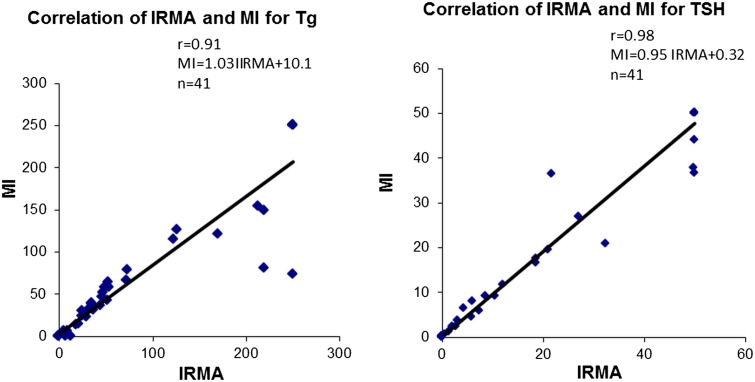
The accuracy of the MI was assessed by comparing the MI results obtained on forty-one human serum samples, obtained from routine clinic to IRMA, an accepted reference standard. The results obtained correlated well with the TSH IRMA (MI = 0.95IRMA + 0.32, r = 0.98, p < 0.001, n = 41) as well as with Tg IRMA (MI = 1.03IRMA + 10.1, r = 0.91, p < 0.001, n = 41) (Fig. 4).
Fig. 4.
Graph showing the comparison of Tg and TSH concentration in forty-one human serum samples measured by MI and IRMA
Discussion
We have developed a MI for the quantitation of Tg and TSH as a proof of concept of multi-analyte immunoassays, and validated it using human serum samples obtained from patients undergoing clinical follow-up at our center. The microarray format was chosen to allow multiplexed detection of the analytes related to thyroid cancer. As an initial proof of concept, MI was developed for analysis of couple of analytes, but further multiplexing can be done by adding more analytes relevant to thyroid cancer.
Development of MI requires rigorous validation of analytical performance to minimize assay imprecision and inaccuracy. Considerations associated with multiplex immunoassays analysis include selection of suitable immobilization support and chemistry to immobile antibodies at high density, in a small area in fully functional form, calibration, interference between antibodies and non- specific proteins, and compatibility of assay sensitivities and working ranges. Based upon these needs, we have used PC-TEM as an immobilization support, which provides high binding and low background support for immobilization of the antibodies because of its highly micro-porosity. Radioiodine (125I) was used as a reporter molecule because of availability at our centre and ease of detection on PC-TEM, because of its low background. Other advantages of using isotopic labels includes low background as these molecules hardly exists in biological fluids as opposed to natural existence of fluorophores or enzymes in biological fluids which interfere in signal measurements using non-isotopic reporters. PC-TEM is not compatible with fluorescence detection system, since it shows high autofluorescence, but is compatible with chemiluminescence. Moreover, fluorescence measurements are interfered by dust and lumiphores signals are short lived cannot be re-measured in case of doubt, which is possible with radioactivity.
One of the important parameter in obtaining high assay sensitivity is support background. By using PC-TEM which provides low background support for antibody immobilization along with 125I which again provides low background as compared to non-isotopic reporter molecules, high sensitivity MI was developed. Sensitivity of the TSH and Tg obtained using MI was 0.07 µIU/ml and 0.13 ng/ml respectively, which was little higher than tube IRMA for TSH (0.025 µIU/ml) and Tg (0.02 ng/ml) but adequate for clinical use. Both the sensitivity and linear ranges of MI are satisfactory for clinical utility.
The developed MI compares significantly with IRMA, a clinically well-established technique used for the detection of TSH and Tg showing high correlation. Moreover, MI was reproducible as both intra-assay and inter-assay CVs obtained were below 20 %. In general, CV values of <20 % are considered indicative of good assay precision, though the recommended acceptance criterion for the evaluation of inter-assay precision is <25 % CV [19]. Although the CV is higher than obtained for corresponding RIA and IRMA but can be improved with better antibody immobilization using microarray spotter and automation of the assay steps involved and automated quantification methods.
Recovery of analytes from spiked samples and linearity of sample is matrix dependent. The recoveries of TSH and Tg as well as linearity of high concentration sample in standard matrix were typically within 25 % of the expected concentration, showing that the assay is suitable for quantitative measurement of TSH and Tg in serum samples. We have not seen any interference between the antibodies while developing MI. Each of the 125I labeled antibody is matched pair to corresponding capture antibodies and MI was cross-talk free.
Although MI was performed manually using radioisotopes, it is expected that use of non-isotopic tracers along with automation will result in faster detection and the sensitivity and precision would be better obtained presently. Simultaneous measurement of two analytes is first step toward MI. These results suggest that the degree of multiplexing on this microarray platform can be increased beyond duplex.
Conclusion
In summary, we have developed a MI, as a proof of concept, for the simultaneous detection TSH and Tg in serum samples, fulfilling all the validation criteria. PC-TEM is a novel immobilization support for antibodies allowing convenient detection TSH and Tg with acceptable sensitivity, accuracy and reproducibility. MI has the advantage of using one small sample aliquot for multiple analytes. It is expected that with the use of automation, the precision would be better than obtained presently. By multiplexing immunoassays for multiple targets of interest, increased amount of information can be acquired from a sample and, thus, increasing the diagnostic specificity and sensitivity. Although only two analytes are addressed in this work, our aim is a similar MI approach for other thyroid cancer specific biomarkers, performed on PC TEM.
Acknowledgments
The authors gratefully acknowledge the support from Dr. S. K. Ghorui and Dr. N.V. Patil, from NRCC, Bikaner for the cameline anti-Tg antisera.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Contributor Information
Bharti Jain, Email: bharti.jain09@gmail.com.
J. Kumarasamy, Email: kumarbarc@gmail.com
Chandrakala Gholve, Email: kalagholve@gmail.com.
Savita Kulkarni, Email: savita.kulkarni1@gmail.com.
M. G. R. Rajan, Phone: +91-22-24157098, Phone: +91-9820409123, Email: mgr.rajan@gmail.com
References
- 1.Ekins RP. Multi-analyte immunoassay. J Pharm Biomed Anal. 1989;7(2):155–168. doi: 10.1016/0731-7085(89)80079-2. [DOI] [PubMed] [Google Scholar]
- 2.Ekins R, Chu F, Biggart E. Fluorescence spectroscopy and its application to a new generation of high sensitivity, multi-microspot, multianalyte, immunoassay. Clin Chim Acta. 1990;194(1):91–114. doi: 10.1016/0009-8981(90)90305-C. [DOI] [PubMed] [Google Scholar]
- 3.Huang R-P. Simultaneous detection of multiple proteins with an array-based enzyme-linked immunosorbent assay (ELISA) and enhanced chemiluminescence (ECL) Clin Chem Lab Med. 2001;39(3):209–214. doi: 10.1515/CCLM.2001.032. [DOI] [PubMed] [Google Scholar]
- 4.Schweitzer B, Roberts S, Grimwade B, Shao W, Wang M, Fu Q, et al. Multiplexed protein profiling on microarrays by rolling-circle amplification. Nat Biotechnol. 2002;20(4):359–365. doi: 10.1038/nbt0402-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam SW, Wiese R, Lee S, Gilmore J, Kumble KD. Simultaneous analysis of eight human Th1/Th2 cytokines using microarrays. J Immunol Meth. 2002;261(1):157–165. doi: 10.1016/S0022-1759(01)00572-5. [DOI] [PubMed] [Google Scholar]
- 6.Knight PR, Sreekumar A, Siddiqui J, Laxman B, Copeland S, Chinnaiyan A, et al. Development of a sensitive microarray immunoassay and comparison with standard enzyme-linked immunoassay for cytokine analysis. Shock. 2004;21(1):26–30. doi: 10.1097/01.shk.0000101668.49265.19. [DOI] [PubMed] [Google Scholar]
- 7.Lochhead MJ, Todorof K, Delaney M, Ives JT, Greef C, Moll K, et al. Rapid multiplexed immunoassay for simultaneous serodiagnosis of HIV-1 and coinfections. J Clin Microbiol. 2011;49(10):3584–3590. doi: 10.1128/JCM.00970-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumachi F, Marino F, Orlando R, Chiara GB, Basso SMM. Simultaneous multianalyte immunoassay measurement of five serum tumor markers in the detection of colorectal cancer. Anticancer Res. 2012;32(3):985–988. [PubMed] [Google Scholar]
- 9.Shimizu Y, Furuya H, Greenwood PB, Chan O, Dai Y, Thornquist MD, et al. A multiplex immunoassay for the non-invasive detection of bladder cancer. J Transl Med. 2016;14(1):31. doi: 10.1186/s12967-016-0783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann M, Roeraade J, Stoll D, Templin MF, Joos TO. Protein microarrays for diagnostic assays. Anal Bioanal Chem. 2009;393(5):1407–1416. doi: 10.1007/s00216-008-2379-z. [DOI] [PubMed] [Google Scholar]
- 11.Rivas LA, Aguirre J, Blanco Y, González-Toril E, Parro V. Graph-based deconvolution analysis of multiplex sandwich microarray immunoassays: applications for environmental monitoring. Environ Microbiol. 2011;13(6):1421–1432. doi: 10.1111/j.1462-2920.2011.02442.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Li Y, Wang A, Slavik M. Rapid, sensitive, and simultaneous detection of three foodborne pathogens using magnetic nanobead-based immunoseparation and quantum dot-based multiplex immunoassay. J Food Prot. 2011;74(12):2039–2047. doi: 10.4315/0362-028X.JFP-11-144. [DOI] [PubMed] [Google Scholar]
- 13.Ellington AA, Kullo IJ, Bailey KR, Klee GG. Antibody-based protein multiplex platforms: technical and operational challenges. Clin Chem. 2010;56(2):186–193. doi: 10.1373/clinchem.2009.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tighe PJ, Ryder RR, Todd I, Fairclough LC. ELISA in the multiplex era: potentials and pitfalls. PROTEOMICS-Clin Appl. 2015;9(3–4):406–422. doi: 10.1002/prca.201400130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molloy PJ, Mc Connell RI, Lamont JV, FitzGerald SP. Automation of biochip array technology for quality results. Clin Chem Lab Med. 2005;43(12):1303–13. doi: 10.1515/CCLM.2005.224. [DOI] [PubMed] [Google Scholar]
- 16.Rajan MGR, Gupta B. Immobilization of antibody spots on glass surfaces-potential use as antibody chips for multianalyte assays. In: 9th APCCB; New Delhi: Trends in Clinical Biochemistry and Laboratory Medicine proceedings of 9th APCCB published by IJCB (ACBI); 2002. p. 66–9.
- 17.Rajan MGR, Gupta B, Iyer RH, Samuel AM, editors. Track-etched membranes- A novel substrate for “antibody chips” for multi analyte immunoassays. Annual Conference of Clinical Biochemists 2004; Bangalore.
- 18.Gupta B, Rajan MGR, Iyer RH, Nair JP, editors. Track etched membranes using heavy particle beam-a novel substrate for multianalyte immunoassays. In: DAE-BRNS Indian particle accelerator conference-2006; 2006.
- 19.Findlay JWA, Smith WC, Lee JW, Nordblom GD, Das I, DeSilva BS, et al. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J Pharm Biomed Anal. 2000;21(6):1249–1273. doi: 10.1016/S0731-7085(99)00244-7. [DOI] [PubMed] [Google Scholar]