Abstract
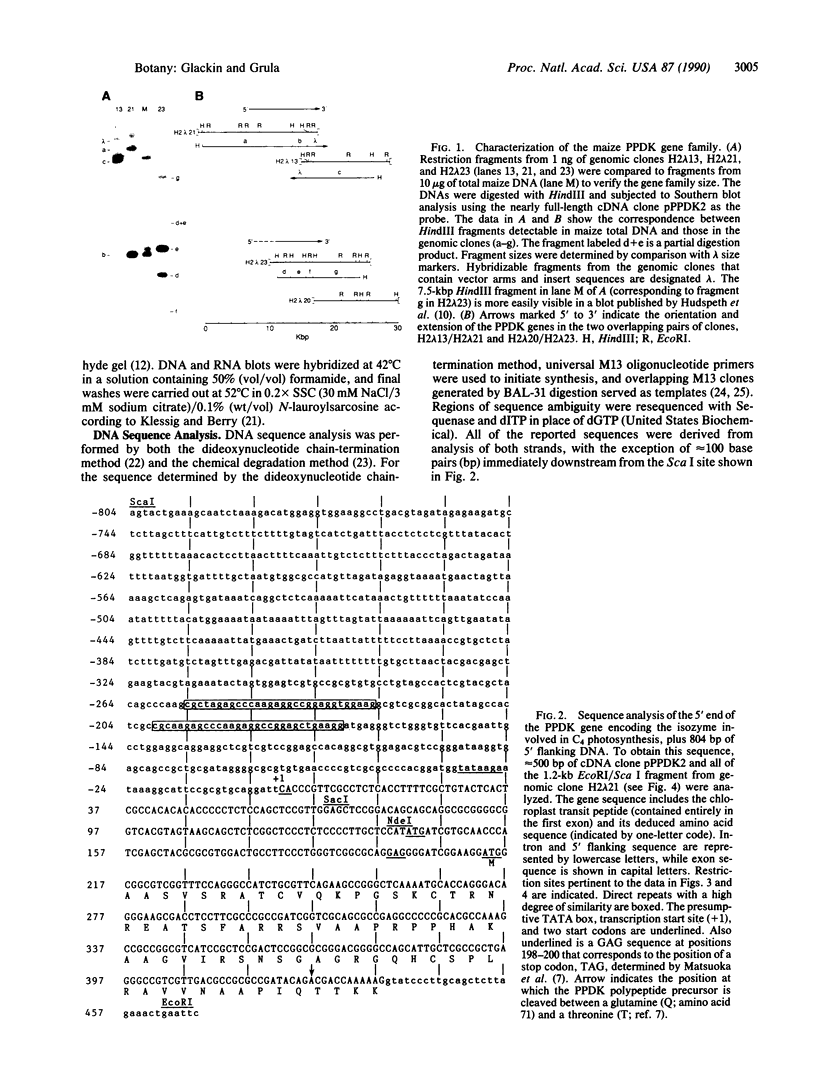
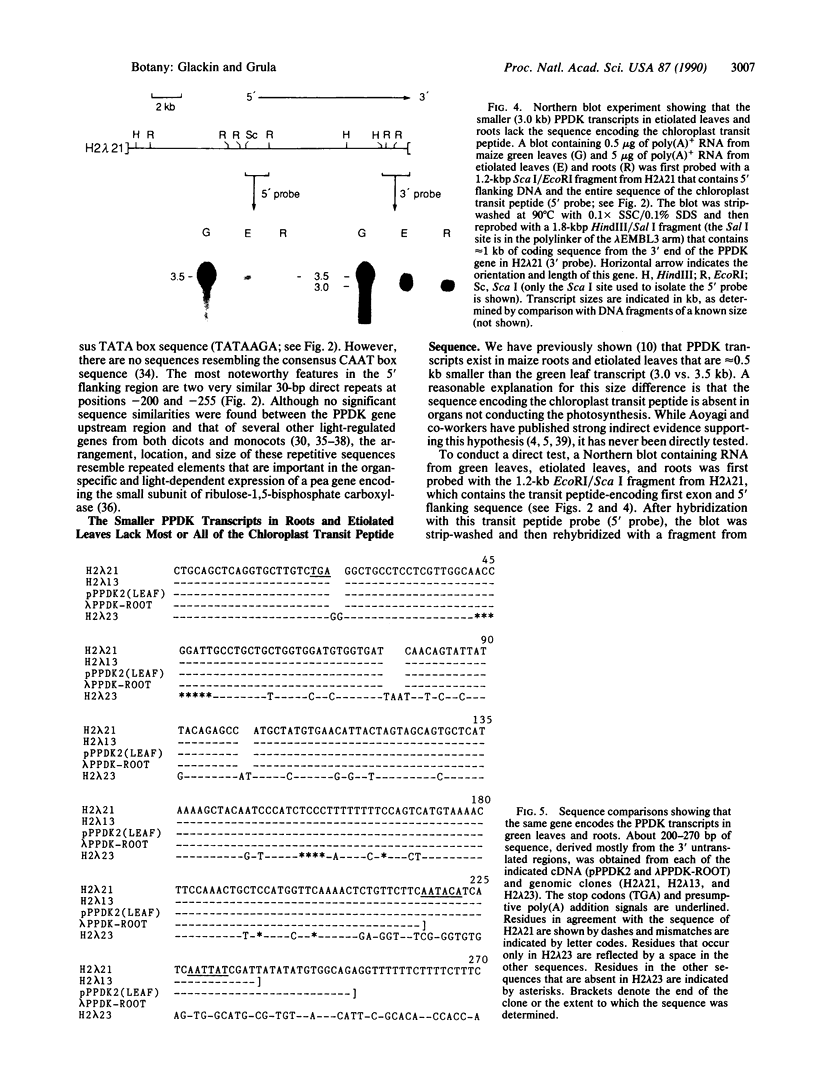
Analyses of genomic DNA and clones indicate that the pyruvate, orthophosphate dikinase (PPDK; ATP: pyruvate, orthophosphate phosphotransferase, EC 2.7.9.1) gene family of maize (Zea mays L. subsp. mays, line B73) contains two members. Restriction site and DNA sequence comparisons between PPDK genomic and leaf cDNA clones have revealed which gene encodes the isozyme involved in C4 photosynthesis. The region flanking the 5' end of this gene contains two 30-base-pair (bp) repetitive elements that may be involved in its light-regulated expression. Sequence analysis of genomic and leaf cDNA clones has also shown that the entire 7.3-kDa PPDK chloroplast transit peptide is encoded in the 436-bp first exon. Northern blot experiments with probes specific for the first exon and the 3' end of the gene showed that the smaller PPDK transcripts in roots and etiolated leaves [3.0 kilobases (kb) vs. the 3.5-kb green leaf transcript] lack the sequence encoding the chloroplast transit peptide. In addition, results from cDNA library screens have confirmed that the root transcript is approximately 50-fold less abundant than the green leaf transcript. Finally, sequence comparisons among cDNA clones from green leaves and roots and genomic clones representing both members of the PPDK gene family demonstrate that the green leaf transcript encoding the C4 isozyme and the root transcript are derived from the same gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyagi K., Bassham J. A., Greene F. C. Pyruvate orthophosphate dikinase gene expression in developing wheat seeds. Plant Physiol. 1984 Jun;75(2):393–396. doi: 10.1104/pp.75.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi K., Bassham J. A. Pyruvate orthophosphate dikinase mRNA organ specificity in wheat and maize. Plant Physiol. 1984 Sep;76(1):278–280. doi: 10.1104/pp.76.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi K., Bassham J. A. Pyruvate orthophosphate dikinase of c(3) seeds and leaves as compared to the enzyme from maize. Plant Physiol. 1984 Jun;75(2):387–392. doi: 10.1104/pp.75.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi K., Bassham J. A. Synthesis and uptake of cytoplasmically synthesized pyruvate, pi dikinase polypeptide by chloroplasts. Plant Physiol. 1985 Aug;78(4):807–811. doi: 10.1104/pp.78.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi K., Chua N. H. Cell-specific expression of pyruvate, pi dikinase : in situ mRNA hybridization and immunolocalization labeling of protein in wheat seed. Plant Physiol. 1988 Feb;86(2):364–368. doi: 10.1104/pp.86.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hague D. R., Uhler M., Collins P. D. Cloning of cDNA for pyruvate, Pi dikinase from maize leaves. Nucleic Acids Res. 1983 Jul 25;11(14):4853–4865. doi: 10.1093/nar/11.14.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Hudspeth R. L., Glackin C. A., Bonner J., Grula J. W. Genomic and cDNA clones for maize phosphoenolpyruvate carboxylase and pyruvate,orthophosphate dikinase: Expression of different gene-family members in leaves and roots. Proc Natl Acad Sci U S A. 1986 May;83(9):2884–2888. doi: 10.1073/pnas.83.9.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin-Neumann G. A., Kohorn B. D., Thornber J. P., Tobin E. M. A chlorophyll a/b-protein encoded by a gene containing an intron with characteristics of a transposable element. J Mol Appl Genet. 1985;3(1):45–61. [PubMed] [Google Scholar]
- Kuhlemeier C., Cuozzo M., Green P. J., Goyvaerts E., Ward K., Chua N. H. Localization and conditional redundancy of regulatory elements in rbcS-3A, a pea gene encoding the small subunit of ribulose-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4662–4666. doi: 10.1073/pnas.85.13.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun M., Waksman G., Freyssinet G. Nucleotide sequence of a gene encoding corn ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit (rbcs). Nucleic Acids Res. 1987 May 26;15(10):4360–4360. doi: 10.1093/nar/15.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P., Rutherford G., Banks J. A., Fedoroff N. Essential large transcripts of the maize Spm transposable element are generated by alternative splicing. Cell. 1989 Aug 25;58(4):755–765. doi: 10.1016/0092-8674(89)90109-8. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Ozeki Y., Yamamoto N., Hirano H., Kano-Murakami Y., Tanaka Y. Primary structure of maize pyruvate, orthophosphate dikinase as deduced from cDNA sequence. J Biol Chem. 1988 Aug 15;263(23):11080–11083. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meyer A. O., Kelly G. J., Latzko E. Pyruvate orthophosphate dikinase from the immature grains of cereal grasses. Plant Physiol. 1982 Jan;69(1):7–10. doi: 10.1104/pp.69.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V. Rapid and simple removal of contaminating RNA from plasmid DNA without the use of RNase. Anal Biochem. 1981 May 1;113(1):34–42. doi: 10.1016/0003-2697(81)90040-3. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O., Cannon L. E. Presecretory and cytoplasmic invertase polypeptides encoded by distinct mRNAs derived from the same structural gene differ by a signal sequence. Proc Natl Acad Sci U S A. 1982 Feb;79(3):781–785. doi: 10.1073/pnas.79.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitout M. J., Potgieter D. J. The isolation of highly polymerized deoxyribonucleic acid from maize. Biochim Biophys Acta. 1968 Jun 18;161(1):188–196. doi: 10.1016/0005-2787(68)90308-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stayton M. M., Black M., Bedbrook J., Dunsmuir P. A novel chlorophyll a/b binding (Cab) protein gene from petunia which encodes the lower molecular weight Cab precursor protein. Nucleic Acids Res. 1986 Dec 22;14(24):9781–9796. doi: 10.1093/nar/14.24.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke J. M., Chatfield J. M., Ogren W. L. Alternative mRNA splicing generates the two ribulosebisphosphate carboxylase/oxygenase activase polypeptides in spinach and Arabidopsis. Plant Cell. 1989 Aug;1(8):815–825. doi: 10.1105/tpc.1.8.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Tzagoloff A. Mitochondrial and cytoplasmic fumarases in Saccharomyces cerevisiae are encoded by a single nuclear gene FUM1. J Biol Chem. 1987 Sep 5;262(25):12275–12282. [PubMed] [Google Scholar]





