Abstract
Acute lung injury (ALI) is characterized by rapid alveolar injury, vascular leakage, lung inflammation, neutrophil accumulation, and induced cytokines production leading to lung edema. The mortality rate of patients suffering from ALI remains high. Epoxyeicosatrienoic acids (EETs) are cytochrome P450-dependent derivatives of polyunsaturated fatty acid (PUFA) with anti-hypertensive, profibrinolytic and anti-inflammatory functions. EETs are rapidly hydrated by soluble epoxide hydrolase (sEH) to their less potent diols. The aim of this study was to investigate the role of sEH inhibitor TPPU and EETs in lipopolysaccharide (LPS)-induced ALI of mice. Our studies revealed that inhibition of sEH with TPPU attenuated the morphological changes in mice, decreased the neutrophil infiltration to the lung, pro-inflammatory cytokine levels (IL-1β and TNF-α) in serum and bronchoalveolar lavage fluid (BALF), and alveolar capillary leakage (lung wet/dry ratio and total protein concentration in BALF). TPPU improved the survival rate of LPS-induced ALI. In addition, in vitro experiments revealed that both TPPU and EETs (11,12-EET and 14,15-EET) suppressed the expression of IL-1β and TNF-α, and LDH release in RAW264.7 cells. These results indicate that EETs play a role in dampening LPS-induced acute lung inflammation, and suggest that sEH could be a valuable candidate for treatment of ALI.
Keywords: acute lung injury, soluble epoxide hydrolase inhibitor, epoxyeicosatrienoic acids, lipopolysaccharide, inflammation
Introduction
Acute lung injury (ALI) and its severe form acute respiratory distress syndrome (ARDS) continue to be major causes of mortality in the intensive care units (ICU). ALI is characterized by pulmonary edema, inflammation, intrapulmonary hemorrhage, and severely impaired gas exchange (1). Many conditions, such as sepsis, pancreatitis, multiple trauma, pneumonia, lung transplantation, or inhalation of injurious gases can lead to ALI (2). Although ventilation with low tidal volume, early neuromuscular blockade and prone positioning have been shown to reduce mortality of ARDS, the mortality of overall ARDS in ICU and hospital remain 42.7% and 47.8%, respectively (3). To date, no pharmacotherapy has proven effective in decreasing mortality in adult patients with ARDS.
The pathophysiology of ALI/ARDS involves inflammation with diffuse cellular damage, increased capillary permeability, interstitial edema, and influx of circulating inflammatory cells (4). Pro-inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α, are elevated in ALI/ARDS patients. These cytokines activate neutrophils, which release oxidants, proteases, leukotrienes and platelet activating factors, resulting in the development of ALI/ARDS (5).
Eicosanoids play vital roles in physiology including vasodilatory, anti-inflammatory and anti-apoptotic function. Cytochrome P450 oxidases metabolize arachidonic acid, one of the polyunsaturated fatty acids (PUFAs), into several products, including epoxyeicosatrienoic acids (EETs). There are four regioisomeric EETs: 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET. EETs exert anti-inflammatory, anti-fibrotic, anti-hypertensive effect, acting in both autocrine and paracrine manners (6). However, once formed, EETs are rapidly hydrolyzed in vivo by epoxide hydrolases, primarily soluble epoxide hydrolase (sEH) in the cytosol, to their corresponding less potent 1,2-diols, termed dihydroxyeicosatrienoic acids (DHETs). Pharmacological inhibition of sEH, such as 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU) (7) and trans-4-{4-[3-(4-trifluoromethoxy-phenyl)-ureido]-cyclohexyloxy}-benzoic acid (tTUCB) (8), increases EETs levels in tissues and plasma, potentiates the effects of EETs, and thus elicits anti-hypertensive and anti-inflammatory effect. Recently, studies reported inhibition of sEH enzyme has beneficial effects on cardiovascular disorders, including ischemia-reperfusion, heart failure, and atherosclerosis (9).
A line of studies has demonstrated that EETs play an important role in pulmonary physiology and pathology. Our previous study found that TPPU attenuates bleomycin-induced inflammation and collagen deposition, and therefore prevents bleomycin-induced pulmonary fibrosis in a murine model (10). The administration of tTUCB attenuates allergic airway inflammation and airway responsiveness in a murine model (11). In addition, inhibition of sEH attenuates inflammation associated with acute exposure to tobacco smoke (12). So inhibition of sEH may be a therapeutic target for pulmonary diseases. But, the effect of sEH inhibitor or EETs on murine ALI remains unknown.
Intratracheal instillation of lipopolysaccharide (LPS), a bacterial cell wall component, to rodents is a well-accepted and common experimental model for ALI (13). Therefore, we hypothesize that inhibitor of sEH attenuates LPS-induced ALI in mice via increasing the levels of EETs. In this study, we used this model of ALI to investigate the effect of sEH inhibitor on ALI in mice. In addition, we tested TPPU and EETs on LPS-challenged mouse macrophage cell in order to study the mechanism how TPPU and EETs attenuate LPS induced lung injury.
Materials and methods
Animal
Adult male C57BL/6 mice were provided by the laboratory animal unit of Central South University. Mice were maintained in a temperature (22–24 °C), humidity (55% ± 5%) and 12 h dark/light cycle condition, and were free access to water and food. Experimental use of mice in the present study was performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the animal protocol was approved by the institutional animal care committee of Xiangya Medical College.
Lipid mediator analysis in blood
The profiles of lipid mediators were measured using the LC/MS/MS method as previously published (14). Aliquots of plasma (250 µL) were used for the measurements after solid phase extraction protocol.
In vivo treatments
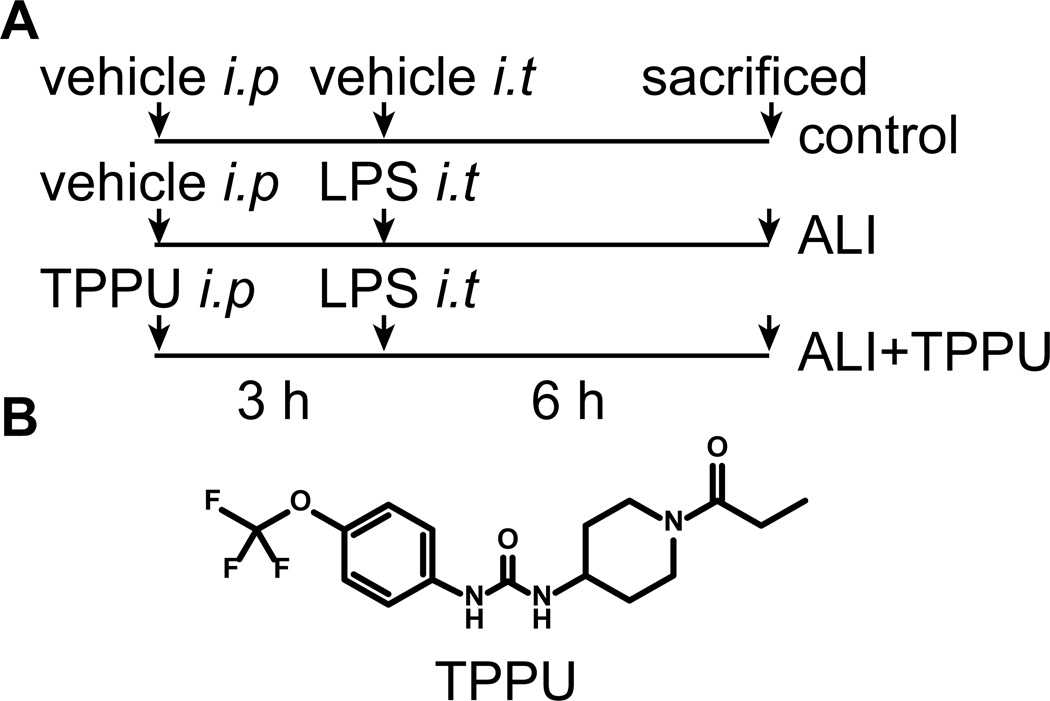
Mice were randomly divided into three groups: control group, ALI + vehicle group, and ALI + TPPU group. The mouse model of ALI was established by an intratracheal injection of O111:B4 LPS from Escherichia coli (5 mg/kg) in 50 µL sterile saline according to a previous published protocol (15). All surgeries were performed under anesthesia with pentobarbital sodium (50 mg/kg). TPPU (1 mg/kg, in 0.5% EtOH) was administered by intraperitoneal injection 3 h before the LPS injection. The mice were sacrificed 6 h after the LPS injection (Figure 1).
Fig. 1.
The experimental design for animal model.
Lung histology and injury score analysis
Right lung lobes were used for histological evaluation. Lungs were fixed in 10% formalin for 48 h, embedded in paraffin, cut into 5 µm section, and then stained with hematoxylin-eosin (HE). HE staining was done by deparafinizing and hydrating the slides to water. Slides were stained with Harris hematoxylin for 15 min and eosin for 30 s. Slides were dehydrated, cleared, and mounted with Cytoseal. A designated scoring system to quantify the extent of histological lung injury in animals was used to assess lung injury (16). Lung injury score was measured by two blinded pathologists. Five independent variables were evaluated with a 0 to 4 point scale. The five variables were neutrophils in the alveolar space, hemorrhage, hyaline membranes, pertinacious debris filling the airspaces, and septal thickening. A score of 0 represented no damage; l represented mild damage; 2 represented moderate damage; 3 represented severe damage and 4 represented very severe histological changes.
Collection of BALF and cell counting
Mice were anesthetized with pentobarbital sodium before sacrifice. The trachea was cannulated, and the lungs were thrice lavaged with 0.8 mL cooled sterile saline to obtain bronchoalvelar lavage fluid (BALF). The recovery of the total lavage exceeded 95%. The samples collected were centrifuged (2000 rpm for 10 min) and cell pellets were resuspended in 0.5 mL PBS for cell counts. Total cells and differential leukocyte counts were counted double-blindly with hemocytometer and Wright-Giemsa staining of cytospin preparations, respectively. The cell-free supernatant was aliquoted and stored at −80 °C for cytokine detection.
Myeloperoxidase (MPO) activity assay
After sacrificed of the mice, the left upper lobe was removed, washed and kept in −80 °C. Then, after weighing, the lungs were homogenized, centrifuged and re-suspended in ice-cold phosphate buffer (50 mM, pH 6.0) containing 0.5% of hexadecyltrimethylammonium bromide. The suspension was freeze-thawed, sonicated and centrifuged. 10 µL of the supernatant was added to 290 µL H2O2/o-dianisidine buffer. The kinetics of absorbance change was measured at 460 nm with a spectrophotometer (Thermo Fisher Scientific, USA). Results are expressed as units of MPO activity per gram of lung tissue.
Lactate dehydrogenase (LDH) activity assay
LDH is a cytoplasmic enzyme, which is released when plasmatic membrane damage occurs. We determined the LDH activity in BALF and serum of mice, or the supernatant of the cell culture. LDH activity was determined using a Sigma Tox-7 toxicology kit and reported as the amount of LDH activity in the medium.
Lung wet/dry weight ratio and protein in BALF
The severity of pulmonary edema was assessed by the wet/dry ratio (W/D ratio). The whole lung was weighed immediately after removal (wet weight). Then, the lung was dehydrated at 80 °C for 48 h in an oven and re-weighed (dry weight). The wet/dry ratio was calculated as the ratio of the wet weight to the dry weight. BALF protein concentrations were determined by BCA kit (Sigma-Aldrich, St. Louis, MO, USA) using BSA as standard.
Western blotting
Lung tissues were homogenized and boiled for 10 min at 100 °C. 50 µg of total protein for each sample was loaded and separated on 12% SDS-PAGE gel. They were then transferred to a PVDF membrane at 280 mA for 2 h. The membrane was blocked in 5% fat-free milk at room temperature and immunoblotted overnight at 4 °C with primary antibody against I-κB (1:1000, Beyotime Biotechnology, China) and β-actin (1:1000, CST, USA) as the internal standard. After washed with TBST, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 2 h. After washed with TBST, the immune complexes were visualized by enhanced chemiluminescent detection (Millipore, USA) using ChemiDoc XRC (Bio-Rad, USA).
Measurement of survival rate in mice
Survival rate is a key indication of therapeutic benefit of TPPU. C57BL/6 mice were used to evaluate survival rate. Our pilot studies demonstrated that LPS at 5 mg/kg (it) caused low death of the mice. According to the report (17), mice were given a lethal dose of O111:B4 LPS from Escherichia coli (40 mg/kg, intraperitoneal, Sigma-Aldrich, St. Louis, MO, USA) to induce endotoxemia. Sixty mice were divided randomly into 3 groups (each group: n=20): control group, ALI + vehicle group, and ALI + TPPU group. TPPU was administered at a dose of 1 mg/kg 3 h prior to the LPS injection. Control group mice were given saline injection. All mice were observed every 4 h for 72 h.
Cell culture and treatment
RAW264.7 is a murine macrophage cell line. RAW264.7 was maintained in culture in DMEM medium with 10% FBS at 37 °C in a humidified 5% CO2 incubator. In order to test whether the TPPU or EETs attenuated LPS-induced inflammation in murine macrophage, TPPU (0.1% DMSO, 0.1, 1, 10 µM) or EET (11,12-EET, 14,15-EET, 1 µM, Cayman Chemical, USA) was added 30 min before LPS stimulation. After 6 h, the supernatant of culture was collected to test inflammatory cytokine content or LDH, and the cell was collected to test mRNA expression of cytokine.
Enzyme-linked immunosorbent assay (ELISA)
The BALF or cell culture supernatant was collected and stored at −80 °C before cytokine assay. IL-1β and TNF-α level in BALF was quantified using a murine ELISA kit (R&D Systems, USA) according to the manufacturer’s recommendations.
Total RNA extraction and Real-Time PCR
Total RNA of lung tissues or murine macrophage was extracted using TRIzol reagent (Invitrogen, USA) and was quantified by spectrophotometric analysis using an ultraviolet spectrophotometer (Thermo Fisher Scientific, USA). The generation of cDNA from RNA (2 µg) was performed using RevertAidTM Reverse Transcriptase (Thermo Fisher Scientific, USA) and random hexamer primers according to the manufacturer’s instructions. Real-Time PCR of cDNA was performed using the iTaq™ Universal SYBR® Green Supermix (Bio-Rad, USA). The sequences of the specific primers were as follows: IL-1β (forward) 5’-GCCCATCCTCTGTGACTCAT-3’ and (reverse) 5’-AGGCCACAGGTATTTTGTCG-3’; TNF-α (forward) 5’-TCTCATTCCTGCTCG TGG-3’ and (reverse) 5’-CTCTGCTTGGTGGTTTGC-3’; β-actin (forward) 5’- TTCCAGCCTT CCTTCTTG-3’ and (reverse) 5’- GGAGCCAGAGCAGTAATC -3’. Relative gene expression was measured by the 2−ΔΔCT method. The mouse β-actin housekeeping gene was used as an internal control.
Statistical analysis
Data are expressed as mean ± SD. Statistical analysis was performed with SPSS17.0. Statistical comparisons among the groups were assessed by one-way analysis of variance (ANOVA). When F ratios were significant (P<0.05), Tukey-Kramer’s post-hoc test, or Log-rank (Mantel-Cox) test was performed (between group comparison). Survival rate was evaluated by the Kaplan-Meier test. P<0.05 was considered a statistically significant.
Results
TPPU attenuated LPS-induced lung morphological injury
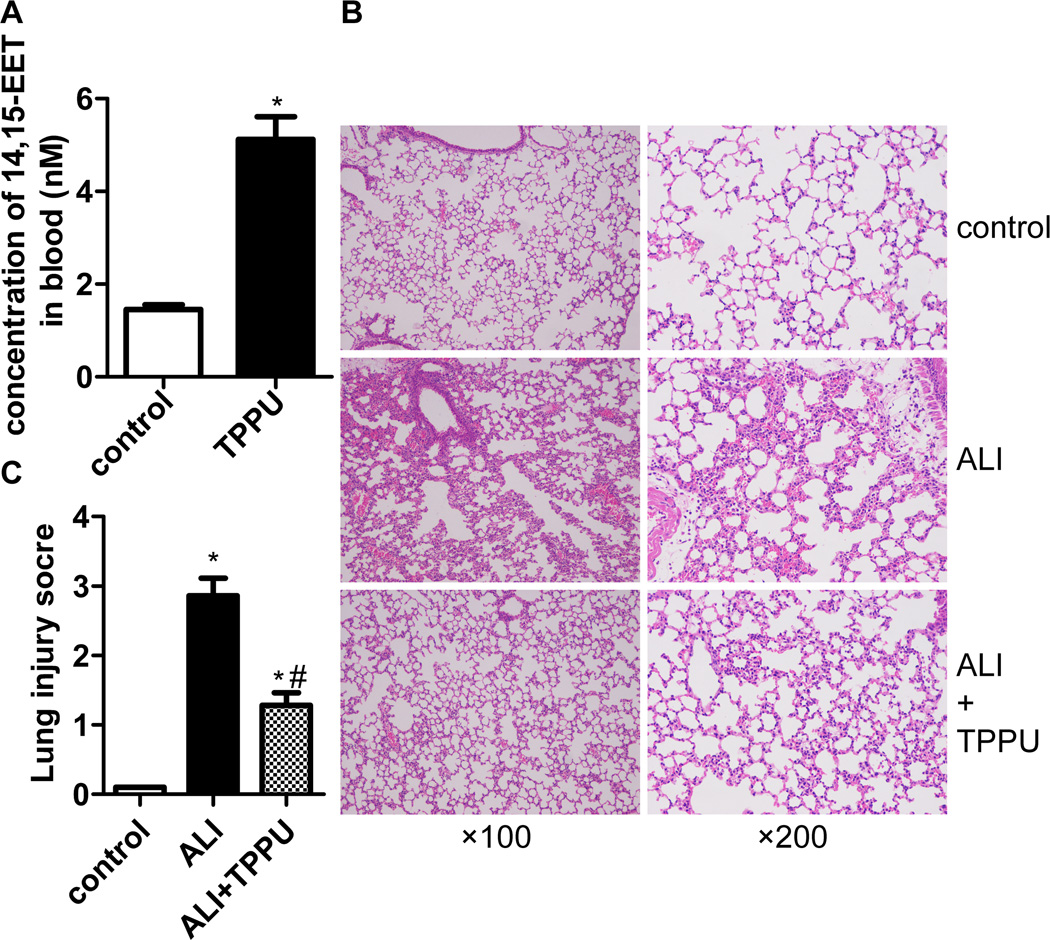
Firstly, we test the 14,15-EET level in the serum of mice. We found TPPU significantly increased 14,15-EET concentration in serum of mice (Figure 2A, P<0.05). Then, HE staining and lung injury score system were used in this study to assess the pathological changes. Six hours after the LPS injection, we found notable inflammatory cell infiltration, interstitial edema, interalveolar and interstitial patchy hemorrhage, and interalveolar septal thickening. TPPU treatment attenuated the pathological changes in the lung tissues (Figure 2B). Accordingly, TPPU treatment significantly decreased the lung injury score compared with the ALI group (Figure 2C, P<0.01).
Fig. 2.
TPPU attenuated pulmonary inflammation in vivo. Six hours after LPS injection with or without TPPU treatments, right lungs were fixed and stained with HE. (A) TPPU increases the concentration of 14,15-EET in blood of mice. (B) The figure demonstrates a representative view (lift column: ×100 magnification; right column: ×200 magnification). (C) Degree of lung injury was measured via the lung injury scoring system. * P<0.05 compared with control group. # P<0.05 compared with ALI group.
TPPU decreased inflammatory cell infiltration in lung
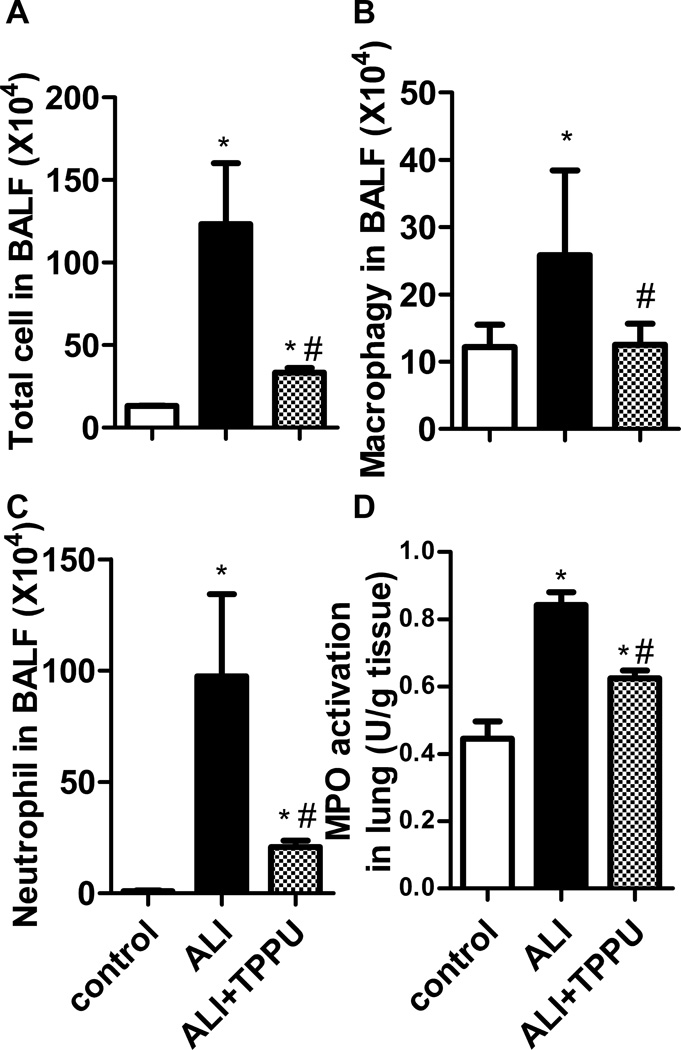
The number of total cells, macrophages and neutrophils in BALF was analyzed 6 h after LPS administration. The results showed that the number of total cells, macrophages and neutrophils increased significantly in ALI group, compared to the control group (P<0.01). TPPU treatment significantly decreased the number of total cell, macrophages and neutrophils in BALF (Figure 3A–3C, P<0.01). MPO is an enzyme expressed abundantly in neutrophil. An increased MPO activity is suggestive of neutrophil accumulation in the lung. Consistent with the changes of neutrophil number in BALF, LPS administration significantly increased MPO activity compared to control group (P<0.01). TPPU treatment significantly inhibited LPS-induced MPO activity (Figure 3D, P<0.01).
Fig. 3.
TPPU decreased inflammatory cell infiltration in lung. Mice were received LPS injection with or without TPPU treatment. 6 h later, BALF was collected, and total cells (A), macrophages (B) and neutrophils (C) were counted. MPO activity was detected to quantify the infiltration of neutrophils in lung (D). * P<0.05 compared with control group. # P<0.05 compared with ALI group.
TPPU ameliorated LPS-induced lung edema and vascular leakage
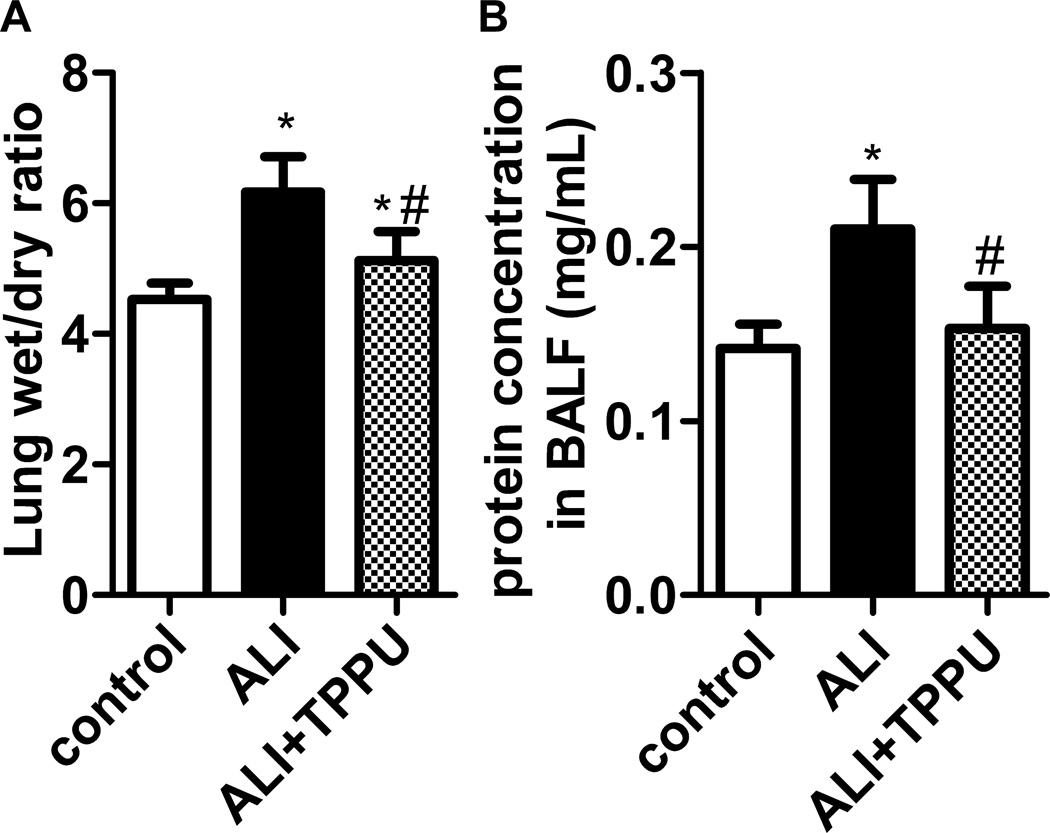
The mice received LPS injection showed higher W/D ratio than in control group (P<0.05). Nevertheless, W/D significantly decreased in TPPU treatment group (Figure 4A, P<0.05). Increased microvascular lung permeability is a cardinal feature of ALI. LPS significantly increased protein levels in BALF of ALI (P<0.05). TPPU treatment decreased the protein concentration in BALF of LPS-challenged mice (Figure 4B, P<0.05). Therefore, TPPU attenuates LPS-induced edema and vascular leakage in murine model of ALI.
Fig. 4.
TPPU alleviated LPS-induced edema and vascular leakage. Mice were received LPS injection with or without TPPU treatment. (A) Six hours later, lung wet/dry ratio was detected to evaluated lung edema. TPPU decreased lung wet/dry ratio in LPS-induced ALI mice. (B) Total protein in BALF was tested to evaluated vascular leakage. * P<0.05 compared with control group. # P<0.05 compared with ALI group.
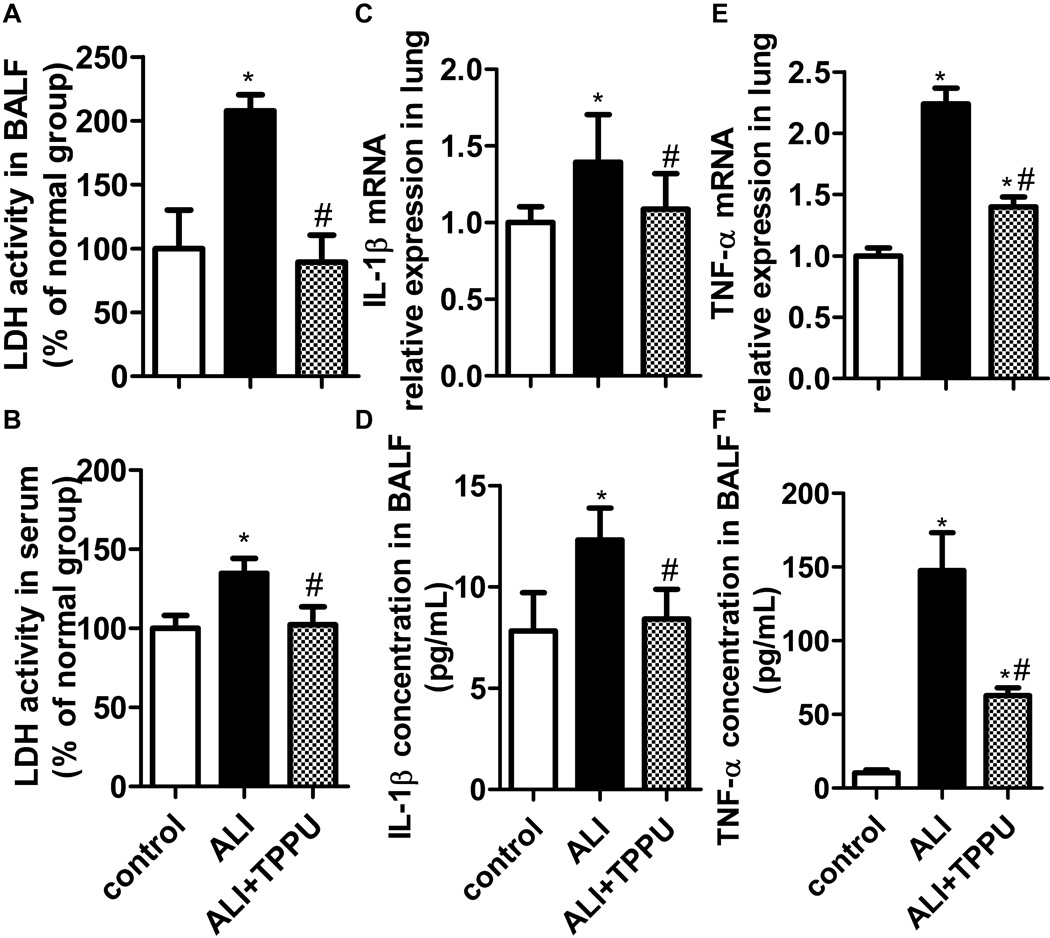
TPPU attenuated cell injury and pro-inflammatory cytokine release in LPS-induced mice
Extracellular LDH can indicate the integrity of cell membrane or cell injury (10). IL-1β and TNF-α are the critical pro-inflammatory cytokines in the development of ALI. Thus, we next tested whether TPPU can attenuate LPS-induced cell injury and pro-inflammatory cytokine release in ALI mice. The results showed that ALI mice demonstrated a significant increase of LDH activity, IL-1β and TNF-α in BALF after LPS challenge (P<0.05). TPPU decreased the LDH activity levels to normal in BALF and serum (Figure 5A, 5B, P<0.05). TPPU also decreased the IL-1β and TNF-α mRNA in lung tissue, and their protein content in BALF (Figure 5C–5F, P<0.05). These results indicate that TPPU attenuates LPS-induced cell injury and pro-inflammatory cytokine release.
Fig. 5.
TPPU attenuated cell injury and pro-inflammatory cytokine release in ALI mice stimulated with LPS. (A–B) LDH levels indicate the integrity of cell membrane or cell injury. TPPU reduced the LDH activity in BALF and serum of ALI mice. (C–D) IL-1β mRNA expression in lung and protein content in BLAF were suppressed by TPPU treatment. (E–F) TPPU treatment decreased TNF-α mRNA expression in lung and protein content in BLAF. * P<0.05 compared with control group. # P<0.05 compared with ALI group.
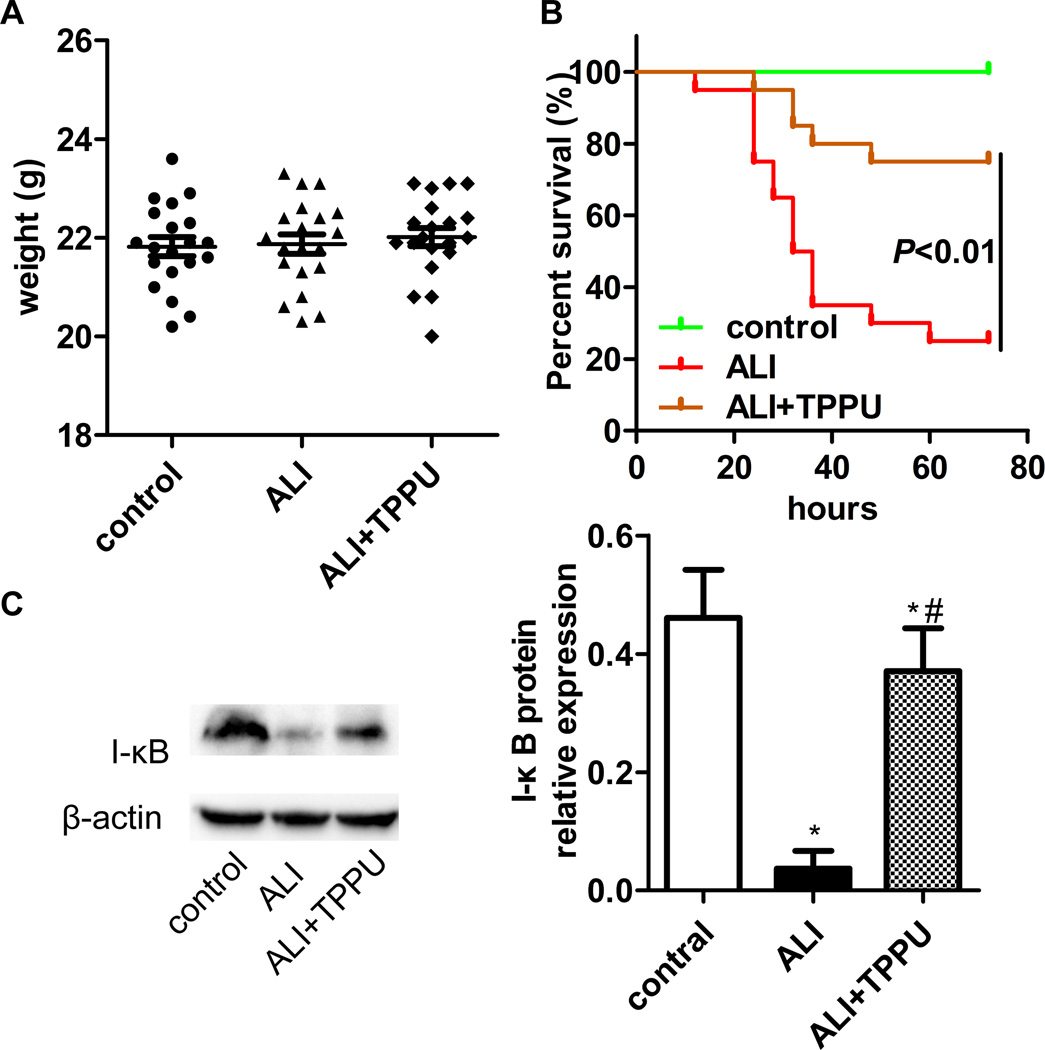
TPPU improved the survival rate of ALI mice stimulated with LPS
As a key indication of therapeutic benefit of TPPU, the survival rate was examined in mice. Mice were given a lethal dose of LPS (40 mg/kg, intraperitoneal injection) to induce endotoxemia. The body weight was not significantly different among the three groups (Figure 6A, P>0.05). Only 25 % of the LPS-stressed mice survived for 72 h in ALI group. The survival rate of the TPPU treatment group improved significantly reaching to 75 % (Figure 6B, P<0.01).
Fig. 6.
TPPU decreased mortality of ALI mice induced by LPS and inhibited the degradation of I-κB. (A) 60 mice were divided into 3 groups: control, ALI and ALI+TPPU. The mice body weight was not significant among groups. (B) Mice were treated with a single intraperitoneal injection of saline, and/or 1 mg/kg TPPU 3 h before 40 mg/kg LPS administration. Data were analyzed by Kaplan Meier survival analysis (n=20). (C) Total lung tissue was detected the expression of I-κB protein by western blotting. * P<0.05 compared with control group. # P<0.05 compared with ALI group.
TPPU inhibited I-κB degradation in LPS-stimulated mice
The NF-κB pathway plays a vital role in the pathological process of ALI. We investigated the effect of TPPU on the activation of NF-κB pathway. LPS treatment (1 µg/mL) for 6 h promoted the degradation of I-κB in the lung of mice (P<0.05). TPPU inhibited the degradation of I-κB (Figure 6C, P<0.05). This result indicates that TPPU attenuates LPS-induced ALI in mice might via suppressing NF-κB pathway.
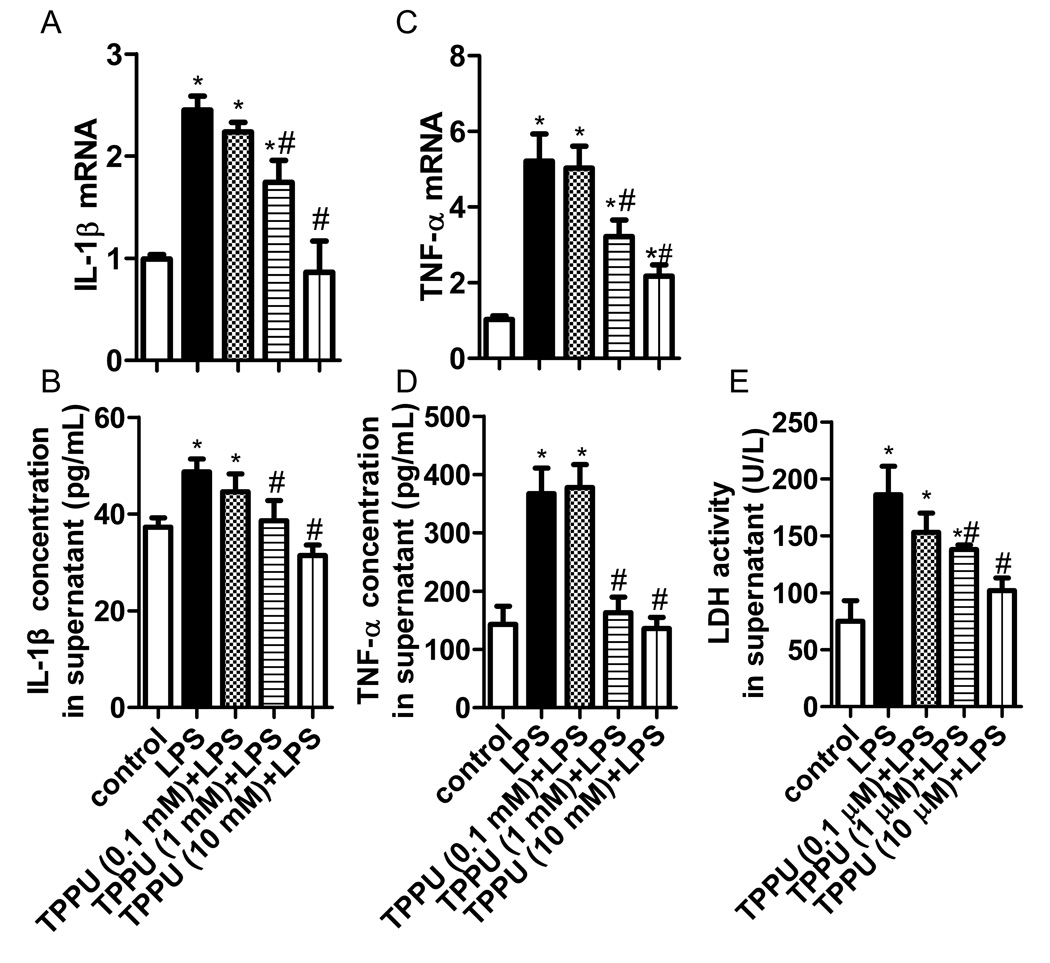
TPPU attenuated inflammatory injury in LPS-challenged murine macrophage
To verify the effect of sEH inhibitor on inflammation, we investigated the role of TPPU in LPS-challenged murine macrophage. We found LPS treatment (1 µg/mL) for 6 h increased the IL-1β and TNF-α expression, and LDH release (P<0.05). Pre-treatment of TPPU attenuated the inflammatory injury in a dose-dependent manner (Figure 7, P<0.05).
Fig. 7.
TPPU attenuated inflammatory injury in LPS-challenged murine macrophage. A series of concentration of TPPU (0.1, 1 and 10 mM) was added to LPS-challenged murine macrophage. (A–B) TPPU inhibited IL-1β mRNA and protein expression in a dose-dependent manner. (C–D) TPPU inhibited TNF-α mRNA and protein expression in a dose-dependent manner. (E) TPPU reduced LDH activity in supernatant. * P<0.05 compared with control group. # P<0.05 compared with ALI group.
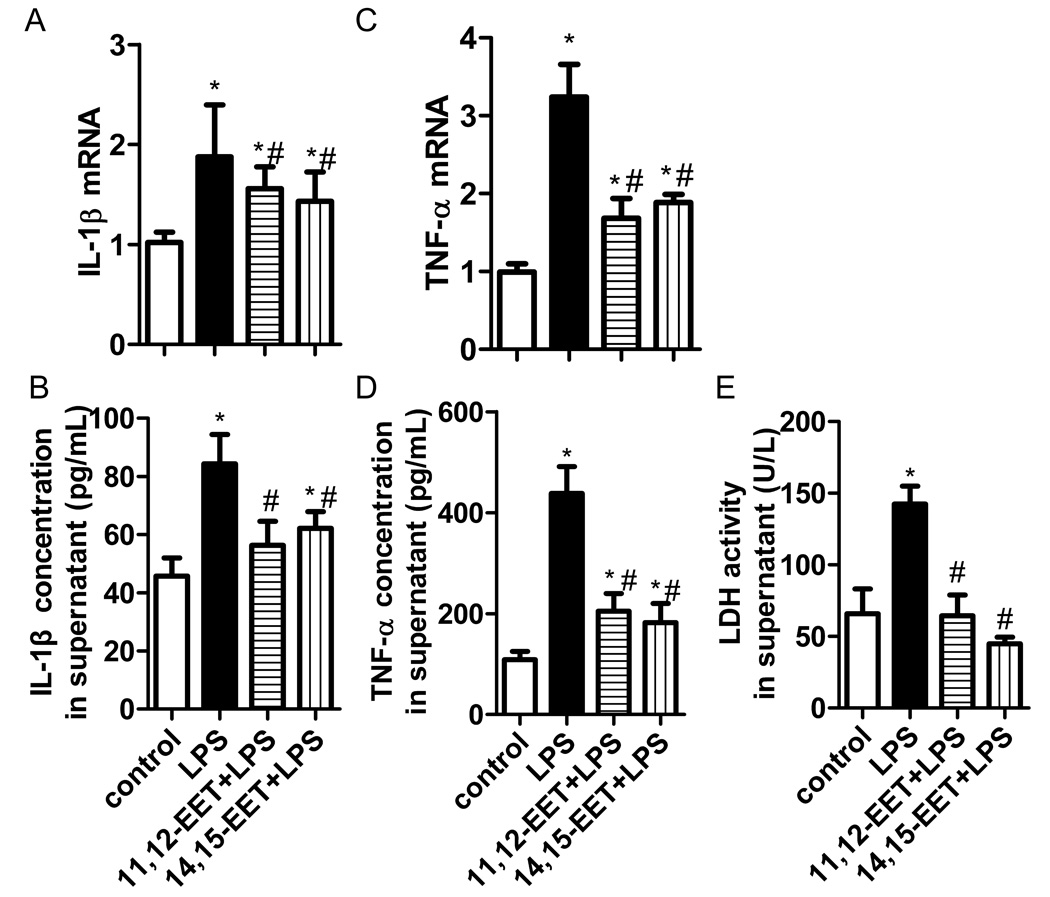
EETs attenuated inflammatory injury in LPS-challenged murine macrophage
We have reported TPPU increased EETs levels in plasma of mice (10). Further, we questioned whether EETs suppressed inflammation induced by LPS. We found both 11,12-EET and 14,15-EET reduced IL-1β and TNF-α expression, and LDH release in LPS-challenged murine macrophage (Figure 8, P<0.05). These results indicate inhibition of sEH results in elevation of EETs and in attenuation of inflammatory injury induced by LPS.
Fig. 8.
EETs attenuated inflammatory injury in LPS-challenged murine macrophage. 11,12-EET (1 µM) or 14,15-EET (1 µM) was administered 30 min before LPS stimulation. (A–B) EETs inhibited IL-1β mRNA and protein expression in a dose-dependent manner. (C–D) EETs inhibited TNF-α mRNA and protein expression in a dose-dependent manner. (E) EETs reduced LDH activity in supernatant. * P<0.05 compared with control group. # P<0.05 compared with ALI group.
Discussion
Infectious etiologies, such as sepsis and pneumonia, are leading causes of ALI/ARDS. LPS is a component of the Gram negative bacterial cell membrane. LPS has been widely used to induce pulmonary inflammation in animal models of ALI (15). In this study, administration of LPS induces severe lung injury, with characteristic symptoms of ALI, including excessive leukocyte accumulation, pulmonary edema, and pro-inflammatory cytokine release. Through these studies, we first demonstrated that inhibition of sEH with TPPU improved the survival rate, attenuated the lung destruction, inhibited inflammatory cell infiltration into lung, and pro-inflammatory cytokines release of LPS-induced ALI in mice. EETs might be the crucial protective factor with the effect of inhibiting inflammation induced by LPS. Notably, inhibitors of sEH could be an efficient therapeutic clinical strategy for ALI.
Our observations are consistent with our hypothesis that EETs, derived from arachidonic acid by cytochrome P450, inhibit inflammation induced by LPS, contributing to the protection of sEH inhibitor against ALI in mice. Arachidonic acid is an essential polyunsaturated fatty acids, which exerts a variety of beneficial effects (18). Accumulated evidence shows that the metabolic network of arachidonic acid is an important part of inflammatory metabolic pathways. Arachidonic acid plays a protective effect on paraquat-induced ALI in mice, and arachidonic acid attenuates paraquat intoxication through antioxidant and anti-inflammatory effects (19). Arachidonic acid exerts their biological activities mainly through the formation of bioactive lipid metabolites (20). However, it has not been elucidated completely which lipid signaling molecule is the essential one in the anti-inflammatory role of arachidonic acid. There are three major pathways within arachidonic acid cascade, such as cyclooxygenase (COX)-derived prostaglandin, lipoxygenase (LOX)-derived leukotrienes (LTs), and CPY450-derived EETs (20). The COX-2 metabolite of arachidonic acid, PGE2, is widely known to promote inflammation but also to initiate a series of events leading to its resolution. COX-2 inhibitors also have been shown to reduced inflammation, contributing to many inflammatory diseases (21). The LTs have roles in many disease states involving in acute or chronic inflammation (22). EETs are autocrine and paracrine lipid signaling molecules produced from arachidonic acid (6). We have reported that inhibition of sEH with TPPU increased EETs levels in plasma of mice and attenuated blomycin-induced pulmonary fibrosis. The anti-inflammation effect of EETs is one of its protective mechanisms (10). This study provides strong evidence that EETs may be the vital anti-inflammatory metabolites of arachidonic acid.
EETs also exert anti-inflammatory effect in many other disease models. Genetic ablation of sEH or its inhibitor enhances levels of EETs, attenuates tubulointerstitial fibrosis and inflammation after unilateral ureteral obstruction (8). In AngII-induced cardic hypertrophy, sEH inhibitor significantly attenuates cardiac inflammatory response and cardiac fibrosis (23). In mechanism, it was firstly reported in 1999 that EETs could inhibit NF-κB activation induced by TNF-α (24). In this study, we found TPPU significantly reduced the intrapulmonary IL-1β expression. IL-1β secretion is mainly dependent on NLRP3 inflammasome. The NLRP3 inflammasome is a molecular platform activated upon signs of cellular ‘danger’ to trigger innate immune defenses through the maturation of pro-inflammatory cytokines such as IL-1β and IL-18 (25). An increasing number of researchers believe that the NLRP3 inflammasome is essential for the development of ALI induced by LPS, mechanical ventilation, hyperoxia or burn (26–29). Inhibition of NLRP3 with various methods could alleviate ALI (30, 31). However, the effect of EETs on activation of NLRP3 inflammasome is poor understood. NF-κB and reactive oxygen species (ROS) are critical factors in the activation of NLRP3 inflammasome (32). Several studies suggest that EETs both reduce ROS production and activation of the ER stress response by ROS and other factors (24, 33). Our data indicate that TPPU and presumably EETs and possibly other epoxy fatty acids attenuate LPS-induced ALI by inhibiting the degradation of I-κB, then suppressing the activation of NLRP3 inflammasome. The effect of EETs on activation of the NLRP3 inflammasome in LPS-induced ALI will be studied in the future to fully reveal the potential molecular mechanism under its protective role in ALI.
As we know, several available sEH inhibitors are potent enough to be moved into the clinic, including TPPU (34). The sEH inhibitor is in clinical development targeting hypertension and type 2 diabetes (35). Pharmacological inhibition of sEH also prevents renal interstitial fibrogenesis in obstructive nephropathy (8). What’s more, no adverse reactions were observed in Phase I and IIA human clinical trials with sEH inhibitors, even when given at high doses (35). The therapeutic index of sEH inhibitors appears large.
Taken together, the selective sEH inhibitor, TPPU, can attenuate the severity of LPS-induced ALI in mice via protective lipid EETs. We suggest that sEH is a valuable candidate for treatment of ALI.
Acknowledgments
This work was supported by the Special Funds for Major State Basic Research Projects (2012CB518104), the National Natural Science Foundation of China (81170059, 81530063, 81500065, 81670014), and Project supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (20130162110052). B.D.H and K.S.S.L is partially supported by the NIEHS grant R01 ES002710 and NIH CounterAct U54 NS079202 and K.S.S.L. has been partially supported by the NIH Pathway to Independence Award from NIH/NIEHS (1K99ES024806-01).
Footnotes
Author Disclosure Statement
The authors declare that no competing financial interest exists.
Author contributions
CXG, JXJ and YZ conceived and designed the experiments. YZ, TL, JXD, LD and PL performed the experiments. YZ, TL, GYS, YPL and JZ analyzed the data. BDH, KSSL and JXJ contributed reagents/materials/analysis tools. YZ and CXG wrote the paper.
References
- 1.Hsu AT, Barrett CD, DeBusk GM, Ellson CD, Gautam S, Talmor DS, Gallagher DC, Yaffe MB. Kinetics and Role of Plasma Matrix Metalloproteinase-9 Expression in Acute Lung Injury and the Acute Respiratory Distress Syndrome. Shock. 2015;44(2):128–136. doi: 10.1097/SHK.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Standiford TJ, Ward PA. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl Res. 2016;167(1):183–191. doi: 10.1016/j.trsl.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, Ambros A, Gandia F, Carriedo D, Mosteiro F, Basaldua S, Fernandez RL, Kacmarek RM. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37(12):1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Liang X, Wang D, Zhang H, Liu L, Chen H, Li Y, Duan Q, Xie K. Combination therapy with nitric oxide and molecular hydrogen in a murine model of acute lung injury. Shock. 2015;43(5):504–511. doi: 10.1097/SHK.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 5.Takashima K, Matsushima M, Hashimoto K, Nose H, Sato M, Hashimoto N, Hasegawa Y, Kawabe T. Protective effects of intratracheally administered quercetin on lipopolysaccharide-induced acute lung injury. Respir Res. 2014;15(1):150. doi: 10.1186/s12931-014-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris TR, Hammock BD. Soluble epoxide hydrolase: gene structure, expression and deletion. Gene. 2013;526(2):61–74. doi: 10.1016/j.gene.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettaieb A, Chahed S, Bachaalany S, Griffey S, Hammock BD, Haj FG. Soluble Epoxide Hydrolase Pharmacological Inhibition Ameliorates Experimental Acute Pancreatitis in Mice. Mol Pharmacol. 2015;88(2):281–290. doi: 10.1124/mol.114.097501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Yoon SP, Toews ML, Imig JD, Hwang SH, Hammock BD, Padanilam BJ. Pharmacological inhibition of soluble epoxide hydrolase prevents renal interstitial fibrogenesis in obstructive nephropathy. Am J Physiol Renal Physiol. 2015;308(2):F131–F139. doi: 10.1152/ajprenal.00531.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8(10):794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Sun GY, Liu T, Duan JX, Zhou HF, Lee KS, Hammock BD, Fang X, Jiang JX, Guan CX. Soluble epoxide hydrolase inhibitor 1-trifluoromethoxyphenyl-3- (1-propionylpiperidin-4-yl) urea attenuates bleomycin-induced pulmonary fibrosis in mice. Cell Tissue Res. 2016;363(2):399–409. doi: 10.1007/s00441-015-2262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Bratt J, Franzi L, Liu JY, Zhang G, Zeki AA, Vogel CF, Williams K, Dong H, Lin Y, Hwang SH, Kenyon NJ, Hammock BD. Soluble epoxide hydrolase inhibitor attenuates inflammation and airway hyperresponsiveness in mice. Am J Respir Cell Mol Biol. 2015;52(1):46–55. doi: 10.1165/rcmb.2013-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci U S A. 2005;102(6):2186–2191. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baudiss K, de Paula Vieira R, Cicko S, Ayata K, Hossfeld M, Ehrat N, Gomez-Munoz A, Eltzschig HK, Idzko M. C1P Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Preventing NF-kappaB Activation in Neutrophils. J Immunol. 2016;196(5):2319–2326. doi: 10.4049/jimmunol.1402681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81(19):8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi D, He J, Wang D, Deng W, Zhao Y, Ye Y, Feng L. 17beta-estradiol suppresses lipopolysaccharide-induced acute lung injury through PI3K/Akt/SGK1 mediated up-regulation of epithelial sodium channel (ENaC) in vivo and in vitro. Respir Res. 2014;15(1):159. doi: 10.1186/s12931-014-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales JN, Gorshkov B, Varn MN, Zemskova MA, Zemskov EA, Sridhar S, Lucas R, Verin AD. Protective effect of adenosine receptors against lipopolysaccharide-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;306(6):L497–L507. doi: 10.1152/ajplung.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King BA, Kingma PS. Surfactant protein D deficiency increases lung injury during endotoxemia. Am J Respir Cell Mol Biol. 2011;44(5):709–715. doi: 10.1165/rcmb.2009-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacerdoti D, Pesce P, Di Pascoli M, Brocco S, Cecchetto L, Bolognesi M. Arachidonic acid metabolites and endothelial dysfunction of portal hypertension. Prostaglandins Other Lipid Mediat. 2015;120:80–90. doi: 10.1016/j.prostaglandins.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Du J, Li X, Lin C, He X. Protective Effects of Arachidonic Acid Against Paraquat-Induced Pulmonary Injury. Inflammation. 2015;38(4):1458–1463. doi: 10.1007/s10753-015-0120-6. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Zhu J, Lyu F, Panigrahy D, Ferrara KW, Hammock B, Zhang G. omega-3 polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014;113–115:13–20. doi: 10.1016/j.prostaglandins.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patrignani P, Patrono C. Cyclooxygenase inhibitors: From pharmacology to clinical read-outs. Biochim Biophys Acta. 2015;1851(4):422–432. doi: 10.1016/j.bbalip.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta. 2015;1851(4):331–339. doi: 10.1016/j.bbalip.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Li N, Pang W, Zhang X, Hammock BD, Ai D, Zhu Y. Opposite effects of gene deficiency and pharmacological inhibition of soluble epoxide hydrolase on cardiac fibrosis. PLoS One. 2014;9(4):e94092. doi: 10.1371/journal.pone.0094092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Node K, Huo YQ, Ruan XL, Yang BC, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285(5431):1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecker A, Kullmar M, Wilker S, Richter K, Zakrzewicz A, Atanasova S, Mathes V, Timm T, Lerner S, Klein J, Kaufmann A, Bauer S, Padberg W, Kummer W, Janciauskiene S, Fronius M, Schweda EK, Lochnit G, Grau V. Phosphocholine-Modified Macromolecules and Canonical Nicotinic Agonists Inhibit ATP-Induced IL-1beta Release. J Immunol. 2015;195(5):2325–2334. doi: 10.4049/jimmunol.1400974. [DOI] [PubMed] [Google Scholar]
- 26.Jones HD, Crother TR, Gonzalez-Villalobos RA, Jupelli M, Chen S, Dagvadorj J, Arditi M, Shimada K. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. Am J Respir Cell Mol Biol. 2014;50(2):270–280. doi: 10.1165/rcmb.2013-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS, Ward PA. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol. 2014;192(12):5974–5983. doi: 10.4049/jimmunol.1400368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han S, Cai W, Yang X, Jia Y, Zheng Z, Wang H, Li J, Li Y, Gao J, Fan L, Hu D. ROS-Mediated NLRP3 Inflammasome Activity Is Essential for Burn-Induced Acute Lung Injury. Mediators Inflamm. 2015;2015:720457. doi: 10.1155/2015/720457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukumoto J, Fukumoto I, Parthasarathy PT, Cox R, Huynh B, Ramanathan GK, Venugopal RB, Allen-Gipson DS, Lockey RF, Kolliputi N. NLRP3 deletion protects from hyperoxia-induced acute lung injury. Am J Physiol Cell Physiol. 2013;305(2):C182–C189. doi: 10.1152/ajpcell.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Li X, Grailer JJ, Wang N, Wang M, Yao J, Zhong R, Gao GF, Ward PA, Tan DX, Li DX. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res. 2016;13(2):148–159. doi: 10.1111/jpi.12322. [DOI] [PubMed] [Google Scholar]
- 31.Galam L, Rajan A, Failla A, Soundararajan R, Lockey RF, Kolliputi N. Deletion of P2X7 attenuates hyperoxia-induced acute lung injury via inflammasome suppression. Am J Physiol Lung Cell Mol Physiol. 2016;310(6):L572–L581. doi: 10.1152/ajplung.00417.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13(2):148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen W, Zheng G, Yang S, Ping W, Fu X, Zhang N, Wang DW, Wang J. CYP2J2 and EETs Protect against Oxidative Stress and Apoptosis in Vivo and in Vitro Following Lung Ischemia/Reperfusion. Cell Physiol Biochem. 2014;33(6):1663–1680. doi: 10.1159/000362950. [DOI] [PubMed] [Google Scholar]
- 34.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen D, Whitcomb R, MacIntyre E, Tran V, Do ZN, Sabry J, Patel DV, Anandan SK, Gless R, Webb HK. Pharmacokinetics and pharmacodynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. J Clin Pharmacol. 2012;52(3):319–328. doi: 10.1177/0091270010397049. [DOI] [PubMed] [Google Scholar]