Abstract
Carboplatin is a chemotherapeutic agent used against various malignancies such as ovarian carcinoma. The aim of this study is to improve the therapeutic efficacy of carboplatin using pegylated liposomal nanocarriers. Nanoparticles were synthesized using thin film hydration technique and characterized for shape morphology, particle size, zeta potential and drug-release properties. In the next step, A2780S and A2780CP ovarian cancer cell lines were used to determine the efficacy of nanodrug by MTT assay. The particle size and zeta potential of nanodrug were measured 244.3 ± 19.6 nm and −22.9 ± 1.7 mV, respectively. High encapsulation capacity (78.6 ± 3.7 %) confirmed the efficiency of technique. The cytotoxicity results also showed that nanodrug compared to free drug improve the efficacy of carboplatin against both A2780S (P < 0.01) and A2780CP (P < 0.05) cell lines. In conclusion, the findings of our study suggested pegylated liposomal nanocarriers are proper for carboplatin delivery to ovarian cancer cell lines A2780S and A2780CP.
Keywords: Ovarian cancer, Carboplatin, Liposome, Cellular uptake
Introduction
Ovarian cancer is one of the most lethal gynecologic malignancies and the seventh most common of cancer among women worldwide [1, 2]. Chemotherapy and surgery are two main treatment options for ovarian cancer [3]. Effectiveness of ovarian cancer chemotherapy is rigidity depended to efficiency of drug delivery. In conventional chemotherapy, therapeutic agents are distributed nonspecifically throughout the body after intravenous injection. Therefore, they affect both malignant and normal cells, and as a result, limited dose of drug reaches to tumor and causes excess toxicity [4].
Nanotechnology materials have strongly improved the targeting of anticancer drugs into tumor. The nanoscale size of particles enables them to passively accumulate into tumor through the so-called enhanced permeability and retention effect [5]. In this way, liposome nanoparticles have aroused considerable attention. Liposomes are composed of an inner aqueous compartment surrounded by one or more concentric bilayer phospholipid [6]. They have marked with excellent features such as biocompatibility, biodegradability and low toxicity. In addition they are able to encapsulate both hydrophilic and hydrophobic drugs and site specific drug delivery to tumors [7].
Carboplatin is a chemotherapeutic agent widely used for treatment of various types of malignancies such as ovarian cancer [8]. Because of systemic administration, drug exerts various adverse effects, including myelosuppression and especially thrombocytopenia which hamper its clinical use [9].
In the present study, carboplatin was encapsulated into pegylated liposomal nanoparticles using thin film hydration technique. To improve the stability of liposomal nanoparticles, polyethylene glycol (PEG) was used [10]. Nanoparticles were characterized in terms of size, zeta potential, drug retention capability and morphology. After that the efficacy of nanodrug was evaluated on the ovarian cancer A2780S and A2780cp cell lines.
Methods and Materials
Carboplatin was purchased from Kunming Precious Metal Institute (Kunming, Yunnan, China). Cholesterol and polyethylene glycol 3350 were prepared from Sigma-Aldrich Co. (UK) and Kimyagaran Emrooz Chemical Ind. Co. (Iran), respectively. Lecithin was obtained from Acros Co. (Belgium) and RPMI 1640 cell culture medium was from Gibco Co. (Germany). 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) was obtained from Sigma-Aldrich (USA). A2780S and A2780CP cell lines were supplied by cell bank of Pasteur Institute of Iran. All materials were analytical grade. In addition, distilled water was used throughout the study.
Nanoliposomal Drug Preparation
Liposomal nanoparticles were synthesized using thin film hydration method. Briefly, carboplatin, lecithin, cholesterol, and polyethylene glycol 3350 (with molar ratio of 1, 10, 7 and 0.8 mM, respectively) were dissolved in 50 ml of ethanol 96 % (45 °C, 60 min, and 150 rpm). After perfect dissolving, the solvent was evaporated using rotary evaporator (Heidolph, Germany) and the thin film was formed on the bottom of vessel. The film was suspended into Phosphate Buffer Saline (PBS, pH 7.4), in that the final concentrations of carboplatin, lecithin, cholesterol, and polyethylene glycol 3350 were estimated 1.5, 13, 9.5 and 1 mM, respectively. Formulation was sonicated by bath ultrasonic (Bandelin Sonorex Digitec, Germany) for 4 min. Blank nanoparticles were prepared with the abovementioned method except that the drug was removed from the process.
Nanoparticle Characterization
The size and zeta potential of nanoparticles were determined by Dynamic Light Scattering (DLS) technique using Zetasizer (Nano ZS3600, Malvern Instruments, UK). Also, nanoparticles were evaluated from the morphological point of view. To perform this, suspension of particles was lyophilized after addition of 3 % mannitol. Powder form of nanoparticles was evaluated by Scanning Electron Microscopy (SEM) (S-4160 -scanning microscope, Hitachi, Japan). Drug loading and encapsulation efficiency were also determined spectrophotometrically. Briefly, suspension of nanodrug was centrifuged (21,000 rpm, 45 min, 4 °C) and supernatant was obtained. The concentration of carboplatin into supernatant was determined spectrophotometrically (UV1800, Shimadzu Co) in 220 nm using standard curve. The drug encapsulation and drug loading efficiency were estimated by following formulas:
| 1 |
| 2 |
In Formula (1), PC: primary carboplatin and CS: carboplatin in supernatant (mg/ml).In Formula (2), C: carboplatin content in the nanoliposomes and W: weight of nanoliposomes (mg).
Also, the stability of nanoparticles was evaluated in which suspension of particles were stored at 4 °C in refrigerator for 3 months. Then, they were characterized once again in terms of size, zeta potential, encapsulation and drug loading efficiency.
Drug Release Study
Release study was performed using dialysis membrane technique. Briefly, sediment of nanodrug was obtained once again using centrifugation process. The sediment was resuspended into PBS and poured into dialysis bag (Sigma, cut off 10,000) and immersed into PBS and stirred (150 rpm, room temperature). In the predetermined time intervals, 2 ml of samples were withdrawn and the concentration of drug was determined. Finally, the drug release profile versus time was plotted.
Cytotoxicity Assay
Cytotoxicity assay was performed using MTT assay. Briefly, A2780CP and A2780S cell lines in the density of 104 were cultured into 96 well plates containing RPMI 1640 supplemented with 10 % fetal bovine serum, 100 μg/ml streptomycin, and 100 U/ml penicillin. After 24 h, culture media were removed, and the cells were incubated with various concentrations of carboplatin (8.7–4480 µg/ml) in the encapsulated or free form. Following 24 h of incubation, the medium was removed and 100 µl of MTT solution (0.5 mg/ml) was added to each well. Formazan crystals were dissolved in isopropanol (100 %) and the optical absorbance was read at 570 nm using a plate reader (Synergy Multi-Mode Reader, Bio-Tek, USA). In addition, IC50 was calculated using the Pharm program.
Statistical Analysis
Results were analyzed by the SPSS software version 18. Data are expressed as Mean ± SD from three independent experiments. Comparisons between groups were analyzed using the t test. The significance level was set at 0.05.
Results
Nanoparticle Characterization
The mean size and zeta potential of nanoparticles were found to be 244.3 ± 19.6 nm and −22.9 ± 1.7 mV, respectively. SEM image also confirmed the preparation of nanoparticles (Fig. 1). Furthermore, the drug encapsulation and loading efficiency were estimated 78.6 ± 3.7 and 2.5 ± 0.1 %, respectively. In addition, results of size, zeta potential, encapsulation and drug loading efficiency after 3 months were confirmed the proper stability of nanoparticles (Table 1).
Fig. 1.

SEM image of carboplatin nanoliposome
Table 1.
Physicochemical characteristics of carboplatin nanoliposomes in the production time and 3 months later
| Time | Zeta potential (mV) | Size (nm) | EE (%) | DLE (%) |
|---|---|---|---|---|
| Production time | −22.9 ± 1.7 | 244.3 ± 19.6 | 78.6 ± 3.7 | 2.5 ± 0.1 |
| 3 months after production | −23.2 ± 1.8 | 250.5 ± 19.2 | 70.7 ± 2.9 | 2.15 ± 0.11 |
EE entrapment efficiency, DLE drug loading efficiency
Data are expressed as mean ± SD from three independent experiments
In Vitro Drug Release Studies
Results of release study were presented in Fig. 2. Although the release pattern was not a regular pattern, it was found that liposome nanoparticles keep carboplatin efficiently, in which only 14 % of encapsulated drug were released after 48 h. In addition, a burst release was observed in the first hour of study.
Fig. 2.
Profile of carboplatin release from liposomal nanoparticles. Data are expressed as mean ± SD from three independent experiments
Cytotoxicity Assay
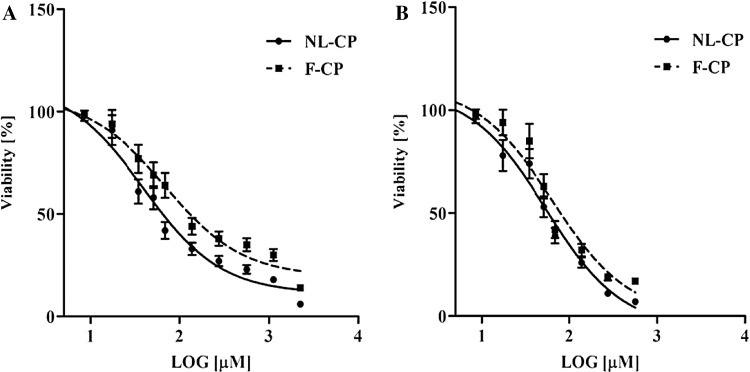
Carboplatin in the standard and encapsulated form was shown dose-dependent cytotoxicity for both A2780CP and A2780S cell lines (Fig. 3). However, nanodrug was significantly reduced the cell viability compared to the standard drug. It was also perceived that A2780CP has more sensitivity to carboplatin (the standard or encapsulated form) than A2780S cell line. Results of IC50 also confirmed the potency of nanodrug compared to the standard drug (Table 2). Nevertheless, these values were also much less for A2780CP compared to A2780S cell line.
Fig. 3.
Cytotoxic effects of free carboplatin (F-CP) and nanoliposomal carboplatin (NL-CP) on A2780S (a) and A2780CP cell lines (b). Data are expressed as mean ± SD from three independent experiments
Table 2.
IC50 values (µg/ml) of carboplatin (in the standard or encapsulated form) on A2780CP and A2780S cell lines
| Cell lines | IC50 of carboplatin | IC50 of nanoliposomal carboplatin | P value |
|---|---|---|---|
| A2780S | 128.8 ± 31.9 | 63.1 ± 20.3 | <0.01 |
| A2780CP | 77.6 ± 16.7 | 57.5 ± 6.0 | <0.05 |
Data are expressed as mean ± SD from three independent experiments
Discussion
It was found thin film hydration technique is a suitable method for preparation of pegylated carboplatin-loaded liposomal nanoparticles. PEG was used in the formulation since it is a water soluble polymer with low immunogenicity and antigenicity, stable in the blood circulation and able to lengthen the drug release time [10]. Use of liposome as carboplatin carrier was reported in some studies [11, 12]. Zhang et al. used of thin film hydration technique to prepare the particles with some modifications. The in vitro and in vivo results of study confirmed the potency of nanodrug compared to the standard drug against gastric cancer [11]. In another study, Chaudhury et al. [12] prepared targeted liposomal nanoparticles containing carboplatin. The particles were targeted using folate ligand. Although nanoparticles prepared by thin film hydration technique, carboplatin was encapsulated into nanoparticles by a passive equilibration technique. They evaluated the efficacy of drug and nanodrug against ovarian cancer in vivo and in vitro environment which indicated carboplatin loaded liposomal nanoparticles have significantly improved the efficacy of drug [12]. The difference between our formulation and Chaudhury et al. [12] formulation is in the materials and methods. We have taken advantage of cheaper materials and easier technique to prepare the nanodrug. Nanoparticles were prepared easily with the size equal to 244 nm. There is a direct relationship between zeta potential and stability of colloids [13]. The zeta potential of −22 mV confirmed the proper stability of nanoparticles. The SEM results also confirmed the preparation of spherical nanoparticles with smooth surface. High encapsulation capacity up to 78 % indicated the efficiency of preparation method. Results of drug release showed that liposome nanoparticles have the suitable capability for retention of carboplatin. However, a burst release in the first hour of study indicated the release of adsorbed drug from nanoparticles. Low and slow release of drug from nanoparticles probably results from the presence of PEG in the formulation. This situation was shown in Cho et al. study [14]. Additionally, PEG increases the stability of nanoparticles and enhances the possibility of drug delivery to tumor, and consequently increases the efficacy of drug [15].
Concerning the cytotoxicity, it was found that nanodrug has more cytotoxicity compared to the standard drug against both A2780CP and A2780S cell lines. This is results from the sustained release of carboplatin from nanoparticles. Interestingly we perceived that the cell toxicity effects of drug and nanodrug was much higher against A2780CP compared to A2780S cells. A2780CP and A2780S ovarian cancer cell lines are resistance and sensitive to cisplatin, respectively [16]. Since the cytotoxicity of carboplatin is directly associated with the amount of drug that enters the cell [17], this phenomenon can be related to the differences in the cell entry mechanism of carboplatin and carboplatin loaded liposomal nanoparticles over the anticancer drug cisplatin. Further research is recommended to obtain more details of this phenomenon. Finally, findings of study suggested liposome nanoparticles are suitable for carboplatin delivery to A2780CP and A2780S cell lines.
Conclusion
It was found that thin film hydration method is proper for construction of carboplatin loaded liposomal nanoparticles. Spherical nanoparticles with smooth surface were obtained. Release study showed nanoparticles have proper carboplatin retention capability. Cytotoxicity evaluation however demonstrated that drug and nanodrug have more potency against cisplatin resistant ovarian cancer cell line A2780CP than A2780S cell line. This phenomenon may indicate that cellular uptake of cisplatin is diverse from carboplatin uptake in these cells. Finally, the results of study suggested liposome nanoparticles are suitable carrier for carboplatin delivery to A2780CP and A2780S cell lines.
Acknowledgments
This study was the M.Sc. thesis of the first author that was approved by institute review board of Islamic Azad University, Shahreza Branch, Shahreza, Iran with code number of (19710603932016) and supported by Department of Pilot Nanobiotechnology, Pasteur Institute of Iran, Tehran, Iran which hereby thanks to all colleagues.
Compliance with Ethical Standard
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Kim A, Ueda Y, Naka T, Enomoto T. Therapeutic strategies in epithelial ovarian cancer. J Exp Clin Cancer Res. 2012;31:14. doi: 10.1186/1756-9966-31-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arab M, Khayamzadeh M, Tehranian A, Tabatabaeefar M, Hosseini M, Anbiaee R, et al. Incidence rate of ovarian cancer in Iran in comparison with developed countries. Indian J Cancer. 2010;47:322–327. doi: 10.4103/0019-509X.64721. [DOI] [PubMed] [Google Scholar]
- 3.Lambert LA. Looking up: recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin. 2015;65:284–298. doi: 10.3322/caac.21277. [DOI] [PubMed] [Google Scholar]
- 4.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu E, Zhou Y, Liu Z, Li J, Zhang D, Chen J, et al. Cisplatin loaded hyaluronic acid modified TiO2 nanoparticles for neoadjuvant chemotherapy of ovarian cancer. J Nanomater. 2015;16:275–282. [Google Scholar]
- 6.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 7.Kopel P, Wawrzak D, Moulick A, Milosavljevic V, Kizek R. Nanotransporters for anticancer drugs, modifications, target molecules. J Metallomics Nanotechnol. 2015;2:32–38. [Google Scholar]
- 8.Della Pepa C, Tonini G, Pisano C, Di Napoli M, Cecere SC, Tambaro R, et al. Ovarian cancer standard of care: are there real alternatives? Chin J Cancer. 2015;34:17–27. doi: 10.5732/cjc.014.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitt A, Gladieff L, Laffont CM, Evrard A, Boyer JC, Lansiaux A, et al. Factors for hematopoietic toxicity of carboplatin: refining the targeting of carboplatin systemic exposure. J Clin Oncol. 2010;28:4568–4574. doi: 10.1200/JCO.2010.29.3597. [DOI] [PubMed] [Google Scholar]
- 10.Shahbazian S, Akbarzadeh A, Torabi S, Omidi M. Anti-cancer activity of pegylated liposomal trans-anethole on breast cancer cell lines MCF-7 and T47D. Biotechnol Lett. 2015;37:1355–1359. doi: 10.1007/s10529-015-1813-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Huang C, Huang H. Antitumor and antimetastasis effects of carboplatin liposomes with polyethylene glycol-2000 on SGC-7901 gastric cell-bearing nude mice. Oncol Lett. 2014;8:2209–2214. doi: 10.3892/ol.2014.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhury A, Das S, Bunte RM, Chiu GN. Potent therapeutic activity of folate receptor-targeted liposomal carboplatin in the localized treatment of intraperitoneally grown human ovarian tumor xenograft. Int J Nanomed. 2012;7:739–751. doi: 10.2147/IJN.S26172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (part 2) Trop J Pharm Res. 2013;12:265–273. [Google Scholar]
- 14.Cho Y, Shi R, Borgens RB, Ivanisevic A. Functionalized mesoporous silica nanoparticle-based drug delivery system to rescue acrolein-mediated cell death. Nanomedicine (Lond) 2008;3:507–519. doi: 10.2217/17435889.3.4.507. [DOI] [PubMed] [Google Scholar]
- 15.Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55:403–419. doi: 10.1016/S0169-409X(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 16.Jamali B, Nakhjavani M, Hosseinzadeh L, Amidi S, Nikounezhad N, Shirazi FH. Intracellular GSH alterations and its relationship to level of resistance following exposure to cisplatin in cancer cells. Iran J Pharm Res. 2015;14:513–519. [PMC free article] [PubMed] [Google Scholar]
- 17.Nejdl L, Kudr J, Blazkova I, Chudobova D, Skalickova S, Ruttkay-Nedecky B, et al. Mechanisms of uptake and interaction of platinum based drugs in eukaryotic cells. Platinum metals in the environment. Berlin: Springer; 2015. pp. 401–415. [Google Scholar]