Abstract
Background/Objective:
Adipokines are involved in the etiology of diabetes, insulin resistance, and the development of atherosclerosis and other latent-onset complications. The objective of this meta-analysis was to determine the effectiveness of exercise interventions on adipokines in pediatric obesity.
Subjects/Methods:
A computerized search was made using three databases. The analysis was restricted to studies that examined the effect of exercise interventions on adipokines (adiponectin, leptin, resistin and visfatin) in pediatric obesity (6–18 years old). Fourteen randomized controlled trials (347 youths) were included. Weighted mean difference (WMD) and 95% confidence intervals were calculated.
Results:
Exercise was associated with a significant increase in adiponectin (WMD=0.882 μg ml−1, 95% CI, 0.271–1.493) but did not alter leptin and resistin level. Likewise, exercise intensity and change in body fat; as well as total exercise program duration, duration of the sessions, and change in body fat all significantly influenced the effect of exercise on adiponectin and leptin, respectively.
Conclusions:
Exercise seems to increase adiponectin levels in childhood obesity. Our results also suggested that exercise on its own, without the concomitant presence of changes in body composition levels, does not affect leptin levels.
Introduction
Obesity is a growing health concern that has become an epidemic in modern-day society. Adipose tissue is a well-known source of inflammation, and is considered as a complex and highly active metabolic endocrine organ 1 which produces various cytokines.2 Adipose tissue-derived cytokines or adipokines are involved in the regulation of many processes such as energy metabolism, inflammation, diabetes and atherosclerosis.3, 4, 5 Indeed, increased levels of adipokines and pro-inflammatory cytokines, such as leptin, adiponectin, resistin, apelin or visfatin, tumor necrosis factor-alpha, and interleukin-6, have prominent roles in the pathogenesis of the metabolic syndrome.2
Leptin and adiponectin both are associated with regulation of energy balance and insulin action6 and obesity negatively affects the levels of these molecules. Leptin also promotes body mass loss decreasing food intake and increasing sympathetic nervous system activity through the hypothalamus.7 Furthermore, adiponectin has anti-atherogenic, anti-diabetic and anti-inflammatory properties8 and also play an essential role in maintaining homeostasis in the human body. Another member of the adipocytokine family, resistin was initially perceived as an insulin resistance inducing hormone in mice, but its associations with altered metabolism states were not confirmed in human studies.9 However, there is growing evidence emphasizing a role of resistin as a pro-inflammatory adipocytokine in humans.10 In addition, visfatin contribute to vascular disease by inducing endothelial dysfunction through a variety of mechanisms.11, 12
Regular exercise has been shown to promote positive adaptations and act as adjuvant for obesity prevention and treatment. Regular exercise can potentially modify metabolic hormones and is considered an important treatment of chronic inflammation13 and obesity-related conditions.14 The magnitude of benefits may vary with the type and amount of exercise. A systematic review in adults showed that the effect of chronic exercises on leptin and adiponectin concentrations revealed disparate findings.15 In patients with type 2 diabetes, a recent meta-analysis showed that aerobic exercise program was associated with a significant change in leptin (−3.72 ng ml−1), but did not alter adiponectin levels.16 Furthermore, a review on pediatric obesity indicated that exercise has an impact on the adipose tissue and the release of adiponectin, resistin, and visfatin.17 However, several studies also reported inconsistent results in the pediatric population.15 Given this latter point we chose to carried-out a meta-analytic approach to examine the effects of exercise interventions compared with a control group on adipokines in overweight and obese youth. Our intent being to provide clarity on the role exercise plays in influencing the critical adipocytokines associated with obesity in a pediatric population.
Materials and methods
The study was undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.18 The review was registered with PROSPERO (CRD42016039025) at the University of York, United Kingdom. PROSPERO provides a comprehensive listing of systematic reviews registered at inception to help avoid unplanned duplication and enable comparison of reported review methods with what was planned in the protocol.
Literature search
Articles published before 10 May 2016, were retrieved by using searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (2002 to 10 May 2016), EMBASE (1980 to 10 May 2016) and MEDLINE (1965 to 10 May 2016) online databases. The search strategy included the topic's specialist journals. The search was conducted between the 1th and the 10th of May 2016. The terms used were: ('Obesity' and 'Overweight' OR), ('Exercise' and 'Training' and 'physical activity' and 'sport' OR). All Medical Subject Headings terms were combined with leptin*, adiponectin*, resistin*, visfatin*, adipokines*, and publication type (randomized controlled trials [RCT]) as limiter. Also, the reference lists were examined to detect studies potentially eligible for inclusion. Studies reported in languages other than English were not explored.
Study selection and inclusion criteria
Two authors (RC and CP) independently screened the titles and abstracts of potentially eligible studies identified by the search strategy. Discrepancies between the two reviewers about study conditions were resolved by consensus with the third author (AG-H). The a priori inclusion criteria for this study were as follows: (a) children and adolescents classified as overweight or obese; (b) randomized controlled trials (RCT) studies in which the control group received no type of physical exercise or dietary restriction intervention; (c) interventions of supervised exercise (without hypocaloric diet intervention); and (d) evaluations of adipokines (adiponectin, leptin, resistin and/or visfatin).
Data collection
Two investigators (RC & CP) independently abstracted all data. Data were extracted regarding the year of publication, the characteristics of participants, exercise programs (type, frequency, duration and intensity), assessments and results. In cases in which duplicate research was published using the same population, the data from the study with the longest follow-up duration were used for the meta-analysis. A request asking for missing data was sent to each of the corresponding authors where appropriate.19
Risk of bias
Two investigators (RC and CP) independently performed the quality assessment. For the quality assessment of RCTs, we used the Delphi list as described by Verhagen et al.,20 which includes eight questions with three response options ‘yes', ‘no', or ‘do not know' depending on the compliance with key methodological components, and produces a quality score that provides an overall estimate of RCTs' quality.
Meta-analysis calculation
For the data analysis, we used Review Manager (Update Software, Oxford, UK) to calculate the weighted mean difference (WMD). The WMD of the adipokines from pre- to post-intervention between groups (exercise vs control)21 in each study was calculated and pooled using the random effects model (DerSimonian–Laird approach). The underlying assumption of the random effects model is that samples are drawn from populations with different effect sizes, and that true effects differ between studies (that is, interventions, duration and so on).
Assessment of heterogeneity
The percentage of total variations across the studies due to heterogeneity (Cochran's Q-statistic)22 was determined using I2. I2 values of <25, 25–50 and >50% are considered to represent small, medium and large amounts of inconsistency.23
Publication bias and sensitivity
Each study was deleted from the model once in order to analyze the influence of each study on the overall results. The Egger test was used to examine publication bias.24 In this case, the funnel plot test as a subsequent follow up was performed only in adiponectin due to in leptin and resistin the number of studies was less than the recommended arbitrary minimum number of ten studies.25
Meta-regression and subgroups analysis
The heterogeneity between studies using meta-regression was analyzed. We used covariates that may influence the association between exercise and adipokines: (a) total exercise program duration of each study (weeks); (b) frequency of sessions per week; (c) duration of exercise per session (minutes); and (d) changes in body fat (BF) post intervention. Also, subgroup moderator analyses were conducted to determine whether exercise effects differed according to intensity of the exercise (moderate, moderate-to-vigorous, and vigorous) according to American College of Sports Medicine cutoffs recommendations.26
Results
Study selection
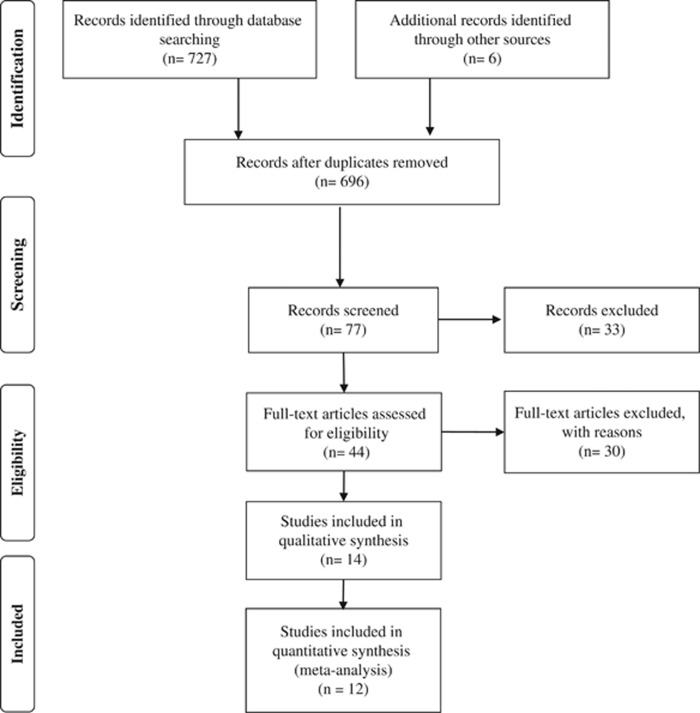
The flow chart relative to data collection is shown in Figure 1. The literature search resulted in 733 studies. Titles and abstracts of returned articles were searched for suitability, leading to the retrieval of 44 full texts. Of those, 30 were rejected—23 for failing the study design criterion (no control group or RCT) and seven due to the type of intervention criterion (interventions with diet or no programmed exercise) (Supplementary Material 1). Finally, 14 RCTs met the inclusion criteria and were included in the meta-analysis.13, 19, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 In the included 14 trials, 5 RCTs analyzed leptin,19, 30, 31, 34, 36 10 analyzed adiponectin,13, 28, 29, 31, 32, 34, 35, 36, 38 2 analyzed resistin,31, 36 and only 1 visfatin.33
Figure 1.
Flow chart for identification of trials for inclusion in the meta-analysis.
Description of the included studies
The characteristics of all included studies are shown in Table 1. The final analysis included a total of 347 youth (190 and 157 in exercise and control group, respectively). The youths were overweight/obese28, 29, 31, 33, 34, 37 or obese.13, 19, 27, 30, 35, 36, 38 Three studies included only boys28, 32, 37 and three only girls,33, 34, 35 and the remaining studies included both boys and girls.13, 19, 27, 29, 30, 31, 36, 38 Participants in three studies were children (6–12 years old),28, 29, 31 in eight adolescents (13–17 years old),13, 19, 32, 33, 34, 35, 36, 37, 38 and in the other both age groups were included.27
Table 1. Characteristics of the studies included in the meta-analysis.
| Study |
EG |
CG |
BMI (percentile or kg m−2) |
Intervention characteristics |
Assessment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age (years) | Type | n | Age (years) | Duration (weeks) | Frequency (Se/W) | Se duration (min) | Intensity | Compliance (%) | |||
| Balagopal et al.19 | 8 | 15.6 | Aerobic+Resistance | 7 | 15.9 | ⩾p97 | 12 | 3 | 45 | NR | NR | Leptin |
| Balagopal et al.13 | 8 | 15.6 | Aerobic+Resistance | 7 | 15.9 | ⩾p97 | 12 | 3 | 45 | NR | NR | Adiponectin |
| Chae et al.27 | 19 | 10.4 | Aerobic+Resistance | 19 | 10.6 | ⩾p97 | 12 | 2 | 90 | NR | NR | Adiponectin |
| Fazelifar et al.28 | 12 | 11–13 | Aerobic+Resistance | 12 | 11–13 | ⩾28 | 12 | 3 | 10–30 | 50–85 HRmax | NR | Adiponectin |
| Jeon et al.29 | 8 | 11.0 | Aerobic+Resistance | 7 | 11.0 | ⩾p85 | 12 | 2 | 50 | 55–75 HRmax | NR | Adiponectin |
| Karacabey30 | 20 | 11.8 | Aerobic | 20 | 11.2 | >30 | 12 | 3 | 30–65 | 60–65 HRmax | NR | Leptin |
| Kelly et al.31 | 9 | 10.8 | Aerobic | 10 | 11.0 | ⩾p85 | 8 | 4 | 30 | 50–60 HRmax | NR | Leptin, Adiponectin, Resistin |
| Kim et al.32 | 14 | 17.0 | Aerobic | 12 | 17.0 | NR | 6 | 5 | 40 | NR | NR | Adiponectin |
| Kim et al.37 | 18 | 17.6 | Aerobic | 12 | 17.4 | ⩾25 | 12 | 5 | 50 | NR | NR | Adiponectin |
| Lee et al.33 | 11 | 16.9 | Aerobic | 7 | 16.9 | ⩾25 | 12 | 3 | 30–40 | 60–80 HRmax | NR | Visfatin |
| Nunes et al.38 | 17 | 16.8 | Aerobic+Resistance | 8 | 15.4 | ⩾p95 | 24 | 4 | 60 | 60–100 VO2peak | NR | Adiponectin |
| Park et al.34 | 15 | 12.1 | Aerobic+Resistance | 14 | 12.2 | ⩾p85 | 12 | 3 | 80 | 50–70 HRreserve | 90 | Leptin, Adiponectin |
| Racil et al.35 | 11 11 | 15.6 16.3 | Anerobic Aerobic | 12 | 15.9 | ⩾p97 | 12 | 4 | 20 | 100–110 VO2peak 70–80 VO2peak | NR | Adiponectin |
| Vasconcellos et al.36 | 10 | 14.1 | Aerobic | 10 | 14.8 | ⩾2 s.d. | 12 | 3 | 60 | 84.8 HRmax | NR | Leptin, Adiponectin, Resistin |
Abbreviations: CG, control group; EG, experimental group; HIIT, high-intensity interval training; HR, heart rate; MIIT, moderate-intensity interval training; NR, not reported; p, percentile; Se, session; W, week.
The type of the programs was based on aerobic,30, 31, 32, 33, 35, 36, 37 anaerobic35 or aerobic plus resistance exercise.13, 19, 27, 29, 34, 38 The intensity of the exercise was moderate,29, 30, 31, 32, 35 moderate-to-vigorous,28, 34, 36, 38 or vigorous35 according to American College of Sports Medicine cut-offs recommendations.26 Finally, adherence to the exercise programs was only reported in one study34 (90%).
Risk of bias
Among the included studies, all satisfied four quality criteria: allocation randomized, inclusion criteria specified, baseline similar, and point estimate and variability (Supplementary Material 2).
Association of exercise intervention with adiponectin
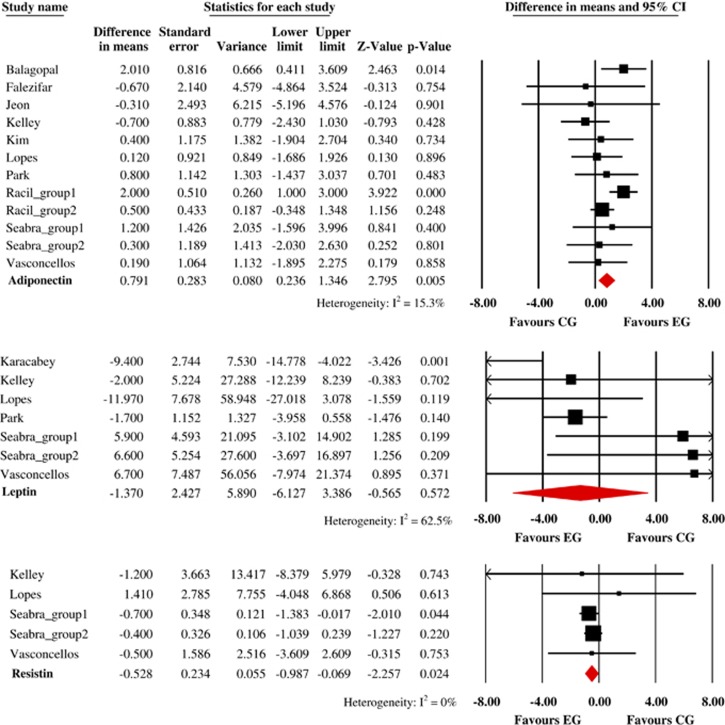
Overall, exercise significantly increased adiponectin levels (n=10 studies and 246 youths) by 0.882 μg/ml (95% CI, 0.271–1.493 μg ml−1, P=0.005; I2=23.3% Figure 2). However, meta-regression analyses revealed a statistically significant relationship between adiponectin and change in BF (ß=−0.072; 95% CI, −0.173 to −0.020; P=0.013), but not for the others covariates. Interestingly, in subgroup analyses, we observed a non-significant change in adiponectin levels for moderate-intensity exercise (WMD=0.248 μg ml−1; 95% CI, −0.417 to 0.913 μg ml−1, P=0.465; I2=0%) and moderate-to-vigorous intensity (WMD=0.745 μg ml−1; 95% CI, −0.519 to 2.010 μg ml−1, P=0.248; I2=0%).
Figure 2.
Absolute changes in adiponectin, leptin, and resistin levels in individual studies of exercise group vs control group. HIIT indicates High-Intensity Interval Training. MIIT indicates Moderate-Intensity Interval Training.
Association of exercise intervention with leptin
Exercise non-significantly changed leptin levels (n=5 studies and 94 youths) by −3.848 μg ml−1 (95% CI, −8.191 to 0.496 μg ml−1, P=0.083; I2=55.5% Figure 2). Meta-regression analyses found statistically significant relationship between leptin, duration of the intervention (ß= −0.708; 95% CI, −1.276 to −0.140; P=0.014), duration of the exercise per session (ß=−0.156; 95% CI, −0.297 to −0.015; P=0.030), and change in BF (ß=−0.729; 95% CI, −1.374 to −0.081; P=0.027). In subgroup analyses, we observed a significant change in leptin levels by moderate-intensity exercise (WMD=−8.179 μg ml−1; 95% CI, −12.719 to −3.640 μg ml−1, P<0.001; I2=0%), but not for moderate-to-vigorous intensity (WMD=−0.758 μg ml−1; 95% CI, −5.953 to 4.438 μg ml−1, P=0.775; I2=18.7%).
Association of exercise intervention with resistin
Significant association was observed between exercise (n=2 studies and 39 youths) and resistin levels by −0.611 ng ml−1 (95% CI, −3.463 to 2.242 ng ml−1, P=0.675; I2=0% Figure 2). None of our covariates significantly explained our pooled analysis of resistin. Due to the limited number of studies, we did not conduct any subgroup analyses.
Publication bias and sensitivity analysis
Both funnel plot asymmetry and Egger test show no significant publication bias for adiponectin (Egger regression intercept, −3.55 (P=0.015)) and leptin (Egger regression intercept, −0.42 (P=0.381)). Due to limited number of studies, we did not conduct the Egger test for resistin.
Finally, in the sensitivity analysis, with each study removed from the model individually, the results remained constant across deletions.
Discussion
The most prominent finding from this meta-analysis was that exercise training substantially increases adiponectin in childhood obesity. Also, exercise programs of longer duration as well as changes in BF seemed to favor a reduction in leptin levels. Similar conclusions have been reported in previous experimental studies29, 31, 34 and narrative reviews.15, 17 Moreover, it is important to highlight that this is the first meta-analysis that has summarized the effectiveness of exercise training in modulating the adipokines levels in pediatric obesity populations. However, the heterogeneity in the exercise programs (length of intervention, frequency, type of exercise and so on) and the limited number of youths could influence the final results, so we must carefully interpret these findings.
Exercise may modulate adipokines levels in childhood obesity
Most of the clinical recommendations for treatment of childhood obesity and its associated comorbidities are based on the combination of several interventions, such as changing eating habits, medication use, regular physical exercise and others items.39 Thus, a number of studies have established an inverse relationship between the amount of physical activity or lifestyle intervention and increased release of pro-inflammatory adipokines by white adipose tissue in childhood obesity.40, 41, 42 Likewise, exercise has been shown to be a safe and effective adjuvant therapy for influencing adiposity and overall body composition.15, 17 However, the role of different types of exercise in the specific reduction of the adipose tissue adipocytokines is unclear due to only a limited number of well-controlled long-term studies being available.41, 43, 44 The type of exercise did not appear to affect any putative association; however, it is highly probable that different exercise modalities cause different responses in adipokines levels. Future research needs to address this point.
Effects of exercise on adiponectin
Adiponectin may be the most biologically active form regulating glucose homeostasis and evidence suggests that adiponectin is an important regulator of insulin sensitivity and glucose homeostasis.45 Further studies suggested an inverse relationship between insulin resistance and type II diabetes with plasma adiponectin level.8 In adults, a systematic review showed that exercise increases serum adiponectin, demonstrating small-to-moderate effect sizes.46 The present meta-analysis confirm this adult study findings in childhood obesity; that is, showing a significantly increase of adiponectin levels. Therefore, there is some support for the use of physical exercise at an adequate duration and intensity to produce substantive changes in fitness levels and raise circulating adiponectin levels in children.15 However, we also pooled data from non-RCT47, 48, 49 and an increase was not observed in adiponectin levels (WMD=0.483 μg ml−1, 95% CI, −0.527 to 1.493 μg ml−1, P=0.349; I2=0%).
In addition, our meta-regression analyses found a statistically significant relationship between adiponectin and change in BF (ß=−0.097), that is, exercise was more effective in influencing adiponectin in those children with a greater reduction in BF levels. This result was experimental showed recently by Lopes and colleges49 in a RCT in thirty-three overweight girls, where a combined training program consisted of six resistance exercises (three sets of 6–10 repetitions at 60–70% 1 RM) followed by 30 min of aerobic exercise (walking/running) at 50–80% VO2peak, performed in the same 60 min session, 3 days/weeks, for 12 weeks. Dâmaso and colleges41 showed a significant increase in adiponectin levels after 1 year of combined (aerobic plus resistance exercise) training included in a multidisciplinary program, possibly due to the significant reduction in body mass (Δ=−12.3 kg) and BF (Δ=−14.2 kg) found after the intervention. Our findings, consistent with these studies, were also observed by Nascimento and colleges47 in a more recent non-RCT with a similar intervention using 5 h per week of moderate-to vigorous intensity physical exercise over eight weeks compared to a sedentary control group. Interestingly, these authors reported reductions in body mass index z-score and BF that were accompanied by an improvement in lipid profile and insulin resistance, a reduction in C-reactive protein, TNF-alpha, and an increase in adiponectin levels, suggesting a possible link between changes in adiponectin and body composition in pediatric overweight and obesity. Finally, the greater increases observed in the Racil et al.35 study, which analyzed 12-week interval training of high-intensity exercise in 34 adolescent females, highlighting the benefits of high-intensity interval exercise interventions in obese population.50 In sum evidence suggests that adipokines are strongly correlated with BF51 and that adequate amounts of exercise reduce BF,14 therefore it is possible that any associations found in such an analysis would be due to decreases in BF alone.
Effects of exercise on leptin
Leptin is one of the best-known hormone markers for obesity and is very sensitive to levels of energy intake, particularly in energy deficient state.52 Epidemiological studies indicate that increased leptin levels are associated with a higher frequency of adverse health consequences including obesity, systemic low-grade inflammation, and insulin resistance in obese youth.53, 54 Our pooled analysis demonstrated that exercise did not reduced leptin concentrations in childhood obesity. However, we pooled data from non-RCT47, 48, 49 and observed a significant reduction in leptin levels (WMD=−5.537 μg/ml, 95% CI, −10.133 to −0.942 μg ml−1, P=0.018; I2=0%). Therefore, the evidence shows somewhat controversial results. For example, data from a Balagopal et al.,19 confirm a decrease in leptin levels (from 22.1±2.9 to 15.6±2.0 ng/ml; P=0.001) in response to lifestyle intervention, accompanied by decrease in fat mass further suggesting a potential role of the leptin-inflammatory axis in obese children. Recently, Lopes et al.49 found a significant reduction in leptin (effect size: −0.95, 95% CI: −1.66 to −0.20 P=0.001) in overweight training after the experimental period. Another experimental therapy, regarding combined training as an adjuvant weight loss therapy for the treatment of chronic low-grade inflammation in obese adolescents, Dâmaso and colleges41 found a significant reduction in leptin after 1 year of combined exercise training in obese adolescents. In contrast, no significant change leptin level could be detected by Vasconcellos et al.36 after 12 weeks of a recreational soccer program in obese adolescents. The discrepancy between these results may be related to the type of the length of intervention (8 vs12 vs 24 weeks) and design.
In addition, acute and short-term bouts of exercise do not appear to affect leptin levels. For example, short-term exercise (⩽60 min), in obese females, walking at 60–80% of the heart rate maximum for 45 min did not alter leptin concentrations, although it decreased insulin resistance.55 Contrastingly, longer durations of exercise (⩾60 min) that are associated with increased energy expenditure (⩾800 kcal) can decrease leptin concentrations.45 This finding confirms our meta-regression analyses showing that duration of the intervention and duration of the exercise per session are negatively related to leptin levels.
On the order hand, an important point of interest in measuring leptin levels is paying attention to diurnal variations in its blood levels. Kraemer and colleges56 determined leptin levels in 15 healthy postmenopausal women at baseline, exercise, and recovery point intervals. Blood sampling with the same time intervals but without exercise was performed one month later as a control group. Even though no difference was detected between two groups, there was a gradual decrease from baseline levels to post-exercise and recovery period. Kraemer et al.56 as well as Golbidi and Laher45 emphasized the need to account for diurnal variations in measuring leptin levels over the course of exercise trials.
Effects of exercise on resistin
Resistin is produced by white and brown adipose tissues and is elevated in obesity.57 It seems that resistin is involved in glucose homeostasis, lipid metabolism, and insulin action.58 Our pooled analysis demonstrated that exercise not reduced resistin concentrations in pediatric obesity, confirming the existing dispute.31, 59, 60 The small number of youths and studies included in the analysis could be explains the non-significant effects. All of the RCTs had small sample sizes (n<100). Therefore, additional intervention on this topic is needed, including longitudinal interventions in this population and taking into account the limitations observed in this meta-analysis.
In contrast with the meta-analysis results, data from non-RCT47, 48, 49 showed a significant decrease in resistin levels (WMD=−5.510 ng ml−1, 95% CI, −0.963 to −0.058 ng ml−1, P=0.027; I2= 0%). Specifically, Seabra et al.48 showed a significant decreases in resistin levels (effect size: −0.22 and 95% CI: −0.48 to −0.91) in 33 overweight girls (13–17 years). Also, data from the ACORDA study47 also confirm reductions; in this study, the authors found a 4% reduction in resistin in an intervention group composed of 117 overweight and obese children and adolescents that completed 5 h per week of moderate-to vigorous intensity physical exercise during 8 weeks compared with a control group (that is, regular classes of physical education at school 3 times a week). Another consideration in assessing studies using an exercise intervention is the timing of blood sampling in relation to the exercise.61 Most studies that have demonstrated a post-exercise increase in resistin found an immediate post-exercise spike followed by a gradual return to baseline or lower than baseline resistin levels over the next 30 min to several hours into recovery.62
Effects of exercise on visfatin
Visfatin is an adipokine that contributes to glucose and obesity-related conditions.63 It is expressed in visceral adipose tissue and has been shown to exert insulin-mimetic effect. We found only one RCT that examined the effects of physical exercise on visfatin levels in obese female adolescents.33 The results suggest that aerobic exercise resulting in an energy expenditure of 1,200–1,600 kcal per week for 12 weeks decreased plasma visfatin and insulin resistance. Another recent non-RCT suggests that regular exercise has positive effects on obesity in Korean children by improving glycemic control and reducing body weight, thereby lowering visfatin levels (from 247.72±14.95 to 184.22±7.75; P<0.05).60 Congruent with these findings, Lai et al.64 reported that a substantial decrease in HOMA-IR after exercise, might indicate that visfatin rs4730153 GG genotype (polymorphism), could possibly improve glucose metabolism in obese children and adolescents by enhancing insulin sensitivity to exercise. Due to the limited number of studies, ultimately, we did not conduct the meta-analysis on this hormone. Therefore, a greater number of RCT studies are required to making safe conclusions as to what the effects of physical exercise would be on visfatin levels in childhood obesity.
Strengths and limitations
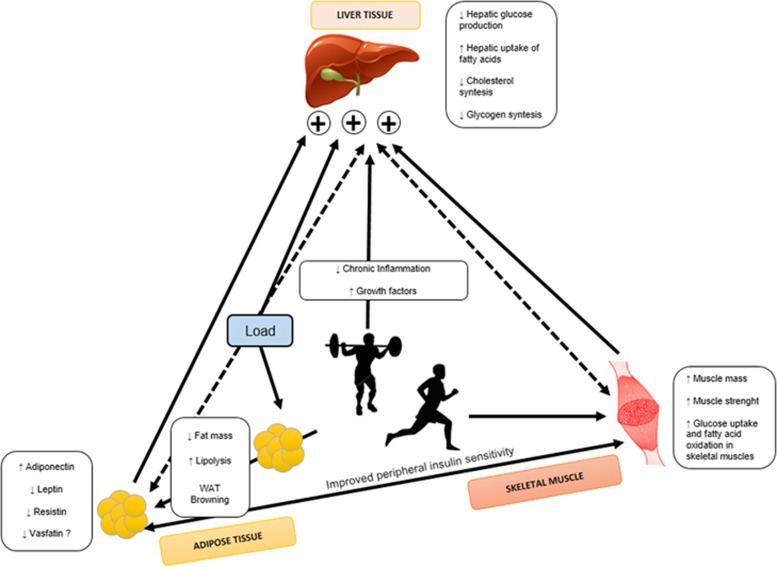
To our knowledge, this is the first meta-analysis that evaluates the changes on adipokines after exercise training in overweight and obese youths. Our results provide novel insight regarding the role of exercise as a non-pharmacological effective intervention in modulating the metabolic environment as well as in the management of childhood obesity. In addition, there were numerous methodological limitations that impacted the generalizability of studies, including a lack of adjustment for confounding factors (for example, plasma volume, participant age or body composition) and a lack of consideration of effect modification. Furthermore, our findings have crucial implications on interventional program in overweight/obese children and adolescents improved the adipokine profile, reducing pro-inflammatory molecules, such as leptin and resistin, and increasing adiponectin, important anti-inflammatory and anti-diabetic adipokines (Figure 3). In addition, all studies included exhibited moderate to high methodological quality and low risk of bias, which is an important issue in terms of external validity of our findings.
Figure 3.
Exercise training–induced adipokines have an endocrine effect and improve whole-body metabolism. We propose a model whereby exercise causes release adipokines (decrease leptin and resistin, and increasing adiponectin), which can act in an endocrine manner to improve metabolism in skeletal muscle, white adipose tissue and liver.
Nevertheless, there are some limitations with regard to our study exist that are important to state. The overall effects estimates were increased due to different modes of exercise across the studies included, although such differences were approached through subgroup analysis according to the mode of exercise. Statistical heterogeneity levels were detected for most of the effect estimates, which suggests some caution when interpreting our findings. This evidence of heterogeneity was counteracted by a random effects model of analysis and can be explained by differences in some characteristics of the exercise employed such as intensity, duration, intervention length, follow-up periods and adherence rates across studies. Finally, we must carefully interpret the findings due to the limited number of youths included in the meta-analysis and meta-regression.
In conclusion, our meta-analysis indicates that exercise was associated with increased adiponectin levels, while no significantly associations with leptin and resistin in overweight/obese children and adolescents were found. However, programs of longer duration as well as changes in BF seem favor a reduction in leptin levels. These findings will aid pediatricians and other health professionals with counseling patients and parents on physical activity and exercise prescription guidelines. Based on our results, we recommend exercise programs that involve both aerobic and resistance exercise on a regular basis, and that last longer than 24 weeks. Therefore, the data presented in this meta-analysis support current physical activity recommendations and suggest that physical exercise could be a critical strategy to control of obesity and inflammatory state progress in the pediatric population relative to some adipocytokines.
Footnotes
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
The authors declare no conflict of interest.
Supplementary Material
References
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89: 2548–2556. [DOI] [PubMed] [Google Scholar]
- Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol 2006; 64: 355–365. [DOI] [PubMed] [Google Scholar]
- Barseghian A, Gawande D, Bajaj M. Adiponectin and vulnerable atherosclerotic plaques. J Am Coll Cardiol 2011; 57: 761–770. [DOI] [PubMed] [Google Scholar]
- Sabbatini AR, Fontana V, Laurent S, Moreno H. An update on the role of adipokines in arterial stiffness and hypertension. J Hypertens 2015; 33: 435–444. [DOI] [PubMed] [Google Scholar]
- Gulcelik NE, Usman A, Gürlek A. Role of adipocytokines in predicting the development of diabetes and its late complications. Endocrine 2009; 36: 397–403. [DOI] [PubMed] [Google Scholar]
- Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol 2002; 13: 51–59. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 2001; 60: 329–339. [DOI] [PubMed] [Google Scholar]
- Ouwens DM, Bekaert M, Lapauw B, Van Nieuwenhove Y, Lehr S, Hartwig S et al. Chemerin as biomarker for insulin sensitivity in males without typical characteristics of metabolic syndrome. Arch Physiol Biochem 2012; 118: 135–138. [DOI] [PubMed] [Google Scholar]
- Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab 2003; 88: 4848–4856. [DOI] [PubMed] [Google Scholar]
- McTernan PG, Kusminski CM, Kumar S. Resistin. Curr Opin Lipidol 2006; 17: 170–175. [DOI] [PubMed] [Google Scholar]
- Northcott JM, Yeganeh A, Taylor CG, Zahradka P, Wigle JT. Adipokines and the cardiovascular system: mechanisms mediating health and disease. Can J Physiol Pharmacol 2012; 90: 1029–1059. [DOI] [PubMed] [Google Scholar]
- Chen C, Jiang J, Lü JM, Chai H, Wang X, Lin PH et al. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol 2010; 299: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopal P, George D, Yarandi H, Funanage V, Bayne E. Reversal of obesity-related hypoadiponectinemia by lifestyle intervention: a controlled, randomized study in obese adolescents. J Clin Endocrinol Metab 2005; 90: 6192–6197. [DOI] [PubMed] [Google Scholar]
- McMurray RG, Hackney AC. Interactions of metabolic hormones, adipose tissue and exercise. Sports Med 2005; 35: 393–412. [DOI] [PubMed] [Google Scholar]
- Bouassida A, Chamari K, Zaouali M, Feki Y, Zbidi A, Tabka Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br J Sports Med 2008; 44: 620–630. [DOI] [PubMed] [Google Scholar]
- Hayashino Y, Jackson JL, Hirata T, Fukumori N, Nakamura F, Fukuhara S et al. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Metabolism 2014; 63: 431–440. [DOI] [PubMed] [Google Scholar]
- Jamurtas AZ, Stavropoulos-Kalinoglou A, Koutsias S, Koutedakis Y, Fatouros I. Adiponectin, Resistin, and Visfatin in Childhood Obesity and Exercise. Pediatr Exerc Sci 2015; 27: 454–462. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopal PB, Gidding SS, Buckloh LM, Yarandi HN, Sylvester JE, George DE et al. Changes in circulating satiety hormones in obese children: a randomized controlled physical activity-based intervention study. Obesity 2010; 18: 1747–1753. [DOI] [PubMed] [Google Scholar]
- Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998; 51: 1235–1241. [DOI] [PubMed] [Google Scholar]
- Morris SB. Estimating effect sizes from the pretest-posttest-control group designs. Organ Res Methods 2008; 11: 364–386. [Google Scholar]
- Higgins J, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions: Wiley Online Library: New Jersey, USA; 2008.
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359. [DOI] [PubMed] [Google Scholar]
- Chae HW, Kwon YN, Rhie YJ, Kim HS, Kim YS, Paik IY et al. Effects of a structured exercise program on insulin resistance, inflammatory markers and physical fitness in obese Korean children. J Pediatr Endocrinol Metab 2010; 23: 1065–1072. [DOI] [PubMed] [Google Scholar]
- Fazelifar S, Ebrahim K, Sarkisian V. Effect of concurrent training and detraining on anti-inflammatory biomarker and physical fitness levels in obese children. Rev Bras Med Esporte 2013; 19: 349–354. [Google Scholar]
- Jeon J-Y, Han J, Kim H-J, Park MS, Seo DY, Kwak Y-S. The combined effects of physical exercise training and detraining on adiponectin in overweight and obese children. Integr Med Res 2013; 2: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karacabey K. The effect of exercise on leptin, insulin, cortisol and lipid profiles in obese children. J Int Med Res 2009; 37: 1472–1478. [DOI] [PubMed] [Google Scholar]
- Kelly AS, Steinberger J, Olson TP, Dengel DR. In the absence of weight loss, exercise training does not improve adipokines or oxidative stress in overweight children. Metabolism 2007; 56: 1005–1009. [DOI] [PubMed] [Google Scholar]
- Kim ES, Im JA, Kim KC, Park JH, Suh SH, Kang ES et al. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity 2007; 15: 3023–3030. [DOI] [PubMed] [Google Scholar]
- Lee K-J, Shin Y-A, Lee K-Y, Jun T-W, Song W. Aerobic exercise training-induced decrease in plasma visfatin and insulin resistance in obese female adolescents. Int J Sport Nutr Exerc Metab 2010; 20: 275–281. [DOI] [PubMed] [Google Scholar]
- Park T-G, Hong H-R, Lee J, Kang H-S. Lifestyle plus exercise intervention improves metabolic syndrome markers without change in adiponectin in obese girls. Ann Nutr Metab 2007; 51: 197–203. [DOI] [PubMed] [Google Scholar]
- Racil G, Ben Ounis O, Hammouda O, Kallel A, Zouhal H, Chamari K et al. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol 2013; 113: 2531–2540. [DOI] [PubMed] [Google Scholar]
- Vasconcellos F, Seabra A, Cunha F, Montenegro R, Penha J, Bouskela E et al. Health markers in obese adolescents improved by a 12-week recreational soccer program: a randomised controlled trial. J Sports Sci 2016; 34: 564–575. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim ES, Jeon JY, Jekal Y. Improved insulin resistance, adiponectin and liver enzymes without change in plasma vaspin level after 12 weeks of exercise training among obese male adolescents. Korean J Obes 2011; 20: 138–146. [Google Scholar]
- Nunes JE, Cunha HS, Freitas ZR, Nogueira AM, Dâmaso AR, Espindola FS et al. Interdisciplinary therapy changes superoxide dismutase activity and adiponectin in obese adolescents: a randomised controlled trial. J Sports Sci 2016; 34: 945–950. [DOI] [PubMed] [Google Scholar]
- Martin A, Saunders DH, Shenkin SD, Sproule J. Lifestyle intervention for improving school achievement in overweight or obese children and adolescents. Cochrane Database Syst Rev 2014; 3: CD009728. [DOI] [PubMed] [Google Scholar]
- Blüher S, Panagiotou G, Petroff D, Markert J, Wagner A, Klemm T et al. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity 2014; 22: 1701–1708. [DOI] [PubMed] [Google Scholar]
- Dâmaso AR, da Silveira Campos RM, Caranti DA, de Piano A, Fisberg M, Foschini D et al. Aerobic plus resistance training was more effective in improving the visceral adiposity, metabolic profile and inflammatory markers than aerobic training in obese adolescents. J Sports Sci 2014; 32: 1435–1445. [DOI] [PubMed] [Google Scholar]
- Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism 2005; 54: 1472–1479. [DOI] [PubMed] [Google Scholar]
- Croymans DM, Paparisto E, Lee MM, Brandt N, Le BK, Lohan D et al. Resistance training improves indices of muscle insulin sensitivity and β-cell function in overweight/obese, sedentary young men. J Appl Physiol 2013; 115: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Heijden GJ, Wang ZJ, Chu Z, Toffolo G, Manesso E, Sauer PJ et al. Strength exercise improves muscle mass and hepatic insulin sensitivity in obese youth. Med Sci Sports Exerc 2010; 42: 1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbidi S, Laher I. Exercise induced adipokine changes and the metabolic syndrome. J Diabetes Res. 2014; 2014: 726861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KA, Singh MAF. Effects of exercise on adiponectin: a systematic review. Obesity 2008; 16: 241–256. [DOI] [PubMed] [Google Scholar]
- Nascimento H, Alves AI, Medeiros AF, Coimbra S, Catarino C, Bronze-da-Rocha E et al. Impact of a School-Based Intervention Protocol - ACORDA Project - On Adipokines in an Overweight and Obese Pediatric Population. Pediatr Exerc Sci. 2016; 28: 407–416. [DOI] [PubMed] [Google Scholar]
- Seabra A, Katzmarzyk P, Carvalho MJ, Seabra A, Coelho-E-Silva M, Abreu S et al. Effects of 6-month soccer and traditional physical activity programmes on body composition, cardiometabolic risk factors, inflammatory, oxidative stress markers and cardiorespiratory fitness in obese boys. J Sports Sci 2016; 1–8. [DOI] [PubMed]
- Lopes WA, Leite N, da Silva LR, Brunelli DT, Gáspari AF, Radominski RB et al. Effects of 12 weeks of combined training without caloric restriction on inflammatory markers in overweight girls. J Sports Sci 2016; 34: 1902–1912. [DOI] [PubMed] [Google Scholar]
- García-Hermoso A, Cerrillo-Urbina A, Herrera-Valenzuela T, Cristi-Montero C, Saavedra J, Martínez-Vizcaíno V. Is high-intensity interval training more effective on improving cardiometabolic risk and aerobic capacity than other forms of exercise in overweight and obese youth? A meta-analysis. Obes Rev 2016; 17: 531–540. [DOI] [PubMed] [Google Scholar]
- Sawicka M, Janowska J, Chudek J. Potential beneficial effect of some adipokines positively correlated with the adipose tissue content on the cardiovascular system. Int J Cardiol 2016; 222: 581–589. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab 2011; 301: e567–e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S1, Liu R, Arguelles L, Wang G, Zhang J, Shen X et al. Adiposity trajectory and its associations with plasma adipokine levels in children and adolescents—A prospective cohort study. Obesity 2015; 24: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani RM, Rocha NP, Magalhães DM, Barbosa IG, Teixeira AL, e Silva ACS. Early changes in adipokines from overweight to obesity in children and adolescents. J Pediatr (Rio J) 2016; 92: 624–630. [DOI] [PubMed] [Google Scholar]
- Kanaley JA, Heden TD, Liu Y, Whaley-Connell AT, Chockalingam A, Dellsperger KC et al. Short-term aerobic exercise training increases postprandial pancreatic polypeptide but not peptide YY concentrations in obese individuals. Int J Obes (Lond) 2014; 38: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer RR, Johnson LG, Haltom R, Kraemer GR, Hebert EP, Gimpel T et al. Serum leptin concentrations in response to acute exercise in postmenopausal women with and without hormone replacement therapy. Exp Biol Med 1999; 221: 171–177. [DOI] [PubMed] [Google Scholar]
- Adeghate E. An update on the biology and physiology of resistin. Cell Mol Life Sci 2004; 61: 2485–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DA, Hackney AC Inflammatory cytokines and metabolic risk factors during growth and maturation: influence of physical activity In: Cytokines, Growth Mediators and Physical Activity in Children during Puberty. Karger Publishers: Basel, Switzerland, 2010. pp 43–55. [DOI] [PubMed] [Google Scholar]
- Ben Ounis O, Elloumi M, Lac G, Makni E, Van Praagh E, Zouhal H et al. Two-month effects of individualized exercise training with or without caloric restriction on plasma adipocytokine levels in obese female adolescents. Ann Endocrinol (Paris) 2009; 70: 235–241. [DOI] [PubMed] [Google Scholar]
- Lee SS, Kang S. Effects of regular exercise on obesity and type 2 diabete mellitus in Korean children: improvements glycemic control and serum adipokines level. J Phys Ther Sci 2015; 27: 1903–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney AC, Viru A. Research methodology: endocrinologic measurements in exercise science and sports medicine. J Athl Train 2008; 43: 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggeloussi S, Theodorou AA, Paschalis V, Nikolaidis MG, Fatouros IG, Owolabi EO et al. Adipocytokine levels in children: effects of fatness and training. Pediatr Exerc Sci 2012; 24: 461–471. [DOI] [PubMed] [Google Scholar]
- Fukuhara A, Matsuda M, Nishizawa M et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 2005; 307: 426–430. [DOI] [PubMed] [Google Scholar]
- Lai A, Chen W, Helm K. Effects of visfatin gene polymorphism RS4730153 on exercise-induced weight loss of obese children and adolescents of Han Chinese. Int J Biol Sci 2013; 9: 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.