Abstract
Background
Accumulating evidence suggests that thiazolidinediones (TZDs) may exert protective effects in atrial fibrillation (AF). The present meta-analysis investigated the association between TZD use and the incidence of AF in diabetic patients.
Methods
Electronic databases were searched until December 2016. Of the 346 initially identified records, 3 randomized clinical trials (RCTs) and 4 observational studies with 130,854 diabetic patients were included in the final analysis.
Results
Pooled analysis of the included studies demonstrated that patients treated with TZDs had approximately 30% lower risk of developing AF compared to controls [odds ratio (OR): 0.73, 95% confidence interval (CI): 0.62 to 0.87, p = 0.0003]. This association was consistently observed for both new onset AF (OR =0.77, p = 0.002) and recurrent AF (OR =0.41, p = 0.002), pioglitazone use (OR =0.56, p = 0.04) but not rosiglitazone use (OR =0.78, p = 0.12). The association between TZD use and AF incidence was not significant in the pooled analysis of three RCTs (OR =0.77, 95% CI = 0.53–1.12, p = 0.17), but was significantly in the pooled analysis of the four observational studies (OR =0.71, p = 0.0003).
Conclusions
This meta-analysis suggests that TZDs may confer protection against AF in the setting of diabetes mellitus (DM). This class of drugs can be used as upstream therapy for DM patients to prevent the development of AF. Further large-scale RCTs are needed to determine whether TZDs use could prevent AF in the setting of DM.
Keywords: Atrial fibrillation, Diabetes mellitus, Thiazolidinediones, Pioglitazone, Rosiglitazone, Meta-analysis
Background
Atrial fibrillation (AF) is the most prevalent arrhythmia observed in clinical practice, and is associated with significant morbidity and mortality in the popuation. The burden of AF increases over time mainly due to an aging population and to the increasing prevalence of cardiovascular comorbidities. However, strategies to predict and prevent AF are not fully effective [1]. Diabetes mellitus (DM) is one of the strongest independent risk factors for AF incidence, conferring an approximate 40% higher risk of subsequent AF development [2, 3]. It also predicts the recurrence of AF following a successful direct current cardioversion [4]. Moreover, DM increases the risk of developing stroke, heart failure, and cardiovascular death in patients with AF [5]. Although the exact pathophysiological mechanisms linking DM and AF remain incompletely elucidated, an increasing body of evidence suggests that inflammation and oxidative stress may play an important role [6–8].
Thiazolidinediones (TZDs), a class of peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists, are among the most potent insulin-sensitizing drugs [9]. Apart from their anti-diabetic activity, TZDs display several pleiotropic effects including anti-inflammatory and antioxidant actions that may have potential benefits for AF prevention [10, 11]. However, inconsistent results have been reported regarding TZDs use and AF incidence [12–18]. In light of such conflicting data, we performed a comprehensive meta-analysis to evaluate the present evidence and investigate whether the use of TZDs confers benefits in preventing AF.
Methods
This systematic review was conducted according to the Quality of Reports of Meta-Analyses of Randomized Controlled Trials (QUOROM) recommendations [19] and the guidelines of the Meta-analysis of Observational Studies in Epidemiology Group (MOOSE) [20].
Inclusion criteria
The studies considered for this meta-analysis were either randomized clinical trials (RCTs) or observational studies that investigated the potential effects of TZDs on AF. The inclusion criteria were as follows: RCTs: 1) randomized controlled human trials with a parallel design; 2) comparison of TZDs with control; 3) collecting data on new or recurrent AF during follow-up. Observational Studies: 1) comparison of TZDs with control; 2) evaluating new or recurrent AF as an outcome. In the studies of interventions with TZDs no limit in the length of follow-up period was set due to the paucity of relevant studies.
Search strategies
A systematic literature search was performed by two investigators (Z. Z. and X. Z.) using the online databases of PubMed and Embase to identify relevant studies published before December 2016. The following key terms were used: “thiazolidinediones”, “pioglitazone”, “rosiglitazone”, “troglitazone”, and “atrial fibrillation”. Both investigators independently evaluated the search results and identified potential studies for further assessment. Disagreements were resolved by a third reviewer (T. L.).
Quality assessment and data extraction
As quality scoring in meta-analyses of RCTs and observational studies is controversial, several key points of study quality were assessed according to a critical review checklist of Wynn et al. [21]. The key points of this checklist and quality assessments of included studies are listed in Table 1.
Table 1.
Quality assessments of included studies
| Study, year | Study type |
Randomisation Method |
Blinding | Eligibility criteria reported | Study Population representative of normal practice | Method of follow-up properly defined | Equal follow-up between groups | Was loss to follow-up reported or explained |
Prospective recruitment |
Consecutive recruitment |
|---|---|---|---|---|---|---|---|---|---|---|
| PROactive, 2005 [12] | RCT | Randomised permuted blocks | Double | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Anglade, 2007 [13] | Case control |
NA | NA | Yes | Yes | Yes | Yes | No loss to follow-up | No | Yes |
| RECORD, 2009 [14] | RCT | Randompermuted blocks |
None | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Gu, 2011 [15] | Cohort | NA | NA | Yes | Yes | Yes | Yes | No loss to follow-up | Yes | Yes |
| Chao, 2012 [16] | Case control |
NA | NA | Yes | Yes | Yes | Yes | No loss to follow-up | No | Yes |
| Liu, 2014 [17] | RCT | Computer | Double | Yes | Yes | Yes | Yes | No loss to follow-up | Yes | Yes |
| Pallisgaard, 2016 [18] | Cohort | NA | NA | Yes | Yes | Yes | Yes | No loss to follow-up | Yes | Yes |
Abbreviations: RCT randomized controlled trial, NA not applicable
Two investigators (Z. Z. and X. Z.) independently extracted the relevant data using a pre-defined spreadsheets. The extracted data elements of the meta-analysis included information on the inclusion criteria, publication details, study design, follow-up duration, daily dosage of TZDs, definition of AF, methods of AF detection, baseline patient characteristics, the variables of multivariate model used in observational studies and results. Disagreements were resolved through discussion or consensus with a third reviewer (T. L.).
Statistical analysis
Results of the AF outcome are expressed as odds ratio (OR) with 95% confidence interval (CI) for each study using generic inverse-variance method. The hazard ratio value using multivariate Cox proportional hazards model in the primary study was directly considered as OR [22]. Raw event numbers were extracted from the RCTs and adjusted effect estimates from the observational studies to calculate the overall effects. Statistical heterogeneity was assessed by the χ2 test and quantified by using the I2 statistic. An I2 > 50% is indicative of at least moderate heterogeneity [23]. A random-effects model was used. Subgroup analyses regarding AF subtypes (new onset AF or recurrent AF), different TZDs (solely pioglitazone or solely rosiglitazone), study designs (RCTs or observational studies), and different follow-up duration (>5 years or ≤5 years) were additionally performed. Sensitivity analysis was done by removing one study at a time and checking the consequent effects on the effect estimate. Publication bias was evaluated using a funnel plot. Two-tailed p values of <0.05 were considered statistically significant. The statistical analysis was performed using the Review Manager (RevMan, version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
A total of 346 records were identified initially through our literature search strategy. After careful assessment, seven studies (three RCTs [12, 14, 17] and four observational studies [13, 15, 16, 18]) comprising 130,854 diabetic patients (11,781 in the treatment and 119,073 in the control group) were included in the final meta-analysis (Fig. 1).
Fig. 1.
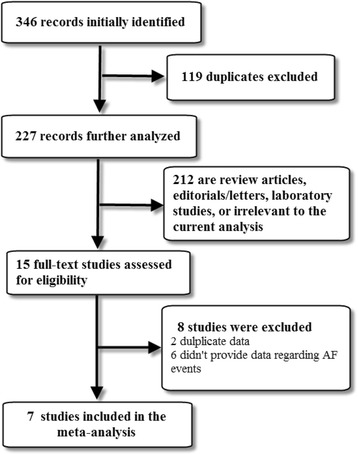
Flow diagram of the study selection process
Three studies [12, 15, 17] examined the relationship between pioglitazone use and AF, while two other [14, 16] studied rosiglitazone use. The remaining two studies [13, 18] reported data regarding the use of pioglitazone, rosiglitazone and troglitazone. The characteristics of each study are listed in Table 2, and the patients’ characteristics in each study are shown in Table 3.
Table 2.
The characteristics of 7 included studies
| Study, year | Study population | Patients (n) | Comparators | Daily dosage of TZDs | Follow-up | Definition of AF | Methods of AF detection | The variables of multivariate model |
|---|---|---|---|---|---|---|---|---|
| PROactive, 2005 [12] | Patients with type 2 diabetes who had evidence of macrovascular disease | 5238 | Pioglitazone (n = 2605) vs. placebo (n = 2633) | Titrated from 15 to 45 mg | 34.5 months | New-onset AF |
NA | NA |
| Anglade, 2007 [13] | Diabetic patients who underwent CABG and/or valvular surgery | 184 | Pioglitazone (n = 14), rosiglitazone (n = 24) and troglitazone (n = 2) vs. No TZD (n = 140) | Pioglitazone: average 30 mg Rosiglitazone: average 6 mg, Troglitazone: average 525 mg |
30 days | Postoperative AF | NA | NA |
| RECORD, 2009 [14] | Patients with type 2 diabetes | 4447 | Rosiglitazone + metformin or sulfonylurea (n = 2220) vs. metformin and sulfonylurea (n = 2227) | Titrated from 4 to 8 mg | 5.5 years | New-onset AF |
NA | NA |
| Gu, 2011 | Type 2 diabetic patients with paroxysmal AF undergoing catheter ablation | 161 | Pioglitazone (n = 51) vs. No pioglitazone (n = 99) | 30 mg | 22.9 ± 5.1 months | Recurrent ATa (AF, AT, AFL) | ECG and Holter recording | Duration of PAF, LAD, treatment with ACEI/ARB |
| Chao, 2012 [16] | Patients with non-insulin dependent diabetes. | 12,065 | Rosiglitazone (n = 4137) vs. No rosiglitazone (n = 7928) | NA | 63 ± 25 months | New-onset AF | NA | Age, HTN, CAD, chronic renal disease and use of statins or alpha-glucosidase inhibitors |
| Liu, 2014 [17] | Diabetic patients with the first presence of persistent AF | 146 | Pioglitazone (n = 70) vs. placebo (n = 76) | 30 mg | 20.1 months | Recurrent AF | ECG, history of arrhythmia-related symptoms, and Holter monitoring | NA |
| Pallisgaard, 2016 [18] | Diabetic patients of Danish nationwide registries | 108,624 | TZD (n = 2658) vs. other second-line antidiabetic drugs (n = 105,966) | NA | 12 years | New-onset AF |
NA | Age, sex, stroke, HF, all cancer, hyperthyroidism, IHD, COPD, CKD, liver disease, vascular disease, HTN, statin use, prior CABG, and prior PCI |
Abbreviations: AF atrial fibrillation, PAF paroxysmal atrial fibrillation, ATa atrial tachyarrhythmias, AT atrial tachycardia, AFL atrial flutter, ECG electrocardiograph, CABG coronary artery bypass graft, TZDs thiazolidinediones, LAD left atrial diameter, ACEI angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker, HTN hypertension, CAD coronary arterial disease, IHD ischaemic heart disease, COPD chronic obstructive pulmonary disease, CKD chronic kidney disease, PCI percutaneous coronary intervention, NA not applicable
Table 3.
Patients characteristics of 7 included studies
| Study, year | Design | Age (years) T/C |
Male T/C |
HF T/C |
HTN T/C |
CAD T/C |
HbA1c (%) T/C | β-blocker T/C |
CCB T/C |
ACEI/ARB T/C |
Statin T/C |
Insulin T/C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PROactive, 2005 [12] | RCT | 61.9/61.6 | 67%/66% | NA | 75%/76% | 48%/48% | 7.8/7.9 | 55%/54% | 34%/37% | 70%/70% | 43%/43% | 33.2%/34% |
| Anglade, 2007 [13] | Nested case control study of patients from the AFIST I, II and III trials | 65.8/67.2 | 72.5%/71.5% | 15.0%/18.8% | 90.0%/75.7% | NA | NA | 75.0%/75.0% | 12.5%/21.5% | 75.0%/56.9% | 77.5%/61.8% | NA |
| RECORD, 2009 [14] | RCT | 58.4/58.5 | 51.4%/51.7% | 0.5%/0.4% | NA | NA | 7.9/7.9 | 22.6%/20.9% | 19.1%/21.6% | 43.1%/42.1% | 18%/19.2% | NA |
| Gu, 2011 | Prospective cohort study | 59.6/58.7 | 52.9%/45.5% | 0/0 | 62.7%/72.7% | 5.9%/5.1% | 6.2/6.4 | 35.3%/37.4% | 35.3%/28.3% | 56.9%/45.5% | 13.7%/12.1% | 3.9%/2.0% |
| Chao, 2012 [16] | Nested case control study of patients from NHIRD | 53.7/54.1 | 52.9%/53.6% | 4.1%/4.7% | 38.1%/44.5% | 16.9%/18.4% | NA | 45.5%/46.4% | NA | 68.6%/68.3% | 59%/57.4% | 0/0 |
| Liu, 2014 [17] | RCT | 60.70/62.25 | 74.3%/ 65.8% | 0/0 | 28.6%/30.3% | 28.6%/30.3% | 6.41/6.19 | 41.4%/38.2% | 20%/17.1% | NA | 31.4%/34.2% | NA |
| Pallisgaard, 2016 [18] | Prospective cohort study | 59.59/62.40 | 56.7%/ 58.1% | 2.3%/4.9% | 50.2%/48.4% | NA | NA | 31.5%/31.5% | NA | 58.8%/55.9% | 58.0%/53.0% | NA |
Abbreviations: RCT randomized controlled trial, HF heart failure, HTN hypertension, CAD Coronary arterial disease, HbA1c haemoglobin A1c, CCB calcium channel blocker, ACEI angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker, T/C thiazolidinediones group/control group, NA not applicable
Of the seven studies, four [15–18] studies showed that TZDs use attenuated either the risk of new-onset or recurrent AF, whereas the other three [12–14] studies did not indicate a statistically significant difference. Overall, the pooled analysis of the seven included studies suggested that patients treated with TZDs have nearly 30% lower risk of AF compared with controls (OR =0.73, 95% CI = 0.62–0.87, p = 0.0003; Fig. 2). No significant heterogeneity between the individual studies was observed (P = 0.36, I2 = 9%).
Fig. 2.
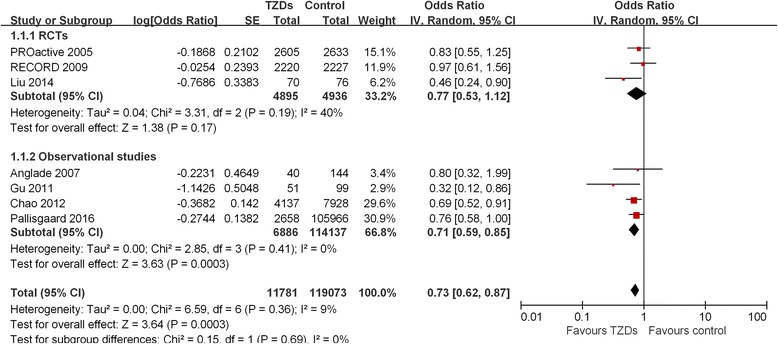
Forest plot showing the association association between thiazolidinediones (TZDs) and atrial fibrillation (AF)
Subgroup analyses according to AF types, different TZDs, follow-up duration, and study designs were subsequently performed (Fig. 2, Table 4). TZDs use was associated with a decrease in the risk of both new-onset [12, 14, 16, 18] (OR =0.77, 95% CI = 0.65–0.91, p = 0.002) and recurrent AF [13, 15, 17] (OR =0.41, 95% CI = 0.24–0.72, 0.002) without any heterogeneity across the studies. Regarding different TZDs, pioglitazone use [12, 15, 17] (OR =0.56, 95% CI = 0.32–0.98, p = 0.04; I2 = 54%) was associated with a lower risk of AF incidence, whereas rosiglitazone use [14, 16] was not significantly associated with a decreasing AF incidence (OR =0.78, 95% CI = 0.57–1.07, p = 0.12; I2 = 34%). Regarding the subgroup analysis on different follow-up duration, there was no significant difference between the 3 studies [14, 16, 18] with a follow-up duration >5 years (OR =0.76, 95% CI = 0.63–0.91, p = 0.002; I2 = 0%) and the 4 studies [12, 13, 15, 17] with a follow-up duration ≤5 years (OR =0.62, 95% CI = 0.41–0.94, p = 0.02; I2 = 34%). Finally, the pooled analysis of the 4 [13, 15, 16, 18] observational studies showed a strong association between TZDs use and risk reduction of AF (OR =0.71, 95% CI = 0.59–0.85, p = 0.0003; I2 = 0%), whereas the pooled analysis of the three RCTs showed a non-statistically significant 23% reduction in the odds of developing AF (OR =0.77, 95% CI = 0.53–1.12, p = 0.10; I2 = 40%).
Table 4.
Subgroup analyses of the association between TZDs and AF
| Subgroup | Study | Number of studies | Heterogeneity | Meta-analysis | |||
|---|---|---|---|---|---|---|---|
| I2 | P-Value | OR | 95% CI | p-Value | |||
| AF types | New-onset AF | 4 | 0% | 0.64 | 0.77 | 0.65–0.91 | 0.002 |
| Recurrent AF | 2 | 0% | 0.54 | 0.41 | 0.24–0.72 | 0.002 | |
| TZDs | Solely pioglitazone | 3 | 54% | 0.11 | 0.56 | 0.32–0.98 | 0.04 |
| Solely rosiglitazone | 2 | 34% | 0.22 | 0.78 | 0.57–1.07 | 0.12 | |
| Follow-up duration | ≤ 5 years | 4 | 34% | 0.21 | 0.62 | 0.41–0.94 | 0.02 |
| > 5 years | 3 | 0 | 0.47 | 0.76 | 0.63–0.91 | 0.002 | |
| Study design | RCTs | 3 | 40% | 0.10 | 0.77 | 0.53–1.12 | 0.17 |
| Observational studies | 4 | 0% | 0.41 | 0.71 | 0.59–0.85 | 0.0003 | |
Abbreviations: TZDs thiazolidinediones, AF atrial fibrillation, RCTs randomized controlled trials, OR odds ratio, CI confidence interval
Besides, due to different pathophysiologic mechanisms of AF, a sensitivity analysis was performed by removing the studies evaluated post-operation AF [13] and post-AF [15] ablation recurrences, no significant differences were found in the heterogeneity (P = 0.44; I2 = 0%) among the remaining five studies [12, 14, 16–18], and the overall outcome remained the same (OR =0.75, 95% CI = 0.64–0.88, p = 0.0003).
Discussion
The main findings of this comprehensive meta-analysis on 130,854 diabetic patients are the following: i. TZDs may confer protection against AF incidence; ii. the beneficial effects of TZDs were consistently observed in both new onset and recurrent AF; iii. Pioglitazone use was associated with a statistically reduced risk of incident AF, whereas rosiglitazone use showed no statistically significant difference; and iv. the protective effects of TZDs were only observed in the pooled analysis of the observational studies rather than the RCTs.
The PROactive [12] and RECORD [14] RCTs showed that pioglitazone or rosiglitazone use does not provide any benefit in preventing AF incidence among high-risk patients with type 2 DM. However, in these two RCTs, AF was reported as an adverse event rather than a predefined endpoint. Furthermore, these trials displayed a very low AF incidence in both intervention and control groups (1.5–2%), and thus AF detection may be underpowered.
Moreover, in the present meta-analysis, we observed that pioglitazone use was associated with beneficial effects on AF prevention compared with rosiglitazone use. Similarly, previous study suggested that pioglitazone has a beneficial effect on cardiovascular disease, whereas rosiglitazone seemed to increase cardiovascular risk [24]. By assembling a diabetic cohort of older than 65 years, Winkelmayer et al. [25] demonstrated greater risk of mortality and congestive heart failure among patients who initiated therapy with rosiglitazone compared with pioglitazone, however, there were no differences in their incidences of myocardial infarction or stroke. Previous data [26] also showed similar effects on glycemic control between pioglitazone and rosiglitazone, as well as on other parameters such as C-reactive protein (CRP), plasminogen activator inhibitor-1 and indices of insulin secretion and sensitivity. However, pioglitazone treatment was associated with greater beneficial changes on plasma lipids than rosiglitazone treatment [26], which may partly explain the advantage of pioglitazone in reducing AF incidence.
Recently, the IRIS trial [27] demonstrated that pioglitazone can prevent fatal or nonfatal stroke or myocardial infarction among patients who have insulin resistance along with cerebrovascular disease. However, the underlying mechanism for these beneficial effects of pioglitazone remains incompletely elucidated. AF is a known risk factor of morbidity and mortality by predisposing to strokes and acute coronary syndrome [28]. Thus, it is possible to postulate that pioglitazone reduces the stroke or MI events partly through the reduction of AF burden.
Accumulating evidence supports the role of inflammation and immune response activation in the genesis and perpetuation of AF in different clinical settings, including cardiac surgery, electrical cardioversion and catheter ablation [29]. Oxidative stress has been suggested to play an important role in AF incidence [30]. Numerous studies have demonstrated that TZDs may attenuate inflammation and oxidative stress as well as atrial electrophysiological and structural remodeling in different animal models.
In a ventricular tachypacing-induced CHF rabbit model, Shimano et al. [31] showed that pioglitazone prevents atrial structural remodeling and inhibits AF promotion. Also, similarly to candesartan, pioglitazone suppresses transforming growth factor-β1 (TGF-β1) and tumor necrosis factor-α (TNF-α) expression in atrial tissue, molecules that are inflammatory mediators related to fibrosis-mediated AF incidence [29]. More recently, Kume et al. [32] suggested that pioglitazone effectively attenuates inflammatory profibrotic signals and vulnerability to AF in a pressure overload AF rat model, possibly via its suppression in monocyte chemoattractant protein (MCP-1) expression. PPAR-γ agonists have been shown to attenuate Angiotensin II (Ang II) -induced atrial electrical and structural remodeling in cellular models [33]. These effects are mediated by prevention of ICa-L remodeling by inhibiting CAMP responsive element binding protein (CREB) phosphorylation, as well as by suppression of connective tissue growth factor (CTGF) expression and cell proliferation via inhibiting TGF-β1/Smad2/3 and TGF-β1/tumor necrosis factor receptor associated factor 6 (TRAF6)/TGF-β-associated kinase 1 (TAK1) signaling pathways. In addition, Pioglitazone exhibits beneficial effects on Ang II-induced potassium channel remodeling [34]. More recently, Chen et al. [35] further indicated that pioglitazone inhibits Ang II-induced atrial fibroblasts proliferation through nuclear factor-κB (NF-κB)/TGF-β1/Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF)/TRAF6 signaling pathway. Additionally, Xu et al. [36] suggested that pioglitazone prevents age-related arrhythmogenic atrial remodeling and AF incidence by improving heat shock protein (HSP) 70 expression and antioxidant capacity, and by inhibiting the mitochondrial apoptotic signaling pathway. In an alloxan-induced diabetic rabbit model, we have shown that rosiglitazone attenuates arrhythmogenic atrial structural remodeling and AF incidence via anti-inflammatory and antioxidant effects [37]. In keeping with these findings, the IRIS trial found lower CRP levels in the pioglitazone group than in the placebo group. Indeed, increased CRP levels have been associated with greater risk of AF [38].
Finally, the treatment of hyperglycemia may have favorable effects on AF burden. In other words, treatment of DM may ameliorate atrial remodeling [7]. Haemoglobin A1c levels have been associated with the occurrence and recurrence of AF [7, 39, 40], and therefore TZDs may exert their favorable effects through HbA1c level reduction.
Study limitations
The present meta-analysis has potential limitations. Firstly, due to the small number of included studies we analyzed observational studies and RCTs together while 2 included RCTs reported AF as an adverse event rather than a predefined endpoint, and the favorable effects of TZDs use on preventing AF incidence were predominately driven by observational studies, whereas data from the 2 RCTs were unable to draw unanimous conclusion. Secondly, information regarding methods of AF detection, cardiac substrate, ejection fraction and atrial volume were not fully presented in our analysis due to the lack of relative data. Thirdly, the heterogeneous types of patient populations (ranging from uncomplicated type 2 diabetics to post-CABG or post-AF ablation patients) may indicate latent bias in this meta-analysis. Fourthly, “gray” literature (primarily conference abstracts/presentations, ongoing studies, communication with investigators) was not searched. Finally, the results of the funnel plot suggested that publication bias may be present, although the small number of studies made this somewhat difficult to interpret (Fig. 3).
Fig. 3.
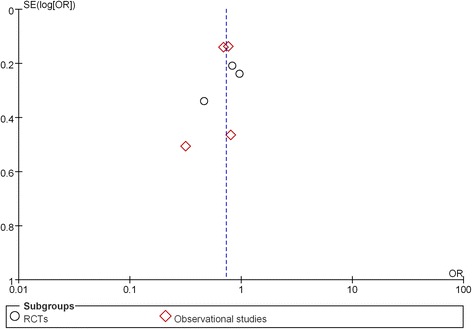
Funnel plot of meta-analysis
Conclusions
In summary, this meta-analysis suggests that TZDs may be effective in AF prevention in the setting of DM. Therefore, TZDs may be considered as the treatment of choice in diabetic patient with high risk features for AF incidence. Since the overall conclusion was mainly drawn from the observational studies, further large-scale prospective RCTs that assessed AF as a predefined outcome are needed to determine whether TZDs use could prevent AF in the setting of DM.
Acknowledgments
Not applicable.
Funding
This work was supported by grants (81,570,298, 30,900,618, 81,270,245 to T.L.) from the National Natural Science Foundation of China, Tianjin Natural Science Foundation (16JCZDJC34900 to T.L.).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Authors’ contributions
ZZ and ZX participated in study design, searched databases, extracted and assessed data, carried out the statistical analysis and drafted the manuscript. LM and MG performed statistical analyses. TL and GL conceived the design of the study, selected the included studies and drafted the review. KP, KPL and GT revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AF
Atrial fibrillation
- CHF
Congestive heart failure
- CI
Confidence interval
- CRP
C-reactive protein
- DM
Diabetes mellitus
- OR
Odds ratio
- PPAR-γ
Peroxisome proliferator-activated receptor-γ
- RCTs
Randomized clinical trials
- TZDs
Thiazolidinediones
Footnotes
Zhiwei Zhang and Xiaowei Zhang contributed equally to this work.
Contributor Information
Zhiwei Zhang, Email: zhangzhiweidoc@126.com.
Xiaowei Zhang, Email: upto2006@aliyun.com.
Panagiotis Korantzopoulos, Email: p.korantzopoulos@yahoo.gr.
Konstantinos P. Letsas, Email: letsaskpa@yahoo.co.uk
Gary Tse, Email: tseg@cuhk.edu.hk.
Mengqi Gong, Email: qituzi@vip.qq.com.
Lei Meng, Email: 1194667662@qq.com.
Guangping Li, Email: gp_limail@aliyun.com.
Tong Liu, Phone: +86-22-88328648, Email: liutongdoc@126.com.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108(1):56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tse G, Lai ET, Tse V, Yeo JM. Molecular and electrophysiological mechanisms underlying cardiac arrhythmogenesis in diabetes mellitus. J Diabetes Res. 2016; [DOI] [PMC free article] [PubMed]
- 4.Soran H, Younis N, Currie P, Silas J, Jones IR, Gill G. Influence of diabetes on the maintenance of sinus rhythm after a successful direct current cardioversion in patients with atrial fibrillation. QJM. 2008;101(3):181–187. doi: 10.1093/qjmed/hcm123. [DOI] [PubMed] [Google Scholar]
- 5.Du X, Ninomiya T, de Galan B, Abadir E, Chalmers J, Pillai A, et al. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE study. Eur Heart J. 2009;30(9):1128–1135. doi: 10.1093/eurheartj/ehp055. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Liu T, Ng CY, Li G. Diabetes mellitus and atrial remodeling: mechanisms and potential upstream therapies. Cardiovasc Ther. 2014;32(5):233–241. doi: 10.1111/1755-5922.12089. [DOI] [PubMed] [Google Scholar]
- 7.Goudis CA, Korantzopoulos P, Ntalas IV, Kallergis EM, Liu T, Ketikoglou DG. Diabetes mellitus and atrial fibrillation: Pathophysiological mechanisms and potential upstream therapies. Int J Cardiol. 2015;184:617–622. doi: 10.1016/j.ijcard.2015.03.052. [DOI] [PubMed] [Google Scholar]
- 8.Tse G, Yan BP, Chan YW, Tian XY, Huang Y. Reactive oxygen species, endoplasmic reticulum stress and mitochondrial dysfunction: the link with cardiac arrhythmogenesis. Front Physiol. 2016;7:313. doi: 10.3389/fphys.2016.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338(13):867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 10.Liu T, Li G. Thiazolidinediones as novel upstream therapy for atrial fibrillation in diabetic patients: a review of current evidence. Int J Cardiol. 2012;156(2):215–216. doi: 10.1016/j.ijcard.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Liu T, Li G. Pioglitazone may offer therapeutic advantages in diabetes-related atrial fibrillation. Int J Cardiol. 2013;168(2):1603–1605. doi: 10.1016/j.ijcard.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macroVascular events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 13.Anglade MW, Kluger J, White CM, Aberle J, Coleman CI. Thiazolidinedione use and post-operative atrial fibrillation: a US nested case-control study. Curr Med Res Opin. 2007;23(11):2849–2855. doi: 10.1185/030079907X242494. [DOI] [PubMed] [Google Scholar]
- 14.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 15.Gu J, Liu X, Wang X, Shi H, Tan H, Zhou L, et al. Beneficial effect of pioglitazone on the outcome of catheter ablation in patients with paroxysmal atrial fibrillation and type 2 diabetes mellitus. Europace. 2011;13(9):1256–1261. doi: 10.1093/europace/eur131. [DOI] [PubMed] [Google Scholar]
- 16.Chao TF, Leu HB, Huang CC, Chen JW, Chan WL, Lin SJ, et al. Thiazolidinediones can prevent new onset atrial fibrillation in patients with non-insulin dependent diabetes. Int J Cardiol. 2012;156(2):199–202. doi: 10.1016/j.ijcard.2011.08.081. [DOI] [PubMed] [Google Scholar]
- 17.Liu B, Wang J, Wang G. Beneficial effects of pioglitazone on retardation of persistent atrial fibrillation progression in diabetes mellitus patients. Int Heart J. 2014;55(6):499–505. doi: 10.1536/ihj.14-107. [DOI] [PubMed] [Google Scholar]
- 18.Pallisgaard JL, Lindhardt TB, Staerk L, Olesen JB, Torp-Pedersen C, Hansen ML, et al. Thiazolidinediones are associated with a decreased risk of atrial fibrillation compared with other antidiabetic treatment: a nationwide cohort study. Eur Heart J Cardiovasc Pharmacother; 2016. https://doi.org/10.1093/ehjcvp/pvw036. [Epub ahead of print]. [DOI] [PubMed]
- 19.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354(9193):1896–1900. doi: 10.1016/S0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Wynn GJ, Das M, Bonnett LJ, Panikker S, Wong T, Gupta D. Efficacy of catheter ablation for persistent atrial fibrillation: a systematic review and meta-analysis of evidence from randomized and nonrandomized controlled trials. Circ Arrhythm Electrophysiol. 2014;7(5):841–852. doi: 10.1161/CIRCEP.114.001759. [DOI] [PubMed] [Google Scholar]
- 22.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simo R, Rodriguez A, Caveda E. Different effects of thiazolidinediones on cardiovascular risk in patients with type 2 diabetes mellitus: pioglitazone versus rosiglitazone. Curr Drug Saf. 2010;5(3):234–244. doi: 10.2174/157488610791698352. [DOI] [PubMed] [Google Scholar]
- 25.Winkelmayer WC, Setoguchi S, Levin R, Solomon DH. Comparison of cardiovascular outcomes in elderly patients with diabetes who initiated rosiglitazone vs pioglitazone therapy. Arch Intern Med. 2008;168(21):2368–2375. doi: 10.1001/archinte.168.21.2368. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28(7):1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 27.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/paisley study. Am J Med. 2002;113(5):359–364. doi: 10.1016/S0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 29.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12(4):230–243. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 30.Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115(2):135–143. doi: 10.1016/j.ijcard.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Shimano M, Tsuji Y, Inden Y, Kitamura K, Uchikawa T, Harata S, et al. Pioglitazone, a peroxisome proliferator-activated receptor-gamma activator, attenuates atrial fibrosis and atrial fibrillation promotion in rabbits with congestive heart failure. Heart Rhythm. 2008;5(3):451–459. doi: 10.1016/j.hrthm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Kume O, Takahashi N, Wakisaka O, Nagano-Torigoe Y, Teshima Y, Nakagawa M, et al. Pioglitazone attenuates inflammatory atrial fibrosis and vulnerability to atrial fibrillation induced by pressure overload in rats. Heart Rhythm. 2011;8(2):278–285. doi: 10.1016/j.hrthm.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 33.Gu J, Liu X, Wang QX, Guo M, Liu F, Song ZP, et al. Beneficial effects of pioglitazone on atrial structural and electrical remodeling in vitro cellular models. J Mol Cell Cardiol. 2013;65:1–8. doi: 10.1016/j.yjmcc.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Gu J, Hu W, Liu X. Pioglitazone improves potassium channel remodeling induced by angiotensin II in atrial myocytes. Med Sci Monit Basic Res. 2014;20:153–160. doi: 10.12659/MSMBR.892450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen XQ, Liu X, Wang QX, Zhang MJ, Guo M, Liu F, et al. Pioglitazone inhibits angiotensin II-induced atrial fibroblasts proliferation via NF-kappaB/TGF-beta1/TRIF/TRAF6 pathway. Exp Cell Res. 2015;330(1):43–55. doi: 10.1016/j.yexcr.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Xu D, Murakoshi N, Igarashi M, Hirayama A, Ito Y, Seo Y, et al. PPAR-gamma activator pioglitazone prevents age-related atrial fibrillation susceptibility by improving antioxidant capacity and reducing apoptosis in a rat model. J Cardiovasc Electrophysiol. 2012;23(2):209–217. doi: 10.1111/j.1540-8167.2011.02186.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu T, Zhao H, Li J, Korantzopoulos P, Li G. Rosiglitazone attenuates atrial structural remodeling and atrial fibrillation promotion in alloxan-induced diabetic rabbits. Cardiovasc Ther. 2014;32(4):178–183. doi: 10.1111/1755-5922.12079. [DOI] [PubMed] [Google Scholar]
- 38.Liu T, Li G, Li L, Korantzopoulos P. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol. 2007;49(15):1642–1648. doi: 10.1016/j.jacc.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 39.Huxley RR, Alonso A, Lopez FL, Filion KB, Agarwal SK, Loehr LR, et al. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the atherosclerosis risk in communities study. Heart. 2012;98(2):133–138. doi: 10.1136/heartjnl-2011-300503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anselmino M, Matta M, D’Ascenzo F, Pappone C, Santinelli V, Bunch TJ, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus: a systematic review and meta-analysis. Europace. 2015;17(10):1518–1525. doi: 10.1093/europace/euv214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


