Abstract
Chronic respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF), are among the leading causes of mortality and morbidity worldwide. In the past decade, the interest in the role of microbiome in maintaining lung health and in respiratory diseases has grown exponentially. The advent of sophisticated multiomics techniques has enabled the identification and characterisation of microbiota and their roles in respiratory health and disease. Furthermore, associations between the microbiome of the lung and gut, as well as the immune cells and mediators that may link these two mucosal sites, appear to be important in the pathogenesis of lung conditions. Here we review the recent evidence of the role of normal gastrointestinal and respiratory microbiome in health and how dysbiosis affects chronic pulmonary diseases. The potential implications of host and environmental factors such as age, gender, diet and use of antibiotics on the composition and overall functionality of microbiome are also discussed. We summarise how microbiota may mediate the dynamic process of immune development and/or regulation focusing on recent data from both clinical human studies and translational animal studies. This furthers the understanding of the pathogenesis of chronic pulmonary diseases and may yield novel avenues for the utilisation of microbiota as potential therapeutic interventions.
Chronic respiratory diseases
Chronic respiratory diseases, namely asthma, chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF), are among the leading cause of morbidity and mortality worldwide.1 In Australia, deaths due to respiratory diseases are among top five causes of mortality, and COPD alone is the second most common cause of hospitalisation.2 Mortality rates are expected to rise significantly in the next decade, partly due to ageing populations and also as a result of the lack of effective measures to reduce some of the major risk factors such as smoking, air pollution, wood and cooking smoke. There are also likely to be generational effects of exposures prolonging these effects into subsequent generations even if exposures were to decline now. Despite the enormous burden of these conditions, the various causative factors and underlying mechanisms associated with disease progression are still not fully understood. The human microbiome is one such factor, which not only has a pivotal role in maintaining health but also in regulating various inflammatory and metabolic pathways in a range of conditions including gastrointestinal (GI) diseases (inflammatory bowel disease, irritable bowel syndrome), arthritis (rheumatoid arthritis), cancer (colorectal cancer) and recently in chronic pulmonary diseases, such as asthma, COPD and CF.3, 4
Early investigations largely focussed on the roles of intestinal commensal bacteria and their metabolites in health and disease. However, new evidence suggests that non-bacterial microorganisms such as fungi (mycobiome) and viruses (virome), as well as microbiota residing on other tissues such as the lungs, could be critical in modulating health and disease in the host.5, 6 The term ‘microbiota' refers to the entire microbial community (bacteria, archaea, fungi, viruses and protozoa) associated with specific host tissues or organs, whereas the total genome of this community is designated the ‘microbiome'.7, 8 Accordingly, the human microbiome can be defined as the sum total of all forms of microorganisms and their genomes, inhabiting an individual, at a given time. The composition and diversity of microbiota varies greatly between different individuals and is shaped by both environmental and host genetics factors, although it remains relatively stable within a host in the absence of a major change in lifestyle (e.g., diet, disease onset, inhalation and environment exposures etc.).9 Remarkably, the microbiome is often dubbed the ‘new' biomarker of human health as it aids in maintaining normal host physiology, developing and educating the immune system, metabolising complex substrates and providing crucial protection against opportunistic pathogens. With the rapid technological advances in the field of metagenomics and bioinformatics, researchers are now unravelling the effects of both the respiratory and GI microbiota on the immunity of lungs in health and disease.
Here we review the relationships between the host and the microbiota in chronic respiratory disorders (e.g., asthma, COPD and CF), and outline novel microbiome-oriented strategies with therapeutic potential for preventing or treating these diseases, which are currently incurable and often have poor treatment options.
Healthy microbiome in the GI and respiratory tracts
The comprehensive analysis of microbial communities, the implication of individual taxa in biological processes and the identification of interactions between the microbiota, host and the immune response have been driven by the development and application of new technologies and advanced high-throughput bioinformatics techniques. Although a detailed discussion of modern sequencing and analysis would form its own review, familiarity with the basic principles is key to understanding the workflow and potential limitations in the field. The DNA encoding the 16S rRNA gene is amplifiable by PCR, and consists of both conserved regions present in all prokaryotes and variable (V1-V9) sequence regions. Notably, these regions have evolved at different rates, allowing measurement of both close and distant phylogenetic relationships, which enables the taxonomic assignment of a broad range of bacteria to family/genus/species level.10 Similarly, fungal detection by DNA based technologies include sequencing and evaluation of conserved 18S rRNA gene (phylum level differentiation), which can be coupled with highly variable internal transcribed spacer regions (ITS) to achieve inter-species separation.11 It should be noted that the utility of 18S rRNA/ITS sequencing based approaches for fungal identification can be confounded by the specificity and bias of currently available PCR primers, as well as the lack of exhaustive fungal reference libraries.12 Despite some concerns about the variability of experimental protocols or PCR primer bias, 16S rRNA/18S rRNA remain the mainstay of microbiome analysis. Briefly, total DNA is extracted from the sample (e.g., stool, bronchoalveolar lavage fluid, tissue) and the bacterial 16S rRNA gene is PCR-amplified to create a mix of individual amplicons. For eukaryotic microbes (fungi and protozoa), 18S rRNA/ITS genes are amplified and assessed. Similar phylogenetic characterisation of viruses/phages can be undertaken but is difficult owing to the lack of small subunit rRNA (16S/18S), or any conserved genes/proteins. In addition, overlapping of specific marker genes that are frequently transferred between viruses through horizontal gene transfer induces further challenges for the identification and characterisation of viruses.13 However, with the advent of next-generation sequencing and sophisticated methods for purifying virus-like particles, it is now possible to assess viral diversity through shotgun sequencing.14 Then next generation sequencing (NGS) technology is employed to analyse these sequences simultaneously, thus avoiding the pedestrian traditional separation and cloning of individual amplified sequences and reducing the biases. These 16S rRNA gene amplicon sequences are assessed qualitatively and highly similar sequences are clustered into operational taxonomic units (OTUs), which are finally identified against a standard reference database. It may be possible to classify some OTUs to the species level, while others may be classified only to a higher taxonomic level such as family or genus. This is primarily due to the varying resolution in the sequencing reads of specific regions of the 16S rRNA gene used for distinguishing different types of bacteria. The total number of reads assigned to each operational taxonomic unit is indicative of the relative abundance of members of the microbiome, as well as characterising their phylogenetic relationships, that is, taxonomic diversity.15 These microbial profiles can then be correlated with host physiological parameters in both health and disease. More recently, the advent of whole-genome sequencing and functional metagenomics (i.e., genome-based pathway analyses) has enabled the prediction of the functionality of whole microbial communities as well as of individual members.16 The sequencing of 16S rRNA gene amplicons of bacteria, and 18S rRNA/ITS gene amplicons of fungi, allows the cost-effective identification and characterisation of microbial diversity in ecosystems, including the human gut.17 This has proved extremely valuable in enabling the analysis of host-microbe interactions in health and disease. However, several key issues remain to be resolved, such as reducing PCR-bias, accurate projections of microbial diversity, translating microbial taxonomic classification into essential biological parameters, and determining cause and effect of species and population changes. Crucially, shotgun metagenome sequencing provides both the identification of microbes and the information regarding biological function encoded in the total genome. Major challenges still exist with this technique, including analysis of extremely complex data into meaningful conclusions, presence of host DNA in the whole sample, exclusion of sequences generated due to contaminants and high cost. However, both 16S rRNA sequencing and shotgun metagenome sequencing are in use across in many laboratories worldwide, and with advances made in refining these techniques, it will become more sophisticated and affordable in coming years.18 Additional molecular methods such as metatranscriptomics (analysis of mRNA transcripts), metaproteomics (analysis of proteins) and metabolomics (analysis of metabolites) have also proved beneficial in understanding the functional complexity of host–microbe interactions.
The total surface area of the gastrointestinal mucosa in the adult human is between 30 and 80 m2,19 and this harbours large populations of microorganisms (~1014), which are predominantly commensal in nature. To characterise the ‘healthy' microbiome in humans, Qin et al.20 sequenced the total faecal genetic material from 124 European adults and revealed that the gut microbiota is predominantly comprised of bacteria and archaea, representative of >1000 distinct species, with lesser populations of viruses and eukaryotic microbes. These ‘Healthy' gut microbiota are dominated by the phyla Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria and Verrucomicrobia with a lesser proportion of Fusobacteria. Regional tissue variations in microbiome composition exist in the GI tract, which are determined by factors such as oxygen gradient, antimicrobial peptides (bile acids) and pH. Lactobacillaceae and Enterobacteriaceae dominate small intestine (~102 CFU g−1), whereas the colon (~1011 CFU g−1) is inhabited by members of Bacteroidaceae, Prevotellaceae, Rikenellaceae, Lachnospiraceae and Ruminococcaceae.21 Consequently, luminal- (faeces) and mucosal-associated (colonic mucosa) microbiota demonstrate the persistence of distinct microbial taxa, differing in both diversity and composition, with luminal populations showing higher diversity.22
Mouse models are integral to understanding the role of the GI microbiome in immune disorders. Lagkouvardos et al.23 recently established the first public library of the Mouse Intestinal Bacterial Collection.18 Approximately 1500 pure cultures were isolated from the GI tract of mice, and 76 different species from 26 families belonging to the phyla Firmicutes (74% strains), Actinobacteria, Bacteroidetes, Proteobacteria and Verrucomicrobia were identified, characterised and archived. The authors also identified 32 bacterial species shared between both humans and mice, and 16 species that were exclusively present in the murine intestine.23 Other key factors such as variability in the gut microbiome between different laboratory mouse strains and environmental factors (e.g., co-housing, physical/psychological stress) should be considered while referring to miBC library.24 Moreover, further investigations are warranted to resolve differences in the composition of the gut microbiota in different mouse strains, and the influence of their respective microbiota on health and disease.25
Recently, our knowledge of the respiratory microbiome has improved significantly. Previously thought to be sterile, numerous investigators have detected bacterial DNA in the lower respiratory tract of healthy individuals.26, 27, 28 Despite several technical limitations, such as low bacterial load and potential contamination with upper respiratory tract secretions (oral or nasal), it is now widely accepted that a ‘healthy' microbiome exists in the lung. Whether this is resident or is transient and continually reseeded from the environment and cleared by immune responses is currently under debate.4 The bronchial tree consists of a complex and dynamic microbial community (~500 species), which often overlaps with the oral microbiome.27 In contrast to the GI tract, the luminal surface area of lungs is between 50–75 m2,29 and the total bacterial load varies greatly between subjects in both bronchoalveolar lavage fluid (4.5–8.25 log copy numbers per ml)30, 31 and lung tissue samples (10–100 bacterial cells per 1000 human cells). Similar to gut microbiota, the ‘core' airway microbiota in healthy lungs is predominantly comprised of the phyla Bacteroidetes, Firmicutes and Proteobacteria, followed by lesser proportions of Actinobacteria.28, 32, 33 Moreover, the lung bacterial communities of healthy individuals resemble the microbiome of the mouth but not the nose. Indeed, the prevalence of the genus Prevotella, which occurs at high levels in the oral cavity, is low in healthy lungs.27 This suggests that oral microbial communities that transgress into lower respiratory tract are selectively eliminated from healthy lungs.27 It has also been postulated that increases in the abundance of Prevotella may induce low-grade inflammation in lungs.34
Establishment of the microbiome
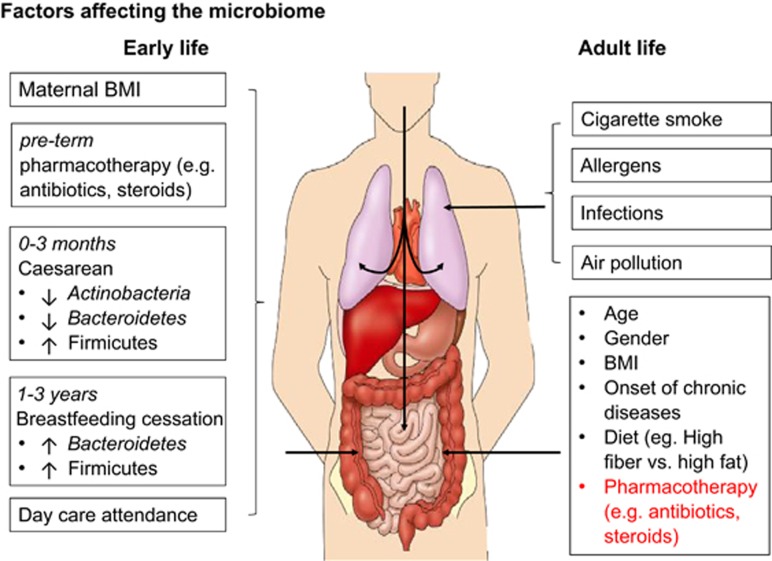
The establishment of the microbiome may commence before an individual is born, as nonpathogenic commensal microbes, largely resembling the oral microbiota, have been detected from placental DNA (Figure 1).35 At birth, the mode of delivery affects early colonisation in the gut, with delivery by caesarean section shown to be significantly associated with lower abundance and diversity of the phyla Actinobacteria and Bacteroidetes, whereas higher abundance and diversity of Firmicutes in the first 3 months of life.36 Other factors including the place of birth, feeding practice, host genetics, siblings, gender and preterm pharmacotherapy (e.g., antibiotics, steroids) could also affect the microbiome substantially.37 At age 1–2 years, the infant gut microbiome changes dramatically, although detectable changes in microbiota may occur as early as 6 weeks post birth as these changes are largely shaped by the cessation of breastfeeding and subsequent alterations in diet.38 During the next 18–36 months, a final shift in microbiota takes place in the majority of individuals, which primarily involves enrichment with bacterial phyla Bacteriodetes and Firmicutes, and leads to a microbiome resembling that of adults.39 Once established, the microbiome remains fairly stable throughout middle age, with only transient changes occurring. A 5-year follow-up study of US adults showed that the relative abundance of the majority of bacterial strains (~60%), especially Bacteroidetes and Actinobacteria, remain fairly stable in an individual.40 Nevertheless, there is a large degree of interpersonal variation of microbiota, which is mainly due to host associated factors, including diet, genetics, age and gender (Figure 1)41, 42 and new taxa continue to be characterised.43 Data generated by the Human Microbiome Project44 reported significant correlations between the first principle coordinates of host genetic variations and first principle coordinate of microbiome composition in stool samples and palatine tonsils (UniFrac distances). Similar statistically nonsignificant correlations between host genome and microbiota composition were also observed for other body sites, such as the skin.41 Another study also used the Human Microbiome Project data to show that mitochondrial haplotypes correlated with abundances of specific microbiota in faeces.44 Other factors can also lead to long-term sustained changes in the composition of gut microbiota, such as the onset of chronic diseases, changes in diet or repeated exposure to environmental insults, such as cigarette smoke, allergens, infections and air pollution (Figure 1).4, 45 Gender-specific differences in microbiota also occur and could possibly explain differences in the prevalence of metabolic and intestinal inflammatory diseases in men compared with women.46 Gut bacterial microbiota differs between men and women at the phylum (Firmicutes:Bacteriodetes ratio higher in men <33 kg m−2 body mass index (BMI); higher in women >33 kg m−2 BMI) as well as genus level (Bacteroides and Bilophila: higher in women; Veillonella and Methanobrevibacter: higher in men).46 In the same study, levels of 66 bacterial genera strongly correlated with both BMI and plasma lipids (triglycerides, high- and low-density lipoproteins and total cholesterol).46 Evidence also suggests that commensal gut microbial communities affect both sex hormone levels and metabolomics in a non-obese mice model of type 1 diabetes (T1D), and also regulates autoimmune diseases in these genetically susceptible animals.47 Furthermore, recent evidence suggests that normal ageing profoundly affects the composition of microbiome, in both humans and mice,17, 48 and is strongly influenced by BMI (Figure 1).
Figure 1.
Factors affecting microbiome in early life and adulthood. Host microbiome in both early and adult life is affected by various factors. Preterm (during gestation) determinants of microbiome include maternal BMI and pharmacotherapy, whereas post-term factors such as mode of delivery, duration of breastfeeding, day-care attendance and diet (throughout life) significantly influence the microbiota composition. Environmental factors, for example, exposure to cigarette smoke/air pollution/allergens, respiratory infections as well as host-associated factors such as age, gender, BMI and onset of chronic diseases could result in ‘dysbiosis'.
Effects of diet and other factors on microbiome composition
Thomson and co-workers analysed stool samples from nine infants (age; 7 days–10.5 months; followed weekly for 16 weeks) and reported that the infant microbiome was affected by age, diet and day-care attendance. They also found a greater plasticity in microbiome composition in exclusively breastfed infants compared with non-exclusively breastfed infants, although the phylogenetic diversity and species richness was diminished in exclusively breastfed infants.49 In 33 children with irritable bowel syndrome, a diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) was found to be effective in reducing the frequency of abdominal pain, compared with a typical American childhood diet.50 Independent of maternal BMI, a maternal high-fat diet was associated with a notable relative depletion of Bacteroidetes in neonates at birth, which persisted up to 6 weeks of age.51 In adults, a short-term (5-day) dietary intervention (entirely plant or animal based) significantly altered gut microbial community structure (with notable increases in the genus Prevotella) and accounted for individual differences in microbiome-associated metabolic gene expression.52 Consumption of fibre is also important in maintaining the composition of the microbiome as a low fibre diet has recently been shown to cause an irreversible loss in gut bacterial diversity over several generations in mice.53
Lee and Ko54 reported that oral administration of retinoic acid significantly increased the abundance of members of Lactobacillaceae families in the murine gut, which inhibited murine noroviral replication in the host through the upregulation of interferon-β. Prebiotic feeding of β(1–4)galacto-oligosaccharides (GOS90) formulation to germ-free (GF) mice colonised with normal mouse gut microbiota resulted in increased abundance of Bifidobacterium adolescentis(B. adolescentis), B. pseudocatenulatum, B. lactis and B. gallicum and decreased abundance of Bacteroidales, Helicobacter and Clostridium. However, the prebiotic diet (GOS90) was not associated with an altered inflammatory profile (interleukin-6 (IL-6), IL-12, IL-1β, interferon-γ and tumour necrosis factor-α).55
Recent evidence also indicates the role of gut microbiota in aggravating metabolic inflammation via toll-like receptor (TLR) signalling (primarily TLR2 and TLR4) induced with a saturated lipid-rich diet. Feeding mice a diet rich in saturated fats resulted in weight gain, higher food consumption and reduced respiratory quotient as well as increased the abundance of genera Bacteroides, Turicibacter and Bilophila. This was in comparison with a diet rich in polyunsaturated lipids in which the genera Actinobacteria (Bifidobacterium and Adlercreutzia), Lactobacillus, Streptococcus, Verrucomicrobia (Akkermansia muciniphila), Alphaproteobacteria and Deltaproteobacteria were increased.56
Effects of microbiome in health and regulation of host immunity
Normal gut microbiota perform a wide range of metabolic activities that benefit the host. Forms of vitamin K synthesised by bacteria are known as menaquinones, and are associated with cardiometabolic health in humans.57 Moreover, Bifidobacteria and Lactobacilli have vital roles in the synthesis of several components of vitamin B complexes.58 Healthy gut bacteria also modulate energy metabolism by increasing the serum concentrations of pyruvic acid, citric acid, fumaric acid and malic acid.59 Specific members of gut microbiota (Bacteroides, Bifidobacterium, Fecalibacterium and Enterobacteria) facilitate the fermentation of indigestible oligosaccharides resulting in the production of short chain fatty acids (SCFAs) such as acetate, propionate and butyrate. These SCFAs are present in the intestinal lumen at a total concentration of ~100 mM at an approximate ratio of 6:3:1, respectively, although this is subject to carbohydrate availability, microbiota composition and intestinal transit time.60 SCFAs serve as the primary energy substrate for colonocytes and also aid in maintaining colonic epithelial integrity, regulating host energy balance, suppressing colonic inflammation and inducing apoptosis in colon cancer cells.61, 62 The gut microbiota has also been shown to modulate lipid metabolism by promoting lipase activity in adipocytes.63 Targeted alterations in the gut microbiome could prove beneficial in controlling body mass, triglycerides and high-density lipoproteins.64
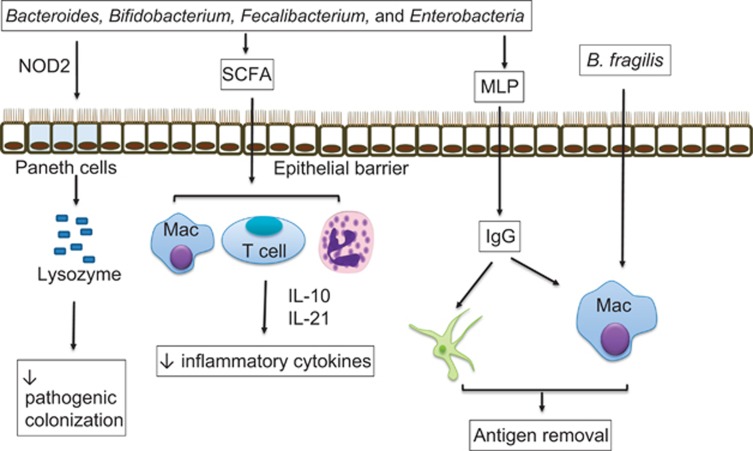
Local intestinal immunity is largely regulated by resident microbiota (and/or microbial metabolites/components) and fails to develop fully in the absence of normal microbiota. This is best demonstrated in GF mice that have underdeveloped gut-associated lymphoid tissues including Peyer's patches, isolated lymphoid follicles and mesenteric lymph nodes.65 Genesis and maturation of lymphoid follicles in mice requires stimulation by bacterial cell wall components from commensals and is mediated through nucleotide-binding oligomerization domain-containing protein 1 (NOD1) signalling.66 Establishment of normal microbiota at an early age is critical for the development of appropriate cellular immune responses, as colonisation of neonatal mice, but not adult GF mice, with conventional microbiota from wild-type mice protected the animals from pathological manifestations of IBD and asthma that were associated with the accumulation of mucosal invariant natural killer T cells.67 Microbiota are involved in the maintenance of innate immunity. Peptidoglycan from intestinal bacteria has been shown to regulate the steady-state cellular lifespan of neutrophils and inflammatory monocytes through NOD1 signalling.68 Commensal bacteria are commonly recognised by pathogen-sensing receptors, such as TLRs under normal conditions, which is essential in maintaining intestinal epithelial homeostasis and protects against dextran sulphate sodium-associated gut injury in mice.69 Commensal bacteria maintain the intestinal barrier in mice by selectively stimulating the release of lysozyme from secretory intestinal epithelial cells (Paneth cells) and protect the host from enteric infections through increased identification of enteric pathogens via NOD2 signalling in IBD.70 Furthermore, individual members of the intestinal microbiome can markedly alter the inflammatory state of the intestinal immune system. Murein lipoprotein from selective gut symbiotic Gram-negative bacteria are capable of inducing systemic immunoglobulin G (IgG) responses in a TLR4-dependent manner, which primarily targets bacterial antigens for removal by phagocytes and protects mice against systemic infections by Escherichia coli and Salmonella.71 Very recently, in an in vitro model of bone marrow-derived macrophages, Deng et al.72 demonstrated that B. fragilis polarises the macrophages into an proinflammatory M1 phenotype, and also enhances its phagocytic activity against pathogenic bacteria (Figure 2). Microbiota also regulate adaptive immunity. Intestinal microbiota promotes the development of CD4+ T cells, regulatory T (Treg) cells, Th1 or Th2 responses and Th17 T cells, all of which are important in the maintenance of systemic immune responses.73 Recently, it has also been shown that resident microbiota can induce high IL-21-producing CD4+ T-follicular helper cells in Peyer's patches that subsequently regulate the development of germinal centres in mice.74
Figure 2.
Roles of commensal bacteria and/or their components/metabolites in regulation of host immunity. Commensal bacteria induce the lysozyme production by Paneth cells that reduces the colonisation of pathogenic bacteria via NOD2 sensing. Moreover, these beneficial bacteria ferment dietary fibres to produce SCFAs, which then promotes the secretion of anti-inflammatory cytokines (IL-10, IL-21) by immune cells. Murein lipoprotein (MLP), a Gram-negative commensal bacterial cell wall component, can induce production of IgG, which primes macrophages and dendritic cells to remove antigens/pathogens. B. fragilis selectively polarises macrophages into proinflammatory M1 phenotype leading to enhanced pathogen clearance/inflammation.
The bacterial metabolites SCFAs bind specifically to the G-protein-coupled receptor 43/free fatty acid receptor 2 (GPR43/FFAR2), GPR41/FFAR3 and GPR109A, and these interactions significantly affect normal inflammatory responses. Similar to GF mice, which have negligible SCFAs, GPR43-deficient (Gpr43−/−) mice showed either heightened or unresolved inflammatory responses in experimental models of colitis, arthritis and asthma.75 GPR43 is expressed on cells of the distal ileum, colon, adipose tissue and immune cells, and intestinal mast cells. GPR41 is localised in the cytoplasm of enterocytes and enteroendocrine cells in human colonic mucosa. GPR41-expressing cells occur at lower levels than GPR43-expressing cells.76 Propionate-GPR41 interactions promote the development of macrophages with enhanced phagocytic activity as well as dendritic cell precursors, which migrate to the lung and possess impaired ability to differentiate naïve T cells into TH2 cells.77 Similarly, butyrate–GPR109A interactions have been shown to induce IL-10 production by colonic Treg cells in mice (Figure 2). Interestingly, a Gpr109A agonist, niacin, also suppressed colitis and colon cancer in a Niacr-knockout mice model.78 Moreover, microbial-derived butyrate has been shown to induce differentiation (but not survival or proliferation) of intestinal Treg cells, in both in vitro and in vivo models, which also ameliorated the development of colitis in Rag1−/− mice. This promotion of Treg differentiation occurred via the activity of butyrate in inhibiting histone deacetylase, leading to enhanced histone H3 acetylation at both promoter and non-coding regions of the Foxp3 locus. In support of this, the intake of butyrylated high-amylose maize starch significantly increased IL-10-producing Treg cells in the colon.79
Microbial components also have the potential to maintain immune homeostasis in the host. Evidence is mounting in support of a ‘common mucosal response', where the GI mucosa may regulate immune responses at distal mucosal sites (e.g., lungs), or vice versa, through the migration of preprogramed lymphoid cells and/or inflammatory mediators.80 Using animal models, it has been demonstrated that perturbation of the normal gut microbiota in inbred mice may promote fungal overgrowth in the gut, and the development of experimental asthma when challenged with fungal spores in later life (allergic airway disease), which is characterised by increased levels of in eosinophils, IgE, IL-5 and IL-13, as well as goblet cell metaplasia.81 In the respiratory tract, upper airway commensals may impart an effective antiviral immune response against influenza A virus in the lower airways through the induction of M2-macrophage-induced anti-inflammatory cytokines and inhibitory ligands.82 Furthermore, commensal neomycin-sensitive bacteria regulate protective immune responses in the lungs against influenza A virus infections by generating virus-specific CD4 and CD8 T cells through inflammasome activation and dendritic cell migration from the site of infection to lymph nodes.83 These findings clearly demonstrate a critical role for normal microbiota, as well as its metabolites, in shaping and modulating host immune responses, not only in intestinal tract but also at other sites such as the lungs.4, 45
Disruption of either the composition or overall numbers of ‘normal' microbiota (in both gut and lungs) is termed as ‘dysbiosis', and may contribute to the development, progression, or exacerbation of various inflammatory disorders of lungs, including asthma, COPD and CF.84, 85, 86, 87 Typically, dysbiosis is associated with the outgrowth of dominant (usually pathogenic) bacterial genera over community diversity. However, in relation to lung diseases, we do not precisely know if any of these observed microbial community changes are causal factors or result of disease, and/or then subsequently contribute to disease progression.
The role of the microbiome in asthma
Asthma is a multifactorial disease, primarily characterised by heightened airway inflammation, associated smooth muscle hyperplasia and airway hyperreactivity.88, 89, 90 Patients are susceptible to infectious exacerbations.91 Allergic asthma develops in responses to host and environmental factors involving infections and other exposures.92, 93, 94 As the microbiota aids in the development and maturation of both innate and adaptive immunity, this potentially influences the allergic responses throughout the life of an individual, including the development of allergic asthma.94, 95, 96 Several studies have observed a positive association between childhood antibiotic exposure, which disrupts the normal microbiome, and risk of early-onset childhood asthma.97 The role of the microbiome appears to relate directly to the type of microbial exposure as opposed to the total microbial load. Indeed, neither personal nor home cleanliness were associated with a risk for asthma and allergies, but exposure to specific microbial markers in the environment, such as muramic acid and endotoxin, correlated with asthma prevalence (odds ratio (OR): 0.59; 95% confidence interval (CI): 0.39–0.90) and allergic sensitisation (OR: 0.73; 95% CI: 0.56–0.96) in school-age children.98
The diversity of nasopharyngeal microbiota has a fundamental role in determining the host susceptibility to febrile lower respiratory infections and the development of asthma later in life.99 In particular, colonisation of the hypopharyngeal region of 1-month-old infants (n=321) with Moraxella catarrhalis, Haemophilus influenzae or Streptococcus pneumoniae was associated with a significantly increased risk for childhood asthma.100 Respiratory H. influenzae, Chlamydia and viral infections are linked to severe asthma.90, 101, 102, 103 In human adults, the composition of the lung microbiome in asthmatics differs significantly from healthy controls,84 which seems to regulate proasthmatic immune responses. Several studies have reported greater abundance of Proteobacteria in the lower airway secretions of asthmatics compared with healthy individuals,84, 104 which was also associated with TH17-associated gene expression.105 Specific pulmonary bacterial communities have been associated with clinical characteristics (e.g., sputum total leucocyte levels, reduced quality of life) in severe asthma (dominated by Actinobacteria) and differs from both healthy individuals and patients with mild-to-moderate asthma.105 Interestingly, asthmatics who displayed improved bronchial reactivity after 6 weeks of macrolide antibiotic (clarithromycin) treatment had higher baseline bacterial diversity,106 potentially implicating the resident microbiota in the outcome of therapeutic interventions. In addition to bacterial communities, the analysis of induced sputum revealed that 90 fungal species were more abundant in asthmatics, whereas 46 were more abundant in the control subjects.107 In particular, members of genera Aspergillus and Penicillium were significantly associated with impaired postbronchodilator reduced expiratory volume in 1 s in asthmatics.108 Mouse models have been developed that replicate features of acute, chronic, mild-to-moderate and severe steroid-resistant asthma.103, 109, 110, 111, 112 Such models have been used to confirm this role for the respiratory microbiota in allergic asthma, as increased infiltration of lymphocytes and eosinophils occurred following ovalbumin administration in GF compared with SPF mice, which could be reversed with recolonising GF mice with normal microflora113 (phenotype-specific).
In addition to the modulation of immunity by the lung microbiome, perturbations in GI microbiota, such as through antibiotic use and poor diet, may disrupt mucosal tolerance. A recent Finnish study showed that the use of macrolides in early life was associated with long-term microbial dysbiosis (depletion of Actinobacteria, increased Bacteroidetes and Proteobacteria), and significantly increased asthma risk and antibiotic-associated weight gain in 2–7-year-old children.114 A mouse model of antibiotic use in early life demonstrated significant changes in gut microbiome composition (both richness and Shannon evenness) that was dependent on the number of courses and class of antibiotic used, resulting in weight gain.115 Similarly, vancomycin-treated neonatal mice exhibited markedly altered gut microbiota and heightened allergic asthma characterised by significant increases in bronchoalveolar lavage fluid eosinophils, serum ovalbumin-specific IgE levels and airway hyperresponsiveness compared with control mice.116 Even in the absence of antibiotics, subtle differences in the GI microbiota in early life can affect asthma risk. Data from Canadian Healthy Infant Longitudinal Development (CHILD) Study (n=319) demonstrated that gut microbial dysbiosis during the first 3 months of life predisposed infants to a relatively high risk of asthma, which was associated with significant decreases in bacterial genera Faecalibacterium, Lachnospira, Veillonella and Rothia as well as reduced faecal acetate levels. GF mice inoculated with faeces lacking these taxa exhibited airway inflammation and pathology in their adult progeny. This was improved in mice inoculated with the same faeces supplemented with the specific bacterial taxa, demonstrating a beneficial role for these bacterial taxa in asthma prevention.117 Furthermore, pregnant mice fed with high-fibre/acetate diet protected the offspring against the development of allergic airway disease, possibly via histone deacetylase 9 inhibition and promoting highly suppressive Treg cells involving acetylation at the Foxp3 promoter region.118
Apart from infancy, the changing microbiota throughout life may influence allergic asthma. Vital et al.119 found that aged mice (9–10 months) that were sensitised and challenged with house dust mite exhibited greater allergic airway responses compared with young similar treated mice (6–8 weeks). Notably, age-related gut microbiota alterations in old mice (decrease in Bacteroidetes:Firmicutes ratio) resemble those in mice challenged with house dust mite. In addition, intestinal microbial dysbiosis was significantly associated with elevated bronchoalveolar lavage fluid IL-17A and increased Th17 cells in the spleen in aged mice, whereas serum IL-17A levels were increased in both groups. This study suggests important links between age-associated gut microbial dysbiosis and allergic airway responses. Moreover, interestingly, pulmonary sensitization with house dust mite may drive alterations in gut microbiome through priming Th17 regulatory cells.
The role of the microbiome in COPD
COPD is a multicomponent disease that involves airway inflammation, mucociliary dysfunction and lung structural changes, which are observed in varying degree of severities in COPD patients, and contribute to airflow obstruction and breathing difficulties.120, 121, 122 The structural and airway changes in COPD patients include small airway fibrosis and emphysema, whereas the large airways demonstrate epithelial goblet cell hyperplasia, epithelial squamous metaplasia and submucosal gland hyperplasia. Emphysema develops usually after a decade and only in a subset of patients.123 Tobacco smoking remains the principal causal factor worldwide, especially in developed countries. In addition to cigarette smoke, exposure to biomass fuel smoke and air pollution contribute significantly in disease prevalence in low- and middle-income countries.124, 125
The clinical course of COPD is complicated by frequent episodes of acute exacerbations, which primarily involves microbial and viral infections.91, 126, 127 In the past two decades, our understanding of the pathogenesis of airway infections has increased substantially. The most common ‘colonisers' in the stable state of COPD are non-typeable H. influenzae (~60%), M. catarrhalis (~48%) and S. pneumoniae (~28%).128 Moreover, bacterial colonisation of the respiratory tract in COPD patients is believed to be dynamic, as the bacterial flora frequently changes in terms of both strains and species, although the above-mentioned bacteria remain prominent.129 These bacterial pathogens undoubtedly worsen a patient's health over a long period of time and may be a major driver of airway luminal inflammation and oxidative stress,128, 130, 131, 132, 133 as well as increased daily symptoms in COPD patients.134 Indeed, COPD patients chronically colonised with H. influenzae during stable phase showed increased airway inflammation and reduced lung volumes when compared with non-chronically colonised patients.135
The influence of cigarette smoke on the microbiome and the role of the microbiome in COPD are relatively new paradigms with limited data.4, 45 However, both of these issues could be relevant in understanding disease development, progression and exacerbations. Results from a multicentre cohort study (n=64), based on modern culture-independent techniques such as quantitative reverse transcription PCR (using 16S rRNA) show that the oropharyngeal microbiome (oral wash) differed in non-smokers compared with smokers, but no difference was found in lung microbial communities using bronchoalveolar lavage.136 In contrast, the microbial communities in the lungs of COPD patients differ significantly from both healthy smokers and non-smokers, primarily due to an enrichment of some taxa including Firmicutes (e.g., Lactobacillus, Streptococcus) and Proteobacteria (e.g., Burkholderia, Campylobacter).32, 33, 137, 138
Studies of the COPD microbiome have also shown significant biogeographical and temporal variations in microbial diversity and relative abundance.32, 33, 138 In a healthy individual, spatial and temporal variation in microbiota composition is significantly less than variation across individuals and is primarily influenced by microbial immigration and elimination, whereas in disease microbial communities are more likely to be affected by local growth conditions.26 In particular, the decline in both the diversity and richness in the respiratory microbiome is shown to be associated with greater emphysema and increased immune cell infiltration in COPD patients.33 Moreover, a recent study by Wang et al.139 showed different phenotypic patterns in COPD exacerbations (e.g., bacterial or eosinophilic), which were associated with distinct lung microbiome profiles at both phylum and genus levels. In patients exhibiting bacterial exacerbations, the microbiome was dominated by the phyla Proteobacteria (mainly Haemophilusspp.), whereas Firmicutes dominated eosinophilic exacerbations. Thus, changes in the lung microbiota composition may be associated with acute exacerbations and could potentially shape host inflammatory responses (especially IL-8), at least in some individuals.
Mouse models have been developed that replicate features of COPD, including exacerbations, in a short time frame and can be used in cause and effect and mechanism studies.110, 121, 122, 126, 140, 141, 142, 143, 144 Several mechanistic studies in animals have explored the modulation of lung immunity by lung microbes. One of the first showed a causal relationship between the bacterial microbiome, inflammation and IL-17A-mediated lymphoid follicle formation.145 In a lipopolysaccharide/elastase-challenged mice model of COPD, Yadava et al.145 showed that treated mice had decreased microbiota richness and diversity compared with SPF mice, with an increased representation of the genera Pseudomonas and Lactobacillus and a reduction in Prevotella. The authors also observed increased inflammation (lymphoid follicle formation), extensive lung damage as well as increased production of antibodies and IL-17A in treated mice. Interestingly, GF mice exhibited reduced inflammatory cells in the airways, indicating that microbial factors modulate lung inflammatory responses.145 In addition, intranasal administration of bronchoalveolar lavage fluid from lipopolysaccharide/elastase-treated mice, but not from phosphate-buffered saline-treated mice, resulted in an increase in IL-17A-producing cells in the lungs of both GF mice and antibiotic-treated mice.145 Besides inflammation, lung microbiome may also affect lung architecture, which could significantly affect both pulmonary function and airflow obstruction. Yun et al.146 reported metabolically active lung microbiota in both SPF- and wild-type outbred mice, but not in GF mice. Although the overall microbiome composition across these mice was similar at the phylum and family level, species richness was significantly different between those housed in SPF and non-SPF facilities. The authors also found a positive correlation between higher bacterial abundance in non-SPF mice and increased number and smaller size alveoli.146
The GI microbiome has not been characterised in COPD patients; however, there is evidence of gut bacterial dysbiosis in response to cigarette smoke in both humans and mice. Compared with non-smoking Crohn's disease patients, gut microbial gene richness, genus and species diversity were reduced in smoking patients, with lower relative abundance of the genera Collinsella, Enterorhabdus and Gordonibacter, and of Faecalibacterium prausnitzii.147 Moreover, significant alterations in microbiota composition has been reported in healthy smokers, which reverses upon smoking cessation, with marked increases in overall microbial diversity and an increase in the phyla Firmicutes and Actinobacteria, as well as a lower proportion of Bacteroidetes and Proteobacteria, compared with continuing smokers and non-smokers.148 Colonic bacterial dysbiosis was also reported in mice chronically (24 weeks) exposed to cigarette smoke, with increases in Lachnospiraceaesp.149
Increased levels of reactive oxygen species likely drives pathophysiology of COPD.150 Bacterial activation of NOD-like receptors in the GI tract enhances production of reactive oxygen species in alveolar macrophages, potentially implicating the GI microbiome in oxidative stress and inflammation in COPD.151 There is evidence for the beneficial use of probiotic bacterial strains to modulate lung immune responses in COPD patients. Phagocytosis of Lactobacillus rhamnosus and Bifidobacterium breve by human macrophages in vitro resulted in the suppression of cigarette smoke-induced nuclear factor-κB activation and associated inflammation.152 In addition, daily intake of Lactobacillus casei Shirota increased the cytotoxic activity of natural killer cells as well as CD16+ cells in current smokers.153 Respiratory syncytial virus infection can lead to significant airspace enlargement and fibrosis in mice exposed to cigarette smoke, as well as heightened disease severity in mice.154 Notably, administration of L. rhamnosus before respiratory syncytial virus infection in mice resulted in elevated antiviral responses via TLR3/RIG-I activation.155 Although the role of microbiome in COPD needs to be further investigated, early studies have suggested an association between both lung and gut microbiota and the outcomes of disease.
The role of the microbiome in CF
CF is caused by mutations in the gene encoding the CF transmembrane conductance regulator (CFTR) protein that affects various organs, including lungs, pancreas, intestines and hepatobiliary tract.156 The predominant bacterial pathogens identified in CF are Pseudomonas aeruginosa and Staphylococcus aureus, whereas H. influenzae and Burkholderia cepacia coinfection also have a role. It is now acknowledged that CF patients harbour a complex polymicrobial community in both the airways and gut, and alterations in these microbial communities significantly affect the disease progression and clinical course.157 Early microbial dysbiosis in the lungs due to mucus hypersecretion and impaired airway clearance may result in chronic aberrant inflammation and damage of the airways. This in turn may lead to chronic colonisation by major bacterial pathogens in CF due to impaired immunity and barrier function. Moreover, variation in CF phenotypes, especially in infants and young children sharing similar CFTR mutations, may be attributed to the differences in microbial composition and its interaction with the host immune system. Recently, Mirkovic et al.158 demonstrated that the abundance of SCFA-producing anaerobic bacterial species are increased in CF airways. Furthermore, SCFAs stimulated excessive IL-8 production by bronchial epithelial cells from CF patients, but not from normal participants. The SCFA receptor GPR41 is overexpressed in epithelial cells from CF patients and blocking its signalling through small interfering RNA inhibited IL-8 production by CF airway cells, implicating GPR41 in microbiota-mediated inflammation in CF patients.158 Additionally, SCFAs also promoted the release of granulocyte–macrophage colony-stimulating factor, granulocyte colony-stimulating factor and IL-6, while the concentration of SCFAs are positively correlated with sputum neutrophil counts in CF patients.159
In other studies, Hoen et al.160 conducted a prospective longitudinal metagenomics analysis (120 samples collected from 13 participating children) of oropharyngeal (n=66) and stool samples (n=54) in infants from birth to 34 months old, and noted a significant reduction in genus Parabacteroides in the gut well before the chronic airway colonisation with P. aeruginosa. The investigators also found significant associations between gut microbial communities (but not respiratory microbiota) and early-life CF exacerbations indicating the crucial role of gut–lung cross-talk in this chronic respiratory disease.160 Apart from lung pathology, elevated levels of intestinal inflammatory markers have been reported in whole gut lavage (e.g., albumin, IgG, IgM, eosinophil cationic protein, neutrophil elastase, IL-1β, IL-8)161 and faeces (e.g., calprotectin)162 from young children with CF. The GI microbiome of CF patients has reduced richness and diversity beginning in early childhood (2 years) and continuing until late adolescence (17 years).163 Other changes that may affect the overall clinical outcome include an increased abundance of P. aeruginosa in the gastric juice of CF patients, and markedly lower abundance of normal gut bacteria (Bacteroides, Faecalibacterium) in digestive tract samples from CF patients compared with non-CF individuals.164 Notably, the severity of CFTR dysfunction was related to dysbiotic faecal bacterial profiles, with an increased abundance of deleterious species (Escherichia coli, Eubacterium biforme) and reduction in normal species (F. prausnitzii, Bifidobacterium spp., Eubacterium limosum).165 Gut microbiota dysbiosis in CF children (<3 years old) has also been linked to significantly altered lipid metabolism, including depleted capacity for overall fatty acid biosynthesis and increased capacity for degrading anti-inflammatory SCFAs, particularly butyrate and propionate,166 which may lead to increased risk of developing allergic responses in the lungs.
In a mouse model of CF (BALB/c Cftrtm1UNC), the administration of streptomycin significantly reduced gut bacterial overgrowth compared with wild-type mice and ameliorated airway hyperresponsiveness. Cftr mutants had reduced lymphocytes in lymph nodes, which could be restored with streptomycin treatment, whereas γδ T cells were not changed in Cftrtm1UNC mice, they were reduced by the same treatment.167 This study further strengthens the notion that gut microbiota indeed influences lung inflammatory responses in CF.
Conclusions and future directions
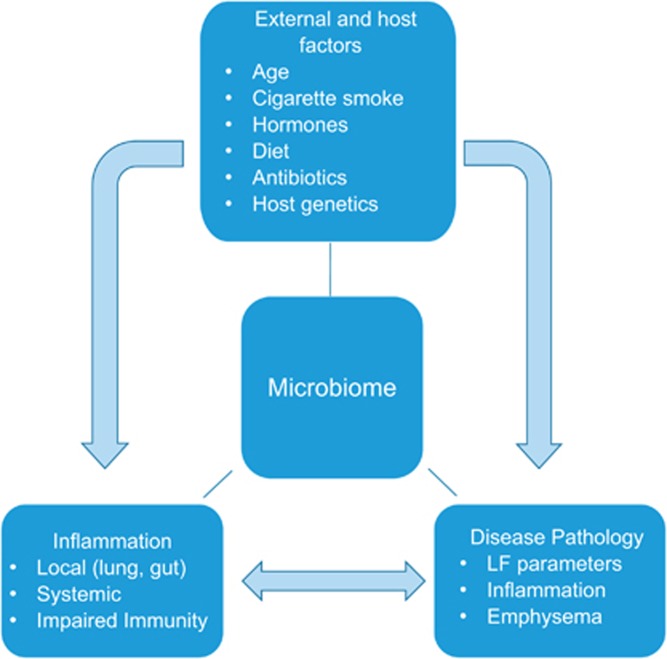
Evidence suggests that certain microbes have pivotal roles in the development of healthy immune responses, and microbial dysbiosis can contribute to chronic inflammatory lung diseases such as asthma, COPD and CF. The cross-talk between mucosal barriers, such as occurs in gut–lung cross-talk, is considered to be mediated by both resident microbes and patrolling immune cells, but this remains to be fully elucidated. Currently, available treatments for major non-communicable lung diseases only focus on alleviating symptoms with poor applicability to completely prevent and/or treat the diseases. A better understanding of microbiome-driven pathophysiology and inflammation, in conjunction with the interaction of major risk factors for chronic lung disease such as host genetics and cigarette smoking, would aid in optimising current treatments and in managing these chronic lung conditions (Figure 3). Furthermore, by improving our understanding of the role of microbiomes in these diseases, novel therapeutic strategies may be developed. The effects of current therapeutics on overall microbiome, and consequently on the disease severity/progression, remains largely unknown and needs to be properly understood to realise the full impact of these treatments. Modification of the microbiome through diet, probiotics, faecal or selected bacterial transfers may supplement currently available treatments or be effective treatments in their own right, but further research into such alternatives is required.
Figure 3.
Interaction matrix: risk factors for chronic respiratory diseases and associated pathology with microbiome. Major risk factors could lead to immune dysregulation, characteristic pathology and ‘dysbiosis'. Altered microbiome could then aggravate the host immunity and disease pathology. Notably, aberrant immune response could further skew the microbiome favoring specific pathogens typically reported in respiratory diseases, such as COPD and CF. LF, lung function.
Acknowledgments
PMH is supported by fellowships and grants from the National Health and Medical Research Council (NHMRC) of Australia and the Brawn Fellowship, Faculty of Health and Medicine, The University of Newcastle, NSW, Australia, and the Rainbow Foundation. We thank F Thomson and M Thomson for their continued support.
Footnotes
The authors declare no conflict of interest.
References
- WHOthe Top 10 Causes Of Death. World Health Organization: Geneva. 2014. [Google Scholar]
- Australian Bureau of StatisticsNational Health Survey: First Results, 2014-15. ABS: Canberra. 2015. (Viewed on 08 July 2016)ABS cat no 4364055001. [Google Scholar]
- O'Dwyer DN, Dickson RP, Moore BB. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol 2016; 196: 4839–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol 2017; 15: 55–63. [DOI] [PubMed] [Google Scholar]
- Nguyen LD, Viscogliosi E, Delhaes L. The lung mycobiome: an emerging field of the human respiratory microbiome. Front Microbiol 2015; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K. The virome in host health and disease. Immunity 2015; 42: 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev 2012; 70: S38–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet 2012; 13: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science 2009; 326: 1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD. The effects of alignment quality, distance calculation method, sequence filtering, and region on the analysis of 16S rRNA gene-based studies. PLoS Comput Biol 2010; 6: e1000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl BoD, Nilsson RH, Tedersoo L, Abarenkov K, Carlsen T, Kjoller R et al. Fungal community analysis by high-throughput sequencing of amplified markers—a user's guide. New Phytol 2013; 199: 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IC, Campbell CD, Prosser JI. Potential bias of fungal 18S rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil. Environ Microbiol 2003; 5: 36–47. [DOI] [PubMed] [Google Scholar]
- Casjens SR. Comparative genomics and evolution of the tailed-bacteriophages. Curr Opin Microbiol 2005; 8: 451–458. [DOI] [PubMed] [Google Scholar]
- Reyes A, Semenkovich NP, Whiteson K, Rohwer F, Gordon JI. Going viral: next-generation sequencing applied to phage populations in the human gut. Nat Rev Microbiol 2012; 10: 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan XC, Segata N, Huttenhower C. Biodiversity and functional genomics in the human microbiome. Trends Genet 2013; 29: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz P, Tyson GW. Microbiology: metagenomics. Nature 2008; 455: 481–483. [DOI] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG et al. Human gut microbiome viewed across age and geography. Nature 2012; 486: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpton TJ. An introduction to the analysis of shotgun metagenomic data. Front Plant Sci 2014; 5: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander HF, Fandriks L. Surface area of the digestive tract—revisited. Scand J Gastroenterol 2014; 49: 681–689. [DOI] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Micro 2016; 14: 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel Y, Maharshak N, Ringel-Kulka T, Wolber EA, Sartor RB, Carroll IM. High throughput sequencing reveals distinct microbial populations within the mucosal and luminal niches in healthy individuals. Gut Microbes 2015; 6: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagkouvardos I, Pukall R, Abt B, Foesel BU, Meier-Kolthoff JP, Kumar N et al. The Mouse Intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat Microbiol 2016; 1: 16131. [DOI] [PubMed] [Google Scholar]
- Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev 2016; 40: 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblum S, Carr R, Borenstein E. Extensive strain-level copy-number variation across human gut microbiome species. Cell 2015; 160: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB et al. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc 2015; 12: 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015; 6: e00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 2011; 184: 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasleton PS. The internal surface area of the adult human lung. J Anat 1972; 112: 391–400. [PMC free article] [PubMed] [Google Scholar]
- Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA et al. Analysis of the lung microbiome in the "healthy" smoker and in COPD. PLoS ONE 2011; 6: e16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ES, Diamond JM, Bittinger K, Fitzgerald AS, Yadav A, Haas AR et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med 2012; 186: 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS ONE 2012; 7: e47305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 185: 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JM, Steen-Jensen DB, Laursen JM, Sondergaard JN, Musavian HS, Butt TM et al. Divergent pro-inflammatory profile of human dendritic cells in response to commensal and pathogenic bacteria associated with the airway microbiota. PLoS ONE 2012; 7: e31976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 2014; 6: 237ra265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants' life: a systematic review. BMC Gastroenterol 2016; 16: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Makino H, Cetinyurek Yavuz A, Ben-Amor K, Roelofs M, Ishikawa E et al. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS ONE 2016; 11: e0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan JC, Hoen AG, Lundgren SN, Farzan SF, Cottingham KL, Morrison HG et al. Association of cesarean delivery and formula supplementation with the intestinal microbiome of 6-week-old Infants. JAMA Pediatr 2016; 170: 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom A, Skov TH, Bahl MI, Roager HM, Christensen LB, Ejlerskov KT et al. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol 2014; 80: 2889–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL et al. The long-term stability of the human gut microbiota. Science 2013; 341: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT et al. Host genetic variation impacts microbiome composition across human body sites. Genome Biol 2015; 16: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzosa EA, Huang K, Meadow JF, Gevers D, Lemon KP, Bohannan BJ et al. Identifying personal microbiomes using metagenomic codes. Proc Natl Acad Sci USA 2015; 112: E2930–E2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod KL, Wood DL, Lachner N, Gellatly SL, Daly JN, Parsons JD et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016; 4: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Chen D, Lu K, Wang L, Han X, Zhao Y et al. Design, synthesis, and structure-activity relationships of novel benzothiazole derivatives bearing the ortho-hydroxy N-carbamoylhydrazone moiety as potent antitumor agents. Eur J Med Chem 2014; 86: 257–269. [DOI] [PubMed] [Google Scholar]
- Chambers DC, Gellatly SL, Hugenholtz P, Hansbro PM. JTD special edition 'Hot Topics in COPD'—The microbiome in COPD. J Thorac Dis 2014; 6: 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro C, Rangel-Zuniga OA, Alcala-Diaz JF, Gomez-Delgado F, Perez-Martinez P, Delgado-Lista J et al. Intestinal microbiota is influenced by gender and body mass index. PLoS ONE 2016; 11: e0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013; 339: 1084–1088. [DOI] [PubMed] [Google Scholar]
- Langille MG, Meehan CJ, Koenig JE, Dhanani AS, Rose RA, Howlett SE et al. Microbial shifts in the aging mouse gut. Microbiome 2014; 2: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AL, Monteagudo-Mera A, Cadenas MB, Lampl ML, Azcarate-Peril MA. Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front Cell Infect Microbiol 2015; 5: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumpitazi BP, Cope JL, Hollister EB, Tsai CM, McMeans AR, Luna RA et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with irritable bowel syndrome. Aliment Pharmacol Ther 2015; 42: 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med 2016; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016; 529: 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Ko GP. Antiviral effect of vitamin A on norovirus infection via modulation of the gut microbiome. Sci Rep 2016; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteagudo-Mera A, Arthur J, Jobin C, Keku T, Bruno-Barcena J, Azcarate-Peril M. High purity galacto-oligosaccharides enhance specific Bifidobacterium species and their metabolic activity in the mouse gut microbiome. Benef Microbes 2016; 7: 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani Patrice D, Backhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metabol 2015; 22: 658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl JP, Fu X, Wang X, Zhao Y, Shen J, Zhang C et al. Changes in fecal vitamin K content are associated with the gut microbiota. The FASEB Journal 2015; 29. [Google Scholar]
- LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol 2013; 24: 160–168. [DOI] [PubMed] [Google Scholar]
- Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res 2010; 51: 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc 2003; 62: 67–72. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr 2008; 100: 297–305. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol 2013; 13: 869–874. [DOI] [PubMed] [Google Scholar]
- Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Scien USA 2014; 111: 7421–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Bonder MJ, Cenit MiC, Tigchelaar EF, Maatman A, Dekens JAM et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res 2015; 117: 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 2009; 9: 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 2008; 456: 507–510. [DOI] [PubMed] [Google Scholar]
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012; 336: 489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergott CB, Roche AM, Tamashiro E, Clarke TB, Bailey AG, Laughlin A et al. Peptidoglycan from the gut microbiota governs the lifespan of circulating phagocytes at homeostasis. Blood 2016; 127: 2460–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004; 118: 229–241. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Pan Y, Yan R, Zeng B, Wang H, Zhang X et al. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat Immunol 2015; 16: 918–926. [DOI] [PubMed] [Google Scholar]
- Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M et al. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 2016; 44: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Li Z, Tan Y, Guo Z, Liu Y, Wang Y, Yuan Y et al. A novel strain of Bacteroides fragilis enhances phagocytosis and polarises M1 macrophages. Sci Rep 2016; 6: 29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol 2015; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Ho WQ, Ying S, Ramakrishna L, Srinivasan KG, Yurieva M et al. A subpopulation of high IL-21-producing CD4(+) T cells in Peyer's Patches is induced by the microbiota and regulates germinal centers. Sci Rep 2016; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009; 461: 1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M et al. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res 2009; 30: 149–156. [DOI] [PubMed] [Google Scholar]
- Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014; 20: 159–166. [DOI] [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014; 40: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504: 446–450. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C, Prince SJ, Michalek SM, Jackson S, Russell MW, Moldoveanu Z et al. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci USA 1987; 84: 2449–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun 2004; 72: 4996–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li F, Sun R, Gao X, Wei H, Li LJ et al. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat Commun 2013; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA 2011; 108: 5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010; 5: e8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Bafadhel M, Haldar K, Spivak A, Mayhew D, Miller BE et al. Lung microbiome dynamics in chronic obstructive pulmonary disease exacerbations. Eur Respir J 2016; 47: 1082–1092. [DOI] [PubMed] [Google Scholar]
- Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML et al. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci USA 2012; 109: 13769–13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GB, Zain NM, Bruce KD, Burr LD, Chen AC, Rivett DW et al. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc 2014; 11: 496–503. [DOI] [PubMed] [Google Scholar]
- Hansbro PM, Scott GV, Essilfie AT, Kim RY, Starkey MR, Nguyen DH et al. Th2 cytokine antagonists: potential treatments for severe asthma. Expert Opin Investig Drugs 2013; 22: 49–69. [DOI] [PubMed] [Google Scholar]
- Thorburn AN, Hansbro PM. Harnessing regulatory T cells to suppress asthma: from potential to therapy. Am J Respir Cell Mol Biol 2010; 43: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansbro PM, Kaiko GE, Foster PS. Cytokine/anti-cytokine therapy-novel treatments for asthma? Br J Pharmacol 2011; 163: 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey MR, Jarnicki AG, Essilfie AT, Gellatly SL, Kim RY, Brown AC et al. Murine models of infectious exacerbations of airway inflammation. Curr Opin Pharmacol 2013; 13: 337–344. [DOI] [PubMed] [Google Scholar]
- Hansbro NG, Horvat JC, Wark PA, Hansbro PM. Understanding the mechanisms of viral induced asthma: new therapeutic directions. Pharmacol Ther 2008; 117: 313–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MJ, Hiebert PR, Park HY, Stefanowicz D, Le A, Starkey MR et al. Mucosal production of uric acid by airway epithelial cells contributes to particulate matter-induced allergic sensitization. Mucosal Immunol 2016; 9: 809–820. [DOI] [PubMed] [Google Scholar]
- Hansbro PM, Beagley KW, Horvat JC, Gibson PG. Role of atypical bacterial infection of the lung in predisposition/protection of asthma. Pharmacol Ther 2004; 101: 193–210. [DOI] [PubMed] [Google Scholar]
- Starkey MR, Nguyen DH, Essilfie AT, Kim RY, Hatchwell LM, Collison AM et al. Tumor necrosis factor-related apoptosis-inducing ligand translates neonatal respiratory infection into chronic lung disease. Mucosal Immunol 2014; 7: 478–488. [DOI] [PubMed] [Google Scholar]
- Starkey MR, Essilfie AT, Horvat JC, Kim RY, Nguyen DH, Beagley KW et al. Constitutive production of IL-13 promotes early-life Chlamydia respiratory infection and allergic airway disease. Mucosal Immunol 2013; 6: 569–579. [DOI] [PubMed] [Google Scholar]
- Ong M-S, Umetsu DT, Mandl KD. Consequences of antibiotics and infections in infancy: bugs, drugs, and wheezing. Ann Allergy Asthma Immunol 2014; 112: 441–445.e441. [DOI] [PubMed] [Google Scholar]
- Weber J, Illi S, Nowak D, Schierl R, Holst O, von Mutius E et al. Asthma and the hygiene hypothesis. Does cleanliness matter? Am J Respir Crit Care Med 2015; 191: 522–529. [DOI] [PubMed] [Google Scholar]
- Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015; 17: 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007; 357: 1487–1495. [DOI] [PubMed] [Google Scholar]
- Wood LG, Simpson JL, Hansbro PM, Gibson PG. Potentially pathogenic bacteria cultured from the sputum of stable asthmatics are associated with increased ;8-isoprostane and airway neutrophilia. Free Radic Res 2010; 44: 146–154. [DOI] [PubMed] [Google Scholar]
- Kim RY, Pinkerton JW, Gibson PG, Cooper MA, Horvat JC, Hansbro PM. Inflammasomes in COPD and neutrophilic asthma. Thorax 2015; 70: 1199–1201. [DOI] [PubMed] [Google Scholar]
- Kim RY, Horvat JC, Pinkerton JW, Starkey MR, Essilfie AT, May all JR et al. MicroRNA-21 drives severe, steroid-insensitive experimental asthma by amplifying phosphoinositide 3-kinase-mediated suppression of histone deacetylase 2. J Allergy Clin Immunol 2016; 139: 519–532. [DOI] [PubMed] [Google Scholar]
- Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol 2013; 131: 346–352 e341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR et al. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J Allergy Clin Immunol 2015; 136: 874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol 2011; 127: 372–381 e371–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerden HC, Gregory C, Brown R, Marchesi JR, Hoogendoorn B, Matthews IP. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: a community based case control study. BMC Infect Dis 2013; 13: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbetile J, Fairs A, Desai D, Hargadon B, Bourne M, Mutalithas K et al. Isolation of filamentous fungi from sputum in asthma is associated with reduced post-bronchodilator FEV1. Clin Exp Allergy 2012; 42: 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essilfie AT, Horvat JC, Kim RY, Mayall JR, Pinkerton JW, Beckett EL et al. Macrolide therapy suppresses key features of experimental steroid-sensitive and steroid-insensitive asthma. Thorax 2015; 70: 458–467. [DOI] [PubMed] [Google Scholar]
- Liu G, Cooley MA, Jarnicki AG, Hsu AC, Nair PM, Haw TJ et al. Fibulin-1 regulates the pathogenesis of tissue remodeling in respiratory diseases. JCI Insight 2016; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn AN, Foster PS, Gibson PG, Hansbro PM. Components of Streptococcus pneumoniae suppress allergic airways disease and NKT cells by inducing regulatory T cells. J Immunol 2012; 188: 4611–4620. [DOI] [PubMed] [Google Scholar]
- Preston JA, Essilfie AT, Horvat JC, Wade MA, Beagley KW, Gibson PG et al. Inhibition of allergic airways disease by immunomodulatory therapy with whole killed Streptococcus pneumoniae. Vaccine 2007; 25: 8154–8162. [DOI] [PubMed] [Google Scholar]
- Herbst T, Sichelstiel A, Schar C, Yadava K, Burki K, Cahenzli J et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med 2011; 184: 198–205. [DOI] [PubMed] [Google Scholar]
- Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun 2016; 7: 10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel YR, Cox LM, Kirigin FF, Bokulich NA, Yamanishi S, Teitler I et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015; 6: 7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 2012; 13: 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 2015; 7: 307ra152. [DOI] [PubMed] [Google Scholar]
- Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 2015; 6. [DOI] [PubMed] [Google Scholar]
- Vital M, Harkema JR, Rizzo M, Tiedje J, Brandenberger C. Alterations of the murine gut microbiome with age and allergic airway disease. J Immunol Res 2015; 2015: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol 2012; 5: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker M, Deane A, Hansbro PM. Animal models of chronic obstructive pulmonary disease. Expert Opin Drug Discov 2014; 9: 629–645. [DOI] [PubMed] [Google Scholar]
- Jones B, Donovan C, Liu G, Gomez HM, Chimankar V, Harrison CL et al. Animal models of COPD: What do they tell us? Respirology 2016; 22: 21–32. [DOI] [PubMed] [Google Scholar]
- Sohal SS, Ward C, Danial W, Wood-Baker R, Walters EH. Recent advances in understanding inflammation and remodeling in the airways in chronic obstructive pulmonary disease. Expert Rev Respir Med 2013; 7: 275–288. [DOI] [PubMed] [Google Scholar]
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD)2017. Available athttp://goldcopd.org (accessed on 22/11/2016).
- Hirota JA, Gold MJ, Hiebert PR, Parkinson LG, Wee T, Smith D et al. The nucleotide-binding domain, leucine-rich repeat protein 3 inflammasome/IL-1 receptor I axis mediates innate, but not adaptive, immune responses after exposure to particulate matter under 10 mum. Am J Respir Cell Mol Biol 2015; 52: 96–105. [DOI] [PubMed] [Google Scholar]
- Hsu AC, Starkey MR, Hanish I, Parsons K, Haw TJ, Howland LJ et al. Targeting PI3K-p110alpha suppresses influenza virus infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 191: 1012–1023. [DOI] [PubMed] [Google Scholar]
- Hsu AC, Parsons K, Moheimani F, Knight DA, Hansbro PM, Fujita T et al. Impaired antiviral stress granule and IFN-beta enhanceosome formation enhances susceptibility to influenza infection in chronic obstructive pulmonary disease epithelium. Am J Respir Cell Mol Biol 2016; 55: 117–127. [DOI] [PubMed] [Google Scholar]
- Barker BL, Haldar K, Patel H, Pavord ID, Barer MR, Brightling CE et al. Association between pathogens detected using quantitative polymerase chain reaction with airway inflammation in COPD at stable state and exacerbations. Chest 2015; 147: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcha DS, Thurston SJ, Patel AR, Mackay AJ, Goldring JJ, Donaldson GC et al. Changes in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPD. Thorax 2012; 67: 1075–1080. [DOI] [PubMed] [Google Scholar]
- King PT, Sharma R, O'Sullivan K, Selemidis S, Lim S, Radhakrishna N et al. Nontypeable Haemophilus influenzae induces sustained lung oxidative stress and protease expression. PLoS ONE 2015; 10: e0120371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JL, Baines KJ, Horvat JC, Essilfie AT, Brown AC, Tooze M et al. COPD is characterized by increased detection of Haemophilus influenzaeStreptococcus pneumoniae and a deficiency of Bacillus species. Respirology 2016; 21: 697–704. [DOI] [PubMed] [Google Scholar]
- Simpson JL, McDonald VM, Baines KJ, Oreo KM, Wang F, Hansbro PM et al. Influence of age, past smoking, and disease severity on TLR2, neutrophilic inflammation, and MMP-9 levels in COPD. Mediators Inflamm 2013; 2013: 462934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay HL, Kaiko GE, Plank M, Li J, Maltby S, Essilfie AT et al. Antagonism of miR-328 increases the antimicrobial function of macrophages and neutrophils and rapid clearance of non-typeable Haemophilus influenzae (NTHi) from infected lung. PLoS Pathog 2015; 11: e1004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai H, Eschberger K, Wrona C, Grove L, Agrawal A, Grant B et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014; 11: 303–309. [DOI] [PubMed] [Google Scholar]
- Tufvesson E, Bjermer L, Ekberg M. Patients with chronic obstructive pulmonary disease and chronically colonized with Haemophilus influenzae during stable disease phase have increased airway inflammation. Int J Chron Obstruct Pulmon Dis 2015; 10: 881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med 2013; 187: 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharkina T, Heinzel E, Koczulla RA, Greulich T, Rentz K, Pauling JK et al. Analysis of the airway microbiota of healthy individuals and patients with chronic obstructive pulmonary disease by T-RFLP and clone sequencing. PLoS ONE 2013; 8: e68302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millares L, Ferrari R, Gallego M, Garcia-Nunez M, Perez-Brocal V, Espasa M et al. Bronchial microbiome of severe COPD patients colonised by Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis 2014; 33: 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Bafadhel M, Haldar K, Spivak A, Mayhew D, Miller BE et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J 2016; 47: 1082–1092. [DOI] [PubMed] [Google Scholar]
- Beckett EL, Stevens RL, Jarnicki AG, Kim RY, Hanish I, Hansbro NG et al. A new short-term mouse model of chronic obstructive pulmonary disease identifies a role for mast cell tryptase in pathogenesis. J Allergy Clin Immunol 2013; 131: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haw TJ, Starkey MR, Nair PM, Pavlidis S, Liu G, Nguyen DH et al. A pathogenic role for tumor necrosis factor-related apoptosis-inducing ligand in chronic obstructive pulmonary disease. Mucosal Immunol 2016; 9: 859–872. [DOI] [PubMed] [Google Scholar]
- Jarnicki AG, Schilter H, Liu G, Wheeldon K, Essilfie AT, Foot JS et al. The inhibitor of semicarbazide-sensitive amine oxidase, PXS-4728A, ameliorates key features of chronic obstructive pulmonary disease in a mouse model. Br J Pharmacol 2016; 173: 3161–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansbro PM, Hamilton MJ, Fricker M, Gellatly SL, Jarnicki AG, Zheng D et al. Importance of mast cell Prss31/transmembrane tryptase/tryptase-gamma in lung function and experimental chronic obstructive pulmonary disease and colitis. J Biol Chem 2014; 289: 18214–18227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singanayagam A, Glanville N, Walton RP, Aniscenko J, Pearson RM, Pinkerton JW et al. A short-term mouse model that reproduces the immunopathological features of rhinovirus-induced exacerbation of COPD. Clin Sci (Lond) 2015; 129: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadava K, Pattaroni C, Sichelstiel AK, Trompette A, Gollwitzer ES, Salami O et al. Microbiota promotes chronic pulmonary inflammation by enhancing IL-17A and autoantibodies. Am J Respir Crit Care Med 2016; 193: 975–987. [DOI] [PubMed] [Google Scholar]
- Yun Y, Srinivas G, Kuenzel S, Linnenbrink M, Alnahas S, Bruce KD et al. Environmentally determined differences in the murine lung microbiota and their relation to alveolar architecture. PLoS ONE 2014; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opstelten JL, Plassais J, van Mil SW, Achouri E, Pichaud M, Siersema PD et al. Gut microbial diversity is reduced in smokers with Crohn's disease. Inflamm Bowel Dis 2016; 22: 2070–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS ONE 2013; 8: e59260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allais L, Kerckhof FM, Verschuere S, Bracke KR, De Smet R, Laukens D et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ Microbiol 2016; 18: 1352–1363. [DOI] [PubMed] [Google Scholar]
- Belchamber K, Singh R, Wedzicha J, Barnes P, Donnelly L. Elevated mitochondrial reactive oxygen species in COPD macrophages at exacerbation. Eur Respir J 2015; 46: PA387. [Google Scholar]
- Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immun 2014; 82: 4596–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E, Adcock IM, Ricciardolo FL, Varahram M, Jamaati H, Velayati AA et al. Anti-inflammatory effects of Lactobacillus rhamnosus and Bifidobacterium breve on cigarette smoke activated human macrophages. PLoS ONE 2015; 10: e0136455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M, Boscolo P, Bellante V, Tarantelli C, Di Nicola M, Forcella L et al. Daily intake of Lactobacillus casei Shirota increases natural killer cell activity in smokers. Br J Nutr 2012; 108: 308–314. [DOI] [PubMed] [Google Scholar]
- Foronjy RF, Dabo AJ, Taggart CC, Weldon S, Geraghty P. Respiratory syncytial virus infections enhance cigarette smoke induced COPD in mice. PLoS ONE 2014; 9: e90567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomosada Y, Chiba E, Zelaya H, Takahashi T, Tsukida K, Kitazawa H et al. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol 2013; 14: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989; 245: 1066–1073. [DOI] [PubMed] [Google Scholar]
- Madan JC, Koestler DC, Stanton BA, Davidson L, Moulton LA, Housman ML et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio 2012; 3: e00251–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovic B, Murray MA, Lavelle GM, Molloy K, Azim AA, Gunaratnam C et al. The role of short-chain fatty acids, produced by anaerobic bacteria, in the cystic fibrosis airway. Am J Respir Crit Care Med 2015; 192: 1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani P, Santhakumar P, Hu Q, Djiadeu P, Wolever TM, Palaniyar N et al. Short-chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur Respir J 2015; 46: 1033–1045. [DOI] [PubMed] [Google Scholar]
- Hoen AG, Li J, Moulton LA, O'Toole GA, Housman ML, Koestler DC et al. Associations between gut microbial colonization in early life and respiratory outcomes in cystic fibrosis. J Pediatr 2015; 167: 138–147 e131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth RL, Croft NM, O'Hea U, Marshall TG, Ferguson A. Intestinal inflammation in cystic fibrosis. Arch Dis Child 2000; 82: 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal J, Leach S, Katz T, Nahidi L, Pang T, Lee JM et al. Intestinal inflammation and impact on growth in children with cystic fibrosis. J Pediatr Gastroenterol Nutr 2015; 60: 521–526. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Needham B, Leach ST, Day AS, Jaffe A, Thomas T et al. Disrupted progression of the intestinal microbiota with age in children with cystic fibrosis. Sci Rep 2016; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-momani H, Perry A, Stewart CJ, Jones R, Krishnan A, Robertson AG et al. Microbiological profiles of sputum and gastric juice aspirates in Cystic Fibrosis patients. Sci Rep 2016; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippa S, Iebba V, Santangelo F, Gagliardi A, De Biase RV, Stamato A et al. Cystic fibrosis transmembrane conductance regulator (CFTR) allelic variants relate to shifts in faecal microbiota of cystic fibrosis patients. PLoS ONE 2013; 8: e61176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor O, Levy R, Pope CE, Hayden HS, Brittnacher MJ, Carr R et al. Metagenomic evidence for taxonomic dysbiosis and functional imbalance in the gastrointestinal tracts of children with cystic fibrosis. Sci Rep 2016; 6: 22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazett M, Bergeron ME, Haston CK. Streptomycin treatment alters the intestinal microbiome, pulmonary T cell profile and airway hyperresponsiveness in a cystic fibrosis mouse model. Sci Rep 2016; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]