Abstract
Background
Umbelliferone, also known as 7-hydroxycoumarin, is a phenolic metabolite found in many familiar plants. Its derivatives have been shown to have various pharmacological and chemo-preventive effects on human health. A uridine diphosphate glycosyltransferase YjiC from Bacillus licheniformis DSM 13, a cytochrome P450BM3 (CYP450 BM3) variant namely mutant 13 (M13) from Bacillus megaterium, and an O-methyltransferase from Streptomyces avermitilis (SaOMT2) were used for modifications of umbelliferone.
Results
Three umbelliferone derivatives (esculetin, skimmin, and herniarin) were generated through enzymatic and whole cell catalysis. To improve the efficiencies of biotransformation, different media, incubation time and concentration of substrate were optimized and the production was scaled up using a 3-L fermentor. The maximum yields of esculetin, skimmin, and herniarin were 337.10 μM (67.62%), 995.43 μM (99.54%), and 37.13 μM (37.13%), respectively. The water solubility of esculetin and skimmin were 1.28-folds and 3.98-folds as high as umbelliferone, respectively, whereas herniarin was 1.89-folds less soluble than umbelliferone. Moreover, the antibacterial and anticancer activities of herniarin showed higher than umbelliferone, esculetin and skimmin.
Conclusions
This study proves that both native and engineered enzymes could be employed for the production of precious compounds via whole cell biocatalysis. We successfully produced three molecules herniarin, esculetin and skimmin in practical amounts and their antibacterial and anticancer properties were accessed. One of the newly synthesized molecules the present research suggests that the combinatorial biosynthesis of different biosynthetic enzymes could rapidly promote to a novel secondary metabolite.
Electronic supplementary material
The online version of this article (doi:10.1186/s13036-017-0056-5) contains supplementary material, which is available to authorized users.
Keywords: Glycosylation, Hydroxylation, Methylation, Umbelliferone
Background
Umbelliferone and its derivatives are compounds derived from coumarin (Fig. 1) with pharmacological and chemopreventive benefits for human health. Umbelliferone is known to have antinociceptive and anti-inflammatory activities in animal models [1]. Moreover, esculetin exhibits various biological activities including antioxidant [2], anti-tumor, anti-metastatic [3], anti-proliferative, pro-apoptotic [4], and neuroprotective activities [5]. Umbelliferone glycosides have been found to possess neuroprotective effects against serum deprivation-induced PC12 cell damage [6] with antidiabetic, antihyperlipidemic, and antioxidative activities [7]. Furthermore, 7-methoxycoumarin showed antifugal and antibacterial activities [8]. Likewise, other umbelliferone derivatives such as scopoletin, scoparone, fraxetin, esculin, and daphnetin have been reported to have antioxidant and intestinal anti-inflammatory activities [9].
Fig. 1.
The chemical structure of the simple coumarins
Coumarins are phenolic metabolites commonly distributed in many plant species [10]. The ortho-hydroxylation of cinnamate is a pivotal step in the biosynthesis of coumarin in plants. The core structure, 2H-1-benzopyran-2-one, of umbelliferone and its derivatives are formed via ortho-hydroxylation of cinnamates which undergo trans/cis isomerization of the side-chain followed by lactonization [11]. Although these compounds exhibit multi-beneficial pharmacological properties, isolation and purification of umbelliferone and its derivatives from plants are problematic due to low concentration and seasonal and regional dependency [12]. On the other hand, chemical synthesis requires the usage of hazardous agents, long synthetic steps, and extreme reaction conditions [13, 14].
Recently, an alternative approach has emerged as a promising method to synthesize small molecules by engineering microbes. Simple coumarins have been obtained from engineered Escherichia coli (E. coli) by combinational expression of artificial pathway enzymes with diverse genetic source including phenylalanine ammonia lyase (PAL) or tyrosine ammonia lyase (TAL), 4-cinnamic acid:coenzyme A ligase (4CL), coumarin synthase (C2´H, coumaroyl-CoA 2′-hydroxylase; or F6´H, feruloyl-CoA 6′-hydroxylase) [15–17]. For example, umbelliferone and scopoletin have been synthesized by using TAL, HpaBC (4-hydroxyphenylacetate 3-hydroxylase), and CCoAOMT (caffeoyl-CoA O-methyltransferase) in conjunction with 4CL and F6´H from p-coumaric acid and ferulic acid, respectively [16]. Moreover, tyrosine can be converted to esculetin by employing TAL, C3H (coumarate 3-hydroxylase from E.coli) or Sam5 (a monooxygenase from Saccharothrix espanaensis), 4CL, and F6´H [17, 18]. These researches demonstrated that hydroxyl cinnamic acid or glucose was the strating material for biosynthesis of simple coumarins. However, low catalytic activity, low final yield, and limited products are the most frequently encountered problems when applying a combinatorial expression approach.
In this study, biocatalyst system was carried out to modify umbelliferone by using glycosyltransferase, cytochrome P450, and O-methyltransferase. Three derivatives of umbelliferone, namely esculetin, skimmin, and herniarin, were successfully synthesized and their antibacterial and anticancer activities were evaluated.
Results
Protein expression and purification
Recombinant proteins CYP450 BM3 (119 kDa), M13 (119 kDa), YjiC (45 kDa), and SaOMT2 (37.5 kDa) were overexpressed in E. coli BL21 (DE3) (Additional file 1: Figure S1). The content acquired from the soluble fractions of proteins CYP450 BM3 and M13 were 477.253 and 1932.069 nM/L, respectively. The concentrations of crude proteins of CYP450 BM3, M13, YjiC, and SaOMT2 were determined to be approximately 147.54, 122.93, 344.28, and 76.99 μg/mL, respectively, based on the Bradford method.
Enzymatic reaction
The four enzymes was performed enzymatic reaction using umbelliferone as standard substrate. The results were analyzed by high performance liquid chromatography-photodiode array (HPLC-PDA) and high-resolution quadruple time-of-flight electrospray ionization-mass spectrometry (HR-QTOF ESI/MS). The retention time (t R) of umbelliferone standard was observed at 13.749 min with UV absorbance at 330 nm (Fig. 2a(i)). The found mass of umbelliferone was ~ 163.0394 [M + H]+ m/z + equivalent to molecular formula C9H7O3, for which the calculated mass was 163.0395 (Additional file 1: Figure S2A). HPLC-PDA chromatogram revealed the appearance of a new peak (P1) in the hydroxylation reaction of both CYP450 BM3 and M13. A new peak t R at 12.730 min (Fig. 2a(ii;iii)) and λ max at 325 nm might be hydroxylated umbelliferone. P1 was further analyzed by HR-QTOF ESI/MS. The mass spectrum displayed a peak with found mass of ∼ 179.0390 [M + H]+ m/z +, resembling the mass of mono-hydroxylated derivative of umbelliferone with a molecular formula C9H7O4, for which the calculated mass was ∼ 179.0344 (Additional file 1: Figure S2B). HPLC-PDA analysis showed that M13 had higher catalytic activity (35.48%) as a monooxygenase than CYP450 BM3 in which the conversion was limited to 8.29% (Table 1, Fig. 2a(iii)). Moreover, the HPLC-PDA analysis of glycosylation reaction mixture showed a new peak at t R of 10.323 min (P2) (Fig. 2a(iv)). P2 exhibited the found mass [M + H]+ m/z + at ~ 325.0912 with λmax:317 nm, corresponding to the calculated mass of the mono-glucoside derivative of umbelliferone with molecular formula C15H17O8 for [M + H]+ m/z + ~ 325.0923 (Additional file 1: Figure S2C). Furthermore, the HPLC-PDA analysis of methylation reaction mixture exhibited an additional peak at at t R of 16.381 min (P3) (Fig. 2a(v)). P3 showed the exact mass [M + H]+ m/z + at ~ 177.0553 with λmax:321 nm, corresponding to the calculated mass of methylated umbelliferone with molecular formula C10H9O3 for [M + H]+ m/z + ~ 177.0552 (Additional file 1: Figure S2D). The result displayed the conversion of umbelliferone to its derivatives were 86.45% and 2.08% in the glycosylation and methylation reactions, respectively (Table 1). These in vitro results indicated that umbelliferone might be a substrate of various transferase enzymes. It was converted more efficiently to glycosylated form than other derivatives. Therefore, umbelliferone was used for the production of its derivatives by using M13, YjiC, and SaOMT2 in whole cells.
Fig. 2.
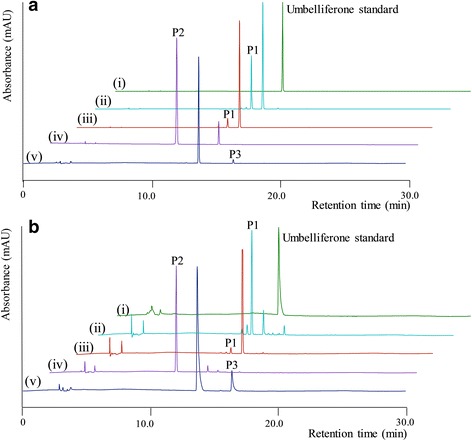
a In vitro reaction mixture and b whole cells bioconversion of umbelliferone on the HPLC-PDA analysis. (i) control reaction of umbelliferone using E. coli BL21 (DE3); (ii) hydroxylation with M13 and (iii) CYP450 BM3 at 48 h; (iv) glycosylation with YjiC at 12 h; and (v) methylation with SaOMT2 at 48 h, respectively
Table 1.
The HPLC-PDA, chemical formula, HR-QTOF ESI/MS, UV maxima and conversion product analyses of umbelliferone in in vitro reaction using CYP450 BM3 and its variant proteins M13, YjiC and SaOMT2
| Name | HPLC (t R) min | Chemical formula | Calculated mass [M + H]+ m/z +~ |
Exact mass [M + H]+ m/z +~ |
UV maxima (nm) | % Conversion | |
|---|---|---|---|---|---|---|---|
| Substrate | Umbelliferone | 13.749 | C9H6O3 | 163.0395 | 163.0394 | 327 | |
| Products | Hydroxylated (P1) by P450 BM3 | 12.730 | C9H6O4 | 179.0344 | 179.0390 | 325 | 8.09 |
| Hydroxylated (P1) by M13 | C9H6O4 | 35.48 | |||||
| Glycosylated (P2) | 10.323 | C15H16O8 | 325.0923 | 325.0912 | 317 | 86.45 | |
| Methylated (P3) | 16.381 | C10H8O3 | 177.0344 | 177.0547 | 321 | 2.08 | |
Bioconversion of umbelliferone using CYP450 BM3 and its variant
The hydroxylated derivative was produced from umbelliferone by using E. coli harboring pCW(Ori+)-CYP450 BM3 wild type and E. coli harboring pCW(Ori+)-mutant 13 (M13). The cells were induced with IPTG and were incubated at 28 °C with 100 μM umbelliferone for 12 h in various media as described in materials and methods. Culture media and cell pellets were extracted with ethyl acetate (EtOAc) and the products were analyzed by HPLC-PDA. HPLC-PDA chromatograms of both strains showed a new peak at t R ~ 12.730 min (P1) in comparison with umbelliferone standard at t R ~ 13.749 min under UV absorbance of 330 nm (Fig. 2b(i; ii)). All detected peaks were further analyzed by HR-QTOF ESI/MS. The found mass of umbelliferone standard was observed at ∼ 163.0394 [M + H]+ m/z + corresponding to molecular formula C9H7O3 with λmax ∼ 327 nm, for which the calculated mass was ∼ 163.0395 (Additional file 1: Figure S2A). The found mass of hydroxylated product P1 at ∼ 179.0390 [M + H]+ m/z + corresponding to molecular formula C9H7O4 with λmax ∼ 325 nm, for which the calculated mass was ∼ 179.0344 (Additional file 1: Figure S2B).
The bioconversion rate of umbelliferone to hydroxylated products was very low with the wild type strain using all three different media. However, the conversion was relatively higher with E. coli BL21(DE3) harboring pCW(Ori+)-mutant 13 (M13). The highest hydroxylated product was recorded to be 84.70 μM (84.7% conversion) in M9 medium, which was 1.85-fold higher than that in Luria-Bertani (LB) medium (45.72 μM) and 2.07-fold higher than that in Terrific Broth (TB) medium (40.84 μM) (Additional file 1: Figure S3A). These results further mean that M9 medium was a suitable medium for the production of hydroxylated product from umbelliferone. Similar trend has been observed with other flavonoid molecules [19]. However, the exact reason behind this is unclear. After that, E. coli BL21(DE3) harboring pCW(Ori+)-mutant 13 was chosen to optimize the substrate conversion with separately supplied 100, 250, 500, 750, and 1000 μM of umbelliferone. Substrate utilization and cell growth were measured at 12 h intervals. The maximum concentration of hydroxylated product was 329.29 μM (65.86%) at 48 h with OD600 ~ 3.949 when 500 μM of umbelliferone was added into the biotransformation reaction. Even though umbelliferone was non-toxic to cells (Additional file 1: Figure S3B), bioconversion was inhibited when a higher amount of substrate was supplied (Additional file 1: Figure S3C). Recombinant strain E. coli BL21(DE3) harboring pCW(Ori+)-mutant 13 were cultured in a 3-L fermentor with 1 × M9 minimal medium supplemented with 500 μM umbelliferone (~243.2 mg in 3-L) and supplied 2% glucose. The temperature and pH of the fermentor were kept constant at 28 °C and 7.6, respectively. The samples were taken at 12 h interval and measured by HPLC-PDA. Only 377.0959 μM (~180.2 mg; 60.1 mg/L) of hydroxylated umbelliferone was produced until 48 h as the maximal yield with bioconversion rate of the substrate at OD600 nm ~ 43.37 of approximately 67.62% (Fig. 3a).
Fig. 3.
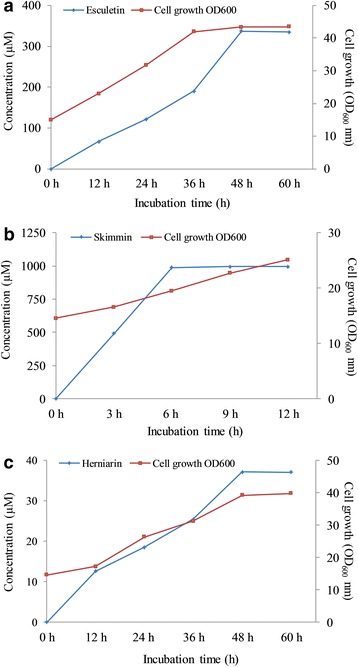
The scale-up of a hydroxylated b glucosylated c methylated umbelliferone and cell growth at OD600 nm in 3-L fermentation at different time intervals
Production of glucosylated umbelliferone
The glucosylated derivative was synthesized from umbelliferone by using E.coli harboring pET28a-YjiC. This E. coli strain was fed with 100 μM substrate at 20 °C for 12 h in various media. The product was extracted with twice volume of EtOAc. HPLC-PDA analysis showed a new peak (P2) with t R ~ 10.323 min compared to t R ~ 13.749 min of a substrate under UV absorbance of 330 nm (Fig. 2b(i; iii)). The found mass of glycosylated product P2 [M + H]+ m/z + was ∼ 325.0912, resembling molecular formula C15H17O8 with λmax ∼ 317 nm. The calculated mass was ∼ 325.0923 (Additional file 1: Figure S2C). While the highest bioconversion of umbelliferone to glucosylated derivative was observed in M9 medium (99.65 μM, 99.65%), which was lower in both LB (58.70 μM, 58.70%) and TB (46.91 μM, 46.91%) media under identical reaction conditions. (Additional file 1: Figure S4A). The different concentration of umbelliferone (100, 500, 1000, 1500 and 2000 μM) was fed into the culture of E.coli harboring pET28a-YjiC in the M9 1x medium. An equal volume of sample was taken and analyzed at 3 h intervals. Time and concentration dependent study showed that the highest concentration of umbelliferone glucoside was 996.72 μM (99.67%) with OD600 ~ 3.06 at 12 h when 1000 μM of substrate was supplemented in the culture. Umbelliferone failed to affect the growth rate of cells (Additional file 1: Figure S4B). However, the amount of glycosylated product was not significantly increased when a higher amount of substrate was supplied (Additional file 1: Figure S4C). Therefore, 1000 μM umbelliferone and 1x M9 minimal medium with 2% glucose were selected for the scale up of bioconversion reaction in 3-L fermentor. Nearly all umbelliferone was transformed into glycosylated product at 6 h, yielding approximately 995.43 μM (~967.6 mg; 322.8 mg/L) of product with OD600 nm ~ 22.7 (Fig. 3b).
Whole cell methylation of umbelliferone
E. coli harboring pRSF-Duet-SaOMT2 was subjected to methylation process of umbelliferone. The biotransformation system was supplied with 100 μM and incubated at 20 °C for 48 h in various media. The product was extracted with EtOAc and analyzed by HPLC-PDA. The result showed methylation product of substrate with a peak at t R ~ 16.381 min whereas the aglycone moiety of umbelliferone appeared at t R ~ 13.749 min (Fig. 2b(iv)). Subsequently, the peak at t R ~ 16.381 min was further analyzed by HR-QTOF ESI/MS. The observed mass [M + H]+ m/z + was ∼ 177.0553, resembling molecular formula C10H19O3 with λmax ∼ 321 nm. The calculated mass of methylated umbelliferone was ∼ 177.0552 (Additional file 1: Figure S2D).
Unlike hydroxylation and glycosylation reactions in whole cell biotransformation, methylated product was produced at maximum concentration (17.61 μM, 17.61% conversion) after 48 h incubation in LB media, followed by that in M9 (2.60 μM, 2.6%) and TB media (1.15 μM, 1.15%) (Additional file 1: Figure S5A). The results of optimized conversion showed that the maximum concentration of methylated product was 17.61 μM (17.61%) at 48 h with OD600 ~ 3.45 when 100 μM of umbelliferone was added into the biotransformation reaction (Additional file 1: Figure S5B). Umbelliferone did not affect the growth rate of cells. However, the bioconversion from a substrate to the methylated product was not meaningfully increased when a higher amount of substrate was supplied (Additional file 1: Figure S5C). To test the reproducibility of the bioconversion process into the large-scale fermentor, batch fermentation of this strain was carried out in a 3-L fermentor with LB medium supplied with umbelliferone at 100 μM (~48.64 mg in 3-L). The temperature and pH of the fermentor were maintained at 25 °C and 7.6, respectively. The samples were detached at 12 h interval and measured by HPLC-PDA. HPLC-PDA analysis revealed that methylated product bioconversion was increased to 37.13 μM (~19.6 mg; 6.5 mg/L) at 48 h with OD600 nm ~ 39.21 (Fig. 3c).
Structural elucidation of umbelliferone and its derivatives
The structures of umbelliferone standard and purified modified products were analyzed by proton nuclear magnetic resonance (1H-NMR) and carbon-13 nuclear magnetic resonance (13C-NMR) at 700 MHz in dimethyl sulfoxide-d6 (DMSO-d 6). We re-confirmed umbelliferone based on the previous report (Additional file 1: Figure S6) [20]. The 1H-NMR spectrum of hydroxylated umbelliferone showed the absence of proton signal at δ = 6.79 ppm (J = 8.4, 2.3 Hz) for C-6. It showed the presence of a new signal at δ = 10.09 ppm (s) for a hydroxyl group. An upfield shift at δ = 139.02 ppm of the C-6 of hydroxylated product was also observed in comparison with the same carbon of umbelliferone at δ = 113.59 ppm accompanied with a downfield shift of the resonances of adjacent carbons C-7 at δ = 158.21 ppm and δ = 160.92 ppm, respectively (Table 2; Additional file 1: Figure S7). Furthermore, the compound was identical to an authentic sample of esculetin, a hydroxylated derivative of umbelliferone [21].
Table 2.
Umbelliferone in comparision with hydroxylated (esculetin), glucosylated (skimmin) and methylated umbelliferone (herniarin) on 1H-NMR and 13C-NMR analyses
| Carbon No. | Umbelliferone | Esculetin | Skimmin | Herniarin | ||||
|---|---|---|---|---|---|---|---|---|
| 1H NMR | 13C NMR | 1H NMR | 13C NMR | 1H NMR | 13C NMR | 1H NMR | 13C NMR | |
| 2 | 160.92 | 158.21 | 158.64 | 160.77 | ||||
| 3 | 6.20 (d, J = 9.4 Hz). | 111.87 | 6.70 (d, J = 2.3 Hz) | 113.61 | 6.33 (d, J = 9.5 Hz) | 113.75 | 6.30 (d, J = 9.4 Hz) | 112.89 |
| 4 | 7.93 (d, J = 9.4 Hz) | 145.00 | 7.35 (d, J = 8.4 Hz) | 127.73 | 8.01 (d, J = 9.5 Hz) | 144.74 | 8.00 (d, J = 9.5 Hz) | 144.84 |
| 4a | 111.75 | 112.72 | 113.61 | 101.19 | ||||
| 5 | 7.52 (s) | 130.18 | 7.06 (s) | 116.69 | 7.65 (d, J = 8.6 Hz) | 129.91 | 7.64 (d, J = 8.5 Hz) | 129.97 |
| 6 | 6.79 (dd, J = 8.4, 2.3 Hz) | 113.59 | 139.02 | 7.05 (d, J = 2.3 Hz) | 114.13 | 6.96 (dd, J = 8.6, 2.4 Hz) | 112.96 | |
| 7 | 161.76 | 159.16 | 160.68 | 162.96 | ||||
| 8 | 6.72 (s) | 102.63 | 6.74 (dd, J = 8.5, 2.3 Hz) | 102.36 | 7.02 (dd, J = 8.6, 2.3 Hz) | 103.63 | 7.01 (d, J = 2.4 Hz) | 75.85 |
| 8a | 155.97 | 151.00 | 155.50 | 155.91 | ||||
| 1’ | 5.03 (d, J = 7.5 Hz) | 100.43 | 56.42 | |||||
| 2’ | 3.44 (dd, J = 10.9, 6.1 Hz) | 73.52 | ||||||
| 3’ | 3.38 – 3.22 (m) | 77.57 | ||||||
| 4’ | 3.13 (s) | 70.03 | ||||||
| 5’ | 3.68 (dd, J = 2.9, 1.1 Hz) | 76.85 | ||||||
| 6’ | 4.28 (d, J = 9.1 Hz) | 61.05 | ||||||
| Hydroxyl group | ||||||||
| 7-OH | 10.59 (s) | 9.84 (s) | ||||||
| 6-OH | 10.09 (s) | |||||||
| Methyl group | ||||||||
| 7-OCH3 | 3.86 (s) | |||||||
s singlet, d doublet, dd doublet of doublet, m multiplet
In addition, 1H-NMR study of the purified glycosylated product displayed the presence of an anomeric proton at δ = 5.03 ppm (d, J = 7.5 Hz, H-1´) with a beta (β) configuration of the sugar moiety, whereas the sugar region observed between δ of 3.13 and 4.28 ppm. In 13C-NMR, 6 carbon signals of the O-glucoside moiety were found at δ = 100.43 (C-1´), δ = 73.52 (C-2´), δ = 77.57 (C-3´), δ = 70.03 (C-4´), δ = 76.85 (C-5´), and δ = 61.05 ppm (C-6´) (Table 2; Additional file 1: Figure S8A&B). The site of glycosylation at C-7 was confirmed by a correlation between the anomeric proton H-1’ at δ = 5.03 ppm and carbon C-7 at δ = 160.68 ppm of umbelliferone in Heteronuclear multiple-bond correlation spectroscopy (HMBC) analysis (Additional file 1: Figure S8C). These analyses identified that the glycosylated product was 7-O-β-D glucopyranosyl umbelliferone, also known as skimmin [22].
Moreover, the 1H-NMR spectrum of the purified methylated product showed a set of signal at δ = 3.86 ppm (s, 3H) corresponding to a methyl group. Furthermore, the 13C-NMR showed ten carbons signals, including one new methyl group at δ = 56.42 ppm representing O-CH3 group in comparison with the nine carbon signals of umbelliferone (Table 2; Additional file 1: Figure S9A&B). The site of methylation at C-7 was confirmed by a correlation between methyl group and carbon C-7 at δ = 162.95 ppm of umbelliferone in HMBC analysis (Additional file 1: Figure S9C). These data confirmed that methylated product was 7-methoxycoumarin, also known as herniarin [21].
Water solubility determination
The data showed that the water solubility of herniarin was decreased by 1.89-folds, whereas esculetin and skimmin were significantly improved by 1.28 and 3.98-folds, respectively, compared to that of umbelliferone. These results indicated that hydroxylation and glycosylation of umbelliferone enhanced its water solubility by attachment of hydrophilic moieties, while methylation decreased its water solubility by attachment of hydrophobic methyl moiety.
Antibacterial activities
Results of disc diffusion assays showed that umbelliferone, esculetin, and skimmin did not exhibit any antibacterial activity against the tested five different human pathogens when 10 μL of 100 mM compound was applied. However, herniarin exhibited antibacterial activity against Gram-positive bacteria Staphylococcus aureus subsp. aureus KCTC 1916 (S. aureus) and Bacillus subtilis KACC 17047 (B. subtilis) with a zone of inhibition values of 9 ± 0.12 mm and 8.5 ± 0.18 mm, respectively (Additional file 1: Table S1). These results revealed that methylation of umbelliferone at hydroxyl group of the C-7 position might be profitable for heightening its antibacterial activity against Gram-positive bacteria.
Anticancer activities
All prepared compounds were further evaluated for their in vitro cytotoxicity by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay against four different cancer cell lines (Fig. 4). Results showed that herniarin exhibited good cytotoxic activities compared to the other three compounds. Cell viability of skin melanoma (B16F10), gastric carcinoma (AGS), epitheliod cervix carcinoma (HeLa) and hepatic carcinoma (HepG2) reduced approximately 32.63%, 20.25%, 12.79%, and 31.28% (p < 0.05), respectively, compared to controls, when treated 400 μM of herniarin. The 50% inhibitory concentration (IC50) values of herniarin for B16F10, AGS, HeLa and HepG2 cells were 197.0, 28.29, 80.21, and 206.1 μM, respectively. Skimmin inhibited AGS cell lines with an IC50 value of 34.42 μM. However, esculetin did not show any activity against the tested cell lines, whereas umbelliferone exhibited effective anticancer activity against AGS and HepG2 cell lines with IC50 values of 129.9 and 222.3 μM, respectively (Additional file 1: Table S2). These results suggest that herniarin and skimmin can remarkable reduce the cell viability of AGS cell line in a dose-dependent manner. This is the first report of the activity of the two compounds against AGS cell line.
Fig. 4.
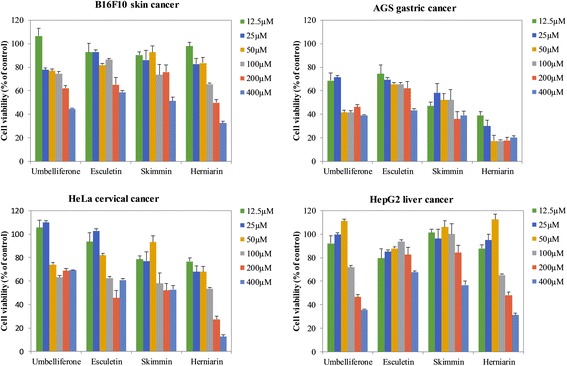
The growth of four cancer cell lines were treated with umbelliferone and its derivatives
Discussion
Post-modifications of natural compounds is one of the approaches to enhance the biological and pharmaceutical properties of parent molecules [23]. In this research, we produced three umbelliferone derivatives using a monooxygenase, a glycosyltransferase, and a methyltransferase enzyme via microbial biotransformation in E. coli. First, umbelliferone was oxidized by CYP450 BM3 from B. megaterium and its variant M13 to produce metabolite esculetin. Second, a flexible glycosyltransferase YjiC from B. licheniformis capable of accepting various small molecules such as chalcone [24, 25], flavonoids [26], stilbene [27], and anthracycline antibiotic were used for efficient glycosylation of umbelliferone to produce skimmin [28]. Finally, we extended acceptor substrate of SaOMT2 from S. avermitilis [29] to produce herniarin. Our results suggest that modification of umbelliferone with different enzymes can be used to develop new compounds with novel biological activities.
Enzymatic methods were considerable contributed to the industrial production of pharmaceutical substances [30]. However, there are several shortcomings when utilizing an in vitro enzymatic synthesis approach. Most biocatalysts are limited by cofactor-dependent enzymes such as CYP BM3 which is NADPH-dependent cytochrome P450 [31] and YjiC, a UDP-dependent glycosyltransferase. In addition, NADPH, UDP-glucose, and S-adenosyl-L-methionine (SAM) not only are high cost but also unstable to limit their apply in small scale stoichiometric reaction [32]. Although alternative cofactors-generating systems have been developed for large-scale reactions, the cost of reaction is still too expensive and reaction yield is deficient [33]. For example, only 8.09% and 35.48% of conversion rates from umbelliferone to esculetin were found in in vitro reactions with CYP450 BM3 and M13, respectively. Similarly, methylation reaction of SaOMT2 are limited convert substrate to herniarin (Table 1). Therefore, metabolic engineering and microbial biotransformation approaches are needed to further enhance the production by utilizing indigenous co-factors, ultimately lowering the cost of target molecule production.
Interestingly, the catalytic efficiency of M13 with umbelliferone was 4.39-folds higher than CYP450 BM3 in in vitro reaction. In addition, protein M13 showed higher catalytic efficiency for umbellifferone than wild-type CYP450 BM3 in whole cells for 48 h under the same condition (Table 2; Additional file 1: Figure S3). The long-distance between the heme iron at ferric resting state in the CYP450 BM3 and oxidizable carbon of umbelliferone is one of the reasons for the low bioconversion efficiency [34]. Furthermore, substitution of several key amino acids in the wild-type protein can affect on the activity and selectivity of the biocatalyst reaction. For example, arginine residues at position 47 is supposed to be noticeable for entering of the substrate to the channel and controlling substrate accessibility to the binding pocket [35]. The leucine residues at position 86 could be effective for conformational changes of amino acids near heme, resulting in alteration of the electron transfer pathway to increase activity [36]. The substitution of phenylalanine 87 can directly interact with bound substrates and affect the activity and stereo-selectivity or regioselectivity of enzymes [37]. It is possible that the substituted key amino acid in the wild type protein might have altered CO difference spectra of M13 in comparison with CYP450 BM3. These data indicate that protein engineering was powerful method for enhance the production of natural molecules. They also suggest that one way to improve the activity of SaOMT2 toward umbelliferone is by engineering via site-saturation mutagenesis based on the structural model of substrate binding site of the enzyme. Moreover, engineering of YjiC could advance metabolic engineering by tailoring compounds with high stereo-selectivity or regio-selectivity to achieve high yields of desired molecules [38].
We also studied the biological activities of umbelliferone and its three derivatives as such antibacterial and anticancer agents. Although the activities of four compounds were tested against five bacteria, only herniarin was effective against two Gram-positive bacteria B. subtilis and S. aureus. This indicated that the methyl substitution at the hydroxyl group of the C-7 position reduced the water solubility and showed significant antibacterial activity. The possible reason could explain that the enhanced lipophilicity of alkyl group leads to improving the ability of herniarin to overcome cell membrane [39], followed by coumarin-based inhibitors of bacteria replicative DNA helicase [40]. Similar to bacterial activity, among the four compounds, herniarin showed the most potential anticancer activity against four tested cancer cell lines. Although we accessed the activity of herniarin and skimmin against AGS cell line for the first time, these compounds could be used in further studies to reveal their exact mechanisms of action and their anti-cancer effects in vivo. The previous study has reported that umbelliferone have growth inhibitory effects on human cancer cell lines such as breast cancer (MCF-7) and lung cancer (H727) [41]. Esculetin did not show activity against the tested cell lines in this study. However, it has anti-proliferative effects on G361 human malignant melanoma [4] and U937 human leukemia cells [42] and. These results provide the ability that modification of umbelliferone might generate a promising anticancer agent against various cancer cell lines.
Conclusion
In conclusion, the results demonstrate that both native enzyme and engineered enzyme expressed in the whole cell allow producing the precious compounds. As a result we successfully produced three different molecules herniarin, esculetin and skimmin from umbelliferone using engineered microbial cells. The employed fermentation approach is cheaper than in vitro reaction and other chemical synthesis methods. The biosynthesized molecules were also accessed for their potential bioactivities against different pathogens and cancer cell lines. The present research suggests that the combinatorial biosynthesis of different biosynthetic enzymes could rapidly promote to a novel secondary metabolite.
Methods
Chemicals and reagents
Standard umbelliferone, δ–aminolevulinic acid hydrochloride (δ–ALA), SAM, deuterium oxide (D2O), and DMSO-d6 were purchased from Sigma-Aldrich (USA). UDP-α-D-glucose, α-D-glucose 1-phosphate, and isopropyl-β-D-thiogalactopyranoside (IPTG) were obtained from Genechem (Korea). NADP oxidized form was provided from Tokyo Chemical Industry Co., Ltd (Japan). HPLC-grade acetonitrile and water were purchased from Mallinckrodt Baker (Phillipsburg, NJ, USA). All other chemicals used were of analytical grade.
Plasmids, microorganisms, and culture conditions
Plasmid pCW(Ori+)-mutant 13 (M13) carrying amino acid substitution relative to wild type CYP450 BM3: M13 (R47L/L86I/F87V/L188Q) [43] and pET28a-YjiC were constructed previously [24]. The sequence of previously characterized S. avermitilis derived OMT [29] was cloned using the first cloning site of pRSF-Duet vector to make recombinant plasmid pRSF-Duet-SaOMT2. These plasmids were transformed into E. coli BL21 (DE3) (Stratagene, USA) using standard procedures. 100 μg/mL of ampicillin and 50 μg/mL of kanamycin were used in LB medium for protein expression and biotransformation assay.
Enzyme expression and purification
E. coli BL21 (DE3) harboring recombinant pCW(Ori+)-P450 BM3 with pCW(Ori+)-mutant 13, pET28a-YjiC, and pRSF-Duet-SaOMT2 were cultured in LB broth containing appropriate antibiotics at 37 °C and 180 rpm for 6 h. 0.5 mM IPTG and 1 mM δ–ALA were used to expression of CYP450 BM3 and mutant 13 when cell optical density at 600 nm (OD600 nm) reached 0.6. These cells were further incubated for an additional 20 h at 28 °C before harvest. Furthermore, 0.5 mM IPTG was used to induced for protein expression of pET28a-YjiC and pRSF-Duet-SaOMT2 at 20 °C for 20 h. These cells were harvested by centrifugation at 842 × g for 10 min and suspended twice in 100 mM Tris–HCl (pH 7.6) buffer containing 10% glycerol. Cells were suspended in 1 mL of the same buffer and lysed by sonication using a Sonosmasher (Ultrasonic, Inc.). Following centrifugation at 13,475 × g for 30 min at 4 °C, the protein fractions were applied across 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Amicon®Ultra-0.5 mL 100 K and 30 K devices (Merck, USA) were used to collect soluble cytosolic fraction of CYP450 BM3, mutant 13, and YjiC, SaOMT2, respectively. 100 mM Tris–HCl (pH 7.6) buffer containing 10% glycerol was used to store solution fractions at −20 °C until next use. CYP450 BM3 protein content (nmol) = [(absorbance difference × 1000)/91 mM−1cm−1] x dilution factor [44]. Protein concentration was determined via Bradford method [45].
Enzyme essay
The reaction mixture was performed in 200 μL with 1 mM substrate. Hydroxylation reaction contained 50 μg/mL CYP450 BM3 or mutant 13, 100 mM potassium phosphate buffer (pH 7.6), and 10 mM MgCl2.6H2O. The sample was pre-incubated at 37 °C for 15 min and the reaction was initiated by the addition of NADPH regenerating system consisting of 10 mM glucose-6-phosphate, 0.5 U glucose-6-phosphate-dehydrogenase, and 0.5 mM NADP+. Glucosylation reaction mixture was incubated with 2 mM UDP-α-D-glucose, 10 mM MgCl2, 50 μg/mL purified YjiC in 100 mM Tris–HCl buffer (pH 7.6). Methylation reaction contained 50 μg/mL of purified SaOMT2 and 10 mM MgSO4 with 2 mM SAM as methyl donor in 100 mM Tris–HCl buffer (pH 7.6). Reactions were incubated at 37 °C for 30 min. These reactions were stopped by adding chilled methanol at twice volume followed by vigorous shaking for 15 min. Mixtures were then centrifuged at 13,475 x g for 30 min. The supernatants were used to HPLC-PDA and HR-QTOF ESI/MS. The conversion percentage of each substrate was calculated through intergrated between substrate and product peak area. A calibration standard was created using different concentration of umbelliferone (10, 25, 50, 100, and 200 μg/mL).
Whole cell biotransformation
Seed cultures of E. coli BL21 (DE3) harboring recombinant plasmids were prepared in 6 mL LB broth with appropriate antibiotics followed by incubation at 37 °C with shaking at 180 rpm for approximately 6 h. For whole cell reaction of CYP450 mutant 13, 200 μL of pre-inoculum was transferred into 250 ml flask containing 50 mL of different media (LB, TB, 1x M9 minimal salt) with appropriate antibiotic and incubated at 37 °C. When OD600 reached 0.8, the culture was induced with 1.0 mM IPTG and 0.5 mM δ-ALA and incubated at 28 °C for 12 h. Biotransformation of E.coli harboring pET28a-YjiC and pRSF-Duet-SaOMT2 was induced by 0.5 mM IPTG at 20 °C for 12 h. 100 mM umbelliferone was supplemented to the same samples. The culture was incubated for an additional 12 h, collected, extracted and analyzed.
Scale-up of whole cell biocatalyst system in a fermentor
Fermentation of three E.coli BL21 (DE3) recombinant strains (CYP450 mutant 13, YjiC, and SaOMT2) was carried out in 3-L media under optimal conditions as described previously [34, 46]. The quantify of hydroxylated and methylated production were taken every 12 h until 48 h. Likewise, glycosylated sample was taken every 3 h up to 15 h. Then, culture broth was centrifuged. Finally, the supernatant was extracted with twice volume of EtOAc.
Analytical methods
The culture broth was extract with double volume of EtOAc (v/v = 2:1) using Soxhlet extractor. Soxhlet extractor was then kept still to separate two layers after the mixture was shaken for 12 h at room temperature. A rotary evaporator was used to dried EtOAc fraction. The products were analyzed by HPLC-PDA using a reversed-phase column (Mightysil RP–18 GP 250–4.6 (5 μm), Kanto Chemical, Japan) at 330 nm. The binary mobile phases were include solvent A [0.05% trifluroacetic acid in HPLC-grade water] and solvent B (100% acetonitrile). The total flow rate was kept at 1 mL/min for 30 min. The percentages of solvent B used were as follows: 0–15% (0–4 min), 45% (4–10 min), 75% (10–14 min), 90% (14–20 min), 10% (20–25 min), 10% (25–30 min). The compounds were purified by preparative HPLC (Shimazu, Tokyo, Japan) with C18 column (YMC–Pack ODS-AQ (250 × 20 mm I.D., 10 μm) linked to a UV detector (330 nm). HR–QTOF ESI/MS analysis using an ACQUITY UPLC® coupled with SYNAPT G2-S (Water Corp., USA). For NMR analysis of the purified product, compounds were dried, lyophilized, and dissolved in DMSO-d6 and subjected to 700 MHz Bruker Biospin NMR for one-dimensional 1H-NMR, 13C-NMR, and two-dimensional HMBC analyses.
Solubility study
50 μM of umbelliferone, esculetin, skimmin and herniarin were separately dissolved in 200 μL phosphate-buffered saline (PBS) at pH 7.6. The mixtures were vortexed for 30 min and centrifuged at 13,475 x g for 15 min. The supernatants were collected and filtered through 0.45-μm syringe filters. Subsequently, aliquots (20 μL) were analyzed by HPLC-PDA at 330 nm. The concentrations of all compounds dissolved in PBS were determined by regression equations.
Antibacterial activity
Three Gram-positive bacteria (Staphylococcus aureus subsp. aureus KCTC 1916, Bacillus subtilis KACC 17047, and Micrococcus luteus KACC 13377) and two Gram-negative bacteria (Pseudomonas aeruginosa KACC 10232 and Enterobacter cloaceae subsp. disolvens KACC 13002) were used to test antibacterial activity of umbelliferone and its derivatives. The paper disc diffusion assay on Mueller-Hinton agar (MHA) plate were carried out. Inocula containing 107 colony forming units (CFU)/mL were spread onto MHA plates. 10 μL of 100 mM compounds were placed on the surface of inoculated agar plates through sterile filter paper discs. The samples were then incubated at 37 °C for 12 h. The zone of inhibition diameter was measured in millimeter [47].
Anticancer activities
Four cencer cell lines (B16F10, AGS, HeLa, and HepG2) grown in Roswell Park Memorial Institute 1640 medium containing 10% fetal bovine serum (FBS) (Invitrogen, USA) were applied to test anticancer activities of umbelliferone and its derivatives. All cells were maintained at 37 °C in a humidified 5% CO2 incubator. For cell growth assay, cells seeded at 2 x 103 cell/well in 96-well plates (SPL Life Sciences, Korea) were treated with each compound after serial dilution (400 μM, 200 μM, 100 μM, 50 μM, 25 μM, 12.5 μM) for 72 h. Cell viability was measured using MTT colorimetric assay [48].
Statistical analysis
Values are mean ± standard deviation (SD). SD was calculated from the results of three independent experiments. Differences with p value < 0.05 were indicated a statistically significant.
Additional files
The disc-diffusion assay showed the inhibition zone diameter (mm) of four compounds against various Gram-positive and Gram-negative bacteria. Table S2. IC50 values of four compounds against B16F10, AGS, HeLa and HepG2 cell lines. Figure S1. SDS-PAGE analysis of four recombinant proteins used in the study. Figure S2. The UV maxima absorbance and exact mass analysis of umbelliferone (A) and reaction products P1 (B), P2 (C), P3 (D). Figure S3. Hydroxylated umbelliferone production optimization and cell growth. Figure S4. Glycosylated umbelliferone production optimization and cell growth. Figure S5. Methylated umbelliferone production optimization and cell growth. Figure S6. 1-Dimensional NMR of umbelliferone standard. Figure S7. 1-Dimensional NMR of esculetin. Figure S8. NMR of skimmin. Figure S9. NMR of herniarin. (DOCX 27595 kb)
Acknowledgements
Not applicable.
Funding
This work was funded by Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01188001), Rural Development Administration, Republic of Korea.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Authors’ contributions
LLC designed, performed the majority of the experiment work and wrote the manuscript. RPP and TSK helped in writing the manuscript. JKS and LCC were responsible for the original concept and supervised the work. HNL, NHT and HJJ did the majority of anticancer activities. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AGS
Gastric carcinoma
- B16F10
Skin melanoma
- DMSO
Dimethyl sulfoxide
- HeLa
Epitheliod cervix carcinoma
- HepG2
Hepatic carcinoma
- HPLC-PDA
High performance liquid chromatography‑photodiode array
- HR-QTOF ESI/MS
High resolution-quadruple time of flight electrospray ionization/mass spectrometry
- IPTG
Isopropyl-β-D-thiogalactopyranoside
- NADP+
β-Nicotinamide adenine dinucleotide phosphate oxidized
- NADPH
β-Nicotinamide adenine dinucleotide phosphate reduced
- NMR
Nuclear magnetic resonance
- δ-ALA
δ-aminolevulinic acid hydrochloride
References
- 1.Rauf A, Khan R, Khan H, Pervez S, Pirzada AS. In vivo antinociceptive and anti-inflammatory activities of umbelliferone isolated from Potentilla evestita. Nat Prod Res. 2014;28:1371–1374. doi: 10.1080/14786419.2014.901317. [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Kang KA, Zhang R, Piao MJ, Ko DO, Wang ZH, Chae SW, Kang SS, Lee KH, Kang HK, Kang HW, Hyun JW. Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta Pharmacol Sin. 2008;29:1319–1326. doi: 10.1111/j.1745-7254.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- 3.Kimura Y, Sumiyoshi M. Antitumor and antimetastatic actions of dihydroxycoumarins (esculetin or fraxetin) through the inhibition of M2 macrophage differentiation in tumor-associated macrophages and/or G1 arrest in tumor cells. Eur J Pharmacol. 2015;746:115–125. doi: 10.1016/j.ejphar.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 4.Jeon YJ, Jang JY, Shim JH, Myung PK, Chae JI. Esculetin, a coumarin derivative, exhibits anti-proliferative and pro-apoptotic activity in G361 human malignant melanoma. J Cancer Prev. 2015;20:106–112. doi: 10.15430/JCP.2015.20.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramaniam SR, Ellis EM. Neuroprotective effects of umbelliferone and esculetin in a mouse model of Parkinson’s disease. J Neurosci Res. 2013;91:453–461. doi: 10.1002/jnr.23164. [DOI] [PubMed] [Google Scholar]
- 6.Shi J, Li CJ, Yang JZ, Yuan YH, Chen NH, Zhang DM. Coumarin glycosides and iridoid glucosides with neuroprotective effects from Hydrangea paniculata. Planta Med. 2012;78:1844–1850. doi: 10.1055/s-0032-1315394. [DOI] [PubMed] [Google Scholar]
- 7.Kumar V, Ahmed D, Verma A, Anwar F, Ali M, Mujeeb M. Umbelliferone β-D-galactopyranoside from Aegle marmelos (L.) corr. an ethnomedicinal plant with antidiabetic, antihyperlipidemic and antioxidative activity. BMC Complement Altern Med. 2013;13:273. doi: 10.1186/1472-6882-13-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Céspedes CL, Avila JG, Martínez A, Serrato B, Calderón-Mugica JC, Salgado-Garciglia R. Antifungal and antibacterial activities of Mexican tarragon (Tagetes lucida) J Agric Food Chem. 2006;54:3521–3527. doi: 10.1021/jf053071w. [DOI] [PubMed] [Google Scholar]
- 9.Witaicenis A, Seito LN, da Silveira Chagas A, de Almeida LD, Jr, Luchini AC, Rodrigues-Orsi P, Cestari SH, Di Stasi LC. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine. 2014;21:240–246. doi: 10.1016/j.phymed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Venugopala KN, Rashmi V, Odhav B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed Res Int. 2013;2013:963248. doi: 10.1155/2013/963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kai K, Mizutani M, Kawamura N, Yamamoto R, Tamai M, Yamaguchi H, Sakata K, Shimizu B. Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J. 2008;55:989–999. doi: 10.1111/j.1365-313X.2008.03568.x. [DOI] [PubMed] [Google Scholar]
- 12.Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;8:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Al-Amiery AA, Musa AY, Kadhum AA, Mohamad AB. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules. 2011;16:6833–6843. doi: 10.3390/molecules16086833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alipour M, Khoobi M, Moradi A, Nadri H, Homayouni Moghadam F, Emami S, Hasanpour Z, Foroumadi A, Shafiee A. Synthesis and anti-cholinesterase activity of new 7-hydroxycoumarin derivatives. Eur J Med Chem. 2014;82:536–544. doi: 10.1016/j.ejmech.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Yan Y. Biosynthesis of caffeic acid in Escherichia coli using its endogenous hydroxylase complex. Microb Cell Fact. 2012;11:42. doi: 10.1186/1475-2859-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y, Sun X, Yuan Q, Yan Y. Combinatorial biosynthesis of plant-specific coumarins in bacteria. Met Eng. 2013;18:69–77. doi: 10.1016/j.ymben.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Yang SM, Shim GY, Kim BG, Ahn JH. Biological synthesis of coumarins in Escherichia coli. Microb Cell Fact. 2015;14:65. doi: 10.1186/s12934-015-0248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berner M, Krug D, Bihlmaier C, Vente A, Müller R, Bechthold A. Genes and enzymes involved in caffeic acid biosynthesis in the actinomycete Saccharothrix espanaensis. J Bacteriol. 2006;188:2666–2673. doi: 10.1128/JB.188.7.2666-2673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan Y, Chemler J, Huang L, Martens S, Koffas MA. Metabolic engineering of anthocyanin biosynthesis in Escherichia coli. Appl Environ Microbiol. 2005;71:3617–3623. doi: 10.1128/AEM.71.7.3617-3623.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson EB, Aynilian GH, Dobberstein RH, Cordell GA, Fong HH, Farnsworth NR. Biological and phytochemical investigation of plants XV. Pteryxia terebinthina var. terebinthina (Umbelliferae) J Nat Prod. 1979;42:120–125. doi: 10.1021/np50001a008. [DOI] [PubMed] [Google Scholar]
- 21.Silván AM, Abad MJ, Bermejo P, Sollhuber M, Villar A. Antiinflammatory activity of coumarins from Santolina oblongifolia. J Nat Prod. 1996;59:1183–1185. doi: 10.1021/np960422f. [DOI] [PubMed] [Google Scholar]
- 22.Okuyama T, Takata M, Shibata S. Structures of linear furano- and simple-coumarin glycosides of Bai-Hua Qian-Hu. Planta Med. 1989;55:64–67. doi: 10.1055/s-2006-961828. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Li W, Yao H, Xu J. Insights into drug discovery from natural products through structural modification. Fitoterapia. 2015;103:231–241. doi: 10.1016/j.fitote.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Pandey RP, Li TF, Kim EH, Yamaguchi T, Park YI, Kim JS, Sohng JK. Enzymatic synthesis of novel phloretin glucosides. Appl Environ Microbiol. 2013;79:3516–3521. doi: 10.1128/AEM.00409-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li HM, Lee JK, Nie LJ, Huo Q, Ma T, Sohng JK, Hong YS, Wu CZ. Enzymatic synthesis of novel isobavachalcone glucosides via a UDP-glycosyltransferase. Arch Pharm Res. 2015;38:2208–2215. doi: 10.1007/s12272-015-0658-8. [DOI] [PubMed] [Google Scholar]
- 26.Gurung RB, Kim EH, Oh TJ, Sohng JK. Enzymatic synthesis of apigenin glucosides by glucosyltransferase (YjiC) from Bacillus licheniformis DSM 13. Mol Cells. 2013;36:355–361. doi: 10.1007/s10059-013-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin JY, Pandey RP, Jung HY, Chu LL, Park YI, Sohng JK. In vitro single-vessel enzymatic synthesis of novel Resvera-A glucosides. Carbohydr Res. 2016;424:8–14. doi: 10.1016/j.carres.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Chu LL, Pandey RP, Shin JY, Jung HY, Sohng JK. Synthetic analog of anticancer drug daunorubicin from daunorubicinone using one-pot enzymatic UDP-recycling glycosylation. J Mol Catal B-Enzym. 2016;124:1–10. doi: 10.1016/j.molcatb.2015.11.020. [DOI] [Google Scholar]
- 29.Kim BG, Jung BR, Lee Y, Hur HG, Lim Y, Ahn JH. Regiospecific flavonoid 7-O-methylation with Streptomyces avermitilis O-methyltransferase expressed in Escherichia coli. J Agric Food Chem. 2006;54:823–828. doi: 10.1021/jf0522715. [DOI] [PubMed] [Google Scholar]
- 30.Meyer HP, Eichhorn E, Hanlon S, Lütz S, Schürmann M, Wohlgemuth R, Coppolecchia R. The use of enzymes in organic synthesis and the life sciences: perspectives from the Swiss Industrial Biocatalysis Consortium (SIBC) Catal Sci Technol. 2013;3:29–40. doi: 10.1039/C2CY20350B. [DOI] [Google Scholar]
- 31.Mazur CS, Kenneke JF, Goldsmith MR, Brown C. Contrasting influence of NADPH and a NADPH-regenerating system on the metabolism of carbonyl-containing compounds in hepatic microsomes. Drug Metab Dispos. 2009;37:1801–1805. doi: 10.1124/dmd.109.027615. [DOI] [PubMed] [Google Scholar]
- 32.Uppada V, Bhaduri S, Noronha SB. Cofactor regeneration-an important aspect of biocatalysis. Curr Sci. 2014;106:946–957. [Google Scholar]
- 33.Zhao H, van der Donk WA. Regeneration of cofactors for use in biocatalysis. Curr Opin Biotechnol. 2003;14:583–589. doi: 10.1016/j.copbio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Chu LL, Pandey RP, Jung N, Jung HJ, Kim EH, Sohng JK. Hydroxylation of diverse flavonoids by CYP450 BM3 variants: biosynthesis of eriodictyol from naringenin in whole cells and its biological activities. Microb Cell Fact. 2016;15:135. doi: 10.1186/s12934-016-0533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitehouse CJ, Bell SG, Wong LL. P450(BM3) (CYP102A1): connecting the dots. Chem Soc Rev. 2012;41:1218–1260. doi: 10.1039/C1CS15192D. [DOI] [PubMed] [Google Scholar]
- 36.Stjernschantz E, van Vugt-Lussenburg BM, Bonifacio A, de Beer SB, van der Zwan G, Gooijer C, Commandeur JN, Vermeulen NP, Oostenbrink C. Structural rationalization of novel drug metabolizing mutants of cytochrome P450 BM3. Proteins. 2008;71:336–352. doi: 10.1002/prot.21697. [DOI] [PubMed] [Google Scholar]
- 37.Graham-Lorence S, Truan G, Peterson JA, Falck JR, Wei S, Helvig C, Capdevila JH. An active site substitution, F87V, converts cytochrome P450 BM-3 into a region- and stereoselective (14S,15R)-arachidonic acid epoxygenase. J Biol Chem. 1997;272:1127–1135. doi: 10.1074/jbc.272.2.1127. [DOI] [PubMed] [Google Scholar]
- 38.Foo JL, Ching CB, Chang MW, Leong SS. The imminent role of protein engineering in synthetic biology. Biotechnol Adv. 2012;30:541–549. doi: 10.1016/j.biotechadv.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 39.John GT. Lipophilicity in drug action and toxicology. In: Pliška V, Testa B, van de Waterbeemd H, editors. Journal medicinal chemistry. Weinheim: VCH; 1996. pp. 5287–5288. [Google Scholar]
- 40.Li B, Pai R, Di M, Aiello D, Barnes MH, Butler MM, Tashjian TF, Peet NP, Bowlin TL, Moir DT. Coumarin-based inhibitors of Bacillus anthracis and Staphylococcus aureus replicative DNA helicase: chemical optimization, biological evaluation, and antibacterial activities. J Med Chem. 2012;55:10896–10908. doi: 10.1021/jm300922h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musa MA, Cooperwood JS, Khan MO. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem. 2008;15:2664–2679. doi: 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park C, Jin CY, Kim GY, Choi IW, Kwon TK, Choi BT, Lee SJ, Lee WH, Choi YH. Induction of apoptosis by esculetin in human leukemia U937 cells through activation of JNK and ERK. Toxicol Appl Pharmacol. 2008;227:219–228. doi: 10.1016/j.taap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Kim DH, Kim KH, Kim DH, Liu KH, Jung HC, Pan JG, Yun CH. Generation of human metabolites of 7-ethoxycoumarin by bacterial cytochrome P450 BM3. Drug Metab Dispos. 2008;36:2166–2170. doi: 10.1124/dmd.108.021220. [DOI] [PubMed] [Google Scholar]
- 44.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 45.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 46.Pandey RP, Malla S, Simkhada D, Kim BG, Sohng JK. Production of 3-O-xylosyl quercetin in Escherichia coli. Appl Microbiol Biotechnol. 2013;97:1889–1901. doi: 10.1007/s00253-012-4438-9. [DOI] [PubMed] [Google Scholar]
- 47.Kuppusamy P, Yusof MM, Parine NR, Govindan N. Evaluation of in vitro antioxidant and antibacterial properties of Commelina nudiflra L. extracts prepared by diffrent polar solvents. Saudi J Biol Sci. 2015;22:293–301. doi: 10.1016/j.sjbs.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung HJ, Lee HB, Lim CH, Kim CJ, Kwon HJ. Cochlioquinone A1, a new anti-angiogenic agent from Bipolaris zeicola. Bioorg Med Chem. 2003;11:4743–4747. doi: 10.1016/S0968-0896(03)00523-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The disc-diffusion assay showed the inhibition zone diameter (mm) of four compounds against various Gram-positive and Gram-negative bacteria. Table S2. IC50 values of four compounds against B16F10, AGS, HeLa and HepG2 cell lines. Figure S1. SDS-PAGE analysis of four recombinant proteins used in the study. Figure S2. The UV maxima absorbance and exact mass analysis of umbelliferone (A) and reaction products P1 (B), P2 (C), P3 (D). Figure S3. Hydroxylated umbelliferone production optimization and cell growth. Figure S4. Glycosylated umbelliferone production optimization and cell growth. Figure S5. Methylated umbelliferone production optimization and cell growth. Figure S6. 1-Dimensional NMR of umbelliferone standard. Figure S7. 1-Dimensional NMR of esculetin. Figure S8. NMR of skimmin. Figure S9. NMR of herniarin. (DOCX 27595 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).


