Abstract
The aim of the current study was to evaluate the role of γ-amino butyric acid (GABA) in insulin disturbance and hyperglycemia associated with brain oxidative damage in streptozotocin-treated rats. Streptozotocin (STZ) was administered to male albino rats as a single intraperitoneal dose (60 mg/kg body weight). GABA (200 mg/Kg body weight/day) was administered daily via gavages during 3 weeks to STZ-treated-rats. Male albino rats Sprague–Dawley (10 ± 2 weeks old; 120 ± 10 g body weight) were divided into 4 groups of 6 rats and treated in parallel. (1) Control group: received distilled water, (2) GABA group: received GABA, (3) STZ group: STZ-treated rats received distilled water, (4) STZ + GABA group: STZ-treated rats received GABA. Rats were sacrificed after a fasting period of 12 h next last dose of GABA. The results obtained showed that STZ-treatment produced hyperglycemia and insulin deficiency (similar to type1 Diabetes). These changes were associated with oxidative damage in brain tissue and notified by significant decreases of superoxide dismutase and catalase activities in parallel to significant increases of malondialdehyde and advanced oxidation protein products levels. The histopathology reports also revealed that STZ-treatment produced degeneration of pancreatic cells. The administration of GABA to STZ-treated rats preserved pancreatic tissue with improved insulin secretion, improved glucose level and minimized oxidative stress in brain tissues. It could be concluded that GABA might protect the brain from oxidative stress and preserve pancreas tissues with adjusting glucose and insulin levels in Diabetic rats and might decrease the risk of neurodegenerative disease in diabetes.
Keywords: Streptozotocin, Diabetes, GABA, Oxidative stress, Brain
Introduction
Diabetes mellitus (DM) or simply diabetes is one of the most frequently occurring chronic diseases worldwide and one of the leading causes of death and disability [1]. DM characterized by hyperglycemia resulting from defects in insulin production and/or insulin action, is generally associated with organ dysfunction and metabolic disturbances [1]. Although the etiology of the disease is not well defined, increasing evidence in both experimental and clinical studies suggests that oxidative stress due to auto-oxidation of glucose and decreased antioxidant defense [2] have a central role in the onset of DM and its complications [3, 4]. Recently, many studies have indicated that DM is also implicated in damage of the central nervous system (CNS) and induced the brain pathological changes, named the diabetic encephalopathy, which is a complication of DM in the CNS characterized by mild cognitive deficits and neuropathology [5, 6]. Diabetic encephalopathy presents many symptoms, which can be described as the features of brain aging including brain atrophy, reactive oxygen species (ROS) accumulation, cerebral vasculopathy, and impairment of cognition [7, 8]. Clinical observation has shown that brain atrophy is more remarkable in diabetic patients than in age-matched controls [9].
Efficient defense and repair mechanisms exist in living cells to protect against oxidant species. Superoxide dismutase (SOD) catalyzes the reduction of superoxide anion to H2O2, which is broken down by catalase and glutathione peroxidase (GSH-Px) [10]. However under abnormal conditions the antioxidant system may not be adequate to protect from oxidative stress and metabolic alterations.
Increased production of ROS can compromise essential cellular functions, and probably contribute to brain injury [11]. Some tissues, especially the brain, are much more vulnerable to oxidative stress because of their elevated consumption of oxygen and the consequent generation of large amounts of ROS [12], which are closely implicated in several diseases of the nervous system including Parkinson’s disease, schizophrenia and Alzheimer’s disease [13]. Increasing evidence in both experimental and clinical studies suggests that free radicals are formed disproportionately in diabetes by glucose oxidation, nonenzymatic glycation of proteins, and the subsequent oxidative degradation of glycated proteins associated with impaired antioxidant defenses [14].
Gamma amino butyric acid (GABA) is considered to be a multifunctional molecule with various physiological effects throughout the body. It is the major inhibitory neurotransmitter in the central nervous system and a paracrine/autocrine signaling molecule in various peripheral tissues [15]. The action of GABA is mediated by receptors. Three types have been identified: type A (GABAAR), ionotropic receptor- a hetero-pentameric ligand-gated Cl− [16], type B (GABABR) metabotropic receptor- a heterodimeric G-protein-coupled receptor [17] and type C (GABACR), ionotropic receptor- which is also a ligand-gated Cl− channel [18]. It is the major inhibitory neurotransmitter in the central nervous system [19], and is responsible for 40 % of inhibitory synaptic processing in the mammalian brain [20]. Still, it can also act as a trophic factor during nervous system development to influence events such as proliferation [21], migration, differentiation, synapse maturation and cell death. GABA mediates these processes by the activation of traditional ionotropic and metabotropic receptors, and probably by both synaptic and non-synaptic mechanisms. The functional properties of GABA receptor signaling in the immature brain are significantly different from, and in some ways opposite to, those found in the adult brain [22].The presence of GABA receptors has been identified in a wide variety of non-neural tissues including the liver [23], the kidney [24], and the pancreas where it exists at the highest concentration outside of the central nervous system [25]. In the pancreas, GABA is localized in synaptic-like micro vesicles in β-cells in the islets of Langerhans [26]. In view of these considerations the aim of the current study was to evaluate the role of GABA in insulin level, and hyperglycemia associated with brain oxidative stress in STZ-treated rats.
Materials and Methods
Experimental Animals
Male albino rats Sprague–Dawley (10 ± 2 weeks old; 120 ± 10 g) purchased from the Egyptian Holding Company for Biological Products and Vaccines (Helwan, Cairo, Egypt) were used in the current study. Animals were maintained under standard conditions of ventilation, temperature, humidity, lighting (light/dark: 13 h/11 h) and fed on standard pellets diet containing all nutritive elements (proteins, fats, carbohydrates, vitamins, salts and minerals). Food and water were available ad libitum. For biochemical analyses animals were sacrificed at 11:00 a.m. ± 1 h. All animal procedures were performed in accordance with the Ethics Committee of the National Research Centre conformed to the “Guide for the care and use of Laboratory Animals” published by the National Institutes of Health (NIH publication No. 85–23, revised 1996).
Streptozotocin Treatment
Streptozotocin (STZ) was purchased from Sigma chemical company, St. Louis Missouri, USA, in the form of 1 g vials. Diabetes was induced by administering intraperitoneal injection of a freshly prepared solution of STZ (60 mg/kg BW) in 0.1 M cold citrate buffer (PH 4.5) to the overnight fasted rats [27]. Since STZ is capable of producing fatal hypoglycemia as a result of massive pancreatic release of insulin, the rats were kept on 5 % glucose for the next 24 h to prevent hypoglycemia [28]. Blood glucose levels were monitored using an Accu-check blood glucose meter (Roche Diagnostics, Basel, Switzerland) in tail vein blood 72 h after STZ administration. Rats with blood glucose levels ≥250 mg/dl were considered diabetics.
Gamma Amino Butyric Acid Treatment
Gamma amino butyric acid (GABA) purchased from Sigma-Aldrich, St Louis, Missouri, USA in the form of 25 g vials was dissolved in distilled water and administered to rats daily by gastric gavages at doses of 200 mg/Kg body weight/day [29] for a period of 3 weeks.
Animal Groups
Rats were divided into 4 groups of 6 rats and treated in parallel. Control group: administered distilled water during 3 weeks via gavages, GABA group: administered GABA (200 mg/Kg body weight/day) daily during 3 weeks via gavages, STZ group: STZ-induced diabetic rats administered distilled water daily during 3 weeks via gavages, STZ + GABA group: STZ-induced diabetic rats administered GABA (200 mg/Kg body weight/day) daily during 3 weeks via gavages.
Biochemical Analysis
At the end of the third week rats were sacrificed after a fasting period of 12 h next day to the last dose of GABA. Rats were anaesthetized with light ether; Blood sample was obtained via heart puncture by sterilized syringe, brain and pancreas tissues rapidly excised. A part of the blood was taken on sodium fluoride to inhibit enolase enzyme and prevent glucose breakdown and used for the determination of plasma glucose [30]. Another part was left to coagulate to obtain serum after centrifugation at 1000g for 15 min. Brain tissue and pancreas tissue 10 % w/v) were homogenized in physiological saline using Teflon homogenizer (Glass-Col, Terre Haute, Ind., USA) and after centrifugation at 10,000g for 15 min using refrigerated centrifuge (K3 Centurion Scientific, Ltd, London, UK) the supernatant was used for the assessment of oxidative stress.
Chemicals and reagents were purchased from Sigma-Aldrich, St Louis, MO, USA otherwise mentioned. Measurement of absorbance was performed using a T60 UV/VIS spectrophotometer, PG instruments, London, UK.
Plasma glucose content was determined using diagnostic kit purchased from Diamond, Egypt according to the method described by Trinder [31], serum insulin level was determined by a solid phase enzyme linked immunosorbant assay (ELISA) according to Clark and Hales [32]. The extent of lipid peroxidation was assayed as described by Yoshioka et al. [33], based on the determination of malondialdehyde (MDA) an end product of lipid peroxidation, which can react with thiobarbituric acid in acidic medium to yield a pink colored trimethine complex. Advanced oxidation protein products (AOPPs) was determined according to the method of Witko-Sarsat et al. [34] based on the measurement of dityrosine containing cross-linked protein products. Superoxide dismutase activity (SOD) was determined according to the method of Nishikimi et al. [35]. One unit of SOD activity defined as the amount of the enzyme causing half the maximum inhibition of nitro blue tetrazolium reduction. Catalase activity was determined as described by Sinha [36] and expressed as µmol of H2O2 consumed/min/mg protein. For histopathological investigations, portion of the pancreas was fixed in 10 % formalin, embedded in molten paraffin wax and ultra-sectioned (5–6 μm thickness), then stained with hematoxylin and eosin and examined under light microscope [37].
Statistical Analysis
Results are presented as mean ± standard deviation (SD). Groups were compared by one-way analyses of variance (ANOVA), and post hoc multiple comparisons were done with LSD test using SPSS/PC software program (version 21; SPSS Inc., Chicago, IL, USA). The degree of change (percent change) in the present results was illustrated in the accompanied figures.
Result
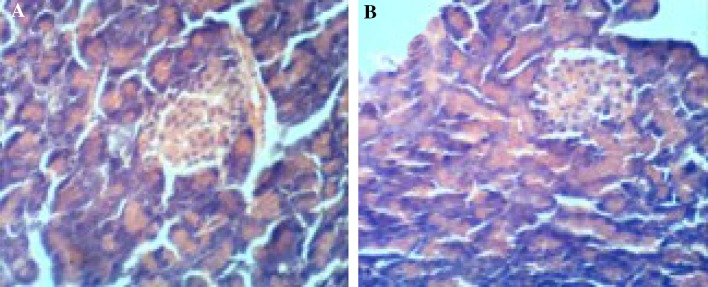
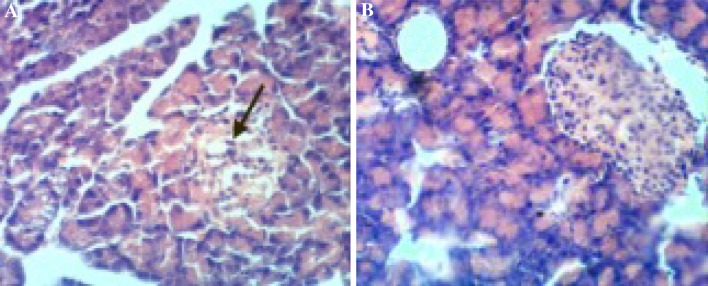
The results obtained in the current study revealed that the administration of gamma amino butyric acid (GABA) (200 mg/Kg/day) (GABA group) to normal rats for 3 weeks, had no significant effect on glucose and insulin. Chemical analysis in the brain tissues showed that superoxide dismutase (SOD) and catalase activities, malondialdehyde (MDA), and advanced oxidation protein products (AOPP) were within normal ranges (Tables 1, 2, 3) and also no histolopathogical changes occurred in pancreas (Fig. 1). The injection of streptozotocin (STZ) 60 mg/kg body weight (STZ group) produced significant hyperglycemia, hypoinsulinemia (similar to type1 Diabetes) and provoked oxidative stress notified by significant decrease in antioxidant enzymes (SOD and catalase) associated to a significant increase in oxidant species (MDA and AOPP) in brain tissues compared to their respective control values (Tables 1, 2, 3). Also Histological investigation revealed damage of islets of Langerhans in STZ group (Fig. 2). Administration of GABA to STZ-induced diabetic rats reduced glucose and elevates insulin level and reduced oxidative stress in brain tissues notified by an increase of antioxidant enzyme activities in brain tissues and decrease of oxidant species compared to their relative values in diabetic rats not receiving GABA. Also administration of GABA preserved pancreas tissue and improved insulin secretion (Fig. 2).
Table 1.
Influence of GABA on plasma glucose and serum insulin levels in different animals groups
| Parameters | Plasma glucose (mg/dl) | Serum insulin (µIU/ml) |
|---|---|---|
| Groups | ||
| Control | 92 ± 6.2 | 13.00 ± 0.81 |
| GABA | 95 ± 4.3 | 12.50 ± 0.65 |
| STZ | 268 ± 12.9a | 4.40 ± 0.36a |
| STZ + GABA | 148 ± 12ab | 8.08 ± 0.6ab |
Values are expressed as mean ± standard deviation (n = 6)
aSignificant versus control, b significant versus respective groups not receiving GABA at P < 0.05
Table 2.
Influence of GABA on antioxidant enzymes in the brain tissues of different animal groups
| Parameters | SOD (U/g tissue) | Catalase (µmol H2O2 consumed/min/mg protein)/g) |
|---|---|---|
| Groups | ||
| Control | 8.1 ± 0.2 | 60 ± 1.4 |
| GABA | 8.3 ± 0.3 | 65 ± 2.9 |
| STZ | 6.7 ± 0.9a | 19 ± 3.3a |
| STZ + GABA | 8.3 ± 0.1b | 53 ± 15b |
Values are expressed as mean ± standard deviation (n = 6)
aSignificant versus control, b significant versus respective groups not receiving GABA at P < 0.05
Table 3.
Influence of GABA on oxidant biomarkers in the brain tissues of different animal groups
| Parameters | MDA (nmol/g) | AOPP (µmol/l) |
|---|---|---|
| Groups | ||
| Control | 92 ± 2.6 | 155 ± 2.4 |
| GABA | 95 ± 1.9 | 161 ± 1.3 |
| STZ | 102 ± 1.6a | 183 ± 3a |
| STZ + GABA | 88 ± 1.6b | 161 ± 3.7b |
Values are expressed as mean ± standard deviation (n = 6)
aSignificant versus control, b significant versus respective groups not receiving GABA at P < 0.05
Fig. 1.
a Photomicrograph of pancreas from control rat showing normal pancreatic acinus with normal pancreatic islets and normal acinar cell. b Photomicrograph of pancreas from rats given GABA showing preservation of pancreatic acinus with normal pancreatic islets and normal acinar cells. (H&E, ×400)
Fig. 2.
a Photomicrograph of pancreas from STZ-treated rat showing ruptured pancreatic acinus, empty pancreatic acinar cells and degenerated islets of pancreas (arrow) b Photomicrograph of pancreas from STZ-treated rat given GABA showing regenerated pancreatic acinus with modulated pancreatic islets. (H&E, ×400)
Discussion
Streptozotocin (STZ) an antibiotic produced by Streptomyces chromogenes is widely used to induce diabetes. Its cytotoxic action has been shown to be mediated through the generation of reactive oxygen species (ROS) causing degeneration of β cells [38]. In the current study, the intraperitoneal administration of 60 mg/kg of STZ induced hyperglycemia associated with hypoinsulinemia (similar to type 1 DM). Hyperglycemia appears to be a consequence of insulin deficiency caused by the degeneration of pancreatic beta cells and reduced insulin synthesis [28]. In the current study administration of 60 mg/kg of STZ induced oxidative stress in brain tissues notified by significant decrease in antioxidant enzymes associated to a significant increase in oxidant species in brain tissues compared to their respective control values with induced degeneration of pancreatic cells notified by photomicrograph of pancreas from STZ-treated rat.
Oxidative stress might be attributed to the state of hyperglycemia [39] and the excessive formation of free radicals [40]. Glucose oxidation produces an elevation in voltage gradient across the mitochondrial membrane and when a critical threshold in voltage gradient is reached, electron transfer is blocked. The electrons accumulate causing overproduction of superoxide [41].The excess of superoxide initiates a cascade of damaging events via the production of more superoxide, hydrogen peroxide, hydroxyl radicals, and peroxynitrite, which injure macromolecules either at or near the site of their formation [42]. The significant decrease in SOD and catalase activities in brain tissues might result from inhibition in the synthesis of antioxidant enzymes [43, 44]. Supporting this postulation the mRNA expressions of SOD and catalase were reported to decrease significantly in the liver and pancreas of diabetic rats [45–47]. On the other hand the decrease of antioxidants might result from their increased utilization to neutralize the excess of free radicals generated in the diabetic body.
In the current study the administration of GABA (200 mg/Kg/day) to normal rats for 3 weeks had no significant effect on glucose, and insulin relative to control values. Chemical analysis in brain tissues showed that SOD and catalase activities, and MDA, and AOPP were within normal ranges with no histopathological changes recorded in pancreas. The results corroborate previous findings that the chronic administration of GABA at up to 1 g/kg/day in rats and dogs was well tolerated without signs of toxicity for a period of up to 1 year [48].
The administration of GABA (200 mg/Kg/day) daily during a period of 3 weeks to STZ-treated rats improved insulin levels, hyperglycemia and significantly attenuated oxidative stress in pancreas tissue supporting that GABA reverses hyperglycemia [49] and ameliorates impaired glucose metabolism [29], which could be attributed to the role of GABA in the regeneration of pancreatic cells where its interaction with GABA receptors in islet β-cells produces membrane depolarization and Ca2+ influx, leading to the activation of PI3-K/Akt–dependent growth and survival pathways, thus preserving β-cells [50, 51]. Moreover GABA causes membrane depolarization and enhances insulin secretion [52]. In addition, the action of GABA on the GABA receptors in the α-cells suppresses glucagon secretion [53] and hence reduced glucose level.
The administration of GABA (200 mg/Kg/day) daily during a period of 3 weeks to STZ-treated rats has significantly attenuated oxidative stress in brain tissue. This is identified by the significant elevations in the activity of the antioxidant enzymes SOD and catalase associated with a significant reduction in the amount of MDA and AOPP, compared to their respective levels in rats not receiving GABA. This may be attributed to the role of GABA in modulating hyperglycemia as mentioned before and the free radicals scavenging activities of GABA [54], and its effectiveness to inhibit the formation of reactive carbonyl intermediates, and to react with MDA to form different conjugated complexes [55]. The results are in agreement with previous findings that GABA reduces the content of MDA in the liver and kidney of diabetic rats [29], and attenuates oxidative stress through increasing SOD and catalase activities, and decreasing lipid peroxidation [56].
The administration of GABA (200 mg/Kg/day) daily during a period of 3 weeks to STZ-treated rats restored the β-cell mass, and rectified histological changes in pancreatic tissues. The regeneration of β- cell could be attributed to β-cell proliferation and decrease of apoptosis, leading to enhanced β-cell mass [57, 58]. According to Soltani et al. [50] in islet β-cells, GABA produces membrane depolarization and Ca2+ influx, leading to the activation of PI3-K/Akt–dependent growth and survival pathways (Phosphatidylinositol-3 kinases- Akt) thus preserving β-cells. Furthermore, Prud’homme et al. [58] show that GABA protects pancreatic islet cells against apoptosis and exerts anti-inflammatory effects. Attention to the role of GABA in human health and disease has been continuously increased following the discovery that GABA possesses antioxidant and free radicals scavenging activities [54, 55], and play a significant role in glucose homeostasis [51, 59].
According to the results obtained in the current study, the administration of GABA to STZ-treated rats regulates insulin and glucose levels, minimizes oxidative stress and reduces the severity of brain and pancreas oxidative damage. It could be concluded that GABA could be a useful adjunct to reduce the risk of brain oxidative damage in Diabetes type 1.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Compliance with Ethical Standards
Conflict of interest
The authors report no conflict of interest.
References
- 1.Chintan AP, Nimish LP, Nayana B, Bhavna M, Mahendra G, Hardik T. Cardiovascular complication of diabetes mellitus. J Appl Pharm Sci. 2011;4:1–6. [Google Scholar]
- 2.Haskins K, Bradley B, Powers K. Oxidative stress in type 1 diabetes. Ann N Y Acad Sci. 2003;1005:43–54. doi: 10.1196/annals.1288.006. [DOI] [PubMed] [Google Scholar]
- 3.Piconi L, Quagliaro L, Ceriello A. Oxidative stress in diabetes. Clin Chem Lab Med. 2003;41:1144–1149. doi: 10.1515/CCLM.2003.177. [DOI] [PubMed] [Google Scholar]
- 4.Wang JY, Zhu C, Qian TW, Guo H, Wang DD, Zhang F, et al. Extracts of black bean peel and pomegranate peel ameliorate oxidative stress-induced hyperglycemia in mice. Exp Ther Med. 2015;9(1):43–48. doi: 10.3892/etm.2014.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis GJ, Martinez JA, Liu WQ, Xu K, Ayer A, Fine J, et al. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabeticencephalopathy. Brain. 2008;131(12):3311–3334. doi: 10.1093/brain/awn288. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Luo Y, Dai J. Axonal and dendritic changes are associated with diabetic encephalopathy in rats: an important risk factor for Alzheimer’s disease. J Alzheimers Dis. 2013;34:937–947. doi: 10.3233/JAD-121762. [DOI] [PubMed] [Google Scholar]
- 7.Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur J Pharmacol. 2002;441:1–14. doi: 10.1016/s0014-2999(02)01486-3. [DOI] [PubMed] [Google Scholar]
- 8.Baquer NZ, Taha A, Kumar P, McLean P, Cowsik SM, Kale RK, et al. A metabolic and functional overview of brain aging linked to neurological disorders. Biogerontology. 2009;10(4):377–413. doi: 10.1007/s10522-009-9226-2. [DOI] [PubMed] [Google Scholar]
- 9.Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, et al. The effects of physical activity, education, and body mass index on the aging brain. Hum Brain Mapp. 2011;32(9):1371–1382. doi: 10.1002/hbm.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J, Chen Y, Li M, Ge Z. Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med. 1998;24:586–593. doi: 10.1016/S0891-5849(97)00291-8. [DOI] [PubMed] [Google Scholar]
- 11.Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Migliore L, Coppede F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Mohamadin AM, Sheikh B. Abd El-Aal AA, Elberry AA, Al-Abbasi FA. Protective effects of Nigella sativa oil on propoxur-induced toxicity and oxidative stress in rat brain regions. Pest Biochem Physiol. 2010;98:128–134. doi: 10.1016/j.pestbp.2010.05.011. [DOI] [Google Scholar]
- 14.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress and antioxidants: a review. J Biochem Mol Toxic. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47. doi: 10.1016/S0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- 16.Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Billinton A, Ige AO, Bolam JP, White JH, Marshall FH, Emson PC. Advances in the molecular understanding of GABAB receptors. Trends Neurosci. 2001;24:277–282. doi: 10.1016/S0166-2236(00)01815-4. [DOI] [PubMed] [Google Scholar]
- 18.Chebib M, Johnston GA. The ‘ABC’ of GABA receptors: a brief review. Clin Exp Pharmacol Physiol. 1999;26:937–940. doi: 10.1046/j.1440-1681.1999.03151.x. [DOI] [PubMed] [Google Scholar]
- 19.Krnjevic K. How does a little acronym become a big transmitter? Biochem Pharmacol. 2004;68:1549–1555. doi: 10.1016/j.bcp.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 20.Bowery NG. GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol. 2006;6:37–43. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Soi-ampornkul R, Junnu S, Kanyok S, Liammongkolkul S, Katanyoo W, Umpornsirirat S. Antioxidative and neuroprotective activities of the pre-germinated brown rice extract. Food Nutr Sci. 2012;3(1):135–140. doi: 10.4236/fns.2012.31020. [DOI] [Google Scholar]
- 22.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3(9):715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 23.Minuk GY. Gamma-aminobutyric acid and the liver. Dig Dis. 1993;11(1):45–54. doi: 10.1159/000171400. [DOI] [PubMed] [Google Scholar]
- 24.Erdo SL, Wolf JR. γ-Aminobutyric acid outside the mammalian brain. J Neurochem. 1990;54:363–372. doi: 10.1111/j.1471-4159.1990.tb01882.x. [DOI] [PubMed] [Google Scholar]
- 25.Braun M, Ramracheya R, Bengtsson M, Clark A, Walker JN, Johnson PR, et al. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes. 2010;59:1694–1701. doi: 10.2337/db09-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergersen LH, Storm-Mathisen J, Gundersen V. Immunogold quantification of amino acids and proteins in complex subcellular compartments. Nat Protoc. 2008;3:144–152. doi: 10.1038/nprot.2007.525. [DOI] [PubMed] [Google Scholar]
- 27.Erejuwa OO, Sulaiman SA, Wahab MSA, Sirajudeen KNS, Salleh MSM, Gurtu S. Glibenclamide or metformin combined with honey improves glycemic control in streptozotocin-induced diabetic rats. Int J Biol Sci. 2011;72:244–252. doi: 10.7150/ijbs.7.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi S, Farhangi A, Allah A, et al. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007;22(2):60–64. doi: 10.1007/BF02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa T, Yokozawa T, Kim HJ, Shibahara N. Protective effect of gamma aminobutyric acid in rats with streptozotocin-induced diabetes. J Nutr Sci Vitaminol. 2005;51:278–282. doi: 10.3177/jnsv.51.278. [DOI] [PubMed] [Google Scholar]
- 30.Mikesh LM, Bruns DE. Stabilization of glucose in blood specimens: mechanism of delay in fluoride inhibition of glycolysis. Clin Chem. 2008;54(5):930–932. doi: 10.1373/clinchem.2007.102160. [DOI] [PubMed] [Google Scholar]
- 31.Trinder P. Enzymatic colorimetric determination of glucose. Ann Clin Biochem. 1969;6(2):24–27. doi: 10.1177/000456326900600108. [DOI] [Google Scholar]
- 32.Clark PMS, Hales CN. How to measure plasma insulin. Diabetes Metab Rev. 1994;10:79–90. doi: 10.1002/dmr.5610100203. [DOI] [PubMed] [Google Scholar]
- 33.Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated oxygen toxicity in the blood. Am J Obstet Gynecol. 1979;135:372–376. doi: 10.1016/0002-9378(79)90708-7. [DOI] [PubMed] [Google Scholar]
- 34.Witko-Sarsat V, Friendlander M, Capelliere-Blandin C, Nguyen-KhoaT Zing J, Jungers P, et al. Advanced oxidation protein products as a noval marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 35.Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 36.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 37.Bancroft JD, Gamble M. Theory and practice of histological techniques. In: 5th ed. Edinburgh. New York, London, Philadelphia, Churchill Livingstone Pub; 2002. pp. 172–175 and 593–620.
- 38.Szkudelski T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med. 2012;237:481–490. doi: 10.1258/ebm.2012.011372. [DOI] [PubMed] [Google Scholar]
- 39.Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, et al. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48(4):927–932. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 40.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 41.Young TA, Cunningham CC, Bailey SM. Reactive oxygen species production by the mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria: studies using myxothiazol. Arch Biochem Biophys. 2002;405(1):65–72. doi: 10.1016/S0003-9861(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 42.Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des. 2004;10(14):1611–1626. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- 43.Davi G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005;7:256–268. doi: 10.1089/ars.2005.7.256. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida S, Hashimoto T, Kihara M, Imai N, Yasuzaki H, Nomura K, et al. Urinary oxidative stress markers closely reflect the efficacy of Candesartan treatment for diabetic nephropathy. Nephron Exp Nephrol. 2008;111:20–30. doi: 10.1159/000178764. [DOI] [PubMed] [Google Scholar]
- 45.Sadi G, Yilmaz O, Güray T. Effect of vitamin C and lipoic acid on streptozotocin-induced diabetes gene expression: mRNA and protein expressions of Cu–Zn SOD and catalase. Mol Cell Biochem. 2008;309:109–116. doi: 10.1007/s11010-007-9648-6. [DOI] [PubMed] [Google Scholar]
- 46.Sadi G, Guray T. Gene expressions of Mn-SOD and GPx-1 in streptozotocin-induced diabetes: effect of antioxidants. Mol Cell Biochem. 2009;327:127–134. doi: 10.1007/s11010-009-0050-4. [DOI] [PubMed] [Google Scholar]
- 47.Matsunami T, Sato Y, Sato T, Ariga S, Shimomura T, Yukawa M. Oxidative stress and gene expression of antioxidant enzymes in the streptozotocin-induced diabetic rats under hyperbaric oxygen exposure. Int J Clin Exp Pathol. 2010;3(2):177–188. [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshikuni Y. FDA GRAS Notice for gamma-amino butyric acid (GABA) Kyoto: Pharma Foods International Co. Ltd; 2008. [Google Scholar]
- 49.Palak P, Dipa I. GABA as potential target in the treatment of Type-1 diabetes mellitus. IAJPR. 2013; 3(3):2636–2642. www.scopemed.org/?mno=155108. Accessed 11 July 2016.
- 50.Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci. 2011;108(28):11692–11697. doi: 10.1073/pnas.1102715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Purwana I, Zheng J, Li X, Deurloo M, Son DO, Zhang Z, et al. GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes. 2014;63(12):4197–4205. doi: 10.2337/db14-0153. [DOI] [PubMed] [Google Scholar]
- 52.Bansal P, Wang S, Liu S, Xiang YY, Lu WY, Wang Q. GABA coordinates with insulin in regulating secretory function in pancreatic INS-1 β-cells. PLoS One. 2011;6(10):26225. doi: 10.1371/journal.pone.0026225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58. doi: 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Hou CW. Pu-Erh tea and GABA attenuates oxidative stress in kainic acid-induced status epilepticus. J Biomed Sci. 2011;18:75. doi: 10.1186/1423-0127-18-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng Y, Wang W, Yu P, Xi Z, Xu L, Li X, et al. Comparison of taurine, GABA, Glu, and Asp as scavengers of malondialdehyde in vitro and in vivo. Nanoscale Res Lett. 2013;8:190. doi: 10.1186/1556-276X-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasaki S, Yokozawa T, Cho EJ, Oowada S, Kim M. Protective role of gamma-aminobutyric acid against chronic renal failure in rats. J Pharm Pharmacol. 2006;11:1515–1525. doi: 10.1211/jpp.58.11.0013. [DOI] [PubMed] [Google Scholar]
- 57.Liu B, Barbosa-Sampaio H, Jones PM, Persaud SJ, Muller DS. The CaMK4/CREB/IRS-2 cascade stimulates proliferation and inhibits apoptosis of β-cells. PLoS One. 2012;7(9):e45711. doi: 10.1371/journal.pone.0045711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prud’homme GJ, Glinka Y, Udovyk O, Hasilo C, Paraskevas S, Wang Q. GABA protects pancreatic beta cells against apoptosis by increasing SIRT1 expression and activity. Biochem Biophys Res Commun. 2014;452(3):649–654. doi: 10.1016/j.bbrc.2014.08.135. [DOI] [PubMed] [Google Scholar]
- 59.Tian J, Dang HN, Yong J, Chui WS, Dizon MP, Yaw CK, et al. oral treatment with γ-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS One. 2011;6(9):25338. doi: 10.1371/journal.pone.0025338. [DOI] [PMC free article] [PubMed] [Google Scholar]