Abstract
Posttraumatic stress disorder (PTSD) is a mental disorder developing after exposure to traumatic events. Although psychotherapy reveals some therapeutic effectiveness, clinically sustainable cure is still uncertain. Some Chinese herbal formulae are reported to work well clinically against mental diseases in Asian countries, but the safety and their mode of action are still unclear. In this study, we investigated the mechanisms of Chinese remedy free and easy wanderer (FAEW) on PTSD. We used a reverse pharmacology approach combining clinical data to search for mechanisms of PTSD with subsequent in vitro verification and bioinformatics techniques as follows: (1) by analyzing microarray-based transcriptome-wide mRNA expression profiling of PTSD patients; (2) by investigating the effect of FAEW and the antidepressant control drug fluoxetine on the transcription factor NF-κB using reporter cell assays and western blotting; (3) by performing molecular docking and literature data mining based on phytochemical constituents of FAEW. The results suggest an involvement of inflammatory processes mediated through NF-κB in the progression of PTSD. FAEW was non-cytotoxic in vitro and inhibited NF-κB activity and p65 protein expression. FAEW's anti-inflammatory compounds, i.e., paeoniflorin, isoliquiritin, isoliquiritin apioside and ononin were evaluated for binding to IκK and p65-RelA in a molecular docking approach. Paeoniflorin, albiflorin, baicalin, isoliquiritin and liquiritin have been reported to relieve depression in vivo or in clinical trials, which might be the active ingredients for FAEW against PTSD.
Keywords: free and easy wanderer, inflammation, NF-κB, pharmacognosy, posttraumatic stress disorder
Introduction
During the past decades, the developments in science and technology have driven medical research further toward “disease-based” approaches, which may be critical for some chronical mental diseases, since no significant morphological changes can be observed, while patients are suffering for a long time. In 1977, a bio-psycho-social medical theory was postulated by the psychiatrist George L. Engel at the University of Rochester (White, 2005). He suggested holistic approaches to handle medical issues, because health is a comprising status of physical, mental and social well-being and not merely the absence of diseases or infirmity, indicating the new direction to look for new approaches from complementary medicine.
With a specific philosophical background and treatment principles, traditional Chinese medicine (TCM) emphasizes the balance of yin and yang to achieve harmony between body, mind and soul. With thousands of years of clinical practice and written documentation, TCM is especially well suited to treat difficult and complex diseases. The success should be partly attributed to diverse medicinal plants, which have been a valuable source of therapeutic agents in the treatment of cancer, neurodegenerative diseases and malaria (Houghton and Howes, 2005; Kong and Tan, 2015; Khandelwal et al., 2016). The enormous structural and chemical diversity of natural products are evolutionarily optimized for serving different biological functions (Mishra and Tiwari, 2011). In addition, the traditional knowledge on plants and well documented ethno-pharmacological information can be taken as reliable hints for further pharmacological research.
Free and Easy Wanderer (FAEW), is a Chinese herb formula, used in China for hundreds of years in the treatment of mood disorders, and in vivo results proved to reverse anxiety-like behavior and cognitive impairments after stress exposure (Wang et al., 2009). To explain how herbal formulae work in human body against mental diseases, some challenges should be discussed:
Mental diseases appear to us with dissociation symptoms and loss of capabilities, such as the interruption of the normally integrative functions of consciousness, memory, the identity, or perception of the environment. There are no significant pathology and morphological changes, which indicates that it may be difficult to identify suitable cellular targets for treatment. The current research mainly focuses on the investigation between neural circuits on fear conditioning and extinction (Rey et al., 2014), epigenetic changes on the hypothalamus-pituitary (HPA) axis (Vukojevic et al., 2014) and various genetic polymorphisms, e.g., FKBP5, 5-HTTLPR, DRD2 (Kolassa et al., 2010; Walsh et al., 2014; Wilker et al., 2014). Until now, no specific pathogenic mechanism has been identified, although some progress was achieved. To solve this problem, genomic analyses of clinical patients may be one potential approach to gain insight into the pathophysiology of such diseases.
Chinese herbal formulae are poly-herbal preparations, frequently consisting of dozens to hundreds of chemical compounds, which might increase safety risks and hamper the agreement on their clinical usage, especially in western countries. To understand how they work in the human body, the molecular mechanisms should be clarified and possible active compounds have to be identified for further drug development.
In this paper, we first analyzed microarray-based transcriptome-wide mRNA expression profiles of patients suffering from posttraumatic stress disorder (PTSD). Based on gene network analyses and associated binding motifs of transcription factors in gene promoters, we hypothesized that inflammatory process may represent a major cause of PTSD. We investigated the cytotoxicity of FAEW and compared its effect with the antidepressant drug fluoxetine on inflammation by assessing the activity of NF-κB and the protein expression of p65. Furthermore, we identified the active compounds of FAEW by molecular docking in silico. Finally, we compared our results with reports in the literature and confirmed some pharmaceutical activities of the compounds.
Materials and methods
Gene expression profiling and network analysis of PTSD patients
Gene expression profiling from PTSD patients were searched from Pubmed, GEO dataset and Google Scholar database with the key word “PTSD” and “gene expression profiling.” Among all the results, only four were related to our analysis with data available (Segman et al., 2005; Su et al., 2008; Neylan et al., 2011; Tylee et al., 2015). Among the datasets we have chosen for our study, three were derived from blood samples (Segman et al., 2005; Neylan et al., 2011; Tylee et al., 2015), and one was originated from post mortem collected brain tissue biopsies (Su et al., 2008). To allow comparisons between the four datasets, consistent fold-change ratios were calculated between the control and PTSD groups. Gene symbols (or IDs) and fold-change values were uploaded into the Ingenuity Pathway Analysis (IPA) software (Jimenez-Marin et al., 2009) to determine canonical signal transduction pathways, gene functions and signaling networks predicted by dysregulated gene expressions.
Binding motif search for transcription profiles in gene promoter sequences
Transcription factor binding site analyses were performed by the Cistrome analysis software (Liu et al., 2011). Briefly, regulated genes were input and BED formats, a tab-delimited text file defining data lines displayed in an annotation track, were retrieved with an upstream setting (promoter region) at 2 kb (Karolchik et al., 2014). SeqPos motif analyses were used to screen for enriched motifs in given regions (http://cistrome.org). Using SeqPos, we scanned all the motifs not only in Transfac, JASPAR, UniPROBE (pbm), hPDI database, but also attempted to identify de novo motifs using MDscan algorithm. The output of genes was ranked by −10×log(p-value).
Chemicals and extracts
The FAEW extract was prepared from commercial pills (Xiaoyao wan) purchased from Wanxi Pharmaceutical Company (Henan Province, China). They were dissolved in H2O: MeOH: DCM in a ratio of 1: 4: 5 for 3 days. A rotary evaporator was used to remove the solvents and the final extracts were stored at −20°C. Fluoxetine was purchased from Sigma-Aldrich (Steinheim, Germany). Tumor necrosis factor-α (TNF-α) was purchased from Sino Biological Inc. (Peking, China). Quanti-Blue was purchased from Invitrogen and prepared according to the instructions of the manufacture.
Cell cultures of T98G brain cells
T98G brain cells were obtained from the German Cancer Research Center (DKFZ, Heidelberg, Germany). The original source of this cell line is the American Type Culture Collection (ATCC® number: CRL-1690™). T98G cells were cultured under standard conditions (37°C, 5% CO2) in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were passaged twice a week. All experiments were performed with logarithmically growing cells.
Cell viability assay
Cell viability was evaluated by the resazurin assay. One hundred microliters of cell suspension were sowed into 96-well plates 1 day before the treatment with different concentrations of FAEW and fluoxetine. After 72 h, 20 μl resazurin (Sigma-Aldrich, Germany) 0.01% w/v in ddH2O was added to each well and the plates were incubated at 37°C for 4 h. The fluorescence was measured with an Infinite M200 Proplate Reader (Tecan, Crailsheim, Germany) using an excitation wavelength of 544 nm and an emission wavelength of 590 nm. The cytotoxic effect of the treatment was determined as a percentage compared with the untreated cells after reducing the background value caused by the medium.
NF-κB reporter assay
HEK293 cells stably expressing the HEK-Blue-Null vector and secreted embryonic alkaline phosphatase (SEAP) reporter gene on NF-κB promoter were purchased from Invitrogen. The cells were cultured according to the recommendations from the company and passaged twice per week. The cells were treated with different concentrations of FAEW (60, 200, 600, and 2000 μg/ml) and fluoxetine (2, 6, and 20 μM) for 24, 48, or 72 h followed by TNF-α induction for 3 h. MG-132 was used as a positive control with treatment for only 1 h. The fluorescence was measured with an Infinite M200 Proplate Reader (Tecan, Crailsheim, Germany) with a wavelength of 630 nm. Background noise caused by the culture medium was subtracted from all wells. The inhibition effect of FAEW and fluoxetine toward NF-κB activity were calculated by comparison with untreated control on the basis of TNF-α induction.
Western blotting
T98G cells were treated with FAEW and fluoxetine for 24, 48, and 72 h, MG-132 for 1 h, followed by TNF-α induction for 3 h. MG-132 was used as positive control drug. Nuclear protein extracts were prepared according to the NE-PER nuclear and cytoplasmic extraction reagent (Thermo Scientific, USA) supplemented with EDTA-free Halt Protease Inhibitor Cocktail (Thermo Scientific). Protein concentrations were measured with Nano drop 1000 spectrophotometry (Thermo Scientific). The densities of the protein bands were quantified by FluorChemQ software (Biozym Scientific Company, Oldendorf, Germany). Nuclear p65 levels were determined with an anti-NF-κB monoclonal antibody (1:3000, Cell signaling). Histone H3 protein levels served as the internal control, using the anti-Histone H3 monoclonal antibody (1:3000, Cell Signaling). The inhibition effects of FAEW and fluoxetine toward p65 protein expression were calculated by comparison with untreated TNF-α induction group after using the control group without TNF-α for the quantification of western blots.
Molecular docking
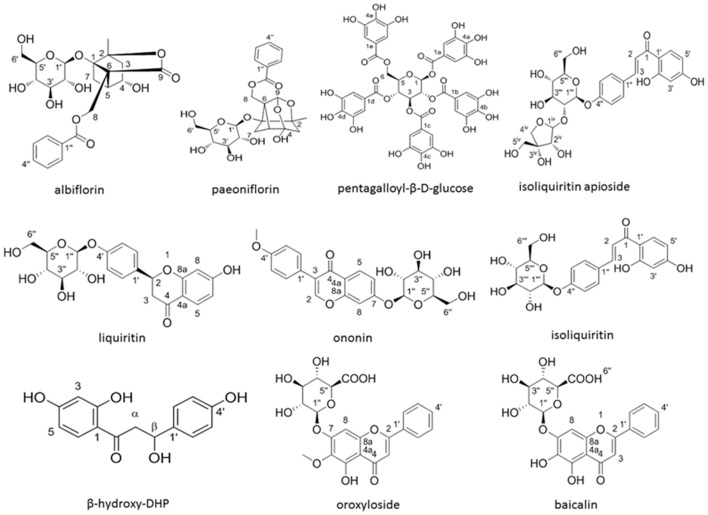
The ligands were selected according to the compounds in the FAEW extract identified by us using 2D-NMR- and HPLC-MS techniques (Figure 1). Together with the anti-depressant drug fluoxetine and MG-132, 12 ligands were evaluated for their binding energies to different proteins of the NF-κB pathway predicted in silico. PDB files of proteins were downloaded from RCSB Protein Data Bank (http://www.rcsb.org/pdb/home/home.do). The defined residues were chosen from the previous work in our laboratory (Kadioglu et al., 2016). The known inhibitor for IκK, MG-132 (PubChem ID: 462382) was selected as a standard to compare its binding modes with other compounds (Wang et al., 2013). 3D structures of these compounds were downloaded from PubChem, ChemSpider was used to convert mol files to pdb file after checking absolute and relative configuration. Molecular docking was then carried out with Auto-Dock 4.2 (The Scripps Research Institute, La Jolla, CA) following a protocol previously reported by us (Qiaoli Zhao et al., 2013). VMD (Visual Molecular Dynamics) was used for visualization of the binding modes obtained from docking. The average of the lowest binding energy of three runs was taken into account.
Figure 1.
The structures of 10 compounds isolated from FAEW.
Statistical analysis
T-test was used to calculate the significance with SPSS statistics 22, i.e., p-values between treatment groups and control group of three independent experiments are shown (*p < 0.05, **p < 0.01).
Literature review
The PubMed database was searched with the corresponding compound name as key word. Identified literature, which were related to the pharmacological activities of the compound, was classified into in vitro, in vivo or clinical trial reports to give a retrospective summary of the current state of knowledge. To search the published studies of the compounds from FAEW on NF-κB, NF-κB and the corresponding compound name were used as the key words.
Results
Canonical pathways, diseases and functions, and networks
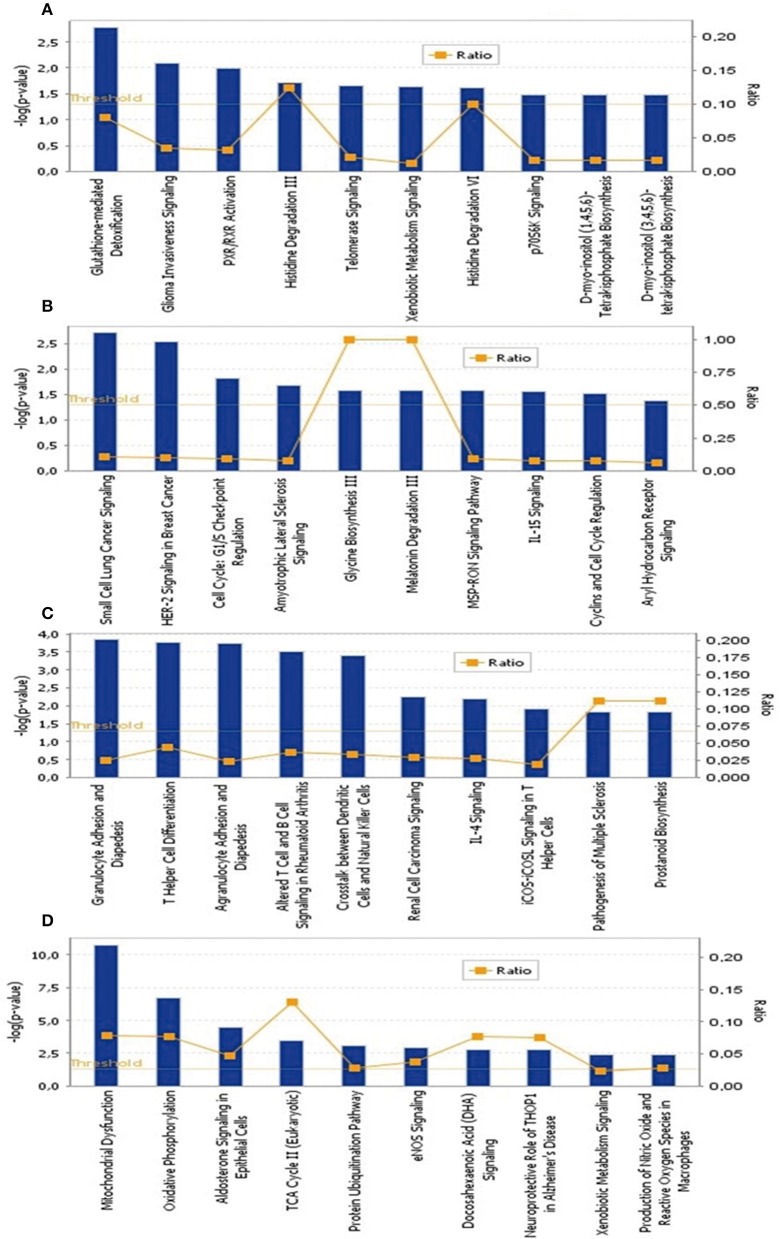
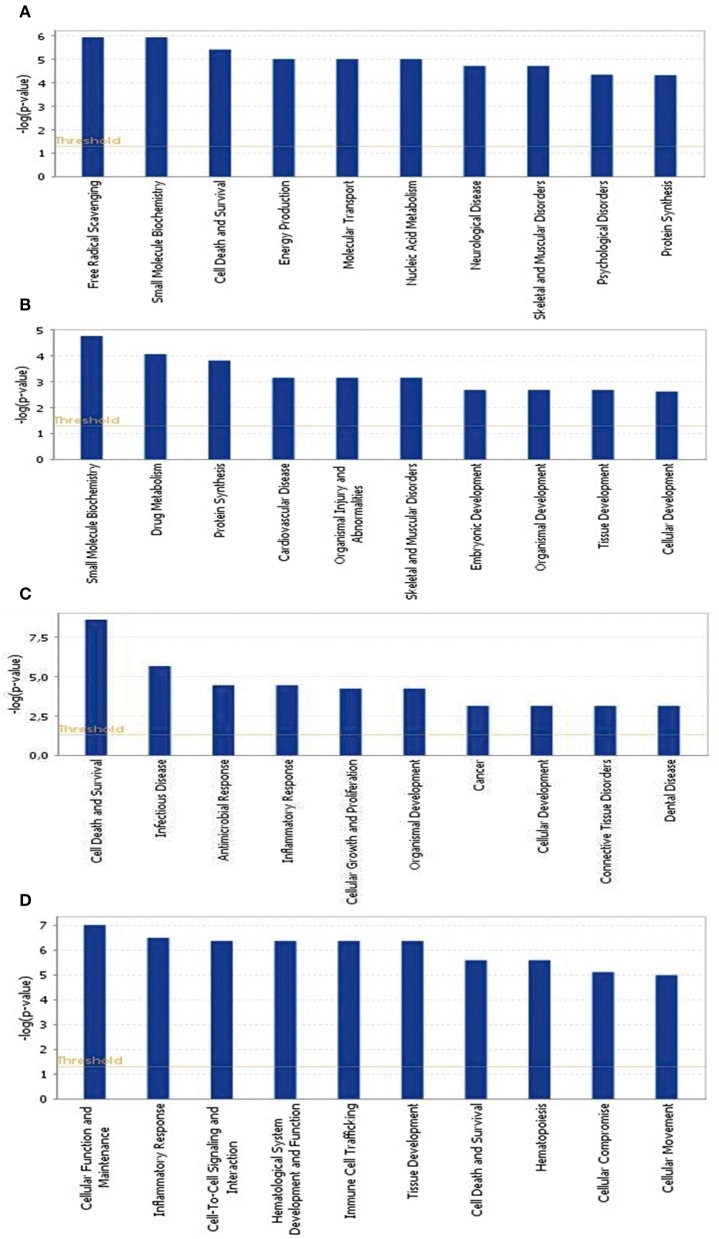
To identify the common pathways related to PTSD, the top 10 canonical pathways of each of the four datasets were displayed by means of IPA analyses (Figure 2). In datasets 1 and 2, the gene profiles were involved with the cancer and tumor pathway. In dataset 3, cellular pathways “change of immune system” and “monocyte among the immune system” were significantly related. In dataset 4, the main pathways occurred in mitochondria, such as “mitochondrial dysfunction” and “oxidative phosphorylation.” Figure 3 shows the top 10 diseases and functions related to the dysregulated genes. Cellular dysfunctions, inflammation and free radical scavenging were highly involved. Cardiovascular diseases, cancer, organismal development and neurological diseases were also predicted by the dysregulated genes. Network analyses were performed to investigate the relationship between the dysregulated genes (Figure 4). Among the four datasets, NF-κB played a central role in all networks of dysregulated genes.
Figure 2.
The top 10 canonical pathways significantly affected by PTSD. (A) Blood-based gene-expression according to [15]; (B) Peripheral blood mononuclear cell gene expression profiles according to [16]; (C) Monocyte gene expression profiles according to [13]; (D) Post mortem brain biopsy gene expression profiles according to [14].
Figure 3.
Top 10 diseases and functions significantly affected by PTSD. (A) Blood-based gene-expression according to [15]; (B) Peripheral blood mononuclear cell gene expression profiles according to [16]; (C) Monocyte gene expression profiles according to [13]; (D) Post mortem brain biopsy gene expression profiles according to [14].
Figure 4.
Deregulated genes among PTSD patients. Red colored genes were up-regulated, green colored ones were down-regulated. The arrows indicted effects of deregulated genes on other genes. Continuous lines show direct interactions, dotted lines indirect interactions. (A) Blood-based gene-expression according to [15]; (B) Peripheral blood mononuclear cell gene expression profiles according to [16]; (C) Monocyte gene expression profiles according to [13]; (D) Post mortem brain biopsy gene expression profiles according to [14].
Analysis of binding motifs for transcription factors in gene promoters
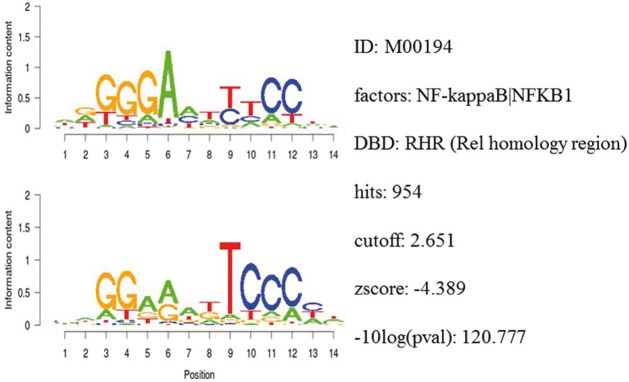
Integrative gene promoter analyses were performed to investigate common binding motifs for transcription factors in the promoter sequences of dysregulated genes among the datasets. Table 1 shows the top ranked transcription factors. Among them was the NF-κB binding motif with a z-score of −4.39. Figure 5 shows detailed information for NF-κB.
Table 1.
The most pronounced gene promoter binding motifs depending on the integrative analysis of Galaxy/Cistrome.
| ID | Factor | Hits | Cutoff | Zscore | −10×log(p-value) |
|---|---|---|---|---|---|
| 1 | IKZF2|Ikzf2 | 33 | 8.733 | −5.25 | 163.693 |
| 2 | CAT8 | 21 | 8.035 | −5.16 | 159.248 |
| 3 | CCDC16 | 70 | 6.592 | −5.09 | 155.157 |
| 4 | HOXA1 | 285 | 5.666 | −4.94 | 147.672 |
| 5 | HOXD10|Hoxd10 | 175 | 7.493 | −4.90 | 145.427 |
| 6 | Meox1 | 235 | 6.41 | −4.87 | 144.123 |
| 7 | E2F1::TFDP1 | 569 | 4.626 | −4.85 | 143.061 |
| 8 | Sfpi1 | 179 | 6.952 | −4.72 | 136.281 |
| 9 | Mox1|MEOX1|CD200|NOX1 | 245 | 6.333 | −4.66 | 133.664 |
| 10 | CBFA2T2 | 233 | 4.945 | −4.65 | 133.291 |
| 11 | DAL81 | 72 | 4.577 | −4.61 | 130.914 |
| 12 | Etv1 | 137 | 6.035 | −4.60 | 130.538 |
| 13 | Hoxa1 | 267 | 5.713 | −4.57 | 129.11 |
| 14 | FOXP4 | 211 | 5.907 | −4.55 | 128.263 |
| 15 | Nkx1-1 | 104 | 6.226 | −4.54 | 127.587 |
| 16 | lhx6.1|LHX6 | 499 | 4.368 | −4.43 | 122.535 |
| 17 | MET4 | 340 | 5.212 | −4.41 | 121.631 |
| 18 | NF-κB |NFKB1 | 954 | 2.651 | −4.39 | 120.777 |
| 19 | ACE2 | 537 | 6.774 | −4.25 | 114.451 |
| 20 | Muscle TATA box | 1242 | 1.144 | −4.23 | 113.4 |
Bold values are possible transcriptional motif related to NF-κB shown in the networks analysis.
Figure 5.
Detailed information of the NF-κB binding motif in gene promoter sequences.
Cytotoxicity of FAEW
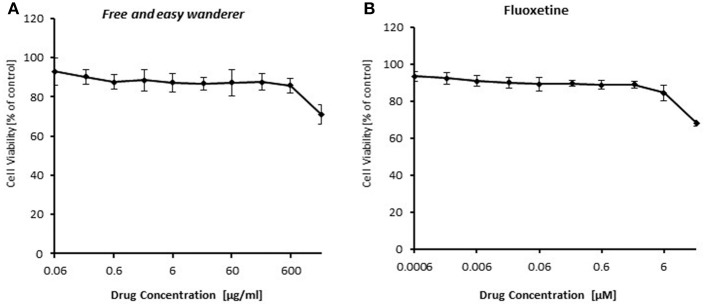
To identify the role of NF-κB in the acting model of FAEW, firstly, resazurin assays in HEK293 cells were performed to investigate, whether or not FAEW reveals cytotoxic effects. As shown in Figure 6, FAEW was indeed not cytotoxic at concentrations up to 2000 μg/ml. For comparison, fluoxetine was non-toxic up to 6 μM and inhibited HEK293 cells at higher concentrations.
Figure 6.
Cytotoxicity of FAEW (A) and fluoxetine (B) as determined by the resazurin assay. Shown are mean values ± SD of three independent experiments.
Inhibition of NF-κB by FAEW
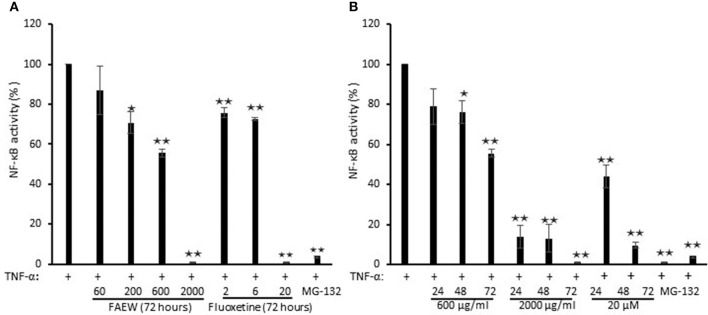
To investigate whether FAEW affects PTSD through NF-κB-mediated inflammatory effects, NF-κB reporter cell assays were performed. As shown in Figure 7, FAEW showed a dose-dependent inhibition and significantly inhibited NF-κB activity at higher concentrations. In addition, the inhibitory effect strengthened in a time-dependent manner from 24 to 72 h, reaching the similar inhibitory level caused by MG-132. Fluoxetine revealed the same trend as FAEW.
Figure 7.
Inhibition of NF-κB activity by FAEW and fluoxetine in HEK293 reporter cells. (A) Concentration kinetics and (B) time kinetics shown are mean values ± SD of three independent experiments. *p < 0.05, **p < 0.01. The inhibition effects of FAEW and fluoxetine toward NF-κB activity were calculated by comparison with untreated TNF-α induction group.
Inhibition of P65 expression by FAEW
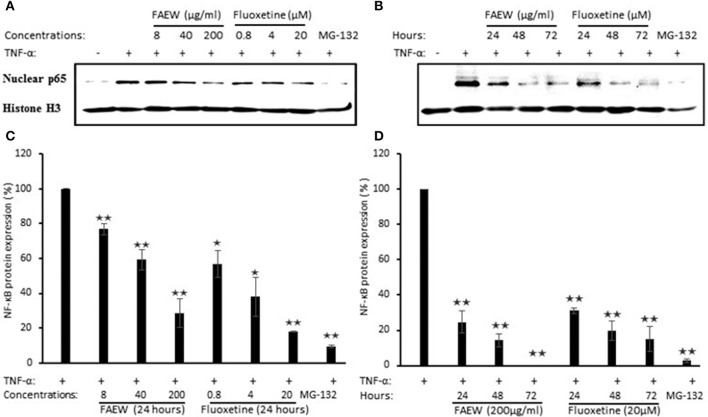
To further confirm the inhibition of NF-κB, western blot assays were performed to investigate the role of FAEW on p65 protein expression in T98G brain cells. According to the cytotoxic assays in Figure 8, three non-cytotoxic concentrations (200, 40, and 8 μg/ml) were selected. As shown in Figures 8A,C, with treatment for 24 h, both FAEW and fluoxetine inhibited p65 expression in a dose-dependent manner. Fixed concentrations of 200 μg/ml FAEW or 20 μM fluoxetine inhibited p65 expression in a time-dependent manner from 24 to 72 h (Figures 8B,D).
Figure 8.
Inhibition of p65 expression by FAEW and fluoxetine in T98G brain cells. (A) Concentration kinetics and (B) time kinetics. Histone was used as loading control. (C,D) show the quantification of western blots shown in (A,B), respectively. The inhibition effects of FAEW and fluoxetine toward p65 protein expression were calculated by comparison with untreated TNF-α induction group after using the control group without TNF-α for the quantification of western blots. Shown are mean values ± SD of three independent experiments. *p < 0.05, **p < 0.01.
Molecular docking of compounds from FAEW to the proteins of the NF-κB pathway
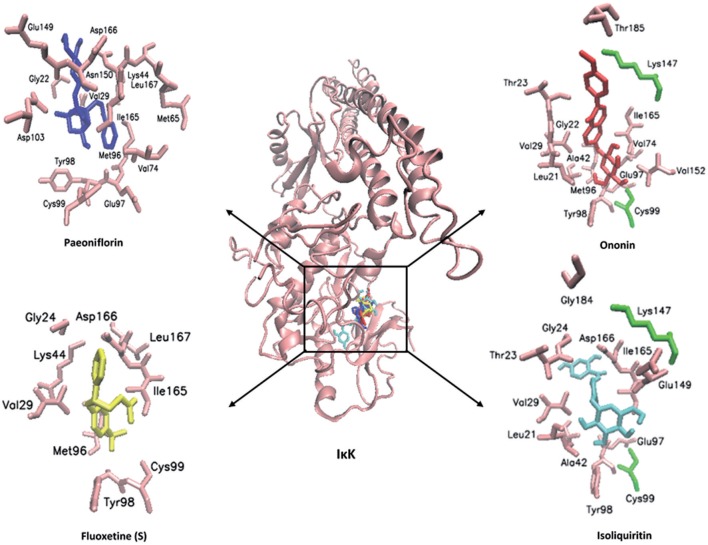
In order to explore possible interactions of compounds known to be in FAEW with the proteins of the NF-κB pathway, in silico molecular docking analysis were performed with 10 compounds (shown in Figure 1) in the remedy to IκK-NEMO, IκK and p65-RelA. As shown in Tables 2, 3, paeoniflorin and ononin bound to IκK and p65-RelA with low binding energies, which were comparable to the binding of MG-132. In addition, isoliquiritin apioside, albiflorin, baicalin, isoliquiritin, liquiritin and oroxyside mainly bound to p65-RelA through DNA and ATP binding sites (Figures 9, 10). The control fluoxetine (S) bound to p65-RelA with high affinity.
Table 2.
Binding energies of molecular docking of chemical compounds of FAEW to proteins of the NF-κB pathway.
| Ligand | IκK-NEMO | IκK | p65-Rel A | |||
|---|---|---|---|---|---|---|
| Lowest binding energy (kcal/mol) | pKi (μM) | Lowest binding energy (kcal/mol) | pKi (μM) | Lowest binding energy (kcal/mol) | pKi (nM) | |
| Baicalin | −3.79 ± 0.50 | 1690 ± 155.56 | −5.97 ± 0.04 | 42.47 ± 2.43 | −9.68 ± 0.09 | 79.88 ± 11.99 |
| β-hydroxy-DHP | −5.04 ± 0.05 | 203.52 ± 18.09 | −5.87 ± 0.18 | 52.24 ± 14.46 | −8.54 ± 0.04 | 553.70 ± 33.52 |
| Isoliquiritin | −3.59 ± 0.17 | 2390.00 ± 692.97 | −7.04 ± 0.08 | 6.92 ± 0.98 | −9.97 ± 0.22 | 61.00 ± 4.24 |
| Isoliquiritin apioside | −1.20 ± 0.12 | 132.78 ± 28.65 | −6.90 ± 0.15 | 9.00 ± 2.28 | −10.10 ± 0.74 | 56.59 ± 8.56 |
| Liquiritin | −4.12 ± 0.28 | 1155.00 ± 247.49 | −6.10 ± 0.19 | 34.91 ± 11.08 | −9.20 ± 0.04 | 180.31 ± 14.99 |
| Ononin | −3.82 ± 0.02 | 1600.00 ± 70.71 | −7.33 ± 0.01 | 4.24 ± 0.07 | −11.55 ± 0.08 | 3.43 ± 0.50 |
| Oroxyloside | −3.32 ± 0.08 | 3690.00 ± 551.54 | −6.16 ± 0.04 | 30.84 ± 1.90 | −9.41 ± 0.02 | 127.50 ± 4.95 |
| Fluoxetine (R) | −4.35 ± 0.05 | 652.97 ± 54.98 | −5.61 ± 0.06 | 77.65 ± 8.25 | −7.35 ± 0.07 | 4093.33 ± 475.01 |
| Fluoxetine (S) | −4.14 ± 0.05 | 928.59 ± 79.41 | −5.90 ± 0.03 | 47.15 ± 2.48 | −7.63 ± 0.03 | 2543.33 ± 123.42 |
| Albiflorin | −5.05 ± 0.30 | 211.37 ± 52.00 | −6.63 ± 0.16 | 14.23 ± 3.77 | −10.66 ± 0.50 | 23.85 ± 3.12 |
| Paeonflorin | −4.41 ± 0.04 | 591.13 ± 29.66 | −7.83 ± 0.21 | 2.09 ± 0.27 | −11.25 ± 0.03 | 5.45 ± 0.07 |
| Pentagalloyl-β-D-glucose | −0.67 ± 0.01 | 320580.00 ± 150.00 | −2.39 ± 0.49 | 19090.00 ± 3490.00 | −6.09 ± 0.21 | 35.50 ± 1.27 |
| MG-132 | −3.50 ± 0.63 | 47053.00 ± 1090.00 | −7.75 ± 0.35 | 2.31 ± 0.33 | −9.07 ± 0.29 | 172.10 ± 27.01 |
IκK, IκB kinase; IκK, NEMO. NF-κB essential modulator; NF-κB, nuclear factor kappa; light, chain-enhancer of activated B cells.
Figure 9.
Visualization of molecular docking of chemical compounds isolated from FAEW to IκK.
Figure 10.
Visualization of molecular docking of chemical compounds isolated from FAEW to p65-RelA.
Table 3.
Hydrogen bonds and amino acid residues identified by molecular docking of chemical compounds of FAEW to proteins of the NF-κB pathway.
| Protein | Ligand | Residues making H bonds | Residues involved in hydrophobic interactions |
|---|---|---|---|
| IκK | Baicalin | Thr23, Val29, Ala42, Lys44, Met65, Met96, Glu97, Tyr98, Cys99, Asp103, Val152, Ile165, Asp166, Leu167 | |
| β-hydroxy-DHP | Lys44 | Thr23, Val29, Ala42, Lys44, Met96, Glu97, Ile165, Asp166, Leu167 | |
| Isoliquiritin | Cys99, Lys147 | Leu21, Thr23, Gly24, Val29, Ala42, Glu97, Tyr98, Cys99, Lys147, Glu149, Ile165, Asp166, Gly184 | |
| Isoliquiritin apioside | Lys44, Cys99 | Thr23, Gly24, Val29, Ala42, Lys44, Met96, Glu97, Tyr98, Cys99, Lys147, Glu149, Ile165, Asp166, Leu167, Gly184 | |
| Liquiritin | Lys147 | Leu21, Thr23, Gly24, Ala42, Glu97, Cys99, Lys147, Glu149, Val152, Ile165, Asp166 | |
| Ononin | Cys99, Lys147 | Leu21, Gly22, Thr23, Val29, Ala42, Val74, Met96, Glu97, Tyr98, Cys99, Lys147, Val152, Ile165, Thr185 | |
| Oroxyloside | Cys99 | Thr23, Val29, Ala42, Lys44, Met65, Met96, Glu97, Tyr98, Cys99, Asp103, Glu149, Val152, Ile165, Asp166, Leu167 | |
| Fluoxetine (R) | Gly24, Val29, Ala42, Lys44, Met65, Met96, Tyr98, Ile165, Asp166, Leu167 | ||
| Fluoxetine (S) | Gly24, Val29, Lys44, Met96, Tyr98, Cys99, Ile165, Asp166, Leu167 | ||
| Albiflorin | Cys99 | Gly22, Val29, Lys44, Met 65, Val74, Met96, Glu97, Tyr98, Cys99, Asp103, Glu149, Asn150, Ile165, Asp166, Leu167 | |
| Paeoniflorin | Gly22, Val29, Lys44, Met65, Val74, Met96, Glu97, Tyr98, Cys99, Asp103, Glu149, Asn150, Ile165, Asp166, Leu167 | ||
| Pentagalloyl-β-D-glucose | Glu100 | Leu21, Gly22, Thr23, Val29, Ala42, Lys44, Glu61, Met65, Val73, Val74, Ala76, Leu94, Met96, Tyr98, Cys99, Glu100, Gly102, Asp103, Glu149, Asn150, Ile151, Val152, Ile165, Asp166, Leu167 | |
| MG-132 | Leu21, Thr23, Val29, Ala42, Met65, Val73, Val74, Met96, Glu97, Tyr98, Asp103, Val152, Ile164, Ile165, Asp166, Leu167 | ||
| p65-RelA | Baicalin | DA18, Lys122 | DT8, DT9, DT10, DA18, DG19, DT20, DC21, Lys122, Arg124 |
| β-hydroxy-DHP | DG19 | DT8, DT9,DT10, DA18, DG19, DT20, DC21 | |
| Isoliquiritin | DA18, DG19 | DC7, DT8, DT9, DT10, DA18, DG19, DT20, DC21, Lys123 | |
| Isoliquiritin apioside | DA18 | DT8, DT10, DA18, DG19, DC21 | |
| Liquiritin | DG19 | DT8, DT9, DA18, DG19, DT20, DC21, Lys123 | |
| Ononin | DG19 | DC7, DT8, DT9, DT10, DA18, DG19, DT20, DC21, Lys445 | |
| Oroxyloside | DG19, Lys122, Arg124 | DT8, DT9, DT10, DG19, DT20, DC21, Lys122, Lys123, Arg124 | |
| Fluoxetine (R) | DA18, DG19 | DC7, DT8, DT9, DT10, DA18, DG19, DT20, DC21 | |
| Fluoxetine (S) | DG19 | DC7, DT8, DT9,DT10, DG19, DT20, DC21 | |
| Albiflorin | DG19 | DC7, DT8, DT9, DT10, DA18, DG19, DT20, DC21, Arg124 | |
| Paeoniflorin | DG19 | DC7,DT8, DT9, DT10, DA18, DG19, DC21, DC22, Arg124 | |
| Pentagalloyl-β-D-glucose | DG19 | DC7, DC8, DT9, DG19, DT20, DC21, DC22, Lys123, Arg124 | |
| MG-132 | DG19 | DA6, DC7, DT8, DT9, DT10, DA18, DG19, DT20, DC21, DC22, Lys123 |
Literature review on the pharmacological activity of the compounds from FAEW
To further characterize the therapeutic potential of the candidate compounds investigated by molecular docking, we performed a literature review of the current research on the isolated compounds of FAEW. As shown in Table 4, paeoniflorin, albiflorin and baicalin were reported to act in vitro against inflammation and oxidative stress, and to be effective in vivo against depression, and Parkinson's disease. Furthermore, clinical trials were carried out among herbal formulae in inflammation-related diseases, such as Japanese traditional recipe Shakuyakukanzoto, and the Chinese herbal formula Xiongshao capsule. In vivo studies on isoliquiritin and liquiritin showed their activity against depression and cognitive-related diseases, for instance, Parkinson's disease. As shown in Table 5, paeoniflorin, baicalin and liquiritin have been reported in many in vitro and in vivo studies to reduce inflammatory processes realted to numerous diseases through inhibiting NF-κB, which implies their role as constituents of FAEW acting against inflammatory reactions related to PTSD.
Table 4.
Literature review on the pharmacological activity of the compounds from FAEW.
| Compounds | Pharmacological activity | ||
|---|---|---|---|
| In vitro | In vivo | Clinical trial | |
| Paeoniflorin | Anti-inflammation and oxidative stress (Dong et al., 2015); neuroprotection (Mao et al., 2012); apoptosis (Yang et al., 2016a) | Parkinson's disease (Gu et al., 2016); depression (Huang et al., 2015); neuropathic pain (Zhou et al., 2016a); non-alcoholic steatohepatitis (Ma et al., 2016) | Rheumatoid arthritis (Chen et al., 2013); muscle cramps and abdominal pains (Sadakane et al., 2015); restenosis after percutaneous coronary intervention (Shang et al., 2011) |
| Albiflorin | Oxidative stress (Suh et al., 2013); neuroprotection (Kim et al., 2009) | Anti-depression (Song et al., 2015); Parkinson's disease (Ho et al., 2015); neuropathic pain (Zhou et al., 2016a; Zhang et al., 2016b) | Muscle cramps and abdominal pains (Sadakane et al., 2015) |
| Baicalin | Inflammation (Min et al., 2015); apoptosis (Li et al., 2015b); oxidative stress (Zheng et al., 2014) | Alcoholic liver injury (Wang et al., 2016b); depression/anxiety (Zhang et al., 2016a); renal damage (Zhang et al., 2016d); memory impairment (Wang et al., 2016c) | Ulcerative colitis (Yu et al., 2015) |
| Isoliquiritin | Anti-allergic activity (Kaur et al., 2009) | Depression (Wang et al., 2008); antifungal activity (Luo et al., 2016) | Depression (Su et al., 2014) |
| Liquiritin | Neuroprotection (Teng et al., 2014) | Cognitive deficits (Jia et al., 2016); Parkinson's disease (Huang et al., 2016); depression (Farahani et al., 2015); focal cerebral ischemia (Sun et al., 2010) | Melisma (Amer and Metwalli, 2000) |
| β-hydroxy-DHP | Apoptosis (Rafi et al., 2002) | ||
| Isoliquiritin apioside | Anti-genotoxic (Kaur et al., 2009) | ||
| Oroxyloside | Colitis (Wang et al., 2016d); inflammation-related diseases (Fong et al., 2015) | ||
Table 5.
Literature review of the effect of chemical constituents of FAEW against NF-κB.
| Compounds | Pharmacological activity on NF-κB | |
|---|---|---|
| In vitro | In vivo | |
| Paeoniflorin | Parkinson's disease (Liu et al., 2016a); immunomodulation (Zhai et al., 2016); Alzheimer's disease (Liu et al., 2015); apoptosis (Dong et al., 2015); morphine tolerance (Jiang et al., 2015); anti-inflammation and immunomodulation (Gan et al., 2014); sepsis (Zhang et al., 2014b); obesity (Kong et al., 2013); cardiac remodeling (Zhou et al., 2013) | Renal function (Liu et al., 2016c); non-alcoholic steatohepatitis(Ma et al., 2016); cardiac dysfunction (Zhai and Guo, 2016); vascular dementia (Zhang et al., 2015b); Alzheimer's Disease (Zhang et al., 2015a); arthritis (Yi, 2014); hepatitis (Chen et al., 2015); colitis (Zhang et al., 2014a); learning dysfunction and brain damage (Guo et al., 2012); lung injury (Zhou et al., 2011) |
| Baicalin | Myofibroblast differentiation (Shin et al., 2016); atherosclerosis (Wang et al., 2016a); haemophilus parasuis infection (Fu et al., 2016); mastitis (Guo et al., 2013); apoptosis (Yang et al., 2016b) | Asthma (Liu et al., 2016b); periodontitis (Sun et al., 2016); allergic diseases (Zhou et al., 2016b); arthritis (Wang et al., 2014); brain edema (Zhou et al., 2014); lung injury (Ding et al., 2016); liver injury (Wan et al., 2008); ischemic stroke (Singh and Chopra, 2014); cerebral ischemia (Tu et al., 2011) |
| Isoliquiritin | Inflammatory responses (Kim et al., 2008) | |
| Liquiritin | Endothelial dysfunction (Zhang et al., 2013) | Myocardial fibrosis (Zhang et al., 2016c); acute Lung Injury (Tao et al., 2016) |
| Oroxyloside | Colitis (Wang et al., 2016d) | |
Discussion
Posttraumatic stress disorder (PTSD) is a delayed and lasting psychological stress disorder after traumatic events. It was initially diagnosed among veterans of the Vietnam War. Subsequent studies indicated that victims of disasters were also potentially suffering from PTSD (Cavanagh et al., 2014). It has been calculated that life time prevalence of PTSD in adults is 7.8%, while women have higher risk than men (20.4 vs. 8.2%) despite experiencing fewer traumas (Kessler et al., 2005; Ditlevsen and Elklit, 2012). Pre-trauma factors, such as lower socioeconomic status, parental neglect and poor social support increase the risk (Hong and Efferth, 2015). In addition, more and more studies reported the correlation between PTSD and other physiological diseases, such as diabetes (Agyemang et al., 2012), cancer (Abbey et al., 2015) and cardiovascular diseases (Boscarino, 2008), or psychiatric diseases, such as depression and anxiety (Farr et al., 2014). In our studies, we analyzed the microarray-based transcriptome-wide mRNA expression profiling of patients with PTSD. As shown in the present investigation, the top 10 pathways and functions, dysregulated genes of PTSD patients were related to oxidative stress and inflammatory response, and involved with a wide array of psychological or physiological diseases, which might be taken as a general hint for common pathogenic factors between PTSD and metabolic syndrome, cardiovascular diseases, cancer, as well as psychological diseases, e.g., depression, suicidality and anxiety, implying that a holistic way to investigate PTSD might be one approach to achieve multiple-targets.
Increasing evidence indicated an involvement of immune system in fear- and anxiety-based disorders. Recent studies suggested that inflammation is associated with increased basal ganglia glutamate in patients in depression (Haroon et al., 2016). Furthermore, inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition, and suggesting that the gut microbiota-inflammasome-brain axis could be novel therapeutic targets for psychiatric disorders (Wong et al., 2016). In our studies, network analyses revealed that NF-κB was activated in both the peripheral and central nervous system. In addition, promoter binding motif search of genes revealed that NF-κB was among the most important transcription factors. These results indicated that NF-κB may be an important immunological component of inflammatory processes in PTSD. Therefore, we hypothesized a link between the therapeutic effect of FAEW on PTSD, and NF-κB as relevant underlying mechanism.
Free and Easy Wanderer (FAEW) is a poly-herbal preparation, which is widely used in Chinese clinics for the treatment of depression, premenstrual dysphoric disorder, climacteric syndrome, and Parkinson's disease induced by antipsychotic drugs. An in vivo study indicated that FAEW ameliorated PTSD-like behavior and cognitive impairments in stressed rats (Wang et al., 2009). Subsequent clinical studies also reported good efficacy, safety, and tolerability in post-stroke depression patients (Li et al., 2008). In our studies, we observed that FAEW had a wide safety range and showed a dose and time-dependent inhibition of NF-κB activity in HEK293 cells as well as of protein expression of NF-κB in T98G brain cells. Hence, FAEW might exert anti-anxiety effects through NF-κB-mediated anti-inflammatory process. Among a panel of 10 compounds, paeoniflorin, isoliquiritin, isoliquiritin apioside, and ononin exerted high affinity to IκK and p65-RelA. Albiflorin, baicalin, liquiritin, and oroxyloside were predicted to strongly bind to p65-RelA. Apart from lipophilic interactions, which widely occur among the interactions between lipophilic groups of molecules and the nonpolar side-chains of residues, such as Ile, Leu, Val, and Phe, hydrogen bonds are the main interaction force to promote molecules bind to proteins. For example, paeoniflorin exerted strong affinity with IκK and p65 through multiple hydrogen bonds involving the OH groups of its glucose moiety. The hemiacetal OH-group in the core (position 5) and the 3′- and 4′-OH of paeoniflorin were predicted to bind to IκK residues Thr23, Cys99 and Glu 97, respectively. In the interaction of the same compound with p65, the 5-OH and the glucose OH groups in positions 3′, 4′, and 6′ bound to the p65 residues DT9, DC22, DT8, and DC21, respectively. Besides, p65 residue DG19 donates a hydrogen bond to the benzoate carbonyl of paeoniflorin. The same type of interactions for the other compounds from FAEW can be applied to explain their activities toward NFκB, such as ononin, isoliquiritin. Furthermore, paeoniflorin, albiflorin, baicalin, isoliquiritin, and liquiritin were reported to be active against depression and Parkinson's disease in in vivo studies and clinical trials, and paeoniflorin, baicalin and liquiritin were reported to inhibit NF-κB in vitro and in vivo. These data demonstrate that FAEW is constituted by a wide array of diverse anti-depressant natural drugs with strong anti-inflammatory activity. Importantly, some compounds have been indeed demonstrated to pass the blood-brain barrier and reach brain tissue, e.g., albiflorin, paeoniflorin, liquiritin, which may might explain the effect of FAEW in the central nervous system (Li et al., 2015a). In addition, it is also quite interesting to observe that some compounds were used to treat colitis in vivo and in clinic studies, which might imply their participation in the balance of the gut microbiota-inflammasome-brain axis (Yu et al., 2015; Wang et al., 2016d).
Fluoxetine first emerged in the 1970s as selective serotonin uptake inhibitor for the treatment of depression due to its safer profile, fewer side effects, and greater tolerability than former drugs (Wilde and Benfield, 1998). Subsequent studies indicated that it also reveals strong anti-inflammatory properties, and its effect on NF-κB was recognized recently (Mackay et al., 2009; Cui et al., 2016). In addition to depression, clinical studies demonstrated that fluoxetine also significantly improved symptom clusters of PTSD (Martenyi and Soldatenkova, 2006). Therefore, in our study, fluoxetine was selected as standard control drug, and it was proved that fluoxetine decreased NF-κB levels, which contributes to the explanation of its effects in the treatment of PTSD.
Different from Western medicine with one drug- one target action model, TCM pursues a multi-target model in different organs and tissues of the body and achieved a lot in the application of acupuncture worldwide. Although Chinese herb formulae are still a matter of discussion, their clinical effects in Asian countries have a high reputation. In our studies, we observed that, with diverse active compounds, FAEW exerted strong anti-inflammatory effects toward NF-κB, which were comparable to the antidepressant drug, fluoxetine. It is safe and approachable for the treatment of inflammation-related diseases. A limitation of this investigation represents the lack of in vivo PTSD models to validate the effect of FAEW in more detail and investigate possible clinical effects. The literature review provided us some hints for the future studies, to confirm and investigate the activity of different components of FAEW. Future studies will focus on the performance of in vivo validation and clinical trials to further evaluate the therapeutic potential of FAEW to treat PTSD-related diseases.
Author contributions
TE came up the idea and designed the experiment. CH conducted the biological experiments. JC and CW assisted the bioinformatic stuff. ET and TO were responsible for the isolation and identification of the compounds, respectively. AS and UK performed the studies, accordingly.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, GRK 2015/1)-Research Training Group “Life Science-Life Writing” at Johannes Gutenberg University, Mainz. Financial support by the Carl Zeiss-foundation (project ChemBioMed) is gratefully acknowledged.
Glossary
Abbreviations
- FAEW
Free and Easy Wanderer
- HPLC
high pressure liquid chromatography
- IκK
inhibitor kappa B kinase
- IPA
ingenuity pathway analysis
- MS
mass spectrometry
- NF-κB
nuclear factor “kappa light chain enhancer” of activated B-cells
- NMR
nuclear magnetic resonance-mass spectrometry
- PTSD
posttraumatic stress disorder
- TCM
traditional Chinese medicine.
References
- Abbey G., Thompson S. B., Hickish T., Heathcote D. (2015). A meta-analysis of prevalence rates and moderating factors for cancer-related post-traumatic stress disorder. Psychooncology 24, 371–381. 10.1002/pon.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agyemang C., Goosen S., Anujuo K., Ogedegbe G. (2012). Relationship between post-traumatic stress disorder and diabetes among 105,180 asylum seekers in the Netherlands. Eur. J. Public Health 22, 658–662. 10.1093/eurpub/ckr138 [DOI] [PubMed] [Google Scholar]
- Amer M., Metwalli M. (2000). Topical liquiritin improves melasma. Int. J. Dermatol. 39, 299–301. 10.1046/j.1365-4362.2000.00943.x [DOI] [PubMed] [Google Scholar]
- Boscarino J. A. (2008). A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom. Med. 70, 668–676. 10.1097/PSY.0b013e31817bccaf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh S. R., Fitzgerald E. J., Urry H. L. (2014). Emotion reactivity and regulation are associated with psychological functioning following the 2011 earthquake, tsunami, and nuclear crisis in Japan. Emotion 14, 235–240. 10.1037/a0035422 [DOI] [PubMed] [Google Scholar]
- Chen L., Qi H., Jiang D., Wang R., Chen A., Yan Z., et al. (2013). The new use of an ancient remedy: a double-blinded randomized study on the treatment of rheumatoid arthritis. Am. J. Chin. Med. 41, 263–280. 10.1142/S0192415X13500195 [DOI] [PubMed] [Google Scholar]
- Chen M., Cao L., Luo Y., Feng X., Sun L., Wen M., et al. (2015). Paeoniflorin protects against concanavalin A-induced hepatitis in mice. Int. Immunopharmacol. 24, 42–49. 10.1016/j.intimp.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Cui J., Yang K., Yu X., Wang J. L., Li J., Zhang Y., et al. (2016). Chronic fluoxetine treatment upregulates the activity of the ERK1/2-NF-κB signaling pathway in the hippocampus and prefrontal cortex of rats exposed to forced-swim stress. Med. Princ. Pract. 25, 539–547. 10.1159/000449165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X. M., Pan L., Wang Y., Xu Q. Z. (2016). Baicalin exerts protective effects against lipopolysaccharide-induced acute lung injury by regulating the crosstalk between the CX3CL1-CX3CR1 axis and NF-κB pathway in CX3CL1-knockout mice. Int. J. Mol. Med. 37, 703–715. 10.3892/ijmm.2016.2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditlevsen D. N., Elklit A. (2012). Gender, trauma type, and PTSD prevalence: a re-analysis of 18 nordic convenience samples. Ann. Gen. Psychiatry 11:26. 10.1186/1744-859X-11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Li R., Yu C., Xu T., Zhang X., Dong M. (2015). Paeoniflorin inhibition of 6-hydroxydopamine-induced apoptosis in PC12 cells via suppressing reactive oxygen species-mediated PKCδ/NF- κB pathway. Neuroscience 285, 70–80. 10.1016/j.neuroscience.2014.11.008 [DOI] [PubMed] [Google Scholar]
- Farahani M. S., Bahramsoltani R., Farzaei M. H., Abdollahi M., Rahimi R. (2015). Plant-derived natural medicines for the management of depression: an overview of mechanisms of action. Rev. Neurosci. 26, 305–321. 10.1515/revneuro-2014-0058 [DOI] [PubMed] [Google Scholar]
- Farr O. M., Sloan D. M., Keane T. M., Mantzoros C. S. (2014). Stress- and PTSD-associated obesity and metabolic dysfunction: a growing problem requiring further research and novel treatments. Metab. Clin. Exp. 63, 1463–1468. 10.1016/j.metabol.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong S. Y., Wong Y. C., Xie C., Zuo Z. (2015). Herb-drug interactions between scutellariae radix and mefenamic acid: simultaneous investigation of pharmacokinetics, anti-inflammatory effect and gastric damage in rats. J. Ethnopharmacol. 170, 106–116. 10.1016/j.jep.2015.04.036 [DOI] [PubMed] [Google Scholar]
- Fu S., Xu L., Li S., Qiu Y., Liu Y., Wu Z., et al. (2016). Baicalin suppresses NLRP3 inflammasome and nuclear factor-kappa B (NF-κB) signaling during Haemophilus parasuis infection. Vet. Res. 47:80. 10.1186/s13567-016-0359-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y., Cui X., Ma T., Liu Y., Li A., Huang M. (2014). Paeoniflorin upregulates β-defensin-2 expression in human bronchial epithelial cell through the p38 MAPK, ERK, and NF-κB signaling pathways. Inflammation 37, 1468–1475. 10.1007/s10753-014-9872-7 [DOI] [PubMed] [Google Scholar]
- Gu X., Cai Z., Cai M., Liu K., Liu D., Zhang Q., et al. (2016). Protective effect of paeoniflorin on inflammation and apoptosis in the cerebral cortex of a transgenic mouse model of Alzheimer's disease. Mol. Med. Rep. 13, 2247–2252. 10.3892/mmr.2016.4805 [DOI] [PubMed] [Google Scholar]
- Guo M., Zhang N., Li D., Liang D., Liu Z., Li F., et al. (2013). Baicalin plays an anti-inflammatory role through reducing nuclear factor-κB and p38 phosphorylation in S. aureus-induced mastitis. Int. Immunopharmacol. 16, 125–130. 10.1016/j.intimp.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Guo R. B., Wang G. F., Zhao A. P., Gu J., Sun X. L., Hu G. (2012). Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses. PLoS ONE 7:e49701. 10.1371/journal.pone.0049701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E., Fleischer C. C., Felger J. C., Chen X., Woolwine B. J., Patel T., et al. (2016). Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatry 21, 1351–1357. 10.1038/mp.2015.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. L., Poon C. Y., Lin C., Yan T., Kwong D. W., Yung K. K., et al. (2015). Inhibition of β-amyloid aggregation by albiflorin, aloeemodin and neohesperidin and their neuroprotective effect on primary hippocampal cells against β-amyloid induced toxicity. Curr. Alzheimer Res. 12, 424–433. 10.2174/1567205012666150504144919 [DOI] [PubMed] [Google Scholar]
- Hong C., Efferth T. (2015). Systematic review on post-traumatic stress disorder among survivors of the wenchuan earthquake. Trauma Violence Abuse 17, 542–561. 10.1177/1524838015585313 [DOI] [PubMed] [Google Scholar]
- Houghton P. J., Howes M. J. (2005). Natural products and derivatives affecting neurotransmission relevant to Alzheimer's and Parkinson's disease. Neurosignals 14, 6–22. 10.1159/000085382 [DOI] [PubMed] [Google Scholar]
- Huang H., Zhao J., Jiang L., Xie Y., Xia Y., Lv R., et al. (2015). Paeoniflorin improves menopause depression in ovariectomized rats under chronic unpredictable mild stress. Int. J. Clin. Exp. Med. 8, 5103–5111. [PMC free article] [PubMed] [Google Scholar]
- Huang X., Wang Y., Ren K. (2016). Protective effects of liquiritin on the brain of rats with Alzheimer's disease. West Indian Med. J. 64, 468–472. 10.7727/wimj.2016.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S. L., Wu X. L., Li X. X., Dai X. L., Gao Z. L., Lu Z., et al. (2016). Neuroprotective effects of liquiritin on cognitive deficits induced by soluble amyloid-β1-42 oligomers injected into the hippocampus. J. Asian Nat. Prod. Res. 18, 1186–1199. 10.1080/10286020.2016.1201811 [DOI] [PubMed] [Google Scholar]
- Jiang C., Xu L., Chen L., Han Y., Tang J., Yang Y., et al. (2015). Selective suppression of microglial activation by paeoniflorin attenuates morphine tolerance. Eur. J. Pain 19, 908–919. 10.1002/ejp.617 [DOI] [PubMed] [Google Scholar]
- Jimenez-Marin A., Collado-Romero M., Ramirez-Boo M., Arce C., Garrido J. J. (2009). Biological pathway analysis by ArrayUnlock and Ingenuity Pathway Analysis. BMC Proc. 3(Suppl. 4), S6. 10.1186/1753-6561-3-s4-s6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadioglu O., Jacob S., Bohnert S., Nass J., Saeed M. E., Khalid H., et al. (2016). Evaluating ancient Egyptian prescriptions today: anti-inflammatory activity of Ziziphus spina-christi. Phytomedicine 23, 293–306. 10.1016/j.phymed.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Karolchik D., Barber G. P., Casper J., Clawson H., Cline M. S., Diekhans M., et al. (2014). The UCSC Genome Browser database: 2014 update. Nucleic Acids Res. 42, D764–D770. 10.1093/nar/gkt1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P., Kaur S., Kumar N., Singh B., Kumar S. (2009). Evaluation of antigenotoxic activity of isoliquiritin apioside from Glycyrrhiza glabra L. Toxicol. In Vitro 23, 680–686. 10.1016/j.tiv.2009.01.019 [DOI] [PubMed] [Google Scholar]
- Kessler R. C., Berglund P., Demler O., Jin R., Merikangas K. R., Walters E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62, 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Khandelwal A., Crowley V. M., Blagg B. S. (2016). Natural product inspired N-Terminal Hsp90 inhibitors: from bench to bedside? Med. Res. Rev. 36, 92–118. 10.1002/med.21351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Y., Park S. J., Yun K. J., Cho Y. W., Park H. J., Lee K. T. (2008). Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-κB in RAW 264.7 macrophages. Eur. J. Pharmacol. 584, 175–184. 10.1016/j.ejphar.2008.01.032 [DOI] [PubMed] [Google Scholar]
- Kim S. H., Lee M. K., Lee K. Y., Sung S. H., Kim J., Kim Y. C. (2009). Chemical constituents isolated from Paeonia lactiflora roots and their neuroprotective activity against oxidative stress in vitro. J. Enzyme Inhib. Med. Chem. 24, 1138–1140. 10.1080/14756360802667977 [DOI] [PubMed] [Google Scholar]
- Kolassa I. T., Kolassa S., Ertl V., Papassotiropoulos A., De Quervain D. J. (2010). The risk of post-traumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biol. Psychiatry 67, 304–308. 10.1016/j.biopsych.2009.10.009 [DOI] [PubMed] [Google Scholar]
- Kong L. Y., Tan R. X. (2015). Artemisinin, a miracle of traditional Chinese medicine. Nat. Prod. Rep. 32, 1617–1621. 10.1039/C5NP00133A [DOI] [PubMed] [Google Scholar]
- Kong P., Chi R., Zhang L., Wang N., Lu Y. (2013). Effects of paeoniflorin on tumor necrosis factor-alpha-induced insulin resistance and changes of adipokines in 3T3-L1 adipocytes. Fitoterapia 91, 44–50. 10.1016/j.fitote.2013.08.010 [DOI] [PubMed] [Google Scholar]
- Li H., Ye M., Zhang Y., Huang M., Xu W., Chu K., et al. (2015a). Blood-brain barrier permeability of Gualou Guizhi granules and neuroprotective effects in ischemia/reperfusion injury. Mol. Med. Rep. 12, 1272–1278. 10.3892/mmr.2015.3520 [DOI] [PubMed] [Google Scholar]
- Li L. T., Wang S. H., Ge H. Y., Chen J., Yue S. W., Yu M. (2008). The beneficial effects of the herbal medicine Free and Easy Wanderer Plus (FEWP) and fluoxetine on post-stroke depression. J. Altern. Complement. Med. 14, 841–846. 10.1089/acm.2008.0010 [DOI] [PubMed] [Google Scholar]
- Li X., Zou K., Gou J., Du Q., Li D., He X., et al. (2015b). Effect of baicalin-copper on the induction of apoptosis in human hepatoblastoma cancer HepG2 cells. Med. Oncol. 32, 72. 10.1007/s12032-015-0527-9 [DOI] [PubMed] [Google Scholar]
- Liu H., Wang J., Wang J., Wang P., Xue Y. (2015). Paeoniflorin attenuates Aβ1-42-induced inflammation and chemotaxis of microglia in vitro and inhibits NF-κB- and VEGF/Flt-1 signaling pathways. Brain Res. 1618, 149–158. 10.1016/j.brainres.2015.05.035 [DOI] [PubMed] [Google Scholar]
- Liu H., Yu C., Xu T., Zhang X., Dong M. (2016a). Synergistic protective effect of paeoniflorin and β-ecdysterone against rotenone-induced neurotoxicity in PC12 cells. Apoptosis 21, 1354–1365. 10.1007/s10495-016-1293-7 [DOI] [PubMed] [Google Scholar]
- Liu J., Wei Y., Luo Q., Xu F., Zhao Z., Zhang H., et al. (2016b). Baicalin attenuates inflammation in mice with OVA-induced asthma by inhibiting NF-κB and suppressing CCR7/CCL19/CCL21. Int. J. Mol. Med. 38, 1541–1548. 10.3892/ijmm.2016.2743 [DOI] [PubMed] [Google Scholar]
- Liu Q., Lin X., Li H., Yuan J., Peng Y., Dong L., et al. (2016c). Paeoniflorin ameliorates renal function in cyclophosphamide-induced mice via AMPK suppressed inflammation and apoptosis. Biomed. Pharmacother. 84, 1899–1905. 10.1016/j.biopha.2016.10.097 [DOI] [PubMed] [Google Scholar]
- Liu T., Ortiz J. A., Taing L., Meyer C. A., Lee B., Zhang Y., et al. (2011). Cistrome: an integrative platform for transcriptional regulation studies. Genome Biol. 12:R83. 10.1186/gb-2011-12-8-r83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Li Z., Wang J., Weng Q., Chen S., Hu M. (2016). Antifungal activity of isoliquiritin and its inhibitory effect against peronophythora litchi chen through a membrane damage mechanism. Molecules 21, 237. 10.3390/molecules21020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Chu L., Liu H., Li J., Zhang Y., Liu W., et al. (2016). Paeoniflorin alleviates non-alcoholic steatohepatitis in rats: involvement with the ROCK/NF-κB pathway. Int. Immunopharmacol. 38, 377–384. 10.1016/j.intimp.2016.06.023 [DOI] [PubMed] [Google Scholar]
- Mackay G. M., Forrest C. M., Christofides J., Bridel M. A., Mitchell S., Cowlard R., et al. (2009). Kynurenine metabolites and inflammation markers in depressed patients treated with fluoxetine or counselling. Clin. Exp. Pharmacol. Physiol. 36, 425–435. 10.1111/j.1440-1681.2008.05077.x [DOI] [PubMed] [Google Scholar]
- Mao Q. Q., Zhong X. M., Qiu F. M., Li Z. Y., Huang Z. (2012). Protective effects of paeoniflorin against corticosterone-induced neurotoxicity in PC12 cells. Phytother. Res. 26, 969–973. 10.1002/ptr.3673 [DOI] [PubMed] [Google Scholar]
- Martenyi F., Soldatenkova V. (2006). Fluoxetine in the acute treatment and relapse prevention of combat-related post-traumatic stress disorder: analysis of the veteran group of a placebo-controlled, randomized clinical trial. Eur. Neuropsychopharmacol. 16, 340–349. 10.1016/j.euroneuro.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Min W., Ahmad I., Chang M. E., Burns E. M., Qian Q., Yusuf N. (2015). Baicalin protects keratinocytes from toll-like receptor-4 mediated DNA damage and inflammation following ultraviolet irradiation. Photochem. Photobiol. 91, 1435–1443. 10.1111/php.12505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B. B., Tiwari V. K. (2011). Natural products: an evolving role in future drug discovery. Eur. J. Med. Chem. 46, 4769–4807. 10.1016/j.ejmech.2011.07.057 [DOI] [PubMed] [Google Scholar]
- Neylan T. C., Sun B., Rempel H., Ross J., Lenoci M., O'donovan A., et al. (2011). Suppressed monocyte gene expression profile in men versus women with PTSD. Brain Behav. Immun. 25, 524–531. 10.1016/j.bbi.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiaoli Zhao M. Z., Tolga E., Jennifer H., Rolf M., Thomas E. (2013). Molecular docking studies of myxobacterial disorazoles and tubulysins to tubulin. J. Biosci. Med. 3, 37–44. 10.5780/jbm2013.6 [DOI] [Google Scholar]
- Rafi M. M., Vastano B. C., Zhu N., Ho C. T., Ghai G., Rosen R. T., et al. (2002). Novel polyphenol molecule isolated from licorice root (Glycrrhiza glabra) induces apoptosis, G2/M cell cycle arrest, and Bcl-2 phosphorylation in tumor cell lines. J. Agric. Food Chem. 50, 677–684. 10.1021/jf010774e [DOI] [PubMed] [Google Scholar]
- Rey C. D., Lipps J., Shansky R. M. (2014). Dopamine D1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal-amygdala circuits. Neuropsychopharmacology 39, 1282–1289. 10.1038/npp.2013.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadakane C., Watanabe J., Fukutake M., Nisimura H., Maemura K., Kase Y., et al. (2015). Pharmacokinetic profiles of active components after oral administration of a kampo medicine, shakuyakukanzoto, to healthy adult Japanese volunteers. J. Pharm. Sci. 104, 3952–3959. 10.1002/jps.24596 [DOI] [PubMed] [Google Scholar]
- Segman R. H., Shefi N., Goltser-Dubner T., Friedman N., Kaminski N., Shalev A. Y. (2005). Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol. Psychiatry 10, 500–513. 10.1038/sj.mp.4001636 [DOI] [PubMed] [Google Scholar]
- Shang Q. H., Xu H., Lu X. Y., Wen C., Shi D. Z., Chen K. J. (2011). A multi-center randomized double-blind placebo-controlled trial of Xiongshao Capsule in preventing restenosis after percutaneous coronary intervention: a subgroup analysis of senile patients. Chin. J. Integr. Med. 17, 669–674. 10.1007/s11655-011-0843-7 [DOI] [PubMed] [Google Scholar]
- Shin J. M., Kang J. H., Lee S. A., Park I. H., Lee H. M. (2016). Baicalin down-regulates IL-1β-stimulated extracellular matrix production in nasal fibroblasts. PLoS ONE 11:e0168195. 10.1371/journal.pone.0168195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D. P., Chopra K. (2014). Flavocoxid, dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase, exhibits neuroprotection in rat model of ischaemic stroke. Pharmacol. Biochem. Behav. 120, 33–42. 10.1016/j.pbb.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Song J., Hou X., Hu X., Lu C., Liu C., Wang J., et al. (2015). Not only serotonergic system, but also dopaminergic system involved in albiflorin against chronic unpredictable mild stress-induced depression-like behavior in rats. Chem. Biol. Interact. 242, 211–217. 10.1016/j.cbi.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Su G. Y., Yang J. Y., Wang F., Ma J., Zhang K., Dong Y. X., et al. (2014). Antidepressant-like effects of Xiaochaihutang in a rat model of chronic unpredictable mild stress. J. Ethnopharmacol. 152, 217–226. 10.1016/j.jep.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Su Y. A., Wu J., Zhang L., Zhang Q., Su D. M., He P., et al. (2008). Dysregulated mitochondrial genes and networks with drug targets in post-mortem brain of patients with post-traumatic stress disorder (PTSD) revealed by human mitochondria-focused cDNA microarrays. Int. J. Biol. Sci. 4, 223–235. 10.7150/ijbs.4.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh K. S., Choi E. M., Lee Y. S., Kim Y. S. (2013). Protective effect of albiflorin against oxidative-stress-mediated toxicity in osteoblast-like MC3T3-E1 cells. Fitoterapia 89, 33–41. 10.1016/j.fitote.2013.05.016 [DOI] [PubMed] [Google Scholar]
- Sun J. Y., Li D. L., Dong Y., Zhu C. H., Liu J., Li J. D., et al. (2016). Baicalin inhibits toll-like receptor 2/4 expression and downstream signaling in rat experimental periodontitis. Int. Immunopharmacol. 36, 86–93. 10.1016/j.intimp.2016.04.012 [DOI] [PubMed] [Google Scholar]
- Sun Y. X., Tang Y., Wu A. L., Liu T., Dai X. L., Zheng Q. S., et al. (2010). Neuroprotective effect of liquiritin against focal cerebral ischemia/reperfusion in mice via its antioxidant and antiapoptosis properties. J. Asian Nat. Prod. Res. 12, 1051–1060. 10.1080/10286020.2010.535520 [DOI] [PubMed] [Google Scholar]
- Tao J., Nie Y., Hou Y., Ma X., Ding G., Gao J., et al. (2016). Chemomics-integrated proteomics analysis of jie-geng-tang to ameliorate lipopolysaccharide-induced acute lung injury in mice. Evid. Based Complement. Alternat. Med. 2016:7379146. 10.1155/2016/7379146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng L., Meng Q., Lu J., Xie J., Wang Z., Liu Y., et al. (2014). Liquiritin modulates ERK and AKT/GSK3β dependent pathways to protect against glutamateinduced cell damage in differentiated PC12 cells. Mol. Med. Rep. 10, 818–824. 10.3892/mmr.2014.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu X. K., Yang W. Z., Shi S. S., Chen Y., Wang C. H., Chen C. M., et al. (2011). Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebral ischemia. Inflammation 34, 463–470. 10.1007/s10753-010-9254-8 [DOI] [PubMed] [Google Scholar]
- Tylee D. S., Chandler S. D., Nievergelt C. M., Liu X. H., Pazol J., Woelk C. H., et al. (2015). Blood-based gene-expression biomarkers of post-traumatic stress disorder among deployed marines: a pilot study. Psychoneuroendocrinology 51, 472–494. 10.1016/j.psyneuen.2014.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukojevic V., Kolassa I. T., Fastenrath M., Gschwind L., Spalek K., Milnik A., et al. (2014). Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and post-traumatic stress disorder risk in genocide survivors. J. Neurosci. 34, 10274–10284. 10.1523/JNEUROSCI.1526-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K., Uddin M., Soliven R., Wildman D. E., Bradley B. (2014). Associations between the SS variant of 5-HTTLPR and PTSD among adults with histories of childhood emotional abuse: results from two African American independent samples. J. Affect. Disord. 161, 91–96. 10.1016/j.jad.2014.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J. Y., Gong X., Zhang L., Li H. Z., Zhou Y. F., Zhou Q. X. (2008). Protective effect of baicalin against lipopolysaccharide/D-galactosamine-induced liver injury in mice by up-regulation of heme oxygenase-1. Eur. J. Pharmacol. 587, 302–308. 10.1016/j.ejphar.2008.02.081 [DOI] [PubMed] [Google Scholar]
- Wang B., Liao P. P., Liu L. H., Fang X., Li W., Guan S. M. (2016a). Baicalin and geniposide inhibit the development of atherosclerosis by increasing Wnt1 and inhibiting dickkopf-related protein-1 expression. J. Geriatr. Cardiol. 13, 846–854. 10.11909/j.issn.1671-5411.2016.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Zhang Y., Bai R., Wang M., Du S. (2016b). Baicalin attenuates alcoholic liver injury through modulation of hepatic oxidative stress, inflammation and sonic hedgehog pathway in rats. Cell. Physiol. Biochem. 39, 1129–1140. 10.1159/000447820 [DOI] [PubMed] [Google Scholar]
- Wang H. N., Peng Y., Tan Q. R., Wang H. H., Chen Y. C., Zhang R. G., et al. (2009). Free and Easy Wanderer Plus (FEWP), a polyherbal preparation, ameliorates PTSD-like behavior and cognitive impairments in stressed rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1458–1463. 10.1016/j.pnpbp.2009.07.031 [DOI] [PubMed] [Google Scholar]
- Wang H. Z., Wang H. H., Huang S. S., Zhao H., Cao Y. G., Wang G. Z., et al. (2014). Inhibitory effect of baicalin on collagen-induced arthritis in rats through the nuclear factor-κB pathway. J. Pharmacol. Exp. Ther. 350, 435–443. 10.1124/jpet.114.215145 [DOI] [PubMed] [Google Scholar]
- Wang P., Cao Y., Yu J., Liu R., Bai B., Qi H., et al. (2016c). Baicalin alleviates ischemia-induced memory impairment by inhibiting the phosphorylation of CaMKII in hippocampus. Brain Res. 1642, 95–103. 10.1016/j.brainres.2016.03.019 [DOI] [PubMed] [Google Scholar]
- Wang W., Hu X., Zhao Z., Liu P., Hu Y., Zhou J., et al. (2008). Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1179–1184. 10.1016/j.pnpbp.2007.12.021 [DOI] [PubMed] [Google Scholar]
- Wang X., Sun Y., Zhao Y., Ding Y., Zhang X., Kong L., et al. (2016d). Oroxyloside prevents dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB pathway through PPARgamma activation. Biochem. Pharmacol. 106, 70–81. 10.1016/j.bcp.2016.02.019 [DOI] [PubMed] [Google Scholar]
- Wang Y., Sun W., Du B., Miao X., Bai Y., Xin Y., et al. (2013). Therapeutic effect of MG-132 on diabetic cardiomyopathy is associated with its suppression of proteasomal activities: roles of Nrf2 and NF-κB. Am. J. Physiol. Heart Circ. Physiol. 304, H567–H578. 10.1152/ajpheart.00650.2012 [DOI] [PubMed] [Google Scholar]
- White P. (2005). Biopsychosocial Medicine: An Integrated Approach to Understanding Illness. Oxford: Oxford University Press. [Google Scholar]
- Wilde M. I., Benfield P. (1998). Fluoxetine. A pharmacoeconomic review of its use in depression. Pharmacoeconomics 13, 543–561. 10.2165/00019053-199813050-00007 [DOI] [PubMed] [Google Scholar]
- Wilker S., Pfeiffer A., Kolassa S., Elbert T., Lingenfelder B., Ovuga E., et al. (2014). The role of FKBP5 genotype in moderating long-term effectiveness of exposure-based psychotherapy for post-traumatic stress disorder. Trans. Psychiatry 4:e403. 10.1038/tp.2014.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. L., Inserra A., Lewis M. D., Mastronardi C. A., Leong L., Choo J., et al. (2016). Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 21, 797–805. 10.1038/mp.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Cui H., Han F., Zhang L., Huang T., Zhou Y., et al. (2016a). Paeoniflorin inhibits human pancreatic cancer cell apoptosis via suppression of MMP-9 and ERK signaling. Oncol. Lett. 12, 1471–1476. 10.3892/ol.2016.4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Li H., Cong X., Wang X., Jiang Z., Zhang Q., et al. (2016b). Baicalin attenuates lipopolysaccharide induced inflammation and apoptosis of cow mammary epithelial cells by regulating NF-κB and HSP72. Int. Immunopharmacol. 40, 139–145. 10.1016/j.intimp.2016.08.032 [DOI] [PubMed] [Google Scholar]
- Yi J. F. (2014). [Effect of paeoniflorin on level of glucocorticoid receptor of peripheral blood monocytes in rats of collagen-induced arthritis]. Zhongguo Zhong Yao Za Zhi 39, 907–910. 10.4268/cjcmm20140529 [DOI] [PubMed] [Google Scholar]
- Yu F. Y., Huang S. G., Zhang H. Y., Chi H. G., Zou Y., Lu R. X., et al. (2015). [Effect of baicalin on signal transduction and activating transcription factor expression in ulcerative colitis patients]. Zhongguo Zhong Xi Yi Jie He Za Zhi 35, 419–424. [PubMed] [Google Scholar]
- Zhai J., Guo Y. (2016). Paeoniflorin attenuates cardiac dysfunction in endotoxemic mice via the inhibition of nuclear factor-κB. Biomed. Pharmacother. 80, 200–206. 10.1016/j.biopha.2016.03.032 [DOI] [PubMed] [Google Scholar]
- Zhai T., Sun Y., Li H., Zhang J., Huo R., Li H., et al. (2016). Unique immunomodulatory effect of paeoniflorin on type I and II macrophages activities. J. Pharmacol. Sci. 130, 143–150. 10.1016/j.jphs.2015.12.007 [DOI] [PubMed] [Google Scholar]
- Zhang H. R., Peng J. H., Cheng X. B., Shi B. Z., Zhang M. Y., Xu R. X. (2015a). Paeoniflorin atttenuates amyloidogenesis and the inflammatory responses in a transgenic mouse model of Alzheimer's disease. Neurochem. Res. 40, 1583–1592. 10.1007/s11064-015-1632-z [DOI] [PubMed] [Google Scholar]
- Zhang J., Dou W., Zhang E., Sun A., Ding L., Wei X., et al. (2014a). Paeoniflorin abrogates DSS-induced colitis via a TLR4-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G27–G36. 10.1152/ajpgi.00465.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Pan X., Wang F., Ma J., Su G., Dong Y., et al. (2016a). Baicalin promotes hippocampal neurogenesis via SGK1- and FKBP5-mediated glucocorticoid receptor phosphorylation in a neuroendocrine mouse model of anxiety/depression. Sci. Rep. 6:30951. 10.1038/srep30951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. G., Wang L. J., Shen Q. Q., Wang H. F., Zhang Y., Shi C. G., et al. (2015b). Paeoniflorin improves regional cerebral blood flow and suppresses inflammatory factors in the hippocampus of rats with vascular dementia. Chin. J. Integr. Med. [Epub ahead of print]. 10.1007/s11655-015-2124-3 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhou J., Huang M., Bi L., Zhou S. (2014b). Paeoniflorin reduced BLP-induced inflammatory response by inhibiting the NF-κB signal transduction in pathway THP-1 cells. Cent. Eur. J. Immunol. 39, 461–467. 10.5114/ceji.2014.47729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Song Y., Han X., Feng L., Wang R., Zhang M., et al. (2013). Liquiritin attenuates advanced glycation end products-induced endothelial dysfunction via RAGE/NF-κB pathway in human umbilical vein endothelial cells. Mol. Cell. Biochem. 374, 191–201. 10.1007/s11010-012-1519-0 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Sun D., Meng Q., Guo W., Chen Q., Zhang Y. (2016b). Calcium channels contribute to albiflorin-mediated antinociceptive effects in mouse model. Neurosci. Lett. 628, 105–109. 10.1016/j.neulet.2016.03.054 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang L., Zhang Y., Xu J. J., Sun L. L., Li S. Z. (2016c). The protective role of liquiritin in high fructose-induced myocardial fibrosis via inhibiting NF-κB and MAPK signaling pathway. Biomed. Pharmacother. 84, 1337–1349. 10.1016/j.biopha.2016.10.036 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Gao X., Guo M., Jiang H., Cao Y., Zhang N. (2016d). The protective effect of baicalin against lead-induced renal oxidative damage in mice. Biol. Trace Elem. Res. 175, 129–135. 10.1007/s12011-016-0731-2 [DOI] [PubMed] [Google Scholar]
- Zheng W. X., Wang F., Cao X. L., Pan H. Y., Liu X. Y., Hu X. M., et al. (2014). Baicalin protects PC-12 cells from oxidative stress induced by hydrogen peroxide via anti-apoptotic effects. Brain Inj. 28, 227–234. 10.3109/02699052.2013.860469 [DOI] [PubMed] [Google Scholar]
- Zhou H., Bian D., Jiao X., Wei Z., Zhang H., Xia Y., et al. (2011). Paeoniflorin protects against lipopolysaccharide-induced acute lung injury in mice by alleviating inflammatory cell infiltration and microvascular permeability. Inflamm. Res. 60, 981–990. 10.1007/s00011-011-0359-9 [DOI] [PubMed] [Google Scholar]
- Zhou H., Yang H. X., Yuan Y., Deng W., Zhang J. Y., Bian Z. Y., et al. (2013). Paeoniflorin attenuates pressure overload-induced cardiac remodeling via inhibition of TGFβ/Smads and NF-κB pathways. J. Mol. Histol. 44, 357–367. 10.1007/s10735-013-9491-x [DOI] [PubMed] [Google Scholar]
- Zhou J., Wang L., Wang J., Wang C., Yang Z., Wang C., et al. (2016a). Paeoniflorin and albiflorin attenuate neuropathic pain via mapk pathway in chronic constriction injury rats. Evid. Based Complement. Alternat. Med. 2016:8082753. 10.1155/2016/8082753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. B., Jin Y. L., Jia Q., Zhang Y., Li L. Y., Liu P., et al. (2014). Baicalin attenuates brain edema in a rat model of intracerebral hemorrhage. Inflammation 37, 107–115. 10.1007/s10753-013-9717-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. J., Wang H., Sui H. H., Li L., Zhou C. L., Huang J. J. (2016b). Inhibitory effect of baicalin on allergic response in ovalbumin-induced allergic rhinitis guinea pigs and lipopolysaccharide-stimulated human mast cells. Inflamm. Res. 65, 603–612. 10.1007/s00011-016-0943-0 [DOI] [PubMed] [Google Scholar]