Abstract
Objectives
The Northern Plains (NP) and Southwest (SW) American Indian populations differ in their smoking patterns and lung cancer incidence. We aimed to compare CYP2A6 genetic variation and CYP2A6 enzyme activity (representative of the rate of nicotine metabolism) between the two tribal populations, as these have previously been associated with differences in smoking, quitting, and lung cancer risk.
Methods
American Indians (N=636) were recruited from two different tribal populations (NP in South Dakota, SW in Arizona) as part of a study conducted as part of the Collaborative to Improve Native Cancer Outcomes P50 project. A questionnaire assessed smoking-related traits and demographics. Participants were genotyped for CYP2A6 genetic variants *1B, *2, *4, *7, *9, *12, *17, and *35. Plasma and/or saliva samples were used to measure nicotine’s metabolites cotinine and 3′-hydroxycotinine and determine CYP2A6 activity (3′-hydroxcotinine/cotinine, i.e. the nicotine metabolite ratio, NMR).
Results
The overall frequency of genetically reduced nicotine metabolizers, those with CYP2A6 decrease- or loss-of-function alleles, was lower in the NP compared to the SW (P=0.0006). CYP2A6 genotype was associated with NMR in both tribal groups (NP P<0.001, SW P=0.04). Notably, the rate of nicotine metabolism was higher in NP compared to SW smokers (P=0.03), and in comparison to other ethnic groups in the United States. Of the variables studied, CYP2A6 genotype was the only variable to significantly independently influence NMR among smokers in both tribal populations (NP P<0.001, SW P=0.05).
Conclusions
Unique CYP2A6 allelic patterns and rates of nicotine metabolism among these American Indian populations suggest different risks for smoking and tobacco-related disease.
Keywords: CYP2A6, genetic variation, nicotine metabolism, nicotine metabolite ratio (NMR), smoking
INTRODUCTION
Patterns of commercial tobacco use in the United States (U.S.) differ among ethnic groups, with cigarette smoking being more prevalent in American Indian and Alaska Native (AI/AN) populations compared to other U.S. populations [1]. However, between different AI/AN tribal populations, prevalence and smoking patterns are also variable, for example between the Northern Plains (NP) and Southwestern (SW) tribal populations [2, 3]. Despite most AI/ANs wanting to quit smoking, and making quit attempts [4–6], the prevalence of smoking is 50% in the NP tribal population of South Dakota while only being 14% in the SW tribal population of Arizona [2], and cigarette consumption is higher in the NP relative to the SW (13 vs. 7 cigarettes per day, CPD) [3]. Consistent with this, rates of lung cancer incidence and mortality, to which cigarette smoking has been causally linked, are more than 6 times higher in the NP compared to the SW tribal population [7, 8]. The underlying reasons for these large differences in tribal smoking and disease risk, whether biological and/or social, are unknown.
One biological influence on smoking is the activity of the hepatic CYP2A6 (cytochrome P450 2A6) enzyme, which varies substantially between individuals and ethnicities [9]. Nicotine, the primary psychoactive component present in cigarette smoke, is metabolically inactivated by CYP2A6 [10] with the major pathway of inactivation being conversion of nicotine to cotinine (COT) [11]. COT is further metabolized to trans-3′-hydroxycotinine (3HC) exclusively by CYP2A6 [12]. Interindividual variation in CYP2A6 enzymatic activity, and thus the rate of nicotine metabolism and clearance, largely results from genetic variation in CYP2A6, the highly polymorphic gene encoding the CYP2A6 enzyme (variants characterized to date found at http://www.cypalleles.ki.se/cyp2a6.htm). Regular daily smokers alter their levels and intensity of smoking to titrate their nicotine intake such that they maintain desired nicotine levels in the body [13]. Accordingly, CYP2A6 genetic variation and altered rates of nicotine metabolism are associated with differences in smoking behaviors [9]. Smokers with slower rates of nicotine metabolism, those possessing decrease and/or loss-of-function CYP2A6 alleles (referred to as reduced metabolizers), often have lower tobacco consumption, dependence, and difficulty quitting smoking compared to faster metabolizers [14–16]. Among heavy smokers (>10 CPD), genetically reduced metabolizers smoke fewer CPD compared to smokers with wild-type CYP2A6 genotypes [14]. However, in lighter smoking populations (<10 CPD), measurement of CPD is not a sensitive measure of tobacco consumption [17, 18]; while light smokers may consume the same number of CPD, they can differ substantially in their smoking topography (puff volume, duration, velocity), and thus overall tobacco consumption [19]. Measuring urinary total nicotine equivalents (TNE) is a considerably more precise biomarker of nicotine dose compared to CPD and is the current gold standard for assessing intake [20, 21]. TNE was lower among Alaska Native light smokers with reduce-of-function CYP2A6 genotypes, and lower rates of nicotine metabolism, despite no differences in CPD [17].
In addition to CYP2A6 genotype, enzyme activity itself is associated with smoking behaviors [22, 23]. CYP2A6 enzymatic activity can be determined in smokers using a ratio of nicotine’s metabolites 3HC/COT (nicotine metabolite ratio, NMR), which is highly correlated to the rate of nicotine metabolism and clearance [24]. The NMR is a validated biomarker of CYP2A6 activity due to its stability over time and measurement consistency among heavy and light smokers [25, 26]. Previous studies have confirmed the strong concordance between CYP2A6 genotype and NMR in multiple populations and ethnic groups suggesting similar impacts on smoking and lung cancer [18, 27].
Lung cancer risk is also associated with CYP2A6 genetic variation. Smoking fewer CPD, which has been observed among genetically reduced compared to normal nicotine metabolizers [14], is associated with lower lung cancer risk [28], however the relationship between CYP2A6 and lung cancer risk remains even when controlling for cigarette consumption [29]. One reason is that CYP2A6 can also metabolically activate procarcinogenic tobacco-specific nitrosamines; thus being a slower CYP2A6 metabolizer can reduce both tobacco consumption and procarcinogen activation resulting in lower lung cancer risk [30].
Although the impact of individual CYP2A6 alleles/genotypes on the rate of nicotine metabolism has been consistent across ethnicities [18, 27, 31], patterns of CYP2A6 genetic variation and nicotine metabolism vary substantially between different ethnic groups. [32, 33]. [27, 34, 35]. To date, no studies have investigated CYP2A6 genetic variation, and characterized associations between CYP2A6 genotype and the rate of enzymatic activity, among American Indian populations, let alone compared two independent tribal groups with contrasting patterns of smoking. Given the distinct patterns of CYP2A6 genetic variation among different ethnic groups, it is plausible that the NP and SW tribal populations exhibit unique patterns of variation at this locus. Therefore, the aim of the current study was to compare CYP2A6 genetic variation and the rate of nicotine metabolism between the NP and SW tribal populations.
MATERIALS AND METHODS
Study design
Members of American Indian tribal groups were recruited between 2012 and 2014 in South Dakota and Arizona for a study entitled “Topography and Genetics of Smoking and Nicotine Dependence in American Indians”, one of five major research studies conducted by the Collaborative to Improve Native Cancer Outcomes. Recruitment in the NP occurred from a random subset of participants in an earlier study of community health [36]. Recruitment in the SW was conducted using respondent driven sampling among American Indian friends and family members of tribal participants in an earlier randomized clinical trial in the greater Phoenix metropolitan area [37]. Both NP and SW subsamples were stratified by sex and smoking status. The final cohort comprised N=636 American Indians aged 20–88 years, with N=426 in the NP and N=210 in the SW groups. The urban SW and the predominantly rural NP tribal populations are culturally and linguistically distinct, and have considerably different historical experiences [38].
Data were collected by trained research staff who were tribal members. Biological samples (blood or saliva) were collected from all participants to be used for genotyping and phenotyping. Personal interviews were conducted, and questionnaires were administered to all participants. Ethical approval for all study procedures was obtained from the institutional review boards of the Great Plains Area Indian Health Service, the University of Toronto, the University of Washington, and MedStar Health Research Institute, and passed through individual tribal approval processes. All participants provided informed consent.
Measures
Data were obtained on age, gender, body mass index (BMI), smoking status, duration of smoking, cigarettes smoked per day (CPD), nicotine dependence scores (Fagerström Test for Nicotine Dependence, FTND; Hooked On Nicotine Checklist, HONC), and ceremonial traditional tobacco use. Levels of COT and 3HC were measured in blood or saliva samples using liquid chromatography tandem mass spectrometry, as previously described [39]. Our phenotypic measure of CYP2A6 activity, NMR, was defined as the ratio of nicotine’s metabolites, 3HC/COT [24].
Relatedness was determined for all participants based on self-report and genetic analyses. When nuclear families were enrolled, only data on parents (founders) were used to determine allele frequencies; when sibships were enrolled, only one randomly chosen sibling was used. In addition to self-report, relatedness was also assessed by identity-by-descent (IBD) values. These data showed an excellent concordance with the self-reported family data. For individuals who had enrolled as independent, we assessed potential cryptic relatedness, and excluded one member of each pair with IBD values of 0.25 or greater. IBD values were computed using SNP & Variation Suite (SVS; Golden Helix, Inc.). Only unrelated participants were included in the comparison of CYP2A6 gene variant frequencies between the two tribal populations. Only self-reported smokers with COT levels above 10 ng/ml have been included in the NMR analyses to ensure smokers had sufficient COT levels that 3HC was quantifiable and formation dependent [39, 40]. NMR is used as a biomarker of CYP2A6 activity in current regular smokers only as steady state levels of cotinine and 3HC, achieved through regular cigarette smoking, are necessary for accurate measurement of CYP2A6 activity; this is not a feasible measurement of CYP2A6 activity in non-smokers in this study [41].
CYP2A6 Genotyping Assays
DNA was extracted from saliva or blood, and DNA from N=634 participants (NP N=426, SW n=208) was successfully genotyped for the following CYP2A6 alleles: *1B, *2, *4, *7, *9, *12, *17, and *35. Genotyping was performed using a two-step allele-specific polymerase chain reaction approach, or an allele-specific TaqMan single nucleotide polymorphism genotyping assay (Applied Biosystems) and real-time polymerase chain reaction, as described previously [42]. The CYP2A6 alleles *2, *4, *7, *9, *12, *17, and *35 have been associated with a decrease or loss of CYP2A6 enzyme activity and rates of nicotine metabolism [18, 43, 44], whereas the *1B allele associates with faster nicotine metabolism [45]. We have grouped participants as “normal” or “reduced” CYP2A6 metabolizers based on the predicted impact of their CYP2A6 genotype. Normal metabolizers possessed no CYP2A6 decrease- or loss-of-function genetic variants, whereas reduced metabolizers possessed one or more copies of these alleles.
Statistical analyses
NMR was non-normally distributed, thus nonparametric statistical tests were used. Chi-squared tests were used to determine Hardy-Weinberg equilibrium, and to compare the frequencies of CYP2A6 genetic variants, and the overall proportion of subjects who were genetically reduced metabolizers, between the NP and SW tribal populations. We used Kruskal-Wallis and Dunn’s Multiple Comparisons tests to analyze the association of CYP2A6 genotype with NMR. Mann-Whitney tests were conducted to compare NMR between genetically normal versus reduced metabolizers within each tribal population and to compare overall NMR between the NP and SW populations.
Linear regression models were run among smokers with COT>10 to determine the percentage of variation in NMR (the output variable) accounted for. Our first model included the variables CYP2A6 genotype, gender, age, BMI, CPD, ceremonial traditional tobacco use, and tribal population (i.e. NP or SW). We also ran separate regression models for each tribal population, in which model variables included CYP2A6 genotype and BMI. In each model, CYP2A6 genotype was coded as 0 for individuals with no known CYP2A6 reduced activity alleles (i.e. *1A/*1A, *1A/*1B, *1B/*1B genotypes), −1 for those possessing one decrease-of-function allele (i.e. *1/*9 and *1/*12 genotypes), and −2 for those possessing one or more loss-of-function or two decrease-of-function alleles (i.e. *1/*2, *1/*4, and *9/*9 genotypes), as done previously [46]. A linear regression model of NMR was also run in wild-type (CYP2A6*1/*1, i.e. individuals who do not possess any known CYP2A6 reduce activity genetic variants) smokers only to assess the impact of the increase-of-function CYP2A6*1B genotype and tribal population. Additionally, a linear regression model was used to test if CYP2A6*1B genotype interacts with tribal population to influence NMR. The single predictors (CYP2A6*1B genotype and tribal population) were entered into block 1, and the interaction term (CYP2A6*1B genotype x tribal population) was entered into block 2. In both models, CYP2A6 genotype was coded as 0 for individuals with *1A/*1A genotype, 1 for those possessing *1A/*1B genotype, and 2 for those possessing *1B/*1B genotype [45]. Analyses were conducted with GraphPad Prism (v6.0) and SPSS (v22), and statistical tests were considered significant for P<0.05.
RESULTS
Frequencies of CYP2A6 alleles and genetically reduced nicotine metabolizers
CYP2A6 genotype frequencies within each tribal population did not significantly deviate from Hardy-Weinberg equilibrium (P>0.05). The two NP and SW tribal populations exhibited distinct frequencies of CYP2A6 variant alleles from one another and compared to other ethnic groups (Table 1). The frequency of the gain-of-function *1B allele was significantly higher in the NP than the SW (NP 69.7% vs. SW 61.6%, P=0.01), whereas, prevalence of the decrease-of-function *9 allele was significantly lower in the NP compared to the SW (NP 11.9% vs. SW 20.9%, P=0.0002). Several alleles that have been found in other ethnic groups were not present in the NP and SW tribal populations (NP *7, *17, *35; SW *7, *17). Next we categorized individuals into “normal” or “reduced” CYP2A6 activity groups based on genotype, with reduced metabolizers defined as individuals who possess any decrease- or loss-of-function alleles; this included the following genotypes: *1/*2, *1/*4, *1/*9, *9/*9, *1/*12, and *1/*35. A smaller proportion of the NP tribal population were CYP2A6 genotype reduced nicotine metabolizers compared to the SW tribal population (NP 27.5% vs. SW 41.8%, P=0.0006).
Table 1.
Comparison of Northern Plains (n=318a) and Southwestern (n=172a) CYP2A6 allele frequencies, and a summary of frequencies from previously studied populations of different ethnic backgrounds
| CYP2A6 Allele | Activity | Allele Frequency (%)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Northern Plains | Southwest | P value b | Alaska Native | Caucasian | African American | Japanese | Chinese | Korean | ||
| *1B | Increase | 69.7 | 61.6 | 0.01 | 65.3 | 26.7–35.0 | 11.2–18.2 | 25.6–54.6 | 40.6–51.3 | 37.1–57.0 |
| *2 | Inactive | 0.3 | 0.6 | 0.53 | 0.4 | 1.1–5.3 | 0–1.1 | 0 | 0 | 0 |
| *4 | Inactive | 1.6 | 0.3 | 0.07 | 14.5 | 0.13–4.2 | 0.5–2.7 | 17.0–24.2 | 4.9–15.1 | 10.8–11.0 |
| *7 | Inactive | 0 | 0 | – | 0 | 0–0.3 | 0 | 6.3–12.6 | 2.2–9.8 | 3.6–9.8 |
| *9 | Decrease | 11.9 | 20.9 | 0.0002 | 8.9 | 5.2–8.0 | 5.7–9.6 | 19.0–20.7 | 15.6–15.7 | 19.6–22.3 |
| *12 | Decrease | 0.3 | 0.3 | 0.95 | 0.4 | 0–3.0 | 0–0.4 | 0–0.8 | 0 | 0 |
| *17 | Inactive | 0 | 0 | – | 0 | 0 | 7.1–10.5 | 0 | 0 | 0 |
| *35 | Decrease | 0 | 0.3 | 0.17 | 0 | 0 | 2.5–2.9 | 0.8 | 0.5 | – |
Only unrelated tribal participants have been included in this analysis. Relatedness was determined based on self-report and genetic analyses
P values compare NP and SW allele frequencies and are based on Chi-Squared tests
Allele frequency data for Alaska Native, Caucasian, African American, Japanese, Chinese, and Korean populations taken from Tanner et al. 2015 [49]
Genotype frequencies did not deviate significantly from Hardy-Weinberg equilibrium (P>0.05 for each genotypes in each tribe)
Associations of CYP2A6 genotype with CYP2A6 activity (NMR)
We assessed the rate of CYP2A6 activity, determined by the NMR, among smokers to determine if the functional impact of the change in each variant CYP2A6 allele was consistent with the impact observed in other populations, and in in vitro expression systems [47]. The CYP2A6*1B allele had previously been associated with an increased rate of overall nicotine clearance and NMR in vivo [45], and we observed higher NMR among smokers in both the NP (P=0.02) and SW (P=0.002) tribal populations who possessed the *1B allele relative to the wild-type *1A allele (Fig. 1; three-group comparison across *1A/*1A, *1A/*1B, and *1B/*1B genotypes). As many decrease- and loss-of-function alleles exist on a variety of *1A or *1B backbones, we have grouped these as the *1/*1 wild-type group for comparisons to the CYP2A6 genotype reduce activity group (a compilation of all CYP2A6 decrease- and loss-of-function alleles).
Figure 1.
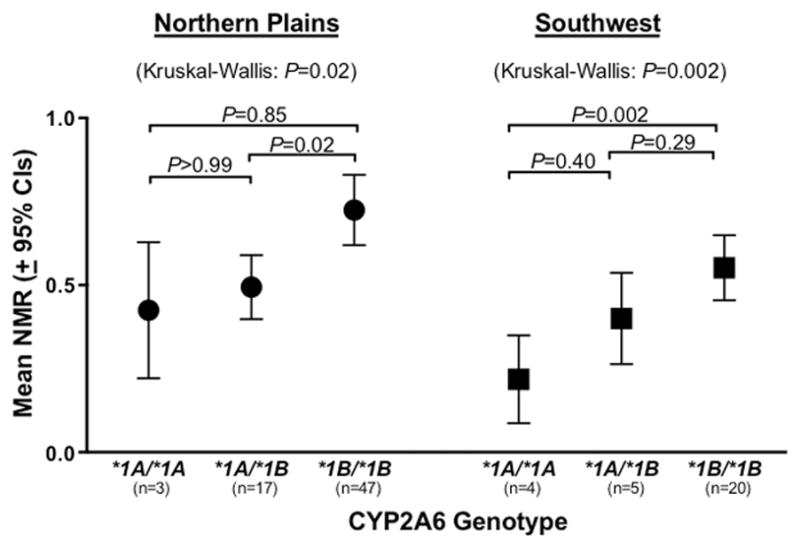
Comparison of CYP2A6 activity (NMR) between the wild-type CYP2A6 genotype groups *1A/*1A, *1A/*1B, and *1B/*1B. Individuals included in this analysis do not possess any other tested variants. P values for multiple group comparisons (across all three genotypes) are based on Kruskal-Wallis tests. P values comparing between groups are based on Dunn’s Multiple Comparisons tests. One outlier, 1.5 standard deviations from the mean, was excluded from the plot for illustration purposes, but was included in the statistical analyses (was one of four members of the Northern Plains *1A*1A group, NMR=1.37).
CYP2A6 genotype was associated with CYP2A6 activity such that smokers possessing one or more decrease- or loss-of-function alleles exhibited lower mean NMR compared to the *1/*1 reference group (NP P<0.0001, SW P=0.04; Fig. 2). We also observed the presence of a gene-dose effect with the prevalent decrease-of-function *9 allele in both the NP and SW tribal populations. In the NP tribal population, relative to the wild-type group (*1/*1 genotype), those with one copy of the *9 allele (*1/*9 genotype) had 63.4% CYP2A6 activity, and those with two copies of *9 (*9/*9 genotype) had 29.6% CYP2A6 activity (Fig. 2). Similarly, in the SW tribal population, smokers with one and two copies of *9 exhibited 84.0% and 52.7% CYP2A6 activity, respectively (Fig. 2).
Figure 2.
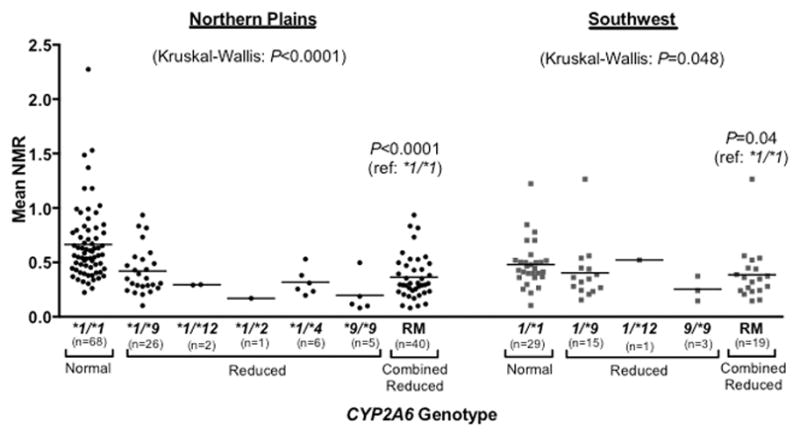
Association between CYP2A6 genotype and CYP2A6 activity (NMR) among smokers. The *1/*1 group represents the normal metabolizers (those who do not possess any known CYP2A6 decrease- or loss-of-function alleles; for simplicity the wild-type *1A and *1B alleles were assessed generically as *1 for these analyses). The reduced metabolizer group (RM) combines all individuals who possess one or more decrease- or loss-of-function alleles (i.e. the following genotypes: *1/*2, *1/*4, *1/*9, *9/*9, and *1/*12). P values for multiple group comparisons (across all genotypes) are based on Kruskal-Wallis tests. P values comparing *1/*1 and RM groups are based on Mann-Whitney tests.
Comparison of the rate of nicotine metabolism across populations
We compared the rate of nicotine metabolism (i.e. CYP2A6 activity), determined by NMR, between NP and SW smokers, and with additional populations of different ethnic backgrounds. Among all smokers (wild-type and CYP2A6 genotype reduced metabolizers combined), NP smokers had a faster mean rate of nicotine metabolism relative to SW smokers (P=0.03; Figs. 3a, Supplementary Figure 1a). NP smokers also had a higher mean rate of nicotine metabolism compared to smokers from other ethnic groups, including Alaska Native, Caucasian, and African American smokers, whose NMR had been previously assessed [18, 27, 48]. When analyses were restricted to wild-type smokers only (those who do not possess any known CYP2A6 decrease- or loss-of-function alleles) to avoid the confound of higher or lower frequencies of CYP2A6 genotype reduced nicotine metabolizers between populations, the rate of nicotine metabolism remained higher among NP smokers compared to SW smokers (P=0.003), and compared to smokers from other ethnic groups (Figs. 3b, Supplementary Figure 1b). In addition, the rate of nicotine metabolism was again higher among NP relative to SW smokers when analyses were restricted to the *1B/*1B group (P=0.02; Fig 1).
Figure 3.
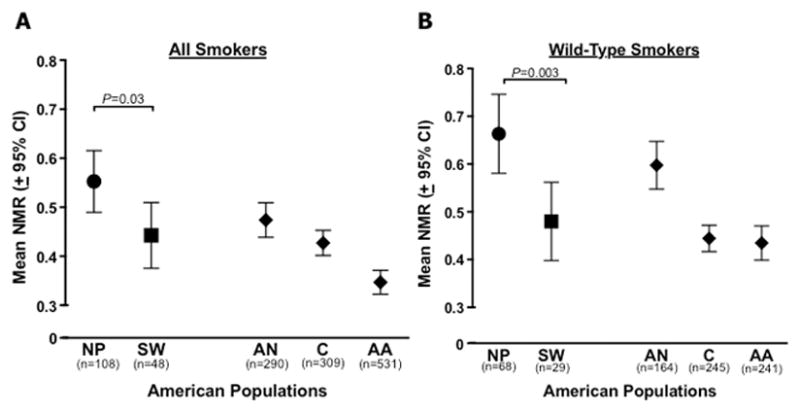
Comparison of CYP2A6 activity (NMR) among the two tribal populations of smokers (NP, Northern Plains; SW, Southwest). (A) All smokers included in analyses, regardless of CYP2A6 genotype. (B) Smokers with wild-type genotypes; *1A/*1A, *1A/*1B, and *1B/*1B genotypes only (CYP2A6 reduced metabolizer (RM) genetic variants were excluded). P values are based on Mann-Whitney tests. Other American smoking populations included for visual comparison (AN, Alaska Native; C, Caucasian; AA, African American). CYP2A6 genotype and NMR data from other American populations taken from the following sources: AN [27]; C [48]; AA [18].
Variables that influence the rate of nicotine metabolism
Using linear regression modeling, we investigated the proportion of variation in the rate of nicotine metabolism (NMR) that was attributable to different predictor variables among smokers in each tribal population, while controlling for the influence of other variables. Both tribal populations were included in a model of NMR in which CYP2A6 genotype, gender, age, ceremonial traditional tobacco use, BMI, CPD, and tribal population (i.e. NP and SW) were assessed as predictor variables of NMR. Only CYP2A6 genotype (P<0.001), BMI (P=0.03), and tribal population (P=0.06) each independently accounted for >1% of the variation in NMR (Table 2a). In a second model we assessed predictors of NMR separately in the NP and SW tribal populations, including only the variables CYP2A6 genotype and BMI (Table 2b and c). These models indicated that, of the variables studied in both tribal populations, CYP2A6 genotype was the only significant independent predictor of NMR among smokers, accounting for 19.71% (P<0.001) and 8.12% (P=0.05) of the variation in NMR in the NP and SW tribal populations, respectively (Table 2).
Table 2.
Linear regression analyses of NMR among Northern Plains and Southwestern smokers
| Variable | B | 95% CI for B | Beta | Variation in NMR accounted for by each variable a | P value |
|---|---|---|---|---|---|
| a. Both Tribal Populations Combined (n=150 included in model) | |||||
| CYP2A6 Genotype b | 0.18 | 0.11 to 0.25 | 0.38 | 14.52% | <0.001 |
| Gender (male=1, female=0) | −0.04 | −0.14 to 0.05 | −0.07 | 0.45% | 0.37 |
| Age (years) | 0.0001 | −0.003 to 0.004 | 0.02 | 0.03% | 0.82 |
| Traditional Tobacco user (yes=1, no=0) | −0.01 | −0.12 to 0.10 | −0.02 | 0.03% | 0.81 |
| Body Mass Index | −0.008 | −0.01 to −0.001 | −0.17 | 1.35% | 0.03 |
| CPD | 0.003 | −0.004 to 0.01 | 0.07 | 0.45% | 0.36 |
| Tribal population (NP=1, SW=2) | −0.10 | −0.20 to 0.002 | −0.15 | 2.05% | 0.06 |
| b. Northern Plains Smokers (n=108 included in model) | |||||
| CYP2A6 Genotype b | 0.21 | 0.13 to 0.30 | 0.45 | 19.71% | <0.001 |
| Body Mass Index | −0.007 | −0.02 to 0.001 | −0.16 | 2.40% | 0.07 |
| c. Southwestern Smokers (n=48 included in model) | |||||
| CYP2A6 Genotype b | 0.11 | 0.0001 to 0.22 | 0.29 | 8.12% | 0.05 |
| Body Mass Index | −0.005 | −0.02 to 0.005 | −0.14 | 1.99% | 0.33 |
Variation in NMR accounted for by each variable is determined by: (Part Correlation)2 x 100
CYP2A6 genotype was coded as 0 for individuals with no known CYP2A6 reduced activity alleles (i.e. *1A/*1A, *1A/*1B, *1B/*1B genotypes), −1 for those possessing one decrease-of-function allele (i.e. *1/*9 and *1/*12 genotypes), and −2 for those possessing one or more loss-of-function or two decrease-of-function alleles (i.e. *1/*2, *1/*4, and *9/*9 genotypes)
Both tribal populations combined: R2=0.222, P<0.001. R2 indicates the proportion of variance in NMR levels (22.2%) that is explained by this model.
NP smokers: R2=0.231, P<0.001. R2 indicates the proportion of variance in NMR levels (23.1%) that is explained by this model.
SW smokers: R2=0.092, P=0.11. R2 indicates the proportion of variance in NMR levels (9.2%) that is explained by this model
As we have observed that NP smokers have a higher overall NMR compared to SW smokers, we aimed to determine if this observation was independent of the effect of the increase-of-function CYP2A6*1B allele, which was expressed at a higher frequency in the NP compared to the SW tribal population. We ran a linear regression model for NMR among wild-type smokers only in which CYP2A6*1B genotype and tribal population were included as predictor variables. Both variables independently accounted for >6% of the variation in NMR (CYP2A6*1B genotype, 7.34%, P=0.0006; tribal population, 6.00%, P=0.01; Table 3a). Additionally, using linear regression analyses, we assessed if there was a significant interaction between CYP2A6*1B genotype and tribal population in influencing NMR. The interaction term (genotype x tribal population) was not significant (P=0.72, model R2 change=0.001; Table 3b), suggesting that the relationship between CYP2A6*1B genotype and NMR is similar in both tribal populations.
Table 3.
Linear regression analyses of NMR among wild-type (*1A/*1A, *1A/*1B, *1B/*1B genotypes) Northern Plains and Southwestern smokers combined into one sample
| Variable | B | 95% CI for B | Beta | Variation in NMR accounted for by each variable a | P value |
|---|---|---|---|---|---|
| a. Original Model (n=97) | |||||
| CYP2A6*1B Genotype b | 0.14 | 0.04 to 0.23 | 0.27 | 7.34% | 0.006 |
| Tribal Population (NP=1, SW=2) | −0.17 | −0.30 to 0.04 | −0.25 | 6.00% | 0.01 |
| b. Original Model Plus Interaction Term “Genotype x Tribe” (n=97) | |||||
| CYP2A6*1B Genotype b | 0.09 | −0.20 to 0.37 | 0.17 | 0.32% | 0.55 |
| Tribal Population (NP=1, SW=2) | −0.23 | −0.56 to 0.11 | −0.33 | 1.64% | 0.19 |
| Genotype x Tribe Interaction c | 0.04 | −0.16 to 0.23 | 0.13 | 0.12% | 0.72 |
Variation in NMR accounted for by each variable is determined by: (Part Correlation)2 x 100
CYP2A6 genotype was coded as 0 for individuals with *1A/*1A genotype, 1 for those possessing *1A/*1B genotype, and 2 for those possessing *1B/*1B genotype.
CYP2A6*1B genotype x tribal population interaction term
Original Model: R2=0.142, P=0.001. R2 indicates the proportion of variance in NMR levels (16.5%) that is explained by this model.
Original Model + Interaction Term: R2 change=0.001, P=0.72. The R2 change tells you the degree of change in your model with the addition of the interaction term (genotype x tribe).
Associations between smoking behaviors and CYP2A6 genotype and activity
Relationships between CYP2A6 genotype and NMR and smoking behaviors were assessed. There was no difference in the proportion of current and former smokers who were CYP2A6 genotype reduced metabolizers in either the NP or SW tribal populations (NP current smokers 31.7%, former smokers 26.7%, P=0.37; SW current smokers 44.9%, former smokers 41.5%, P=0.71). There was no association between the following smoking behaviors and CYP2A6 genotype or NMR among either NP or SW self-reported smokers: mean number of CPD, FTND score, HONC score, and duration of smoking (Supplementary Table 1a). Parallel analyses were run in which smokers were defined by the more stringent cut point of COT>10; these analyses yielded similar results (Supplementary Table 1b).
DISCUSSION
We have demonstrated that the NP and SW tribal populations are genetically distinct at the CYP2A6 locus, with the NP population being comprised of a lower frequency of CYP2A6 genotype reduced metabolizers relative to the SW tribal population, while also exhibiting distinct individual allele frequencies. In both cohorts, CYP2A6 genotype was associated with our phenotypic measure of CYP2A6 activity and rate of nicotine metabolism, NMR. Notably, NP smokers exhibited a faster overall rate of nicotine metabolism compared to smokers in the SW tribal population and other ethnic groups, even among the CYP2A6 wild-type subgroup. CYP2A6 genotype and NMR were not significantly associated with smoking behaviors in either tribal population.
The variety of CYP2A6 alleles that were investigated in this study (*1B, *2, *4, *7, *9, *12, *17, *35) were representative of those with a functional impact and those which were prevalent among different ethnic groups [18, 27, 44]. For example, as illustrated in Table 1, common CYP2A6 alleles (frequency >1%) among different ethnic populations include: Caucasians *1B, *2, *4, *9, *12; African Americans *1B, *2, *4, *9, *17, *35; Asians *1B, *4, *7, *9; Alaska Natives *1B, *4, *9 [49]. Choosing to investigate this variety of alleles allowed us to compare CYP2A6 genetic variation between the two populations, and characterize the tribal populations with respect to other ethnic groups. We had expected that the pattern of CYP2A6 allele frequencies among both the NP and SW tribal populations would most resemble that of Asian populations, as it is postulated that the NP and SW tribal populations have common Asian ancestral origins [50]. However, the CYP2A6*7 allele, which is observed almost exclusively in those of Asian descent, was not present in either tribal population, and both the NP and SW have much lower frequencies of the CYP2A6*4 allele that is common in Asians (see Table 1). Additionally, we observed significantly different CYP2A6 allele frequencies between the NP and SW tribal populations, suggesting that, if these two populations share common ancestry, there have been incidences of genetic divergence between these two populations. There are several potential explanations for their unique genetic patterns. As each tribal population may have been established by a relatively small sample of original ancestors, a founder effect could have contributed to the genetic divergence between tribal populations, as well as from their ancestors [51]. Additionally, because the tribal populations have been geographically separated, forces such as lack of gene flow, random genetic drift, and selective pressures could contribute to development of these unique allele frequency patterns [52, 53].
Not only do the NP and SW tribal populations exhibit different patterns of CYP2A6 allele frequencies, but more specifically, the NP population comprises fewer CYP2A6 genotype reduced nicotine metabolizers. Considering the strong association that we observed between CYP2A6 genotype and activity, this suggests that NP smokers will have a faster rate of nicotine metabolism. As previously stated, faster metabolism has been associated with heavier smoking and higher lung cancer risk [14–16, 29], consistent with characteristics of the NP compared to the SW tribal population [3, 7, 8]. Phenotypic measurements of CYP2A6 activity also confirmed that the NP tribal population has an elevated rate of nicotine metabolism relative to the SW tribal population, and compared to Alaska Native, Caucasian, and African American populations. This observation was present in the overall population of smokers, but also when excluding smokers possessing known CYP2A6 decrease- or loss-of-function alleles. The latter finding suggests that the observed elevated rate of nicotine metabolism among NP smokers was not a result of a lower frequency of reduced metabolizers, but rather other factors are contributing to this, as discussed below. Faster nicotine metabolism may contribute to an elevated risk for smoking and tobacco-related disease in the NP relative to the SW, as well as in comparison to other ethnic populations in the United States.
In order to investigate potential contributors to NMR, we conducted linear regression modeling in the tribal populations. CYP2A6 genotype and BMI were the only significant independent contributors to NMR in the combined population sample. However, in this model, tribal population itself trended toward significance, which suggests that there may be unaccounted for differences between the regional tribal populations that explain the observed higher NMR among the NP compared to the SW tribal population. When we investigated each tribal population separately, we found that CYP2A6 genotype was the only significant independent contributor to NMR in both the NP and SW tribal populations. Therefore, we investigated if a possible explanation for the high NMR in the NP tribal population could be their relatively high frequency of the CYP2A6*1B allele (69.7%) compared to the *1B allele frequency in the SW tribal population (61.6%) and other ethnic groups (Alaska Natives 65%, Caucasians 32%, African Americans 14%, approximately) [27, 54]. CYP2A6*1B was associated with higher NMR in both tribal populations, and in previous studies, with the mechanism believed to be through improving CYP2A6 mRNA stabilization, leading to increased CYP2A6 enzyme expression and activity [45, 55]. However, as NP smokers possessing the *1B/*1B genotype had significantly higher NMR compared to SW smokers with the same genotype, this suggests that the tribal differences in NMR are not resulting from the higher frequency of *1B in the NP compared to the SW tribal population. As further evidence of this, our linear regression model run in wild-type smokers only indicated that CYP2A6*1B genotype and tribal population were significant independent predictors of NMR. This suggests that, independent of CYP2A6*1B genotype, the NP tribal population exhibits higher NMR than the SW tribal population. We also confirmed that there was no interaction between CYP2A6*1B genotype and tribal population in influencing NMR, suggesting that the relationship between CYP2A6*1B genotype and NMR is similar in both tribal populations and therefore other factors, unrelated to CYP2A6*1B, are contributing to tribal differences in NMR.
Considering the highly polymorphic nature of the CYP2A6 gene (http://www.cypalleles.ki.se/cyp2a6.htm), there may be novel CYP2A6 genetic variants present in the NP tribal population that act to increase CYP2A6 gene and protein expression, and/or enzyme activity. For example, in the NP population there may be undetected genetic variation present at the 5′ promoter region of the CYP2A6 gene that interrupts the binding site for an inhibitory transcription factor, such as C/EBPβ, or creates a binding site for an activating transcription factor, such as HNF4α thus decreasing or increasing CYP2A6 levels [56]. This may result in greater CYP2A6 promoter activity and thus gene transcription. Variation in regions 3′ of the CYP2A6 gene, similar to the *1B allele, may contribute to increased mRNA stability and thus increased protein expression [55], contributing to higher NMR among NP relative to SW smokers. The significantly higher frequency of CYP2A6*9 in the SW compared to NP supports the concept of ethnicity, or tribal group, specific genetic variation and variant distribution. Assessment of both common and rare variation at the CYP2A6 locus, and in upstream and downstream sequence, by whole CYP2A6 gene sequencing would improve our understanding of the inter-tribe differences in CYP2A6 and nicotine metabolism. Additionally, environmental factors can also modulate CYP2A6 activity and the rate of nicotine metabolism. For example, CYP2A6 is inducible by dietary and pharmacological agents such as broccoli, rifampin, and phenobarbital [57–59], and therefore potentially by other dietary components of the NP tribal population.
Implications of having a faster rate of nicotine metabolism, as seen in the NP tribal population, include having greater tobacco consumption, nicotine dependence scores, difficulty quitting smoking, and lung cancer risk [14–16, 29]. Greater activation of tobacco-specific procarcinogens, such as nitrosamines, due to faster CYP2A6 activity, may explain the higher lung cancer incidence and mortality rates in the NP compared to the SW [7, 8, 30], however our study did not specifically assess this.
When investigating smoking behaviors, we observed that NP smokers consume more CPD (7 vs. 4) [unpublished], have higher nicotine dependence scores (FTND 2.0 vs. 1.8, HONC 4.5 vs. 3.7) [unpublished], and have longer durations of smoking (23 vs. 20 years) [unpublished] compared to SW smokers. However, we did not observe an association between CYP2A6 genotype or NMR with smoking behaviors in either tribal population. This highlights some limitations of our study. First, this lack of association may have resulted from limited statistical power to detect smoking behavior differences between CYP2A6 genotype or NMR groups due to low sample sizes, particularly of the smokers. The sample size calculations were based on a separate candidate-gene study, conducted as part of the broader “Topography and Genetics of Smoking and Nicotine Dependence in American Indians” study. Additionally, we were limited by available biomarkers and measures of tobacco consumption. The use of a more sensitive biomarker of tobacco consumption, such as TNE, may in the future allow us to detect subtle differences in smoking among these two light smoking populations. Moreover, titration of nicotine intake by CPD according to rate of nicotine metabolism may not be observed in these AI/AN populations, similarly to African American smokers [18, 60], but titration may be detected with TNE as measure of consumption, as seen among Alaska Native smokers [17].
In conclusion, we have identified distinct patterns of CYP2A6 genetic variation and rates of nicotine metabolism between two AI/AN tribal populations with different smoking patterns and risk for lung cancer. Given the association between faster nicotine metabolism and greater smoking and lung cancer risk, these findings suggest that CYP2A6 may contribute to an elevated risk for smoking and tobacco-related disease in the NP compared to the SW, and compared to other ethnic populations in the United States. Future work should focus on determining the cause(s) of the faster nicotine metabolism in the NP and assess any causal relationships between their increased rate of metabolism and lung cancer risk and differences in smoking behaviors including cessation, which may in turn inform strategies to mitigate the elevated risk [16, 22].
Supplementary Material
Acknowledgments
Sources of Funding: We acknowledge the support of the Endowed Chair in Addictions for the Department of Psychiatry (R.F. Tyndale), National Institutes of Health (NIH) Pharmacogenomics Research Network grant DA020830, Canadian Institutes of Health Research grant TMH109787, the Centre for Addiction and Mental Health (CAMH), the Campbell Family Mental Health Research Institute of CAMH, the CAMH Foundation, the Canada Foundation for Innovation (#20289 and #16014), and the Ontario Ministry of Research and Innovation. This research was performed under the auspices of both the Collaborative to Improve Native Cancer Outcomes, a P50 program project sponsored by the National Cancer Institute of the NIH (#P50CA148110; PIs Buchwald and Henderson), and the Native American Research Centers for Health Initiative of the Indian Health Service and NIH (#S06GM092240).
This research was performed under the auspices of the Collaborative to Improve Native Cancer Outcomes (CINCO), a P50 program project sponsored by the National Cancer Institute (#P50CA148110; PIs Buchwald and Henderson). CINCO includes Buchwald D, Flum DR, Garroutte EM, Gonzales AA, Henderson JA, Nez Henderson P, Patrick DL, Tu SP, Winer RL. Additionally, this research was further supported by a grant from the Native American Research Centers for Health initiative of the Indian Health Service and NIH (#S06GM092240; PI Henderson). We acknowledge Maria Novalen and Bin Zhao for performing the analytical chemistry.
Footnotes
Conflicts of Interest: Dr. Tyndale has consulted for Apotex. The remaining authors declare no conflicts of interest.
References
- 1.Centers for Disease C, Prevention. Prevalence of cigarette use among 14 racial/ethnic populations--United States, 1999–2001. MMWR Morbidity and mortality weekly report. 2004;53(3):49–52. [PubMed] [Google Scholar]
- 2.Nez Henderson P, Jacobsen C, Beals J, Team A-S. Correlates of cigarette smoking among selected Southwest and Northern plains tribal groups: the AI-SUPERPFP Study. American journal of public health. 2005;95(5):867–72. doi: 10.2105/AJPH.2004.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eichner JE, Wang W, Zhang Y, Lee ET, Welty TK. Tobacco use and cardiovascular disease among American Indians: the strong heart study. International journal of environmental research and public health. 2010;7(10):3816–30. doi: 10.3390/ijerph7103816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamal A, Agaku IT, O’Connor E, King BA, Kenemer JB, Neff L. Current cigarette smoking among adults--United States, 2005–2013. MMWR Morbidity and mortality weekly report. 2014;63(47):1108–12. [PMC free article] [PubMed] [Google Scholar]
- 5.Gohdes D, Harwell TS, Cummings S, Moore KR, Smilie JG, Helgerson SD. Smoking cessation and prevention: an urgent public health priority for American Indians in the Northern Plains. Public health reports. 2002;117(3):281–90. doi: 10.1016/S0033-3549(04)50162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forster JL, Rhodes KL, Poupart J, Baker LO, Davey C American Indian Community Tobacco Project Steering C. Patterns of tobacco use in a sample of American Indians in Minneapolis-St. Paul. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2007;9(Suppl 1):S29–37. doi: 10.1080/14622200601083434. [DOI] [PubMed] [Google Scholar]
- 7.Bliss A, Cobb N, Solomon T, Cravatt K, Jim MA, Marshall L, et al. Lung cancer incidence among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008;113(5 Suppl):1168–78. doi: 10.1002/cncr.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plescia M, Henley SJ, Pate A, Underwood JM, Rhodes K. Lung cancer deaths among American Indians and Alaska Natives, 1990–2009. American journal of public health. 2014;104(Suppl 3):S388–95. doi: 10.2105/AJPH.2013.301609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malaiyandi V, Sellers EM, Tyndale RF. Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clinical pharmacology and therapeutics. 2005;77(3):145–58. doi: 10.1016/j.clpt.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clinical pharmacology and therapeutics. 1994;56(5):483–93. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 11.Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. The Journal of pharmacology and experimental therapeutics. 1997;282(3):1608–14. [PubMed] [Google Scholar]
- 12.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, et al. Characterization of CYP2A6 involved in 3′-hydroxylation of cotinine in human liver microsomes. The Journal of pharmacology and experimental therapeutics. 1996;277(2):1010–5. [PubMed] [Google Scholar]
- 13.McMorrow MJ, Foxx RM. Nicotine’s role in smoking: an analysis of nicotine regulation. Psychol Bull. 1983;93(2):302–27. [PubMed] [Google Scholar]
- 14.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14(9):615–26. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacology, biochemistry, and behavior. 2009;92(1):6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. Journal of the National Cancer Institute. 2011;103(17):1342–6. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu AZ, Binnington MJ, Renner CC, Lanier AP, Hatsukami DK, Stepanov I, et al. Alaska Native smokers and smokeless tobacco users with slower CYP2A6 activity have lower tobacco consumption, lower tobacco-specific nitrosamine exposure and lower tobacco-specific nitrosamine bioactivation. Carcinogenesis. 2013;34(1):93–101. doi: 10.1093/carcin/bgs306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho MK, Mwenifumbo JC, Al Koudsi N, Okuyemi KS, Ahluwalia JS, Benowitz NL, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clinical pharmacology and therapeutics. 2009;85(6):635–43. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridges RB, Combs JG, Humble JW, Turbek JA, Rehm SR, Haley NJ. Puffing topography as a determinant of smoke exposure. Pharmacology, biochemistry, and behavior. 1990;37(1):29–39. doi: 10.1016/0091-3057(90)90037-i. [DOI] [PubMed] [Google Scholar]
- 20.Benowitz NL, Jacob P, 3rd, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. The Journal of pharmacology and experimental therapeutics. 1994;268(1):296–303. [PubMed] [Google Scholar]
- 21.Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007;47(2):171–83. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Lerman C, Schnoll RA, Hawk LW, Jr, Cinciripini P, George TP, Wileyto EP, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. The Lancet Respiratory medicine. 2015;3(2):131–8. doi: 10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falcone M, Jepson C, Benowitz N, Bergen AW, Pinto A, Wileyto EP, et al. Association of the nicotine metabolite ratio and CHRNA5/CHRNA3 polymorphisms with smoking rate among treatment-seeking smokers. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2011;13(6):498–503. doi: 10.1093/ntr/ntr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dempsey D, Tutka P, Jacob P, 3rd, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clinical pharmacology and therapeutics. 2004;76(1):64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Lea RA, Dickson S, Benowitz NL. Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. Journal of analytical toxicology. 2006;30(6):386–9. doi: 10.1093/jat/30.6.386. [DOI] [PubMed] [Google Scholar]
- 26.Mooney ME, Li ZZ, Murphy SE, Pentel PR, Le C, Hatsukami DK. Stability of the nicotine metabolite ratio in ad libitum and reducing smokers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(6):1396–400. doi: 10.1158/1055-9965.EPI-08-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binnington MJ, Zhu AZ, Renner CC, Lanier AP, Hatsukami DK, Benowitz NL, et al. CYP2A6 and CYP2B6 genetic variation and its association with nicotine metabolism in South Western Alaska Native people. Pharmacogenetics and genomics. 2012;22(6):429–40. doi: 10.1097/FPC.0b013e3283527c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung cancer. 2001;31(2–3):139–48. doi: 10.1016/s0169-5002(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 29.Wassenaar CA, Ye Y, Cai Q, Aldrich MC, Knight J, Spitz MR, et al. CYP2A6 Reduced Activity Gene Variants Confer Reduction in Lung Cancer Risk in African American Smokers - Findings from Two Independent Populations. Carcinogenesis. 2014 doi: 10.1093/carcin/bgu235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong HL, Murphy SE, Hecht SS. Cytochrome P450 2A-catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N′-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem Res Toxicol. 2005;18(1):61–9. doi: 10.1021/tx0497696. [DOI] [PubMed] [Google Scholar]
- 31.Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Molecular psychiatry. 2006;11(4):400–9. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- 32.Mwenifumbo JC, Myers MG, Wall TL, Lin SK, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6*7, CYP2A6*8 and CYP2A6*10 as assessed with a novel haplotyping method. Pharmacogenetics and genomics. 2005;15(3):189–92. doi: 10.1097/01213011-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Nakajima M, Fukami T, Yamanaka H, Higashi E, Sakai H, Yoshida R, et al. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clinical pharmacology and therapeutics. 2006;80(3):282–97. doi: 10.1016/j.clpt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Benowitz NL, Perez-Stable EJ, Herrera B, Jacob P., 3rd Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. Journal of the National Cancer Institute. 2002;94(2):108–15. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Stable EJ, Herrera B, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–6. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 36.Slattery ML, Schumacher MC, Lanier AP, Edwards S, Edwards R, Murtaugh MA, et al. A prospective cohort of American Indian and Alaska Native people: study design, methods, and implementation. American journal of epidemiology. 2007;166(5):606–15. doi: 10.1093/aje/kwm109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howard BV, Roman MJ, Devereux RB, Fleg JL, Galloway JM, Henderson JA, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. Jama. 2008;299(14):1678–89. doi: 10.1001/jama.299.14.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonneborn L. Chronology of American Indian history. New York: Facts On File; 2007. Updated ed. [Google Scholar]
- 39.Tanner JA, Novalen M, Jatlow P, Huestis MA, Murphy SE, Kaprio J, et al. Nicotine Metabolite Ratio (3-hydroxycotinine/cotinine) in Plasma and Urine by Different Analytical Methods and Laboratories: Implications for Clinical Implementation. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015 doi: 10.1158/1055-9965.EPI-14-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St Helen G, Novalen M, Heitjan DF, Dempsey D, Jacob P, 3rd, Aziziyeh A, et al. Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(7):1105–14. doi: 10.1158/1055-9965.EPI-12-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.St Helen G, Jacob P, 3rd, Benowitz NL. Stability of the nicotine metabolite ratio in smokers of progressively reduced nicotine content cigarettes. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2013;15(11):1939–42. doi: 10.1093/ntr/ntt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wassenaar CA, Zhou Q, Tyndale RF. CYP2A6 genotyping methods and strategies using real-time and end point PCR platforms. Pharmacogenomics. 2016;17(2):147–62. doi: 10.2217/pgs.15.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al Koudsi N, Ahluwalia JS, Lin SK, Sellers EM, Tyndale RF. A novel CYP2A6 allele (CYP2A6*35) resulting in an amino-acid substitution (Asn438Tyr) is associated with lower CYP2A6 activity in vivo. Pharmacogenomics J. 2009;9(4):274–82. doi: 10.1038/tpj.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu C, Rao YS, Xu B, Hoffmann E, Jones J, Sellers EM, et al. An in vivo pilot study characterizing the new CYP2A6*7, *8, and *10 alleles. Biochem Biophys Res Commun. 2002;290(1):318–24. doi: 10.1006/bbrc.2001.6209. [DOI] [PubMed] [Google Scholar]
- 45.Mwenifumbo JC, Lessov-Schlaggar CN, Zhou Q, Krasnow RE, Swan GE, Benowitz NL, et al. Identification of novel CYP2A6*1B variants: the CYP2A6*1B allele is associated with faster in vivo nicotine metabolism. Clinical pharmacology and therapeutics. 2008;83(1):115–21. doi: 10.1038/sj.clpt.6100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chenoweth MJ, O’Loughlin J, Sylvestre MP, Tyndale RF. CYP2A6 slow nicotine metabolism is associated with increased quitting by adolescent smokers. Pharmacogenetics and genomics. 2013;23(4):232–5. doi: 10.1097/FPC.0b013e32835f834d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al Koudsi N, Hoffmann EB, Assadzadeh A, Tyndale RF. Hepatic CYP2A6 levels and nicotine metabolism: impact of genetic, physiological, environmental, and epigenetic factors. European journal of clinical pharmacology. 2010;66(3):239–51. doi: 10.1007/s00228-009-0762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clinical pharmacology and therapeutics. 2006;79(6):600–8. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Tanner JA, Chenoweth MJ, Tyndale RF. Pharmacogenetics of nicotine and associated smoking behaviors. Curr Top Behav Neurosci. 2015;23:37–86. doi: 10.1007/978-3-319-13665-3_3. [DOI] [PubMed] [Google Scholar]
- 50.Raghavan M, Steinrucken M, Harris K, Schiffels S, Rasmussen S, DeGiorgio M, et al. POPULATION GENETICS. Genomic evidence for the Pleistocene and recent population history of Native Americans. Science. 2015;349(6250):aab3884. doi: 10.1126/science.aab3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Templeton AR. The reality and importance of founder speciation in evolution. Bioessays. 2008;30(5):470–9. doi: 10.1002/bies.20745. [DOI] [PubMed] [Google Scholar]
- 52.Bamshad M, Wooding SP. Signatures of natural selection in the human genome. Nat Rev Genet. 2003;4(2):99–111. doi: 10.1038/nrg999. [DOI] [PubMed] [Google Scholar]
- 53.Fumagalli M, Sironi M. Human genome variability, natural selection and infectious diseases. Curr Opin Immunol. 2014;30:9–16. doi: 10.1016/j.coi.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Mwenifumbo JC, Tyndale RF. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics. 2007;8(10):1385–402. doi: 10.2217/14622416.8.10.1385. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Pitarque M, Ingelman-Sundberg M. 3′-UTR polymorphism in the human CYP2A6 gene affects mRNA stability and enzyme expression. Biochem Biophys Res Commun. 2006;340(2):491–7. doi: 10.1016/j.bbrc.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 56.Pitarque M, Rodriguez-Antona C, Oscarson M, Ingelman-Sundberg M. Transcriptional regulation of the human CYP2A6 gene. The Journal of pharmacology and experimental therapeutics. 2005;313(2):814–22. doi: 10.1124/jpet.104.081570. [DOI] [PubMed] [Google Scholar]
- 57.Hakooz N, Hamdan I. Effects of dietary broccoli on human in vivo caffeine metabolism: a pilot study on a group of Jordanian volunteers. Curr Drug Metab. 2007;8(1):9–15. doi: 10.2174/138920007779315080. [DOI] [PubMed] [Google Scholar]
- 58.Rae JM, Johnson MD, Lippman ME, Flockhart DA. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. The Journal of pharmacology and experimental therapeutics. 2001;299(3):849–57. [PubMed] [Google Scholar]
- 59.Itoh M, Nakajima M, Higashi E, Yoshida R, Nagata K, Yamazoe Y, et al. Induction of human CYP2A6 is mediated by the pregnane X receptor with peroxisome proliferator-activated receptor-gamma coactivator 1alpha. The Journal of pharmacology and experimental therapeutics. 2006;319(2):693–702. doi: 10.1124/jpet.106.107573. [DOI] [PubMed] [Google Scholar]
- 60.Ross KC, Gubner NR, Tyndale RF, Hawk LW, Jr, Lerman C, George TP, et al. Racial differences in the relationship between rate of nicotine metabolism and nicotine intake from cigarette smoking. Pharmacology, biochemistry, and behavior. 2016;148:1–7. doi: 10.1016/j.pbb.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


