Abstract
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the gene that codes for the CF trans-membrane conductance regulator. These mutations result in abnormal secretions viscous airways of the lungs, favoring pulmonary infection and inflammation in the middle of neutrophil recruitment. Recently it was described that neutrophils can contribute with disease pathology by extruding large amounts of nuclear material through a mechanism of cell death known as Neutrophil Extracellular Traps (NETs) into the airways of patients with CF. Additionally, NETs production can contribute to airway colonization with bacteria, since they are the microorganisms most frequently found in these patients. In this review, we will discuss the implication of individual or mixed bacterial infections that most often colonize the lung of patients with CF, and the NETs role on the disease.
Keywords: neutrophil, NETs, cystic fibrosis, CFTR, Pseudomonas aeruginosa, Staphylococcus aureus, Burkholderia cepacia
Introduction
Cystic Fibrosis (CF) is a lung disease characterized by chronic inflammation of the airways associated with bacterial colonization. Disease occurs by mutations that disrupt the Cystic Fibrosis Transmembrane Regulator (CFTR) gene, a plasma membrane channel that regulates the balance of bicarbonate and chloride secretions across the epithelial layer of the airways. Patients with CF have an elevated presence of thick mucus, DNA and proteins, complexed with bacteria that obstruct airflow due to an inability of the epithelial cilia to beat and mediate mechanical clearance of these structures. More recently, some studies showed that the extracellular DNA levels correlate with neutrophil counts and can be used as a guide of inflammation and lung disease severity (Kirchner et al., 1996; Ratjen et al., 2005).
Neutrophil Extracellular Traps (NETs) release was recently related to the extracellular DNA found in patients with CF in which their presence can exacerbate the disease. The excessive formation of NETs promotes that the mucus in patient's alveoli becomes thicker and sticky, allowing the colonization of bacteria such as Haemophilus influenzae and Staphylococcus aureus, and significantly Pseudomonas aeruginosa (Manzenreiter et al., 2012). Such colonization promotes infiltration of neutrophils that undergo NETosis increasing sputum viscosity and consequently lowering the patient's respiratory capacity. In line with this, a treatment option for patients with CF is the administration of recombinant human DNase to disrupt NETs and so liquefy the sputum and facilitate mucociliary clearance (Papayannopoulos et al., 2011).
The neutrophil and its characteristics
Neutrophils are the most abundant white blood cells in circulation and have an important role in the immune response (Amulic et al., 2012). They possess a segmented nucleus and in their cytoplasm, multiple granules and secretory vesicles can be found. These granules have been subdivided into three classes: azurophilic, specific and gelatinase (Kolaczkowska and Kubes, 2013). Neutrophils also produce many peptides and proteins that directly or indirectly kill microbes divided into three types of antimicrobials: cationic peptides (i.e., defensins and cathelicidins); proteolytic enzymes [i.e., lysozyme, myeloperoxidase and neutrophil elastase (NE)] and, metal chelating proteins (i.e., lactoferrin and calprotectin; Borregaard and Cowland, 1997).
When a pathogen is detected, neutrophils leave the blood vessels and are recruited to the site of infection following a chemotactic gradient produced by microbial and endogenous signals (Papayannopoulos and Zychlinsky, 2009; Brinkmann and Zychlinsky, 2012). In the inflammatory site, these cells employ intracellular and extracellular strategies to contain and eliminate the infection (Kolaczkowska and Kubes, 2013). Intracellularly, eliminate pathogens very effectively by phagocytosis and degradation of the cargo in the lizosome, whereas, extracellular mechanims include degranulation where the lytic granules contained in vacuoles are expelled, production of reactive oxygen species that can affect directly the pathogen proteins and DNA and formation of extracellulat traps which can restrict dissemination at the same time that promote pathogen killing (Brinkmann et al., 2004; Papayannopoulos and Zychlinsky, 2009).
Neutrophil extracellular traps
NETs formation was first described in 2004 and although plenty of information has arised still many aspects of its regulation are unknown (Brinkmann and Zychlinsky, 2012). To release NETs, the activated neutrophil undergoes dramatic morphological changes mediated by signaling pathways that differ depending on the stimulus. It has been found that chemical inductors such as PMA promote “suicidal NETs” formation in which neutrophils extrude their DNA and die, a process that takes of 1.5–2 h to ocurr (Brinkmann and Zychlinsky, 2012; Zawrotniak and Rapala-Kozik, 2013) on the other hand, pathogen inducers promote “vital NETs” formation in matters of minutes (0.5–1 h) in which the cells extrude their DNA but still can migrate and kill pathogens by phagocytosis (Yipp and Kubes, 2016).
In the initial steps of NETs formation, minutes after activation, the neutrophil flattens and adheres firmly to a substrate, such adhesion is mediated by integrin receptors (Neeli et al., 2008). When “suicidal NETs” are induced neutrophil binding to its substrate promotes the activation of the NADPH oxidase complex, when is assembled in the fagosomal cell membrane and reduces the molecular oxygen superoxide anion by electron transfer. Superoxide dismutase (SOD) converts superoxide anion into hydrogen peroxide (H2O2), which acts in turn as a substrate for the enzyme myeloperoxidase (MPO), and also reacts with H2O2 to generate hypochlorous acid (Brinkmann and Zychlinsky, 2012). In contrast, ROS formation is not escential in “vital NETs” since in this a NADPH oxidase independent mechanism has been described to some pathogens (Rochael et al., 2015; Yipp and Kubes, 2016). In a subsequent step, the nucleus loses its lobed shape and the chromatin is decondensed by action of NE and MPO enzymes, that migrate to the nucleus where they exert their proteolytic function on histones (Fuchs et al., 2007; Papayannopoulos et al., 2010). Concomitantly, chromatin decondensation can be increased due to epigenetic mechanisms on histones, as it was found that histones H3 and H4 can be modified by a reaction catalyzed by arginine peptidyl deaminase 4 (PAD4) that converts arginine residues to citrulline (Wang et al., 2009; Leshner et al., 2012). Later, the nuclear envelope disintegrates, nucleoplasm and cytoplasm are mixed forming a homogeneous mass, and finally, rupture of the cell membrane promotes that the cellular contents are expelled into the extracellular space (Fuchs et al., 2007; Brinkmann and Zychlinsky, 2012).
The function of NETs
NETs likely evolved to restrict infections due to its ability to entrap, prevent the spread and exterminate microorganisms (Brinkmann and Zychlinsky, 2012), as they can catch almost all types of pathogens as its presence has been demonstrated in response against gram-positive and gram-negative bacterial infections, yeasts, viruses and protozoan parasites (Lu et al., 2012). Capture within the fibers of DNA prevents propagation of microorganisms on the body and facilitates a higher concentration of antimicrobial factors at the site of infection (Brinkmann et al., 2004). This capture occurs through charge interactions between cell surface components of the pathogen and NETs and its antimicrobial functions are exerted by the granular proteins, primarily by NE, histone and MPO, but also by the action of calgranulin, proteinase 3, lactoferrin, calprotectin, and antimicrobial peptides (AMP) such as defensins or cathelicidin LL-37 (Urban et al., 2009).
In addition to the antimicrobial function of NETs, its ineffective elimination or excessive presence can cause pathological effects. NETs formation has been observed during chronic inflammatory diseases (atherosclerosis), autoimmune diseases such as systemic lupus erythematosus (SLE), in various forms of vasculitis, thrombosis, as well as pulmonary diseases such as CF (Kolaczkowska and Kubes, 2013).
Cystic fibrosis and its relationship with NETs
CF is a disease produced by mutations that disrupt the CFTR gene (Table 1); the effect of these mutations is the production of abnormally viscous secretions in the airways of the lungs causing obstructions, inflammation and tissue destruction (Cutting, 2015). On the other hand, an important role on the immunopathology of the disease has been given to immune cells. Particularly, considerable participation of neutrophils has been proposed, as these cells have an increased influx to the lungs of the patients in response to bacterial colonization (Gray et al., 2015), but present a reduced ability to remove microbes due to an ineffective respiratory burst (Painter et al., 2008) and induce an exacerbated local tissue damage because of uncontrolled degranulation (Rogan et al., 2004; Sagel et al., 2004; Bergsson et al., 2009). Recently, NETs formation has drawn attention as it has been found that once free DNA is present in high quantity of in the sputum, patients present diminished lung function when compared with patients that have mild disease, indicating that the airway obstruction is due to the accumulation of NETs-DNA (Papayannopoulos et al., 2011; Dubois et al., 2012; Dwyer et al., 2014; Marcos et al., 2015). Also, it has been reported that components of NETs (i.e., mieloperoxidase, neutrophil elastase and histones) can induce destruction of epithelial, endothelial and connective tissue worsening the lung pathology (Klebanoff et al., 1993; Manzenreiter et al., 2012; Saffarzadeh et al., 2012). The importance of NETs in this disease is underscored by observations describing that elimination of free DNA from patient's airways constitute an important treatment option for combating CF since administration of recombinant human DNase, leads to a significate improvement on health (Rahman and Gadjeva, 2014).
Table 1.
Classes of CFTR mutations that cause cystic fibrosis.
| Class | Defect | Example | References |
|---|---|---|---|
| I | About half of the CFTR mutations are expected to prevent proper synthesis of full-length, normal CFTR polypeptide because of nonsense, frameshift, or aberrant splicing of mRNA. | G542X | Rowe et al., 2005 |
| II | The defective protein retains substantial chloride-channel function in cell-free lipid membranes. When synthesized by the normal cellular machinery, however, the protein is rapidly recognized as misfolded and is degraded shortly after synthesis, before it can reach its crucial site of action at the cell surface. | ΔF508 N1303K G85E G91R |
O'Sullivan and Freedman, 2009 |
| III | It encodes properly processed, full-length CFTR protein that lacks normal ion-channel activity | G551D G551S G1244E G1255P G1349D |
Rowe et al., 2005 |
| IV | This mutation exhibits only partial CFTR ion-channel activity, a feature that probably explains a less severe pulmonary phenotype. | A455E R117H R334W R347P |
Gan et al., 1995 |
| V | It includes promoter mutations that reduce transcription, nucleotide alterations that promote alternative splicing of the CFTR transcript, and amino acid substitutions that cause inefficient protein maturation. | P574H A455E |
Welsh and Smith, 1993 |
NETs formation by microorganisms of cystic fibrosis
In the lungs, the first point of contact for contaminants and microorganisms is the respiratory tract (Cullen and McClean, 2015). Sophisticated host defense mechanisms in the mucosa of the lungs, especially in healthy individuals, as well as the presence of cilia and mucus epithelial surface prevent infections trapping and removing particles and microorganisms of the healthy lung, even though the microorganisms are constantly inhaled (Eisele and Anderson, 2011). CF is characterized by airway inflammation, increased viscous mucus production and reduced mucociliary clearance, favoring chronic infections that contribute to a rapid decline in lung function and health (Cullen and McClean, 2015).
The alterations in the lung surface provides a suitable environment for the growth of various pathogens, being the most represented bacteria and fungi; among which, Pseudomonas aeruginosa constitutes one of the most prevalent pathogens that colonizes the lungs of CF patients (Cullen and McClean, 2015). Elimination of this bacteria results very difficult for the immune system since it is known that when infects the patient's lungs, this pathogen can migrate to areas with low oxygen concentrations where only few immune cells can exert its function (Young et al., 2011). Among these cells, the neutrophils constitute the primary defense line but P. aeruginosa has developed strategies to evade their destruction, as it has been shown that biofilm formation promotes excessive production of alginate that allows bacterial evasion to neutrophil phagocytosis and degranulation (Mulcahy et al., 2008). Additionally, P. aeruginosa has obtained diverse mechanisms to evade the effects of NETs, among these it is know that they can counteract the capacity of these structures to chelate divalent metal cations by overexpression of genes controlled by the two component systems PhoPQ and PmrAB that sense Mg2+ limitation and at the same time encodes mechanisms to effectively obtain the ion (Mulcahy et al., 2008; Johnson et al., 2012, 2013). Additionally, the bacteria is capable of regulating genes that allow them to tolerate the toxicity caused by the extracellular DNA (Halverson et al., 2015).
Respiratory tract colonization of CF patients by Staphylococcus aureus has drawn increasing attention, as it has been found that they suffer a more aggressive form of the disease. Possible explanations to this are not well understood, but the effect has been attributed to the high production of bacterial toxins that can induce strong damage to the patient's airways and to the resistance that this bacterium has against the immune mechanisms which include phagocytosis and degranulation evasion through production of capsule (Menestrina et al., 2003; Voyich et al., 2005). The role of NETosis in the response against S. aureus in CF is still not completely known but now is suggested that these structures can be more harmful that beneficial for patient's health. It has been reported that toxins produced by this bacterium can induce different types of cell death depending on the concentration including NETs (Genestier et al., 2005), but their antimicrobial effect could be limited due to a production a DNAse by the bacteria (Pilsczek et al., 2010) and moreover could contribute to tissue damage by increasing the local concentration of cytotoxic molecules. Additionally, experiments in vitro have shown that neutrophils infected with S. aureus can induce NETs with a faster kinetic resembling “vital NETs” formation (Pilsczek et al., 2010), and other group demonstrated that these structures are modified in a way to become cytotoxic for macrophages allowing the establishment of chronic infections and increased tissue damage (Thammavongsa et al., 2013).
Other groups of bacteria that have been found colonizing the respiratory tract of CF patients include Haemophilus influenza, Stenotrophomonas maltophilia, Achromobacter xyloxidans, genus Pandoraea and Streptococci (LiPuma, 2010; Callaghan and McClean, 2012). However, it has not been reported the formation of NETs by any of these bacteria in CF and its role on the disease still remains to be explored. A growing concern among clinicians is the observation of adult patient's lungs colonization with Burkholderia cepacia since it has been related to decreased respiratory function and worsening of the disease, the mechanisms behind this are not understood although it has been proposed a possible association with previously colonizing bacteria (Pseudomonas aeruginosa and/or Staphylococcus aureus) to form mixed clusters of pathogens.
NETs and bacterial biofilms can be found in high amounts in CF patient's lung, the possible relationship of these structures induced by bacteria colonizing the alveoli of CF patients is an interesting aspect to study, as it could be possible that the pathogen promotes biofilm and NET formation to use these structures as a niche to stablish the infection and persist at the cost of a detriment in patient's health. The idea results attractive as some groups have shown that in otitis media and supragingival infections some surface bacterial proteins determinants for biofilm formation can also attract neutrophils to the site of infection to induce NETs and at the same time contribute to avoid their elimination by the induced traps and phagocytic killing by other incoming cells (Juneau et al., 2011; Hirschfeld et al., 2015).
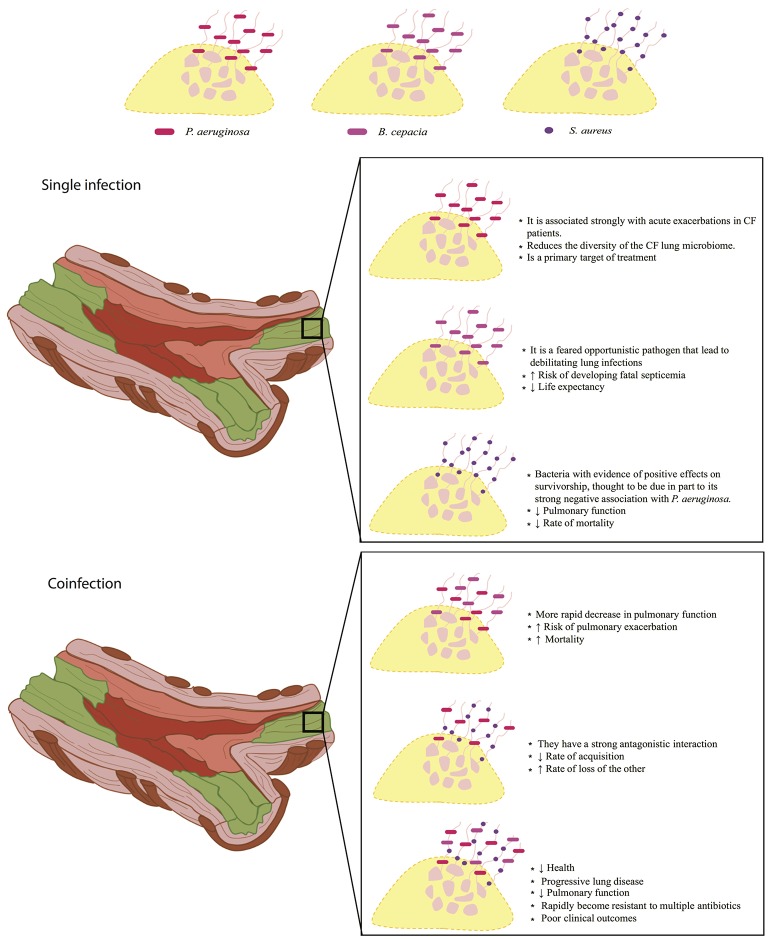
Figure 1 summarizes the information about the effect of mixed or single infections on the CF. P. aeruginosa is considered the main bacterium that affects people with CF, causing chronic lung infections, which leads to high rates of morbidity and mortality, and once the bacteria is established, it is difficult to eradicate it (Winstanley et al., 2016). On the other hand, patients colonized in a chronic manner with B. cepacia present a worse prognosis of the disease, as the use of antibiotics becomes more frequent they also have a greater deterioration of lung function and the rate of mortality is higher compared to that patients colonized by only P. aeruginosa (Gilligan, 2014; Folescu et al., 2015). It has been reported that B. cepacia and P. aeruginosa can form mixed biofilms in the lungs of people with CF, since, P. aeruginosa through its extracellular products can increase the attachment of B. cepacia by modifying the lung epithelial cells on its surface; however, the same does not occur in patients previously colonized with B. cepacia (Saiman et al., 1990). Coinfection between the two bacteria results in a rapid decline in lung function and a high mortality rate (Folescu et al., 2015). In CF the simple colonization by S. aureus is generally considered of better prognosis than those colonized with P. aeruginosa; but in some cases, the presence of small-colony variants (SCV) of S. aureus is associated with the most advanced lung disease in CF, and the phenotype of this bacterium in a coinfection with P. aeruginosa causes disease worsening (Besier et al., 2007; Hubert et al., 2013). In addition, some groups have found that host-pathogen interactions can affect the environment favoring pulmonary disease (Filkins and O'Toole, 2015) and that high levels of inflammation as well as an increase in the accumulation of calprotectin possibly related to NET formation promotes coinfection between P. aeruginosa and S. aureus (Wakeman et al., 2016). Additionally, reports have been published showing that P. aeruginosa, S. aureus, and B. cepacia can cause mixed infections in the respiratory tract of patients with CF; together, lead to pulmonary exacerbations, decreased pulmonary function and destruction of the lung, so the mortality rate is higher compared to simple infections or mixed infections with two different bacteria (Zemanick et al., 2011).
Figure 1.
NET formation in the lung surface of cystic fibrosis patients. Implications on disease prognosis (text box) in bacterial single infections (middle panel) and coinfections (lower panel) are shown. NET formation can constitute a scaffold for the establishment of simple infections or coinfections that can directly influence the elimination of pathogens that have direct repercussions on patient's health. Upper panel, within single infections, P. aeruginosa is the main bacterium that colonizes the lungs of patients with cystic fibrosis, and is associated with decreased microbiome as compared to healthy individuals. Patients colonized with B. cepacia are at higher risk of developing septicemia by decreasing their life expectancy. On the other hand, S. aureus is less aggressive compared to P. aeruginosa and B. cepacia, despite this, it can decrease lung function and increase the mortality rate. Bottom panel, mixed infections caused by P. aeruginosa and B. cepacia may become the most aggressive, due to a rapid loss of lung function, increasing the risk of pulmonary exacerbation and mortality. Coinfections between P. aeruginosa and S. aureus, have a very antagonistic interaction in which they compete for their establishment by decreasing the rate of acquisition and the rate of loss of another, such battle promote increased lung damage and rapid worsening of patient's health. On the other hand, coinfections between P. aeruginosa, S. aureus, and B. cepacia causes progressive lung disease and decreased lung function severely compromising patients' health.
CF investigations have focused on the role that bacteria on the pathogenesis of the disease, although very often other pathogens can be observed and isolated form CF patient's lungs. Of these, filamentous fungi Aspergillus fumigatus is the most commonly isolated (Armstead et al., 2014) and even thou detailed studies regarding its participation on CF and NET formation are scarce there are some data that could suggest an active role in the disease. In vitro it has been reported formation of high amounts of NETs against A. fumigatus hyphae compared to conidia by human neutrophils. Interestingly, NETs are unable to kill and eliminate A. fumigatus infection, but can reduce the growth of this fungus by trapping it into webs preventing further growth and confine the infection (McCormick et al., 2010). Additionally, other groups have related the reduction of NET formation to the presence of hydrophobin RodA, which is the major component of resting conidia surface although the precise mechanism behind this still has to be elucidated (Aimanianda et al., 2009; Bruns et al., 2010). Another fungi species that have been also isolated from CF patients are the hyaline fungi Scedosporium apiospermum (Pseudollescheria boydii) and black yeast Exophiala dermatitidis (Chotirmall and McElvaney, 2014) but NET formation and its importance in the disease has not been reported yet.
Candida albicans can also be isolated from the respiratory tract from CF patients but less frequently than other species and tend to colonize the mucoid membranes of the alimentary tract causing inflammation in the oral cavity (LiPuma, 2010; Chotirmall and McElvaney, 2014). Studies in vitro have shown that C. albicans promotes NET formation in human neutrophils and this structures can entrap and kill both the hyphae and yeast forms, (Urban et al., 2006, 2009). NET killing was mediated by neutrophil calprotectin that chelates essential ions such as Zn2+ and Mn2+ (Sohnle et al., 1996) essential for fungal growth. The last can offer a possible explanation about why this opportunistic fungus is not often found in CF patients but still more studies are needed to truly understand the mechanisms behind this.
Concluding remarks
To date, the NETs remain a mystery and its mechanisms of formation has not been completely deciphered. Clearly, it is important for the clearance and containment of a variety of microorganisms, but equally are involved in the development immunopathology of CF, where exacerbate the disease. It is important to fully elucidate the participation of NETs in CF and its interplay with the pathogens colonizing the patient's lungs for in the future develop proper strategies to control the disease.
Author contributions
Wrote the manuscript: SM and AS. Helped with manuscript preparation: LC, JP, and RH. Helped with commentaries and writing of the manuscript: GG.
Funding
This work was supported by the Autonomous University of Nuevo León in México through its Support Program for Scientific and Technological Research (PAICYT) 2015. We thank to the Ethics and Research committee of the Medicine Faculty and University Hospital “Dr. José Eleuterio González” of the UANL for their help and support for the publication of this work.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Microbiology Department members at the Medicine Faculty of the Autonomous University of Nuevo León in México for their support and advice during the preparation of this review.
References
- Aimanianda V., Bayry J., Bozza S., Kniemeyer O., Perruccio K., Elluru S. R., et al. (2009). Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460, 1117–1121. 10.1038/nature08264 [DOI] [PubMed] [Google Scholar]
- Amulic B., Cazalet C., Hayes G. L., Metzler K. D., Zychlinsky A. (2012). Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30, 459–489. 10.1146/annurev-immunol-020711-074942 [DOI] [PubMed] [Google Scholar]
- Armstead J., Morris J., Denning D. W. (2014). Multi-country estimate of different manifestations of aspergillosis in cystic fibrosis. PLoS ONE 9:e98502. 10.1371/journal.pone.0098502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsson G., Reeves E. P., McNally P., Chotirmall S. H., Greene C. M., Greally P., et al. (2009). LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J. Immunol. 183, 543–551. 10.4049/jimmunol.0803959 [DOI] [PubMed] [Google Scholar]
- Besier S., Smaczny C., Von Mallinckrodt C., Krahl A., Ackermann H., Brade V., et al. (2007). Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J. Clin. Microbiol. 45, 168–172. 10.1128/JCM.01510-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Cowland J. B. (1997). Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89, 3503–3521. [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., et al. (2004). Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Zychlinsky A. (2012). Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell Biol. 198, 773–783. 10.1083/jcb.201203170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns S., Kniemeyer O., Hasenberg M., Aimanianda V., Nietzsche S., Thywissen A., et al. (2010). Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 6:e1000873. 10.1371/journal.ppat.1000873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan M., McClean S. (2012). Bacterial host interactions in cystic fibrosis. Curr. Opin. Microbiol. 15, 71–77. 10.1016/j.mib.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Chotirmall S. H., McElvaney N. G. (2014). Fungi in the cystic fibrosis lung: bystanders or pathogens? Int. J. Biochem. Cell Biol. 52, 161–173. 10.1016/j.biocel.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Cullen L., McClean S. (2015). Bacterial adaptation during chronic respiratory infections. Pathogens 4, 66–89. 10.3390/pathogens4010066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting G. R. (2015). Cystic fibrosis genetics: from molecular understanding to clinical application. HHS Public Access 16, 45–56. 10.1038/nrg3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois A. V., Gauthier A., Bréa D., Varaigne F., Diot P., Gauthier F., et al. (2012). Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. Am. J. Respir. Cell Mol. Biol. 47, 80–86. 10.1165/rcmb.2011-0380OC [DOI] [PubMed] [Google Scholar]
- Dwyer M., Shan Q., D'Ortona S., Maurer R., Mitchell R., Olesen H., et al. (2014). Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J. Innate Immun. 6, 765–779. 10.1159/000363242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele N. A., Anderson D. M. (2011). Host defense and the airway epithelium: frontline responses that protect against bacterial invasion and pneumonia. J. Pathog. 2011:249802. 10.4061/2011/249802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkins L. M., O'Toole G. A. (2015). Cystic fibrosis lung infections: polymicrobial, complex, and hard to treat. PLoS Pathog. 11:e1005258. 10.1371/journal.ppat.1005258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folescu T. W., da Costa C. H., Cohen R. W. F., da Conceição Neto O. C., Albano R. M., Marques E. A. (2015). Burkholderia cepacia complex: clinical course in cystic fibrosis patients. BMC Pulm. Med. 15:158. 10.1186/s12890-015-0148-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T. A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., et al. (2007). Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241. 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan K. H., Veeze H. J., van den Ouweland A. M., Halley D. J., Scheffer H., van der Hout A., et al. (1995). A cystic fibrosis mutation associated with mild lung disease. N. Engl. J. Med. 333, 95–99. [DOI] [PubMed] [Google Scholar]
- Genestier A. L., Michallet M. C., Prévost G., Bellot G., Chalabreysse L., Peyrol S., et al. (2005). Staphylococcus aureus Panton-Valentine leucocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Invest. 115, 3117–3127. 10.1172/JCI22684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan P. H. (2014). Infections in patients with cystic fibrosis: diagnostic microbiology update. Clin. Lab. Med. 34, 197–217. 10.1016/j.cll.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R., McCullagh B., McCray P. (2015). NETs and CF lung disease: current status and future prospects. Antibiotics 4, 62–75. 10.3390/antibiotics4010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson T. W. R., Wilton M., Poon K. K. H., Petri B., Lewenza S. (2015). DNA is an antimicrobial component of neutrophil extracellular traps. PLoS Pathog. 11:e1004593. 10.1371/journal.ppat.1004593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld J., Dommisch H., Skora P., Horvath G., Latz E., Hoerauf A., et al. (2015). Neutrophil extracellular trap formation in supragingival biofilms. Int. J. Med. Microbiol. 305, 453–463. 10.1016/j.ijmm.2015.04.002 [DOI] [PubMed] [Google Scholar]
- Hubert D., Réglier-Poupet H., Sermet-Gaudelus I., Ferroni A., Le Bourgeois M., Burgel P. R., et al. (2013). Association between Staphylococcus aureus alone or combined with Pseudomonas aeruginosa and the clinical condition of patients with cystic fibrosis. J. Cystic Fibros. 12, 497–503. 10.1016/j.jcf.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Johnson L., Horsman S. R., Charron-Mazenod L., Turnbull A. L., Mulcahy H., Surette M. G., et al. (2013). Extracellular DNA-induced antimicrobial peptide resistance in Salmonella enterica serovar Typhimurium. BMC Microbiol. 13:115. 10.1186/1471-2180-13-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L., Mulcahy H., Kanevets U., Shi Y., Lewenza S. (2012). Surface-localized spermidine protects the Pseudomonas aeruginosa: outer membrane from antibiotic treatment and oxidative stress. J. Bacteriol. 194, 813–826. 10.1128/JB.05230-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneau R. A., Pang B., Weimer K. W. D., Armbruster C. E., Swords W. E. (2011). Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect. Immun. 79, 431–438. 10.1128/IAI.00660-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner K. K., Wagener J. S., Khan T. Z., Copenhaver S. C., Accurso F. J. (1996). Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 154, 1426–1429. 10.1164/ajrccm.154.5.8912759 [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Kinsella M. G., Wightt T. N. (1993). Degradation of endothelial cell matrix heparan sulfate proteoglycan by elastase and the myeloperoxidase-H202-chloride system. Am. J. Pathol. 143, 907–917. [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E., Kubes P. (2013). Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- Leshner M., Wang S., Lewis C., Zheng H., Chen X. A., Santy L., et al. (2012). PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 3:307. 10.3389/fimmu.2012.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiPuma J. J. (2010). The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 23, 299–323. 10.1128/CMR.00068-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Kobayashi S. D., Quinn M. T., DeLeo F. R. (2012). A NET outcome. Front. Immunol. 3:365 10.3389/fimmu.2012.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzenreiter R., Kienberger F., Marcos V., Schilcher K., Krautgartner W. D., Obermayer A., et al. (2012). Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J. Cystic Fibros. 11, 84–92. 10.1016/j.jcf.2011.09.008 [DOI] [PubMed] [Google Scholar]
- Marcos V., Zhou-Suckow Z., Önder Yildirim A., Bohla A., Hector A., Vitkov L., et al. (2015). Free DNA in cystic fibrosis airway fluids correlates with airflow obstruction. Mediators Inflamm. 2015:408935. 10.1155/2015/408935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick A., Heesemann L., Wagener J., Marcos V., Hartl D., Loeffler J., et al. (2010). NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microb. Infect. 12, 928–936. 10.1016/j.micinf.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Menestrina G., Dalla Serra M., Comai M., Coraiola M., Viero G., Werner S., et al. (2003). Ion channels and bacterial infection: the case of β-barrel pore-forming protein toxins of Staphylococcus aureus. FEBS Lett. 552, 54–60. 10.1016/S0014-5793(03)00850-0 [DOI] [PubMed] [Google Scholar]
- Mulcahy H., Charron-Mazenod L., Lewenza S. (2008). Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 4:e1000213. 10.1371/journal.ppat.1000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeli I., Khan S. N., Radic M. (2008). Histone deimination as a response to inflammatory stimuli in neutrophils. J. Immunol. 180, 1895–1902. 10.4049/jimmunol.180.3.1895 [DOI] [PubMed] [Google Scholar]
- O'Sullivan B. P., Freedman S. D. (2009). Cystic fibrosis. Lancet 373, 1891–1904. 10.1016/S0140-6736(09)60327-5 [DOI] [PubMed] [Google Scholar]
- Painter R. G., Bonvillain R. W., Valentine V. G., Lombard G. A., LaPlace S. G., Nauseef W. M., et al. (2008). The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J. Leukoc. Biol. 83, 1345–1353. 10.1189/jlb.0907658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V., Metzler K. D., Hakkim A., Zychlinsky A. (2010). Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191, 677–691. 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V., Staab D., Zychlinsky A. (2011). Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving dnase therapy. PLoS ONE 6:e28526. 10.1371/journal.pone.0028526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V., Zychlinsky A. (2009). NETs: a new strategy for using old weapons. Trends Immunol. 30, 513–521. 10.1016/j.it.2009.07.011 [DOI] [PubMed] [Google Scholar]
- Pilsczek F. H., Salina D., Poon K. K., Fahey C., Yipp B. G., Sibley C. D., et al. (2010). A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 185, 7413–7425. 10.4049/jimmunol.1000675 [DOI] [PubMed] [Google Scholar]
- Rahman S., Gadjeva M. (2014). Does NETosis contribute to the bacterial pathoadaptation in cystic fibrosis? Front. Immunol. 5:378. 10.3389/fimmu.2014.00378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratjen F., Paul K., van Koningsbruggen S., Breitenstein S., Rietschel E., Nikolaizik W. (2005). DNA concentrations in BAL fluid of cystic fibrosis patients with early lung disease: influence of treatment with dornase alpha. Pediatr. Pulmonol. 39, 1–4. 10.1002/ppul.20134 [DOI] [PubMed] [Google Scholar]
- Rochael N. C., Guimarães-Costa A. B., Nascimento M. T. C., DeSouza-Vieira T. S., Oliveira M. P., Garcia e Souza L. F., et al. (2015). Classical ROS-dependent and early/rapid ROS-independent release of Neutrophil Extracellular Traps triggered by Leishmania parasites. Sci. Rep. 5:18302. 10.1038/srep18302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan M. P., Taggart C. C., Greene C. M., Murphy P. G., O'Neill S. J., McElvaney N. G. (2004). Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis. J. Infect. Dis. 190, 1245–1253. 10.1086/423821 [DOI] [PubMed] [Google Scholar]
- Rowe S. M., Miller S., Sorscher E. J. (2005). Cystic fibrosis. N. Engl. J. Med. 352, 1992–2001. 10.1056/NEJMra043184 [DOI] [PubMed] [Google Scholar]
- Saffarzadeh M., Juenemann C., Queisser M. A., Lochnit G., Barreto G., Galuska S. P., et al. (2012). Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE 7:e32366. 10.1371/journal.pone.0032366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagel S. D., Sontag M. K., Accurso F. J., Bergsson G., Reeves E. P., McNally P., et al. (2004). Relationship between antimicrobial proteins and airway inflammation and infection in cystic fibrosis. Pediatr. Pulmonol. 44, 402–409. 10.1002/ppul.21028 [DOI] [PubMed] [Google Scholar]
- Saiman L., Cacalano G., Prince A. (1990). Pseudomonas cepacia adherence to respiratory epithelial cells is enhanced by Pseudomonas aeruginosa. Infect. Immun. 58, 2578–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohnle P. G., Hahn B. L., Santhanagopalan V. (1996). Inhibition of Candida albicans growth by calprotectin in the absence of direct contact with the organisms. J. Infect. Dis. 174, 1369–1372. 10.1093/infdis/174.6.1369 [DOI] [PubMed] [Google Scholar]
- Thammavongsa V., Missiakas D., Schneewind O. (2013). Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342, 863–866. 10.1126/science.1242255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban C. F., Ermert D., Schmid M., Abu-Abed U., Goosmann C., Nacken W., et al. (2009). Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5:e1000639. 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban C. F., Reichard U., Brinkmann V., Zychlinsky A. (2006). Neutrophil extracellular traps capture and kill Candida albicans and hyphal forms. Cell. Microbiol. 8, 668–676. 10.1111/j.1462-5822.2005.00659.x [DOI] [PubMed] [Google Scholar]
- Voyich J. M., Braughton K. R., Sturdevant D. E., Whitney A. R., Saïd-Salim B., Porcella S. F., et al. (2005). Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175, 3907–3919. 10.4049/jimmunol.175.6.3907 [DOI] [PubMed] [Google Scholar]
- Wakeman C. A., Moore J. L., Noto M. J., Zhang Y., Singleton M. D., Prentice B. M., et al. (2016). The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat. Commun. 7:11951. 10.1038/ncomms11951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li M., Stadler S., Correll S., Li P., Wang D., et al. (2009). Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 184, 205–213. 10.1083/jcb.200806072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Smith A. E. (1993). Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 73, 1251–1254. 10.1016/0092-8674(93)90353-R [DOI] [PubMed] [Google Scholar]
- Winstanley C., O'Brien S., Brockhurst M. A. (2016). Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 24, 327–337. 10.1016/j.tim.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yipp B. G., Kubes P. (2016). Review Article NETosis : how vital is it ? Blood 122, 2784–2795. 10.1182/blood-2013-04-457671 [DOI] [PubMed] [Google Scholar]
- Young R. L., Malcolm K. C., Kret J. E., Caceres S. M., Poch K. R., Nichols D. P., et al. (2011). Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS ONE 6:e23637. 10.1371/journal.pone.0023637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawrotniak M., Rapala-Kozik M. (2013). Neutrophil extracellular traps (NETs) - formation and implications. Acta Biochim. Pol. 60, 277–284. [PubMed] [Google Scholar]
- Zemanick E. T., Sagel S. D., Harris J. K. (2011). The airway microbiome in cystic fibrosis and implications for treatment. Curr. Opin. Pediatr. 23, 319–324. 10.1097/MOP.0b013e32834604f2 [DOI] [PubMed] [Google Scholar]