Abstract
Mutations in ECHS1 result in short-chain enoyl-CoA hydratase (SCEH) deficiency which mainly affects the catabolism of various amino acids, particularly valine. We describe a case compound heterozygous for ECHS1 mutations c.836T>C (novel) and c.8C>A identified by whole exome sequencing of proband and parents. SCEH deficiency was confirmed with very low SCEH activity in fibroblasts and nearly absent immunoreactivity of SCEH. The patient had a severe neonatal course with elevated blood and CSF lactate and pyruvate concentrations, high plasma alanine and slightly low plasma cystine. 2-Methyl-2,3-dihydroxybutyric acid was markedly elevated as were metabolites of the three branched-chain ketoacids on urine organic acids analysis. These urine metabolites notably decreased when lactic acidosis decreased in blood. Lymphocyte pyruvate dehydrogenase complex (PDC) activity was deficient, but PDC and α-ketoglutarate dehydrogenase complex activities in cultured fibroblasts were normal. Oxidative phosphorylation analysis on intact digitonin-permeabilized fibroblasts was suggestive of slightly reduced PDC activity relative to control range in mitochondria. We reviewed 16 other cases with mutations in ECHS1 where PDC activity was also assayed in order to determine how common and generalized secondary PDC deficiency is associated with primary SCEH deficiency. For reasons that remain unexplained, we find that about half of cases with primary SCEH deficiency also exhibit secondary PDC deficiency. The patient died on day-of-life 39, prior to establishing his diagnosis, highlighting the importance of early and rapid neonatal diagnosis because of possible adverse effects of certain therapeutic interventions, such as administration of ketogenic diet, in this disorder. There is a need for better understanding of the pathogenic mechanisms and phenotypic variability in this relatively recently discovered disorder.
Keywords: short-chain enoyl-CoA hydratase deficiency, pyruvate dehydrogenase complex deficiency, lactic acidosis, ketogenic diet, ECHS1
1. Introduction
Short-chain enoyl-CoA hydratase (SCEH, EC4.2.1.17; also known as crotonase and encoded by ECHS1 on chromosome 10) is a 290 amino acid protein localized in the mitochondrial matrix as a 160 kDa homohexameric enzyme [1]. SCEH functions to hydrate the double bond between the second and third carbons of enoyl-CoAs in many metabolic pathways, including mitochondrial short- and medium-chain fatty acid β-oxidation and branched-chain amino acid catabolic pathways, as well as in catabolism of ornithine, methionine and threonine [1–7]. Human SCEH has broad substrate specificity for acyl-CoAs, including crotonyl-CoA (from β-oxidation), acryloyl-CoA (from metabolism of various amino acids), 3-methylcrotonyl-CoA (from leucine metabolism), tiglyl-CoA (from isoleucine metabolism), and methacrylyl-CoA (from valine metabolism) [8]. Although SCEH binds tiglyl-CoA, the rate of hydration is relatively low [8]. SCEH deficiency was first identified as a disorder of valine metabolism since the main accumulating metabolites are derived from the valine degradation pathway.
Patients with a defect in SCEH can present with encephalopathy, generalized hypotonia, respiratory insufficiency, sensorineural deafness, epilepsy, optic atrophy, cardiomyopathy, and/or developmental delay. Blood and cerebrospinal fluid (CSF) lactate and pyruvate are usually elevated and brain MRI may show white matter changes or a Leigh syndrome-like pattern affecting brainstem and basal ganglia resembling other inherited disorders of energy metabolism [5, 9–13]. A number of metabolites when present in the urine (and presumably blood) are important diagnostic markers for this disorder, including S-(2-carboxypropyl)-L-cysteine and S-(2-carboxypropyl)cysteamine (which are derived from methacrylyl-CoA), S-(2-carboxyethyl)-L-cysteine and S-(2-carboxyethylcysteamine (which are derived from acryloyl-CoA), and 2-methyl-2,3-dihydroxybutyric acid (MDHB) [5, 8, 14]. Although MDHB is thought to be derived from acryloyl-CoA [5, 14] and/or possibly methacrylyl-CoA (also known as 2-methylprop-2-enoyl-CoA), the exact origin of this metabolite is currently unknown. The inclusion of some of the above biomarkers in newborn screening (NBS) panels for early neonatal diagnosis of SCEH deficiency has been suggested [8, 14]. To date, almost half of cases diagnosed with this autosomal recessive disorder perish within the neonatal or infantile period, but survival into adulthood is reported. To date, at least 20 missense exonic, one nonsense, and a few splice site and frame shift mutations in ECHS1 have been reported associated with this relatively novel disorder [5, 8–13, 15–17].
Because almost all individuals affected with this disorder present with lactic acidosis, initial workup often involves investigation(s) into disorders of pyruvate metabolism or other mitochondrial oxidative enzyme defects. Therefore, secondary functional pyruvate dehydrogenase complex (PDC) deficiency has been reported in several cases with primary SCEH deficiency. In some cases, secondary PDC deficiency appeared to be due to reduction of the E2 protein component of PDC [11]. In this report, we describe a patient with severe neonatal primary SCEH deficiency with two pathogenic ECHS1 mutations (one novel) identified by whole exome sequencing (WES) with subsequent biochemical and functional confirmation of SCEH deficiency and low PDC activity in lymphocytes but not fibroblasts. We review reported cases with mutations in ECHS1 where PDC activity was also assayed to determine how common secondary PDC deficiency is associated with primary SCEH deficiency.
2. Case report
The Caucasian proband was a male born to a 35 year-old G1P0–1 mother by C-section due to fetal distress at 37 weeks gestational age with birth weight, height and head circumference 2550 gm (26–50%ile), 48 cm (51–75%ile) and 30 cm (4–10%ile), respectively. Apgar scores were 2, 5 and 8 at 1, 5 and 10 minutes. At birth, he was noted to have mild jaundice, decreased overall tone and activity, especially truncal tone with significant head lag. Labs at day-of-life (DOL) #1–2 showed metabolic acidosis with a very high anion gap and trace ketones but normal liver function on comprehensive metabolic panel testing, high blood lactate 10.6–12.8 mM (RR 0.5–1.6), high blood pyruvate 0.56 mM (5x upper limit of normal), with lactate to pyruvate ratio ranging 19–23, and normal plasma ammonia for a neonate (55 μM). Plasma amino acids (PAA) showed very high alanine 1015 μM (RR 145–480) with no other abnormalities except for slightly low cystine 12 μM (RR 15–55). Other metabolic findings included intermittent borderline elevation of C5:1 on plasma acylcarnitines (PAC). No elevations of C5-OH or C4-OH were noted on PAC. Urine organic acids (UOA) on DOL #2 showed massive amounts of lactate, pyruvate and ketones. Branched-chain ketoacids (BCKAs) and 3-methylglutaconic acid were also present (see also Results 4.3).
His electroencephalogram repeatedly showed burst suppression but no electrographic seizures. His brain MRI scans demonstrated diffuse cortical thinning and T2 hyperintensity of the white matter with sparing of the brainstem, cerebellum and basal ganglia. Over time, diffusion restriction abnormalities were noted, especially over the occipital regions and along the corpus callosum. These findings did not suggest Leigh syndrome, but increased lactate peaks were consistently noted by MRS in the frontal gray and white matters, as well as elevated CSF lactate (8.3 mM; RR 0.8–2.4) and CSF pyruvate (>34 μM; RR 6–19) on DOL #25. CSF neurotransmitter metabolites (5-hydroxyindolacetic acid, homovanillic acid and 3-O-methyldopa) and 5-methyltetrahydrofolate were within their respective reference ranges. CSF amino acids were significant for high alanine 143.8 μM (RR 24.7–39.0), with slightly high glycine 18.5 μM (RR 5.8–9.5), and high branched-chain amino acids (BCAAs) with valine 87.6 μM (RR 19.3–29.7), isoleucine 45.1 μM (RR 4.7–11.7) and leucine 85.1 μM (RR 8.4–20.9). There was no evidence of cardiac dysfunction although serum creatine phosphokinase (CPK) was slightly high at 868 U/L (RR 55–400) on DOL #4.On DOL #25, an ophthalmologic evaluation was normal. On DOL #35, bilateral sensorineural deafness was noted by both otoacoustic emissions and auditory brainstem response. Mitochondrial DNA sequencing (Transgenomic Laboratory, New Haven, CT) identified a homoplasmic m.15434C>T (MT-CYB; L230F), representing a rare but a benign polymorphism.
Family history was non-contributory. Maternal breast milk feeding via NG tube was started on DOL #11, then switched to a ketogenic diet (Nutricia KetoCal 3:1 with PDM/MBM-22 kcal/oz) on DOL #29. Thiamine was started at 100 mg daily on DOL #26, the dosage doubled on DOL #29 and then maintained throughout the remaining hospital course. He intermittently received carnitine (50 mg/kg/day) and was administered folinic acid (1 mg/kg/day) for a brief period. Coenzyme Q10 supplementation was considered but not done after checking the blood CoQ10 level which came back normal.
Although the patient was noted to be hypotonic from birth, he was responsive and moved all extremities spontaneously throughout the majority of his hospital course. Around DOL #35, the neurological status started to rapidly deteriorate becoming less responsive and losing the gag reflex. He developed recurrent apneas at which time comfort measures only were instituted and he eventually succumbed to his disorder on DOL #39.
3. Materials and methods
Informed consent was obtained from the parents/guardians for investigative studies by inclusion in the University Hospitals Cleveland Medical Center IRB-approved Disorders of Pyruvate Metabolism study, for additional functional and/or molecular analyses including trio whole exome sequencing (WES) of proband and parents.
3.1 Whole Exome Sequencing (WES) Analysis
Next-generation sequencing (NGS) of 23 genes associated with pyruvate metabolism was negative for the following genes: BOLA3, DLAT, DLD, LIAS, LIPT1, LIPT2, NFU1, PDHA1, PDHB, PDHX, PDK1, PDK2, PDK3, PDK4, PDP1 (PPM2C), PDP2 (PPM2C2), PC, PCK1, PCK2, SLC19A2, SLC19A3, SLC25A19, and TPK1. This led to performance of trio WES analysis using DNA from parents and proband. The WES pipeline used was described before [18], except for using updated Omicia Opal version 4.23.2 and Omicia VAAST Trio Report algorithm in this case.
3.2 Functional and biochemical assays
Assay of PDC, both activated-dephosphorylated and inactivated phosphorylated, α-ketoglutarate dehydrogenase complex (KDC) and dihydrolipoamide dehydrogenase (E3) activities in disrupted blood lymphocytes and cultured skin fibroblasts were as previously described [19, 20]. Assay of SCEH activity and analysis of SCEH protein content by immunoblotting were as previously described [5, 11]. Quantitative oxidative phosphorylation in harvested cultured skin fibroblasts permeabilized with digitonin was measured as described previously [21]. UOA and PAC analyses were performed by standard clinical gas chromatography-mass spectrometry and tandem mass spectrometry methods.
Results
4.1 Identification of two ECHS1 variants by WES
The depth of coverage for the trio WES analysis identifying the ECHS1 gene variants were as follows: 96.2% average target bases covered at ≥10x (range was 96.0–96.2% for the trio samples) and 144 average depth of coverage (range was 138–152 for the trio samples). Omicia Opal identified 14,349 variants, VAAST Trio Report ranked 268 variants as recessive and 8 as X-linked (with ECHS1 VAAST ranked #3 out of 137), and Phevor ranked 137 genes with ECHS1 as #2, after the input of the following phenotype characteristics: lactic acidosis and decreased activity of PDC. Phevor and phenotype/gene association scores were 3.37 and 0.482, respectively.
The candidate ECHS1 variants identified by VAAST were c.836T>C (p.F279S) and c.8C>A (p.A3D) with Omicia scores of 0.93 and 0.42, respectively. The proband was compound heterozygous for these mutations while his mother and father were carriers of the p.F279S and p.A3D substitutions, respectively. The read depth for the c.836T>C and c.8C>A variants were 156 and 30, respectively, distributed essentially equally (78:78 and 14:16, respectively) between both DNA strands.
The Omicia score represents a composite score using MutationTaster, Polyphen-2, SIFT, and PhyloP-vertebrate in silico prediction algorithms [22]. A score of ≥0.85 ≅ 1% false-positive prediction rate [22]. Furthermore, a variant with an Omicia score of >0.85 is considered likely pathogenic, while one with a score between 0.5 and 0.85 is considered potentially pathogenic [22]. The novel p.F279S substitution was predicted to be pathogenic by all 4 in silico algorithms, with prediction/score value as follows: mutation taster, D; Polyphen-2, 1; SIFT, 0; and PhyloP, 4.19. In contrast, only SIFT predicted p.A3D substitution to be pathogenic while PolyPhen and PhyloP scored 0.622 (score range 0 to 1) and 2.87 (score range −11.764 to +6.424), respectively, and MutationTaster predicted this variant to be a polymorphism. Both variants were not found in the 1000 Genomes, Exome Variant Server (EVS), Exome Aggregation Consortium (ExAC) and other common allele frequency databases implying that both alleles are quite rare.
4.2 Enzyme assays and functional confirmation of SCEH deficiency
4.2.1 Assay of PDC and other mitochondrial oxidative enzymes
Prior to identification of the two ECHS1 mutations by WES and because of lactic acidosis and elevated plasma alanine, workup for defects in pyruvate metabolism and other mitochondrial oxidative enzymes were pursued. Assay of PDC in blood lymphocytes showed low activity with low PDC/E3 ratio consistent with PDC deficiency (Table 1). Follow-up assays of PDC and KDC in cultured skin fibroblasts from the patient showed normal activities (Table 1). These results prompted molecular testing of 23 genes related to pyruvate metabolism by NGS which were all normal and eventual enrollment of the patient in a study protocol for research WES. The identification of the two candidate ECHS1 variants by WES prompted reexamination of urine organic acids (UOA) for pathognomonic metabolites (Results 4.3 below) and testing of SCEH activity.
Table 1.
Summary of functional assays
| Enzyme/Complex/Function | Cell | Activity*
|
||
|---|---|---|---|---|
| Case (%mean) | Control
|
|||
| Mean ± SD, n value | Ref. range | |||
| PDC-activated | Lymph | 0.27 (17%)** | 1.6 ± 0.5, n = 596 | 1.0–2.7 |
| FB | 2.2 (90%) | 2.4 ± 0.9, n= 329 | 1.3–4.4 | |
| KDC | FB | 2.1 (100%) | 2.1 ± 1.0, n = 42 | 0.7–4.6 |
| SCEH | FB | <31 (BLQ) | 379 ± 145 | 179–616 |
| OxPhos (pyruvate, malate and ADP) | FB | 22 (56%) | 39 ± 6, n = 57 | 30–53 |
| OxPhos (palmitoylcarnitine, malate and ADP) | FB | 30 (103%) | 29 ± 4, n = 49 | 22–39 |
PDC, KDC and SCEH activities were in nmol/min/mg protein, and OxPhox activities were in pmol/sec/million cells.
PDC/E3 = 0.4 (control mean ± SD: 2.3 ± 0.6, RR 1.4–3.6, n = 596).
Lymph, blood lymphocytes; FB, cultured fibroblasts; PDC, pyruvate dehydrogenase complex; KDC, alpha-ketoglutarate dehydrogenase complex; SCEH, short-chain enoyl-CoA hydratase; OxPhos, oxidative phosphorylation – O2 consumption assayed in digitonin-permeabilized fibroblasts (i.e., intact cellular mitochondria); and BLQ, below limit of quantitation.
Analysis of integrated oxidative phosphorylation (OxPhos) in digitonin-permeabilized patient fibroblasts (i.e., with intact cellular mitochondria) showed a slight reduction in oxygen consumption in the presence of pyruvate, malate and ADP (Table 1). Changes in oxygen consumption in the presence of pyruvate, malate and ADP reflect composite activity of the mitochondrial pyruvate transporter, production of acetyl-CoA by PDC, coupled production of NADH, and oxidation of the NADH by Complex I. Slight reduction of oxygen consumption was also noted when glutamate was added to the solution (i.e., to the pyruvate, malate and ADP mix with permeabilized fibroblasts) (23 pmol/sec/million cells; control mean ± SD: 40 ± 6, RR 30–56, n = 57). Addition of glutamate in the presence of malate stimulates malate dehydrogenase activity, and coupled production of NADH, aspartate, and α-ketoglutarate. As an alternate source of intra-mitochondrial acetyl-CoA, we also examined oxygen consumption in the presence of palmitoylcarnitine, malate and ADP in the digitonin-permeabilized patient fibroblasts and this was normal (Table 1). Uncoupled Complex I respiration was normal (37 pmol/sec/million cells; control mean ± SD: 55 ± 13, RR 30–83, n = 57). There were no functional abnormalities of the other three (II, III or IV) enzyme complexes of mitochondrial oxidative phosphorylation (data not shown). The carnitine transport system, including carnitine acylcarnitine translocase and carnitine palmitoyltransferase II, and the enzymes for long-chain fatty acid β-oxidation were also normal (data not shown).
4.2.2 SCEH assay and protein expression
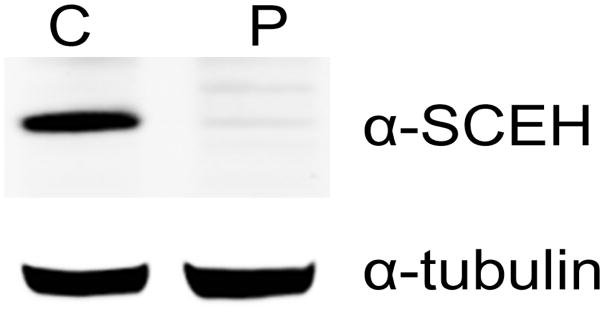
SCEH activity in cultured fibroblasts of our patient was below the limit of quantitation of the enzyme assay (<31 nmol/min/mg protein, RR 179–616), confirming that this patient has primary deficiency of SCEH (Table 1). Furthermore, follow-up protein expression analysis in patient fibroblasts by immunoblotting using antibodies against SCEH revealed a markedly reduced SCEH protein level also supporting the functional SCEH defect (Fig. 1).
Fig. 1.
Protein expression in patient fibroblasts. Immunoblot analysis using antibodies against SCEH and alpha tubulin (loading control). Patient fibroblast (P) shows significantly reduced SCEH protein expression vs a control sample (C).
4.3 Biochemical support for primary SCEH deficiency
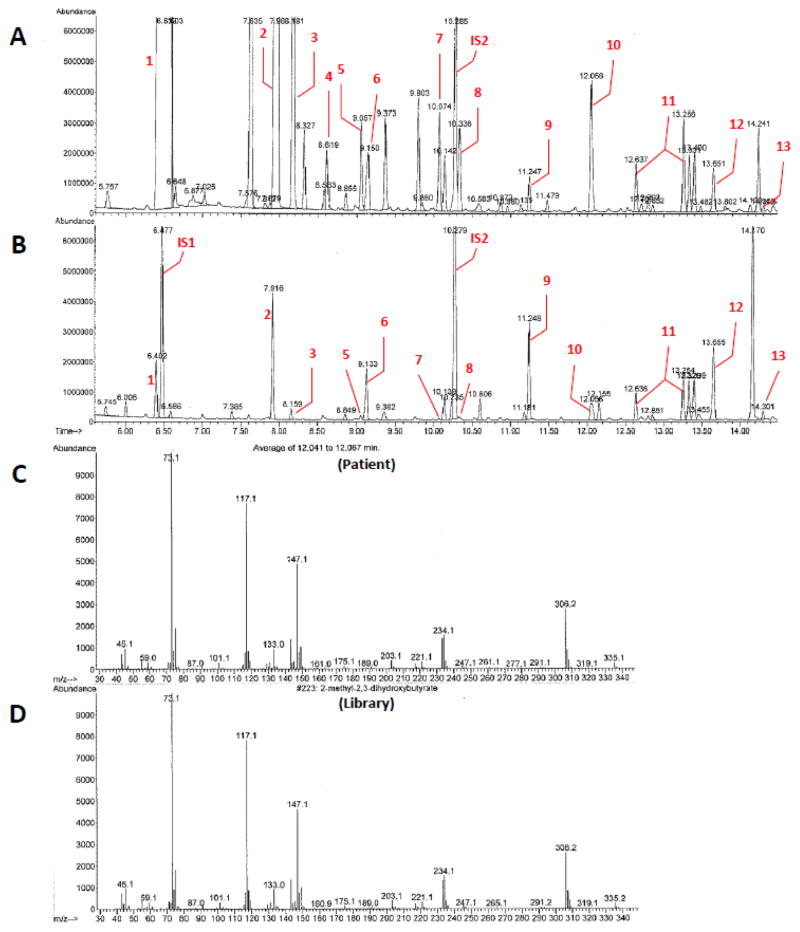
Because many patients with SCEH deficiency show significantly large amounts of 2-methyl-2,3-dihydroxybutyric acid in urine, we re-examined our patient’s UOA for the presence of 2-methyl-2,3-dihydroxybutyric acid. A urine sample collected on DOL #3 when blood lactate concentrations ranged between 10.6–12.8 mM (UOA approximate lactate 15,803 mg/g creatinine; RR <125) showed a large peak of 2-methyl-2,3-dihydroxybutyrate at 12.1 minutes (Fig. 2A). Branched-chain ketoacids (2-ketoisovaleric, 2-ketomethylvaleric and 2-ketoisocaproic acids) were also detected in significant amounts (Fig. 2A). On DOL #19, when lactic acidosis was significantly reduced (UOA lactate 137 mg/g creatinine; RR <125), the 2-methyl-2,3-dihydroxybutyrate peak was noted to be much smaller as were the various branched-chain ketoacid peaks (Fig. 2B). Of note, the tiglylglycine (derived from tiglyl-CoA) peak remained essentially unchanged with different blood lactate levels (Figs. 2A and 2B). In urine of other patients with lactic acidosis but without SCEH deficiency and following ion extraction for masses 306.2 and 234.1, we find only trace amounts of 2-methyl-2,3-dihydroxybutyric acid (data not shown). This supports that identification of a large peak of 2-methyl-2,3-dihydroxybutyric acid is specific for primary deficiency of SCEH [5, 10, 11, 14].
Fig. 2.
Urine organic acid profiles of patient. Total ion chromatograms (A and B from DOL #3 and #19, respectively) and mass spectra (C and D). A, blood lactate 10.6–12.8 mM and UOA lactate 15800 mg/g creatinine (RR <125); B, UOA lactate 137 mg/g creatinine; C, Average of 12.1 minutes, identifying the peak as 2-methyl-2,3-dihydroxybutyric acid; and D, m/z spectrum of 2-methyl-2,3-dihydroxybutyric acid. Noted in red: 1, lactic acid; 2, pyruvic acid; 3, 3-hydroxybutyric acid; 4, acetoacetic acid; 5, 2-ketoisovaleric acid; 6, urea; 7, 2-ketomethylvaleric acid; 8, 2-ketoisocaproic acid; 9, fumaric acid; 10, 2-methyl-2,3-dihydroxybutyric acid; 11, 3-methylglutaconic acid (peaks 1 and 2); 12, adipic acid; and 13, tiglylglycine. Internal standards 1 and 2 (IS1 and IS2) are caproic acid, and cyclohexylacetic acid, respectively.
5. Discussion
5.1 Primary SCEH deficiency
We report a patient compound heterozygous for ECHS1 c.836T>C and c.8C>A mutations with functional deficiency of SCEH whose clinical and biochemical presentation is consistent with other reported severe cases of SCEH deficiency. The pathogenic c.836T>C mutation is novel, but the c.8C>A variant (in a compound heterozygous state with ECHS1 c.389T>A) was described in two siblings with severe lactic acidosis and congenital dilated cardiomyopathy with neonatal demise at ≤2 days of life, without functional studies of SCEH [13]. Although the Omicia score for the ECHS1 c.8C>A mutation was just below the range for a “potentially pathogenic” designation and could have been easily dismissed as not significant in our analysis, the markedly reduced SCEH activity and immunoreactivity supports that the c.8C>A mutation is indeed pathogenic.
Our patient exhibited elevation in all three BCKA metabolites in urine when tested on DOL #2 although plasma BCAAs (leucine, isoleucine and valine) were within their respective reference ranges. CSF BCAAs were elevated on DOL #25. Others have reported elevation of plasma BCAAs in patients with SCEH deficiency [15, 16]. The small amount of tiglylglycine (from isoleucine catabolism) in urine remained essentially unchanged with the degree of lactic acidosis (compare Figs 2A and 2B). This is consistent with the observation that only a small amount of tiglyl-CoA is hydrated by SCEH [8] and the suggestion by others that there must be another hydratase that is more active towards tiglyl-CoA (and crotonyl-CoA) which helps maintain flux through the isoleucine and leucine catabolic pathways [11] in contrast to the valine catabolic pathway.
The slightly low cystine noted on DOL #2 PAA could reflect depletion of cysteine by conjugation with methacrylyl-CoA and acryloyl-CoA. We did not measure S-(2-carboxypropyl)-L-cysteine, S-(2-carboxypropyl)cysteamine, S-(2-carboxyethyl)-L-cysteine, or S-(2-carboxyethyl)cysteamine in this patient but these derivatives have been noted to be elevated in other cases of SCEH deficiency. Although CPK was slightly high in our patient, CPK may be normal or moderately elevated in patients with SCEH deficiency [10, 11] and the reason for this biochemical variability is unclear.
Our patient died from his disorder within 10 days from starting a ketogenic diet when his primary diagnosis was not yet established. Others have also reported lack of success with use of ketogenic diet for this disorder [11]. Use of ketogenic diets is currently the main therapeutic intervention in PDC deficiency, and has been reported to be beneficial: 1) in boys with primary mutations of PDHA1, 2) by parental report in a clinical survey, 3) in other sporadic cases, and 4) in a zebra fish model [23–26]. Blood beta-hydroxybutyrate was infrequently monitored to establish whether “ketosis” was actually established in this case. After 4 days on the ketogenic diet, blood beta-hydroxybutyrate increased from 0.27 to 2.28 mM (RR 0.01–0.29) while blood lactate decreased from 3.6 to 1.4 mM. Administration of a ketogenic diet may not be completely effective to control lactic acidosis and/or may be harmful in cases where PDC deficiency is secondary to impairment of formation of acetyl-CoA in defects of fatty acid β-oxidation (e.g., SCEH deficiency), or decreased oxidation of acetyl-CoA due to primary oxidation defects distal to PDC (e.g., the tricarboxylic acid (TCA) cycle including succinyl-CoA synthetase deficiency [18]), or combined defects of PDC and KDC (e.g., E3, thiamine pyrophosphate, or lipoate deficiencies).
5.2 Secondary PDC deficiencies associated with SCEH deficiency
Decreased PDC activity in cases with ECHS1 mutations (with a few also confirmed to be functionally SCEH deficient) has been reported in fibroblasts as well as in liver and skeletal muscle (SM) tissues (Table 2). Here we report for the first time decreased PDC activity in lymphocytes from a patient with confirmed biochemical, functional and molecular diagnosis of SCEH deficiency. In all, 42% (5/12) of cases with ECSH1 mutations had low PDC activity in fibroblasts. Functional PDC deficiency can be generalized to all tissues tested or appear isolated to one (or a few) specific tissue/cell type(s) tested, with variances observed even between siblings (Table 2). For example, tissue/cell type variability of PDC deficiency is observed in sibs homozygous for ECHS1 c.817A>G and c.88+5G>A (Table 2). In cases with mutations in ECHS1 and with either lactate and/or MDHB elevation, 50% (4/8) of cases showed low PDC activity in fibroblasts, while low PDC activity in skeletal muscle is noted in 33% (2/6) muscle is of cases to date. In those cases where functional SCEH deficiency was noted in fibroblasts, 60% (3/5) also exhibited concurrent functional PDC deficiency in fibroblasts. Therefore, it appears that about half of cases with primary SCEH deficiency also exhibit secondary PDC deficiency.
Table 2.
Reports of patients with ECHS1 mutations where PDC activity in various tissues was evaluated.
| Patient genotype | Elevated lactate | Elevated alanine | Urine MDHB | SCEH | PDC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Tissue | Activity* | Mean ± SD | RR | Tissue | Activity* (%mean) | Mean ± SD | RR | Reference | ||||
| c.817A>G/c.817A>G1 | Yes | Yes | Large | FB | <9 (BLD) | 379 ± 145 | 179–616 | FB | 0.83 (50%)** | 1.66 ± 067 | 0.87–3.03 | 11 |
| FB | 1.11 (46%)** | 2.42 ± 0.88 | 1.26–4.42 | |||||||||
| Liver | 0.33 (15%)** | 2.17 ± 0.77 | 1.23–3.89 | |||||||||
| SM | 0.10 (3%)** | 3.17 ± 1.49 | 1.20–6.52 | |||||||||
|
|
||||||||||||
| c.817A>G/c.817A>G1 | Yes | Yes | Large | FB | <9 (BLD) | 379 ± 145 | 179–616 | FB | 0.88 (53%) | 1.66 ± 067 | 0.87–3.03 | |
| Liver | 0.88 (41)** | 2.17 ± 0.77 | 1.23–3.89 | |||||||||
| SM | 0.29 (9%)** | 3.17 ± 1.49 | 1.20–6.52 | |||||||||
|
|
||||||||||||
| c.433C>T/c.476A>G | Yes | Yes | Large | ND | SM | 5.8 | 5.6–9.4 | |||||
| c.673T>C/c.674G>C | Yes | NR | Large | ND | SM | 129 | 110–130 | |||||
|
| ||||||||||||
| c.197T>C/c.449A>G | Yes | NR | 229 fold | ND | FB | Normal | 10 | |||||
| c.673T>C/ c.673T>C | Yes | Yes | 39 fold | ND | SM | Normal | ||||||
| c.268G>A/c.583G>A | ND | No | 6 fold | ND | FB | Normal | ||||||
| c.161G>A/c.431dup | Yes | No | ND | ND | SM | Normal | ||||||
|
| ||||||||||||
| c.538A>G/c.583G>A | No | Yes | ND | ND | FB | Mildly reduced** | 12 | |||||
| c.538A>G/c.713C>T2 | Mildly increased | No | NR | ND | FB | Normal | ||||||
| c.538A>G/c.713C>T2 | Mildly increased | ND | NR | ND | ND | |||||||
| c.538A>G/c.476A>G | No | No | NR | ND | FB | Normal | ||||||
|
| ||||||||||||
| c.473C>T/c.414+3G>C3 | Yes | NR | 62–100 fold | FB | <9 (BLD) | 379 ± 145 | 179–616 | FB | 0.15** | 0.23–0.53 | 5 | |
| c.473C>T/c.414+3G>C3 | NR | NR | 31–39 fold | FB | <9 (BLD) | 379 ± 145 | 179–616 | FB | 0.04** | 0.23–0.53 | ||
|
| ||||||||||||
| c.88+5G>A/ c.88+5G>A4 | Yes | Yes | NR | ND | FB | 7.6 mU/CS** | 9.7–36 | 16 | ||||
| c.88+5G>A/ c.88+5G>A4 | Yes | Yes | NR | ND | FB | Normal | ||||||
|
| ||||||||||||
| c.8C>A/c.836T>C | Yes | Yes | Large | FB | <31 (BLQ) | 379 ± 145 | 179–616 | Lymph | 0.27 (17%)** | 1.63 ± 0.53 | 0.98–2.72 | This report |
| FB | 2.17 (90%) | 2.42 ± 0.88 | 1.26–4.42 | |||||||||
Corresponding numbered superscripts indicate siblings; * indicates activity reported as nmol/min/mg protein; low/reduced PDC activity are noted in bold and **.
BLD, below limit of detection; BLQ, below limit of quantitation; CS, citrate synthase; FB, fibroblasts; Lymph, lymphocytes; MDHB, 2-methyl-2,3-dihydroxybutyric acid; ND, not determined; NR, not reported; PDC, pyruvate dehydrogenase complex; RR, reference range; SCEH, short-chain enoyl-CoA hydratase; SD, standard deviation; and SM, skeletal muscle.
The mechanism for decreased PDC activity and the reason for this variability are unclear. It is possible that secondary PDC deficiency is only present in severely affected patients irrespective of the degree of functional SCEH deficiency because of additional genetic modifiers of PDC function. Comparative analysis of WES or whole genome sequencing data of patients who underwent both SCEH and PDC functional testing may be informative in identifying potential genetic modifiers that could explain the functional PDC heterogeneity.
The slight reduction in oxygen consumption observed in permeabilized patient fibroblasts in the presence of pyruvate, malate and ADP would be consistent with slight impairment of pyruvate oxidation in intact mitochondria, although assay of PDC activity in disrupted cultured fibroblasts was normal. Because the respiratory chain in intact mitochondria was normal (Results 4.2.1), there is uncertainty about the cause of the observed slight reduction in oxygen consumption from malate oxidation (i.e., decreased NADH production by malate dehydrogenase, MDH) in the presence of glutamate.
The pathogenic mechanism for secondary functional PDC deficiency with primary SCEH deficiency could also be due to inhibitory metabolites. It is hypothesized that toxic enoyl-CoAs such as methacrylyl-CoA and acryloyl-CoA from valine catabolism, at high concentrations could react with lipoyl domain(s) of the E2 subunit or other specific amino acids of PDC, thereby reducing its activity [11, 27]. However, a concurrent reduction of activity of: 1) other dehydrogenase complexes with lipoyl moieties such as KDC, branched-chain ketoacid dehydrogenase complex (BCKDC), and α-ketoadipate dehydrogenase complex (KADC); or 2) the H-protein of the glycine cleavage enzyme (GCE), have either not been observed (in case of KDC and GCE; as in this report for KDC and others for KDC and GCE, [11]) or have not been investigated to our knowledge (in case of BCKDC and KADC).
Others have also found mild to moderate elevations of glycine in patients with SCEH deficiency [16], implying possible intermittent reduction of GCE activity, but this remains to be determined. Reduced immunoreactive E2 in liver and muscle tissues and less so in cultured fibroblasts has been noted as cause of functional PDC deficiency in some cases [11]. 3-Hydroxyisobutyryl-CoA hydrolase (HIBCH), the next distal enzyme to SCEH in the valine catabolic pathway, with secondary functional PDC deficiency has also been reported with primary HIBCH deficiency [27, 28]. Secondary functional PDC deficiency is also reported with primary succinyl-CoA synthetase deficiency and the reason for this also remains unexplained [18]. Therefore, secondary functional PDC deficiency is observed in certain primary defects of valine catabolism, fatty acid β-oxidation and TCA pathway, and the pathophysiologic reason(s) remain to be determined. In all these disorders, there is accumulation of acyl-CoA esters in the mitochondrial matrix where some acyl-CoA esters are known to readily react with certain amino acid groups (e.g., lysine) of some of these complexes, which may affect activity. Table 3 summarizes the currently known genetic etiologies for impaired pyruvate oxidation.
Table 3.
Currently known and potential etiologies of impaired pyruvate oxidation
| Enzyme/complex/function/pathway | Gene
|
|
|---|---|---|
| Known | Potential | |
|
|
|
|
| Pyruvate dehydrogenase complex: | PDHA1, PDHB, DLAT, DLD, PDHX | |
| Pyruvate dehydrogenase phosphatase: | PDP1, PDP2, PDP3 (PDPR)a | |
| Pyruvate carrier (mitochondrial): | MPC1 | |
| Thiamine pyrophosphokinase: | TPK1 | |
| Thiamine/thiamine pyrophosphate transporters: | SLC25A19 | SLC19A2, SLC19A3 |
| Lipoamide synthesis/transfer/degradation: | LIAS, LIPT2, LIPT1, SIRT4 | |
| Fe-S Cluster proteins: | BOLA3, NFU1, GLRX5, IBA57 | ISCA2, ISCU |
| Fatty acid β-oxidation: | ECHS1 | |
| Branched-chain amino acid (valine) metabolism: | ECHS1, HIBCH | |
| Tricarboxylic acid (TCA) cycle: | SUCLA2 | SUCLA1b, SUCLG2b |
| Phosphoenolpyruvate carboxykinase: | PCK2c | |
Christodoulou et al. PgmNr 376 abstract presented at the 2015 ASHG meeting, Baltimore, MD
Presumed, based on PDC deficiency secondary to primary succinyl-CoA synthetase (SUCLA2) deficiency [18].
Bedoyan et al., unpublished data
6. Summary and conclusions
The ECHS1 c.836T>C and c.8C>A mutations in a compound heterozygous state are pathogenic, leading to very low SCEH activity and immunoreactivity of SECH as well as a lethal neonatal phenotype. Lymphocyte PDC activity was low in this patient but PDC and KDC activities in cultured fibroblasts were normal. Oxidative phosphorylation analysis on intact digitonin-permeabilized fibroblasts showed moderate impairment that could be due to reduced pyruvate oxidation in intact mitochondria. Urine 2-methyl-2,3-dihydroxybutyric acid was markedly elevated but markedly decreased when lactic acidosis diminished. Soon after initiation of ketogenic diet the patient’s clinical course deteriorated and he died, prior to his diagnosis with SCEH deficiency. Early and rapid neonatal diagnosis of this disorder through inclusion in NBS panels or by targeted gene-panel or WES testing of ill neonates with lactic acidosis is crucial because of the possible adverse impact of certain therapeutic interventions in outcome. For mechanisms that remain unexplained, about half of previously reported cases with primary SCEH deficiency also exhibit secondary PDC deficiency.
Highlights.
We describe a case compound heterozygous for ECHS1 mutations c.836T>C (novel) and c.8C>A identified by whole exome sequencing. SCEH deficiency was confirmed with very low SCEH activity in fibroblasts and nearly absent immunoreactivity of SCEH.
Lymphocyte pyruvate dehydrogenase complex (PDC) activity was deficient, but PDC and α-ketoglutarate dehydrogenase complex activities in cultured fibroblasts were normal. Oxidative phosphorylation analysis on intact digitonin-permeabilized fibroblasts was suggestive of slightly reduced PDC activity relative to control range in mitochondria.
We reviewed 16 other cases with mutations in ECHS1 where PDC activity was also assayed and we find that about half of cases with primary SCEH deficiency also exhibit secondary PDC deficiency.
This case highlights the importance of early and rapid neonatal diagnosis because of possible adverse effects of certain therapeutic interventions, such as administration of ketogenic diet, in this disorder.
Acknowledgments
This research was supported in part by the Genomics Core Facility of the Case Western Reserve University (CWRU) School of Medicine's Genetics and Genome Sciences Department, and by funds from the Clinical and Translational Science Collaborative (CTSC) CWRU Core Utilization Pilot Grant 2014 (05496) (to JKB) and NIH RDCRN 5U54NS078059-05 project NAMDC 7413 grant (to JKB and SDD). We thank Ms. Sarah Hrabik for her assistance with the informed consent process for this study.
Abbreviations
- SCEH
short-chain enoyl-CoA hydratase
- PDC
pyruvate dehydrogenase complex
- KDC
α-ketoglutarate dehydrogenase complex
- MDHB
2-methyl-2,3-dihydroxybutyric acid
- WES
whole exome sequencing
- RR
reference range
- DOL
day-of-life
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kanazawa M, Ohtake A, Abe H, Yamamoto S, Satoh Y, Takayanagi M, Niimi H, Mori M, Hashimoto T. Molecular cloning and sequence analysis of the cDNA for human mitochondrial short-chain enoyl-CoA hydratase. Enzyme Protein. 1993;47:9–13. doi: 10.1159/000468650. [DOI] [PubMed] [Google Scholar]
- 2.Fong JC, Schulz H. Purification and properties of pig heart crotonase and the presence of short chain and long chain enoyl coenzyme A hydratases in pig and guinea pig tissues. J Biol Chem. 1977;252:542–547. [PubMed] [Google Scholar]
- 3.Fong JC, Schulz H. Short-chain and long-chain enoyl-CoA hydratases from pig heart muscle. Methods Enzymol. 1981;71(Pt C):390–398. doi: 10.1016/0076-6879(81)71049-8. [DOI] [PubMed] [Google Scholar]
- 4.Wanders RJ, Duran M, Loupatty FJ. Enzymology of the branched-chain amino acid oxidation disorders: the valine pathway. J Inherit Metab Dis. 2012;35:5–12. doi: 10.1007/s10545-010-9236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters H, Buck N, Wanders R, Ruiter J, Waterham H, Koster J, Yaplito-Lee J, Ferdinandusse S, Pitt J. ECHS1 mutations in Leigh disease: a new inborn error of metabolism affecting valine metabolism. Brain. 2014;137:2903–2908. doi: 10.1093/brain/awu216. [DOI] [PubMed] [Google Scholar]
- 6.Wanders RJ, Ruiter JP, IJL, Waterham HR, Houten SM. The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J Inherit Metab Dis. 2010;33:479–494. doi: 10.1007/s10545-010-9104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houten SM, Wanders RJ. A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J Inherit Metab Dis. 2010;33:469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada K, Aiba K, Kitaura Y, Kondo Y, Nomura N, Nakamura Y, Fukushi D, Murayama K, Shimomura Y, Pitt J, Yamaguchi S, Yokochi K, Wakamatsu N. Clinical, biochemical and metabolic characterisation of a mild form of human short-chain enoyl-CoA hydratase deficiency: significance of increased N-acetyl-S-(2-carboxypropyl)cysteine excretion. J Med Genet. 2015;52:691–698. doi: 10.1136/jmedgenet-2015-103231. [DOI] [PubMed] [Google Scholar]
- 9.Sakai C, Yamaguchi S, Sasaki M, Miyamoto Y, Matsushima Y, Goto Y. ECHS1 mutations cause combined respiratory chain deficiency resulting in Leigh syndrome. Hum Mutat. 2015;36:232–239. doi: 10.1002/humu.22730. [DOI] [PubMed] [Google Scholar]
- 10.Haack TB, Jackson CB, Murayama K, Kremer LS, Schaller A, Kotzaeridou U, de Vries MC, Schottmann G, Santra S, Buchner B, Wieland T, Graf E, Freisinger P, Eggimann S, Ohtake A, Okazaki Y, Kohda M, Kishita Y, Tokuzawa Y, Sauer S, Memari Y, Kolb-Kokocinski A, Durbin R, Hasselmann O, Cremer K, Albrecht B, Wieczorek D, Engels H, Hahn D, Zink AM, Alston CL, Taylor RW, Rodenburg RJ, Trollmann R, Sperl W, Strom TM, Hoffmann GF, Mayr JA, Meitinger T, Bolognini R, Schuelke M, Nuoffer JM, Kolker S, Prokisch H, Klopstock T. Deficiency of ECHS1 causes mitochondrial encephalopathy with cardiac involvement. Ann Clin Transl Neurol. 2015;2:492–509. doi: 10.1002/acn3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferdinandusse S, Friederich MW, Burlina A, Ruiter JP, Coughlin CR, 2nd, Dishop MK, Gallagher RC, Bedoyan JK, Vaz FM, Waterham HR, Gowan K, Chatfield K, Bloom K, Bennett MJ, Elpeleg O, Van Hove JL, Wanders RJ. Clinical and biochemical characterization of four patients with mutations in ECHS1. Orphanet J Rare Dis. 2015;10:79. doi: 10.1186/s13023-015-0290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tetreault M, Fahiminiya S, Antonicka H, Mitchell GA, Geraghty MT, Lines M, Boycott KM, Shoubridge EA, Mitchell JJ, Michaud JL, Majewski J C Care4Rare Canada. Whole-exome sequencing identifies novel ECHS1 mutations in Leigh syndrome. Hum Genet. 2015;134:981–991. doi: 10.1007/s00439-015-1577-y. [DOI] [PubMed] [Google Scholar]
- 13.Ganetzky RD, Bloom K, Ahrens-Nicklas R, Edmondson A, Deardorff MA, Bennett MJ, Ficicioglu C. ECHS1 Deficiency as a Cause of Severe Neonatal Lactic Acidosis. JIMD Rep. 2016;30:33–37. doi: 10.1007/8904_2016_538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters H, Ferdinandusse S, Ruiter JP, Wanders RJ, Boneh A, Pitt J. Metabolite studies in HIBCH and ECHS1 defects: Implications for screening. Mol Genet Metab. 2015;115:168–173. doi: 10.1016/j.ymgme.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Nair P, Hamzeh AR, Mohamed M, Malik EM, Al-Ali MT, Bastaki F. Novel ECHS1 mutation in an Emirati neonate with severe metabolic acidosis. Metab Brain Dis. 2016;31:1189–1192. doi: 10.1007/s11011-016-9842-x. [DOI] [PubMed] [Google Scholar]
- 16.Al Mutairi F, Shamseldin HE, Alfadhel M, Rodenburg RJ, Alkuraya FS. A lethal neonatal phenotype of mitochondrial short-chain enoyl-CoA hydratase-1 deficiency. Clin Genet. 2016 doi: 10.1111/cge.12891. [DOI] [PubMed] [Google Scholar]
- 17.Olgiati S, Skorvanek M, Quadri M, Minneboo M, Graafland J, Breedveld GJ, Bonte R, Ozgur Z, van den Hout MC, Schoonderwoerd K, Verheijen FW, van IWF, Chien HF, Barbosa ER, Chang HC, Lai SC, Yeh TH, Lu CS, Wu-Chou YH, Kievit AJ, Han V, Gdovinova Z, Jech R, Hofstra RM, Ruijter GJ, Mandemakers W, Bonifati V. Paroxysmal exercise-induced dystonia within the phenotypic spectrum of ECHS1 deficiency. Mov Disord. 2016;31:1041–1048. doi: 10.1002/mds.26610. [DOI] [PubMed] [Google Scholar]
- 18.Huang X, Bedoyan JK, Demirbas D, Harris DJ, Miron A, Edelheit S, Grahame G, DeBrosse SD, Wong LJ, Hoppel CL, Kerr DS, Anselm I, Berry GT. Succinyl-CoA synthetase (SUCLA2) deficiency in two siblings with impaired activity of other mitochondrial oxidative enzymes in skeletal muscle without mitochondrial DNA depletion. Mol Genet Metab. 2016 Nov 12; doi: 10.1016/j.ymgme.2016.11.005. pii: S1096–7192(16)30314–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang DT, Hu CW, Patel MS. Induction of the branched-chain 2-oxo acid dehydrogenase complex in 3T3-L1 adipocytes during differentiation. Biochem J. 1983;214:177–181. doi: 10.1042/bj2140177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerr D, Grahame G, Nakouzi G. Assays of pyruvate dehydrogenase complex and pyruvate carboxylase activity. Methods Mol Biol. 2012;837:93–119. doi: 10.1007/978-1-61779-504-6_7. [DOI] [PubMed] [Google Scholar]
- 21.Ye F, Hoppel CL. Measuring oxidative phosphorylation in human skin fibroblasts. Anal Biochem. 2013;437:52–58. doi: 10.1016/j.ab.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Coonrod EM, Margraf RL, Russell A, Voelkerding KV, Reese MG. Clinical analysis of genome next-generation sequencing data using the Omicia platform. Expert Rev Mol Diagn. 2013;13:529–540. doi: 10.1586/14737159.2013.811907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wexler ID, Hemalatha SG, McConnell J, Buist NR, Dahl HH, Berry SA, Cederbaum SD, Patel MS, Kerr DS. Outcome of pyruvate dehydrogenase deficiency treated with ketogenic diets. Studies in patients with identical mutations. Neurology. 1997;49:1655–1661. doi: 10.1212/wnl.49.6.1655. [DOI] [PubMed] [Google Scholar]
- 24.Taylor MR, Hurley JB, Van Epps HA, Brockerhoff SE. A zebrafish model for pyruvate dehydrogenase deficiency: rescue of neurological dysfunction and embryonic lethality using a ketogenic diet. Proc Natl Acad Sci U S A. 2004;101:4584–4589. doi: 10.1073/pnas.0307074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeBrosse SD, Okajima K, Zhang S, Nakouzi G, Schmotzer CL, Lusk-Kopp M, Frohnapfel MB, Grahame G, Kerr DS. Spectrum of neurological and survival outcomes in pyruvate dehydrogenase complex (PDC) deficiency: lack of correlation with genotype. Mol Genet Metab. 2012;107:394–402. doi: 10.1016/j.ymgme.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Scholl-Burgi S, Holler A, Pichler K, Michel M, Haberlandt E, Karall D. Ketogenic diets in patients with inherited metabolic disorders. J Inherit Metab Dis. 2015;38:765–773. doi: 10.1007/s10545-015-9872-2. [DOI] [PubMed] [Google Scholar]
- 27.Ferdinandusse S, Waterham HR, Heales SJ, Brown GK, Hargreaves IP, Taanman JW, Gunny R, Abulhoul L, Wanders RJ, Clayton PT, Leonard JV, Rahman S. HIBCH mutations can cause Leigh-like disease with combined deficiency of multiple mitochondrial respiratory chain enzymes and pyruvate dehydrogenase. Orphanet J Rare Dis. 2013;8:188. doi: 10.1186/1750-1172-8-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loupatty FJ, Clayton PT, Ruiter JP, Ofman R, Ijlst L, Brown GK, Thorburn DR, Harris RA, Duran M, Desousa C, Krywawych S, Heales SJ, Wanders RJ. Mutations in the gene encoding 3-hydroxyisobutyryl-CoA hydrolase results in progressive infantile neurodegeneration. Am J Hum Genet. 2007;80:195–199. doi: 10.1086/510725. [DOI] [PMC free article] [PubMed] [Google Scholar]