Abstract
Hypercholesterolemia has been implicated in numerous health problems from cardiovascular disease to neurodegeneration. High serum cholesterol levels in midlife have been associated with an increased risk of developing Alzheimer’s disease (AD) later in life which suggests that the pathways leading to AD pathology might be activated decades before the symptoms of the disease are detected. Cholesterol-fed animals, particularly cholesterol-fed rabbits, exhibit brain pathology similar to the changes found in brains of AD patients. Dietary cholesterol, which cannot pass the blood-brain barrier, is thought to influence central nervous system homeostasis by increased transport of its circulatory breakdown product, 27-hydroxycholesterol (27-OHC), into the brain. 27-OHC is an endogenous selective estrogen receptor modulator. Estrogen-mediated non-reproductive functions require estrogen receptors (ERs) and include modulation of mitochondrial function and structure, as well as regulation of synaptogenesis in the brain. ERs are located in brain areas affected early in AD pathogenesis, including the hippocampus. Here we report that increase in serum cholesterol, induced by feeding rabbits a high-cholesterol diet, is associated with higher levels of 27-OHC in the brain as well as increased levels of neurodegeneration in the hippocampus. Furthermore, these results are accompanied by changes in expression of ERs in the hippocampus as well as a decrease in hippocampal mitochondria. These findings provide an important insight into one of the possible mechanisms involved in the development of AD, and shed light on the processes that may antedate amyloid-β and tau phosphorylation changes currently hypothesized to cause AD symptomology and pathology.
Keywords: Alzheimer’s disease, cholesterol, cholesterol-fed rabbit, estrogen receptors, ERα, ERβ, 27-hydroxycholesterol, mitochondria, oxysterol, PSD-95, synapse
INTRODUCTION
As the population of Western countries ages, dementia is becoming a major health concern. Over the last few decades, numerous risk factors contributing to late onset Alzheimer’s disease (AD) development and progression have been investigated. These include diabetes mellitus, hypertension, atherosclerosis, and hypercholesterolemia [1]. High serum cholesterol in midlife is associated with an increased risk of AD [2–4]. Moreover, obese individuals with high blood pressure and high cholesterol are six times more likely to develop AD than individuals without these risk factors [5]. Most existing research investigating the role of cholesterol in increasing the risk of AD has focused on how cholesterol affects amyloid-β protein precursor (AβPP) processing and amyloid-β protein (Aβ) clearance [6–9] even though recent findings in middle-aged neurologically healthy subjects indicate that Aβ accumulation might be a reactive process with little mechanistic connection to disease development [10].
A connection between cholesterol and Aβ aggregation characteristic of AD in an animal model was first shown in cholesterol-fed rabbits [11], and numerous experiments since have found that cholesterol increases Aβ in in-vitro and in-vivo models of AD [12–14]. Treatment of rodents with dietary cholesterol resulted in memory impairment characteristic of AD [15, 16], which we also have shown to be the case in the cholesterol-fed rabbit [17–21]. The inability of cholesterol to cross the blood-brain barrier and the fact that a high-cholesterol diet does not change cholesterol content in the rabbit brain [12, 17] suggest that serum cholesterol by itself does not increase AD risk. This assumption was validated in a study that reported memory impairment in cholesterol-fed mice but not in cholesterol-fed mutant mice lacking the enzyme CYP27A1 that metabolizes cholesterol into 27-OHC [16]. This study suggested that 27-OHC mediated the negative effects of cholesterol on memory. Additionally, increased levels of 27-OHC have been found in AD brains [22]; therefore, increased flux of this cholesterol metabolite into the brain could play an important role in the cascade of events that lead to the development of late-onset AD [23, 24].
A significant insight into a possible mechanism underlying the relationship between 27-OHC and AD came about with the discovery that 27-OHC is an endogenous selective estrogen receptor modulator (SERM) [25, 26]. SERMs are able to act as ligands for different isoforms of the estrogen receptor (ER), including ERα and ERβ, in a tissue-dependent agonist or antagonist manner [27–32].
The purpose of this study was to explore potential 27-OHC-mediated changes in the hippocampus of rabbits fed a high-cholesterol diet. We chose to focus on the hippocampus because it is an area of the brain important for learning and memory and affected early and profoundly in AD pathology [33]. We examined the levels of 27-OHC in hippocampal tissue of hypercholesterolemic and control animals as well as the expression of target ERs, mitochondria, and the postsynaptic marker PSD-95. We hypothesized that higher levels of cholesterol metabolism in the periphery would result in an increased flux of 27-OHC into the brain and that the surge of this SERM into the hippocampus would affect ER signaling and its downstream targets—mitochondria and synapses.
METHODS
Animals, diet, and tissue collection
The subjects were 32 New Zealand White male rabbits (Oryctolagus cuniculus) 3–4 months of age weighing approximately 2 kg upon arrival that were part of a larger study investigating the role of cholesterol on learning and memory [20]. Animals were housed individually with free access to food and water and maintained on a 12-h light–dark cycle. All the experiments followed guidelines of National Institutes of Health and were approved by West Virginia University Animal Care and Use Committee. Rabbits were assigned to two dietary groups: control or a high-cholesterol diet. Control diet animals received Purina 5326 chow, and the high cholesterol diet group received Purina 5326 plus 2% cholesterol chow (T.R. Last Co., Gibsonia, PA). All the rabbits were kept on their respective diets for a total of 11 weeks. At the conclusion of the study, animals were deeply anesthetized with a mixture of ketamine (500 mg/kg) and xylazine (10 mg/kg) and overdosed with Somnasol before transcardial perfusion with either artificial CSF solution or 0.5% paraformaldehyde. Brains were collected and flash frozen or post-fixed in 4% paraformaldehyde solution for further processing. Before euthanasia, transcardial blood was collected in EDTA tubes and centrifuged, and the serum was frozen and stored at –80°C until analysis.
Serum cholesterol levels
Total serum cholesterol levels were assessed at the end of the experiment using a colorimetric kit (BioAssay Systems, ECCH-100) following the manufacturer’s instructions.
Neurodegeneration: Fluoro-Jade C staining
Fluoro-Jade C staining was performed based on previously published methods [34]. Brains stored in paraformaldehyde were cryoprotected in sucrose and formaldehyde solution then sectioned using a freezing microtome (Microm HM450). Coronal 50-μm sections of the dorsal hippocampus were mounted on gelatin-coated slides. Every 3rd section was collected, and an average of 15 sections from each subject were processed. Mounted sections were dried overnight and processed with a Fluoro-Jade C staining kit (Histo-Chem Inc). Slides were incubated in 70% ethanol and sodium hydroxide solution (9 : 1 ratio) for 5 min. They were then rinsed for 2 min in 70% ethanol followed by 2 min in distilled water, then incubated in 0.06% potassium permanganate solution for 10 min and again for 2 min in a distilled water rinse. Slides were then transferred to the Fluoro-Jade C solution with fluorescent DAPI counterstain and incubated for 10 min. This was followed by three 1-min distilled water rinses after which slides were placed on a slide warmer at 50°C for at least 5 min. Semi-dried slides were then cleared in xylenes for 2–3 min each and coverslipped using DPX (Sigma) as the mounting medium. Slides were imaged using a confocal microscope (LSM710, Carl Zeiss International). Digital images were collected and stained cells were counted using Image J software (NIH) by a researcher (SWB) blind to the experimental conditions.
Fluorescent antibody staining
Immunofluorescent staining was performed using manufacturer recommended protocols. Brains stored in paraformaldehyde were cryoprotected in sucrose and formaldehyde solution and then sectioned using a sliding microtome. Coronal 50-μm sections of dorsal hippocampus were mounted on gelled slides. Slides underwent an antigen retrieval protocol in 10 mM citrate buffer pH 6.0 for 40 min at 60°C. The sections were washed in phosphate buffered saline (PBS) plus Tween (pH 7.4) six times for 5 min each at room temperature. After incubation in 5% normal goat serum in PBS+tween for 2 h, sections were incubated in primary antibodies (mouse monoclonal anti-mitochondria (ab3298, Abcam) dilution 1 : 500, mouse monoclonal ERα (MA1-27107, ThermoScientific) dilution 1 : 200, rabbit polyclonal ERβ (PA5-16476, ThermoScientific) dilution 1 : 200) for 48 h at 4°C. Again, the sections were washed with PBS, 6 times for 5 min each before secondary antibody incubation (goat anti-rabbit IgG AF488 (ab150077, Abcam) dilution 1 : 1000, goat anti-mouse IgG AF488 (ab150113, Abcam) dilution 1 : 1000) for 4 h at room temperature. After a final series of rinses (PBS 6 times for 5 min each), slides were coverslipped using fluoromount G containing a DAPI counterstain. Slides were imaged on a LSM710 confocal microscope (Carl Zeiss International) and digital images processed as stated above.
27-OHC extraction from serum samples
Oxysterol extraction methods were adapted from Ahonen et al. [35]. Briefly, 1 mL of methyl t-butyl ether (MTBE) was added to a 150 μL of rabbit serum. Sample was vortexed for 1 min and centrifuged at 2000 rpm for 5 min. MTBE phase was filtered into a glass sample vial through a 0.2 μm syringe filter (Corning Incorporated) and evaporated to dryness. Samples were reconstituted in 100 μL of 5% ammonium acetate (50 mM, pH 4.5 with acetic acid): methanol:acetonitrile (1 : 3:6, v/v) and vortexed just before the analysis by the liquid chromatography-mass spectrometry (LC-MS) system.
27-OHC extraction from hippocampus
Methods of oxysterol extraction from the brain were developed based on Ahonen et al. [35]. Briefly, the intact left hippocampi were weighed and homogenized using ultrasonication, and 0.5 mL dichloromethane (DCM): methanol mixture (1 : 1, v/v) was added to tissue and sonicated on an ice bath for 1 min. Samples were centrifuged at 13200 rpm for 5 min, then supernatants removed and the procedure repeated. After the second extraction, supernatants were collected and evaporated to dryness. Immediately before analysis by LC-MS, the samples were reconstituted in 100 μL of methanol, centrifuged at 13200 rpm for 5 min and the supernatants collected into glass sample vials.
27-OHC levels: Liquid chromatography-mass spectrometry
Extracts from hippocampal tissue and rabbit’s serum (1 μL) were injected into a Dionex UltiMate 3000RS Nano LC system (ThermoScientific) using a custom made 2.5 μm XBridge BEH C8 column, 300 μm × 150 mm (Waters) with a flow rate of 5 μL/min. 27-OHC was eluted using a gradient of 20% A (water with 5 mM of ammonium formate) and 80% B (100% methanol with 5 mM ammonium formate) for 10 min. The gradient was then transitioned from 80 to 99% B for 5 min, then maintained at 99% B for 10 min, followed by a re-equilibration period when the column was returned to 80% B in 5 min and maintained to the end of the 35-min run. 27-OHC eluted at 20.56 min.
Western analysis
Protein levels were quantified using an automated Simple Western “Wes” system from ProteinSimple [36, 37]. It is a capillary electrophoresis assay that automatically loads, separates and detects proteins. Procedures were performed with the manufacturer’s reagents following the manufacturer’s protocol. Briefly, the lysate was mixed with fluorescent standard master mix and heated at 95°C for 5 min. The samples, blocking reagents, primary and secondary antibodies, and chemiluminescent substrate were dispensed into a microplate included in manufacturer’s kit. The prepared microplate and the capillary cartridge were placed into a Wes instrument (ProteinSimple), and the program was run using default settings. During the electrophoresis, proteins were separated by size and immobilized into the capillary wall, and chemiluminescent signals were read by Compass software (version 2.6.5 ProteinSimple) which analyzed the area under the curve for each antibody. The area under the curve represents the signal intensity of the chemiluminescent reaction and is proportional to the amount of target protein in a respective capillary [38]. The data were analyzed by a blinded researcher (SWB) and normalized to β-actin levels (mouse monoclonal anti-β-actin (sc-47778, Santa Cruz Biotechnology, Inc.)).
Antibodies used for western analysis include: mouse monoclonal anti-mitochondria (ab3298, Abcam) dilution 1 : 50, mouse monoclonal ERα (MA1-27107, ThermoScientific) dilution 1 : 50, rabbit polyclonal ERβ (PA5-16476, ThermoScientific) dilution 1 : 50, mouse monoclonal PSD-95 (MA1-046, ThermoScientific) dilution 1 : 50.
Data analysis
All the data analysis was performed by a researcher blinded to experimental conditions (SWB). For statistical analysis one-way ANOVA was performed with significance set at p < 0.05. Data are presented as mean ± SEM.
RESULTS
Serum cholesterol and hippocampal 27-OHC
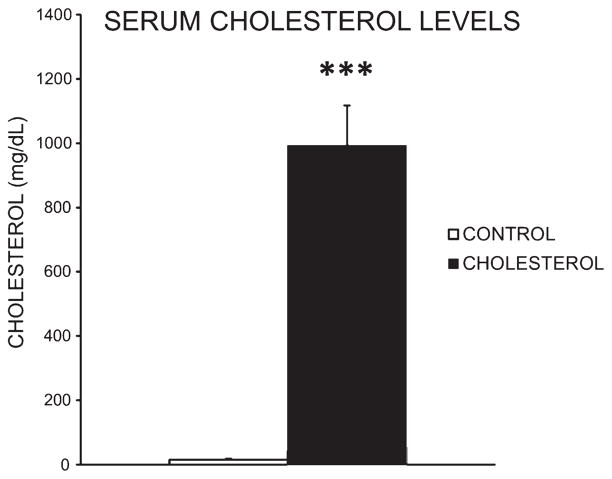
Feeding rabbits a high-cholesterol diet for 11 weeks significantly increased serum cholesterol. The mean serum cholesterol level for cholesterol-fed animals was significantly higher than for animals in the control group. The control group had a mean serum cholesterol level of 15.6 mg/dL whereas the cholesterol-fed group had a mean serum total cholesterol of 992.6 mg/dL as measured by a colorimetric kit [F(1,8) = 61.11, p < 0.001] (Fig. 1).
Fig. 1.
Serum cholesterol. Mean serum cholesterol levels for control group (n = 5) was 15.6 mg/dL while high-cholesterol animals (n = 5) average serum total cholesterol was 992.6 mg/dL as measured by a colorimetric kit (***p < 0.001).
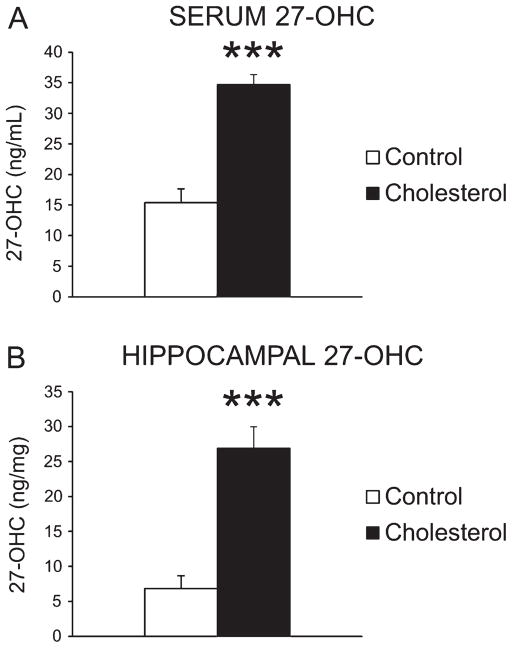
Spectrometric analysis of 27-OHC levels in serum as well as hippocampal tissue showed the level of 27-OHC in serum was significantly increased following a high-cholesterol diet, from 15.37 ng/mL in the control group to 34.71 ng/mL in the cholesterol-fed group [F(1,8) = 47.61, p < 0.001] (Fig. 2A). Similarly the hippocampal level of 27-OHC was significantly increased in the cholesterol-fed group, from 6.82 ng/mg in the control group to 26.94 ng/mg in the cholesterol-fed group [F(1,8) = 32.11, p < 0.001] (Fig. 2B).
Fig. 2.
27-OHC levels in serum and hippocampus. Serum level of 27-OHC was significantly increased following a high-cholesterol diet, from 15.37 ng/mL in control group (n = 5) to 34.71 ng/mL in cholesterol-fed animals (n = 5, ***p < 0.001) (A), and similarly the hippocampal level of 27-OHC increased from 6.82 ng/mg in control group (n = 5) to 26.94 ng/mg in cholesterol-fed group (n = 5, ***p < 0.001) (B).
Neurodegeneration
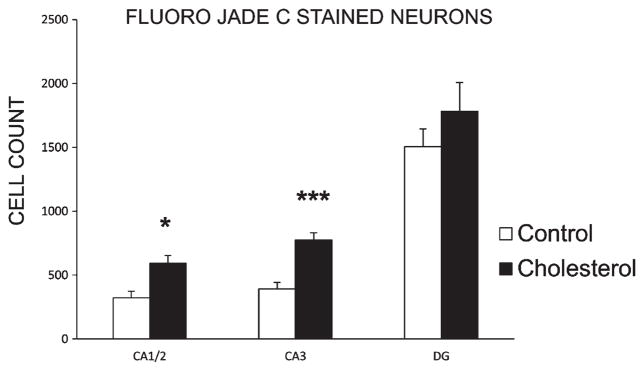
Analysis of Fluoro-Jade C staining in hippocampal tissue indicated that the high-cholesterol diet was associated with significantly increased levels of neurodegeneration in CA1/2 [F(1,8) = 9.60, p < 0.05] and CA3 of hippocampus [F(1,8) = 22.70, p < 0.001] but not in the dentate gyrus (DG) [F(1,8) = 0.85, p = 0.384] (Fig. 3).
Fig. 3.
Neurodegeneration. Fluoro Jade C staining of hippocampal sections. Data are mean (±SEM) counts of stained cells for control (n = 4) and cholesterol-fed (n = 6) groups in each respective region of dorsal hippocampus (CA1/2; *p < 0.05), CA3; ***p < 0.001) and dentate gyrus (DG).
Hippocampal estrogen receptors and their downstream targets
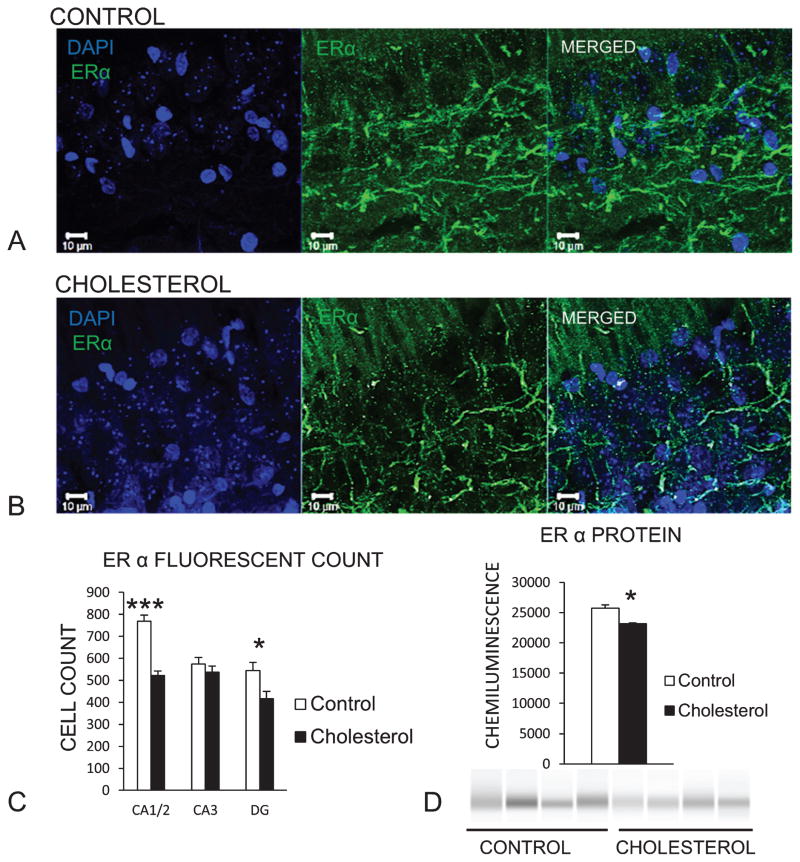
Figure 4A shows representative fluorescent confocal images of the hippocampus of a control rabbit labeled with anti-ERα antibody in green and DAPI staining nuclei blue. Figure 4B depicts the same antibody labeling in the hippocampus of a cholesterol-fed rabbit. In rabbits fed a high-cholesterol diet, immunofluorescent staining of ERα was decreased significantly in CA1/2 [F(1,14) = 55.89, p < 0.001] and DG [F(1,14) = 6.36, p < 0.05] (Fig. 4C). Western blot analysis of whole hippocampal homogenate showed a significant decrease in ERα [F(1,6) = 18.62, p < 0.05] (Fig. 4D).
Fig. 4.
High-cholesterol diet reduces ERα in the hippocampus. Representative confocal images of (A) control hippocampus section with DAPI staining nuclei blue and green fluorescence labeling ERα. B) ERα labeling decreased in rabbits fed a high-cholesterol diet. C) Mean count of stained cells for control (n = 7) and cholesterol (n = 9) groups in each respective region of dorsal hippocampus showed a decrease in the amount of staining in rabbits fed a high-cholesterol diet (CA1/2 (***p < 0.001), CA3 and dentate gyrus (DG) (*p < 0.05)). D) Western blot showed significant downregulation of ER alpha (*p < 0.05) in hippocampal tissue (control group n = 4, cholesterol n = 4).
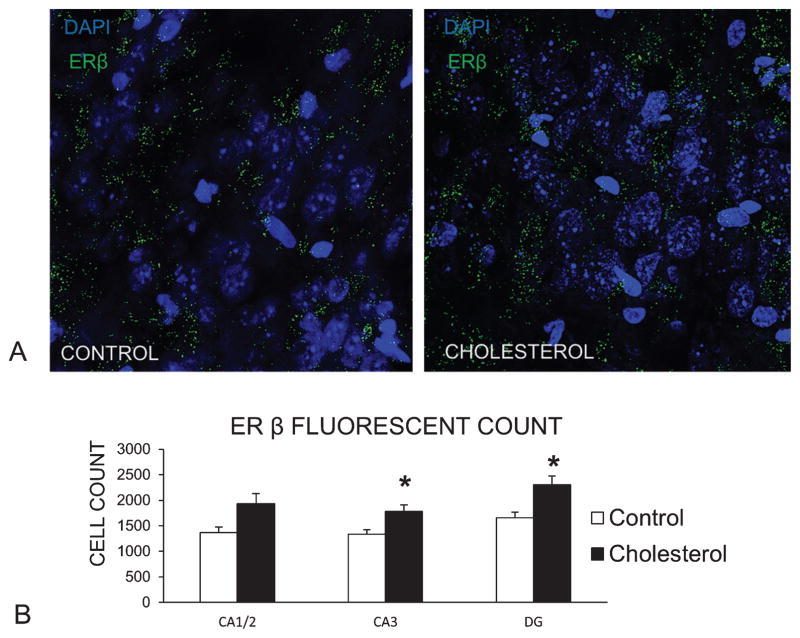
Figure 5A shows representative fluorescent confocal images of labeling of ERβ (green) and the nucleus marker DAPI (blue) in the hippocampus from a control rabbit (left) and in the hippocampus from a cholesterol-fed (right). There was an increase in ERβ immunofluorescent staining in CA3 [F(1,15) = 5.85, p < 0.05] and DG [F(1,14) = 5.68, p < 0.05] in cholesterol-fed rabbits compared to controls (Fig. 5B). In area CA1/2, there was a trend for increased levels of staining for ERβ [F(1,15) = 4.15, p = 0.06] in cholesterol-fed rabbits. Western blot analysis of whole hippocampus homogenates suggested somewhat higher levels of ERβ in hypercholesterolemic subjects although this difference was not statistically significant.
Fig. 5.
ERβ labeling in hippocampus increased following a high-cholesterol diet. A) Representative images of ERβ (green) fluorescent labeling in hippocampus. Cholesterol-fed subject (right) shows and increase in ERβ staining relative to control (left) (DAPI – blue – stains nuclei). B) Fluorescent labeling for ERβ was quantified using Image J and a one-way ANOVA showed a significant effect of the high-cholesterol diet on the number of ERβ in areas CA3 and DG of the hippocampus (p < 0.05). Data from CA1 were also consistent with these findings although not statistically significant (p = 0.06) (control group n = 6, cholesterol n = 11).
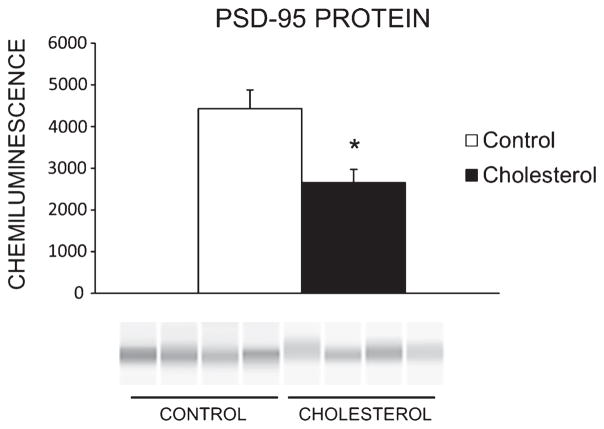
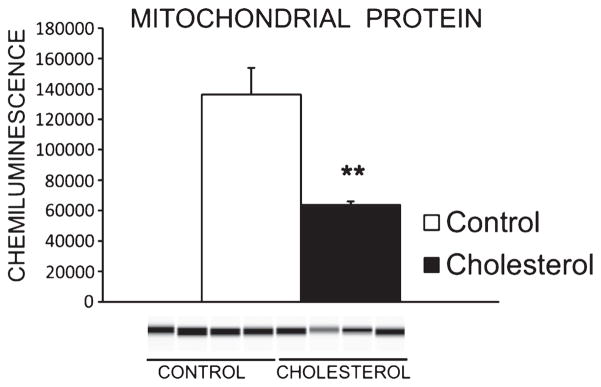
Finally, cholesterol-fed rabbits exhibited lower levels of post-synaptic marker PSD-95 in hippocampus [F(1,6) = 10.40, p < 0.05] (Fig. 6) as well as a decrease in levels of mitochondria [F(1,6) = 17.057, p < 0.01] (Fig. 7) compared to controls.
Fig. 6.
Post-synaptic protein PSD-95 in hippocampus. Western blot showed significant downregulation of post-synaptic marker PSD-95 in rabbits fed the high-cholesterol diet (*p < 0.05) (control group n = 4, cholesterol n = 4).
Fig. 7.
Mitochondrial protein content in hippocampus. Western blot showed significant downregulation of mitochondrial protein in rabbits fed the high-cholesterol diet (**p < 0.01) (control group n = 4, cholesterol n = 4).
DISCUSSION
In this study, we were able to show for the first time that high serum cholesterol in a cholesterol-fed rabbit is accompanied by increased levels of 27-OHC in the hippocampus and provide an indication of possible mechanisms responsible for AD-like pathology related to hypercholesterolemia. Based on our results, we suggest that there may be an association between the levels of 27-OHC, an endogenous estrogen modulator, and expression of ERs in the rabbit hippocampus. Moreover, we report that a high-cholesterol diet is associated with higher levels of neurodegeneration in the hippocampus as well as decreased levels of mitochondria and synaptic marker protein PSD-95, which could be a downstream result of aberrant ER signaling.
The cholesterol-fed rabbit model of AD shows a multitude of pathological findings similar to those seen in AD patients including Aβ deposits, neurofibrillary tangles, apoptosis, microglia activation, and increased ventricular volume [12, 13, 39, 40] as well as cognitive deficits [17–21]. Here we also demonstrated that a high-cholesterol diet is associated with significant levels of neurodegeneration in the hippocampus. This is an important finding because hippocampal neurodegeneration is one of the hallmarks of AD neuropathology and an aspect often lacking in transgenic models of the disease based on autosomal dominant forms of AD [41, 42]. Mutations of AβPP [43, 44], presenilin-1 [45–47], or presenilin-2 [48] all result in an abnormally high Aβ burden as well as some cognitive deficits characteristic in patients with familial AD. Transgenic rodents containing mutations in genes encoding for AβPP and enzymes involved in AβPP processing present with Aβ plaques accompanied by cognitive deficits, but most of these models fail to show a loss of synapses and neurons in the hippocampus [41, 42]. Consequently, clinical trials based on research with genetically modified animals targeting Aβ have been discouraging [49, 50]. Moreover, studies utilizing those models provided very few answers regarding the etiology of late onset (sporadic) AD. This suggests that there might be important mechanistic differences between early and late onset forms of dementia, and there is a need for non-transgenic models such as the cholesterol-fed rabbit for studying the causes of more common sporadic AD.
Many studies have investigated the correlation between oxysterol levels and neurodegenerative disease but their findings are somewhat inconsistent [3, 22, 51–55]. Some of these inconsistencies arise from the fact that subject cohorts comprised different disease stages and subtypes. The appropriate time for clinical assessment and treatment is especially important as midlife cardiovascular risk factors confer increased risk for developing AD, but once dementia begins, these risk factors diminish [56], a finding that might account for inconsistencies in studies of diet affecting AD patients. In this study we used a cholesterol-fed rabbit model of sporadic AD that removes many of these inconsistencies to investigate the role of 27-OHC at a relatively early time point during the progression of well-characterized AD-like pathology [11, 57].
An increase of 27-OHC in hippocampus in this study is especially important in light of recent findings regarding the function of this oxysterol. This biologically-active molecule has the potential to bind to estrogen receptors and either activate or inhibit ER-dependent pathways. Since its identification as the first endogenous estrogen receptor modulator [26], 27-OHC has been extensively studied in known estrogen-regulated tissues and cell types. It has been shown to act as a partial agonist of ERα in breast cancer cells [26, 29] and bone tissue [27, 58], but in the cardiovascular system, 27-OHC competitively antagonizes estrogen’s actions on ERα and ERβ [30] and promotes inflammation and atherosclerosis via ERα signaling [32]. Not much is known at present about the biological actions of 27-OHC in the brain although it has been shown that 27-OHC adversely affects cognition in rodents [15, 16]. Here we report that an increase in 27-OHC in the hippocampus is accompanied by changes in ER expression, a finding that could indicate a mechanistic link between oxysterols and neurodegeneration.
In our study we used male rabbits in order to eliminate the protective effects of estrogen seen in female rabbits with elevated cholesterol [59]. Both ERα and ERβ are localized throughout the hippocampal formation, and there are no significant sex differences in ER distribution in the hippocampus of rodents, primates, or humans [60–64] making our study relevant to both males and females. It has been shown that AD is more common in women [65], and women experience more severe behavioral and cognitive symptoms during the progression of the disease [66, 67]. Early research into possible reasons for this sex difference was based on the fact that women tend to live longer and hence have a higher susceptibility to developing the disease. However, more recent studies indicate that there must be other factors predisposing women to a higher risk for AD [68], and one of these is the loss of neuroprotection mediated by the loss of estrogen at menopause.
Downregulation of ERα in the hippocampus of hypercholesterolemic rabbits in our study could indicate that 27-OHC antagonizes the beneficial effects of estrogen signaling through ERα receptor. A decrease in hippocampal ERα has been associated with estrogen depletion in rodents while ERβ levels remained unchanged [69, 70] implicating ERα as an important element in estrogen-mediated neuroprotection. Neuroprotective roles of estrogen have been extensively studied, and there are many reports that estrogen affects cognitive function in humans and in animal models [61, 62, 71]. The importance of estrogen signaling in the hippocampus came to light with the discovery of estrogen-induced synapse formation [72]. ERα and ERβ receptors function differently in regulating synaptic connectivity in the hippocampus: activating ERα increases the density of dendritic spines in CA1 [73] while treatment with ERβ agonists has the opposite effect [74] suggesting the importance of ERα in synaptogenesis. Moreover, in an organotypic hippocampal culture, estrogen treatment prevented Aβ-induced neuronal death and that same neuroprotective effect was achieved with the use of an ERα selective agonist [75]. The same study also showed that neuronal death was correlated with a decrease in the post-synaptic marker PSD-95. Therefore, we suggest that low levels of ERα associated with the high-cholesterol diet in our study could be responsible for a reduction in the post-synaptic marker PSD-95. Additionally, upregulation of ERβ has been shown to have a negative effect on synaptic function by downregulating synaptopodin, an actin associated post-synaptic protein [76]. The increase in ERβ levels and the decreased number of mitochondria observed in our study are presumably connected because it has been shown that mitochondrial ERβ can have detrimental effects on mitochondrial function [77].
In summary we demonstrate here that diet-induced hypercholesterolemia causes an increase in 27-OHC in hippocampus. Our results suggest that 27-OHC is an active molecule that is associated with downregulation in expression of ERα and the synaptic marker PSD-95, and increased levels of ERβ possibly linked to decreased mitochondria in hippocampal cells. We suggest that 27-OHC modulates ER signaling that leads to the loss of estrogen-related neuroprotection which might explain one of the mechanisms of 27-OHC-related neurodegeneration described in both in-vivo [52] and in-vitro systems [24, 78].
Sporadic AD is a heterogeneous disorder, and there are many well-characterized risk factors influencing an individual’s chance of developing this disease as well as its progression. We conclude that there is sufficient evidence linking life style risk factors like hypercholesterolemia with a biological predisposition to develop AD. In this study we examined some of the elements that could be involved in mechanisms leading to dementia, suggesting a role for high levels of 27-OHC in the hippocampus and associated ER signaling dysfunction.
Acknowledgments
This research was supported by the National Institute on Aging (AG023211 to BGS), funds from the Blanchette Rockefeller Neurosciences Institute, and NIGMS training grant (5T32GM081241 to SWB). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIA. We thank Lauren B. Burhans, Jimena Gonzalez-Joekes, Carrie Smith-Bell, and Desheng Wang for collecting the tissue, Roger Bell for help with serum cholesterol measurement, Thomas J. Nelson for help with HPLC-MS method development, and Lauren B. Burhans for help with statistical analysis and comments on the manuscript.
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0725r1).
References
- 1.Panza F, Frisardi V, Capurso C, Imbimbo BP, Vendemiale G, Santamato A, D’Onofrio G, Seripa D, Sancarlo D, Pilotto A, Solfrizzi V. Metabolic syndrome and cognitive impairment: Current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2010;21:691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- 2.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137:149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 3.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ. 2005;330:1360–1369. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luchsinger J, Gustafson D. Adiposity and Alzheimer’s disease. Curr Opin Clin Nutr Metab Care. 2009;12:15–21. doi: 10.1097/MCO.0b013e32831c8c71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns MP, Rebeck GW. Intracellular cholesterol homeostasis and amyloid precursor protein processing. Biochim Biophys Acta. 2010;1801:853–859. doi: 10.1016/j.bbalip.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posse de Chaves E. Reciprocal regulation of cholesterol and beta amyloid at the subcellular level in Alzheimer’s disease. Can J Physiol Pharmacol. 2012;90:753–764. doi: 10.1139/y2012-076. [DOI] [PubMed] [Google Scholar]
- 8.Maulik M, Westaway D, Jhamandas JH, Kar S. Role of cholesterol in APP Metabolism and its significance in Alzheimer’s Disease Pathogenesis. Mol Neurobiol. 2013;47:37–63. doi: 10.1007/s12035-012-8337-y. [DOI] [PubMed] [Google Scholar]
- 9.Ong WY, Tanaka K, Dawe GS, Ittner LM, Farooqui AA. Slow excitotoxicity in Alzheimer’s disease. J Alzheimers Dis. 2013;35:643–668. doi: 10.3233/JAD-121990. [DOI] [PubMed] [Google Scholar]
- 10.Fornicola W, Pelcovits A, Li BX, Heath J, Perry G, Castellani RJ. Alzheimer disease pathology in middle age reveals a spatial-temporal disconnect between amyloid-beta and phosphorylated tau. Open Neurol J. 2014;8:22–26. doi: 10.2174/1874205X01408010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparks DL, Scheff SW, Hunsaker JC, III, Liu H, Landers T, Gross DR. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 12.Ghribi O, Larsen B, Schrag M, Herman MM. High cholesterol content in neurons increases BACE, beta-amyloid, and phosphorylated tau levels in rabbit hippocampus. Exp Neurol. 2006;200:460–467. doi: 10.1016/j.expneurol.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Jaya Prasanthi RP, Schommer E, Thomasson S, Thompson A, Feist G, Ghribi O. Regulation of beta-amyloid levels in the brain of cholesterol-fed rabbit, a model system for sporadic Alzheimer’s disease. Mech Ageing Dev. 2008;129:649–655. doi: 10.1016/j.mad.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemieux SK, Smith-Bell CA, Wells JR, Ezerioha NM, Carpenter JS, Sparks DL, Schreurs BG. Neurovascular changes measured by time-of-flight MR angiography in cholesterol-fed rabbits with cortical amyloid beta-peptide accumulation. J Magn Reson Imaging. 2010;32:306–314. doi: 10.1002/jmri.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang DD, Yu HL, Ma WW, Liu QR, Han J, Wang H, Xiao R. 27-hydroxycholesterol contributes to disruptive effects on learning and memory by modulating cholesterol metabolism in the rat brain. Neuroscience. 2015;300:163–173. doi: 10.1016/j.neuroscience.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Heverin M, Maioli S, Pham T, Mateos L, Camporesi E, Ali Z, Winblad B, Cedazo-Minguez A, Bjorkhem I. 27-hydroxycholesterol mediates negative effects of dietary cholesterol on cognition in mice. Behav Brain Res. 2015;278:356–359. doi: 10.1016/j.bbr.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Darwish DS, Wang D, Konat Gregory W, Schreurs BG. Dietary cholesterol impairs memory and memory increases brain cholesterol and sulfatide levels. Behav Neurosci. 2010;124:115–123. doi: 10.1037/a0018253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreurs BG. Cholesterol and copper affect learning and memory in the rabbit. Int J Alzheimers Dis. 2013;2013:518780–518792. doi: 10.1155/2013/518780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schreurs BG, Wang D, Smith-Bell CA, Burhans LB, Bell R, Gonzalez-Joekes J. Dietary cholesterol concentration and duration degrade long-term memory of classical conditioning of the rabbit’s nictitating membrane response. Int J Alzheimers Dis. 2012;2012:732634–732644. doi: 10.1155/2012/732634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreurs BG, Smith-Bell CA, Wang D, Burhans LB. Dietary cholesterol degrades rabbit long term memory for discrimination learning but facilitates acquisition of discrimination reversal. Neurobiol Learn Mem. 2013;106:238–245. doi: 10.1016/j.nlm.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparks DL, Schreurs BG. Trace amounts of copper in water induce beta-amyloid plaques and learning deficits in a rabbit model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2003;100:11065–11069. doi: 10.1073/pnas.1832769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heverin M, Bogdanovic N, Lutjohann D, Bayer T, Pikuleva I, Bretillon L, Diczfalusy U, Winblad B, Bjorkhem I. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J Lipid Res. 2004;45:186–193. doi: 10.1194/jlr.M300320-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Marwarha G, Ghribi O. Does the oxysterol 27-hydroxycholesterol underlie Alzheimer’s disease-Parkinson’s disease overlap? Exp Gerontol. 2015;68:13–18. doi: 10.1016/j.exger.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjorkhem I, Cedazo-Minguez A, Leoni V, Meaney S. Oxysterols and neurodegenerative diseases. Mol Aspects Med. 2009;30:171–179. doi: 10.1016/j.mam.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 25.DuSell CD, McDonnell DP. 27-Hydroxycholesterol: A potential endogenous regulator of estrogen receptor signaling. Trends Pharmacol Sci. 2008;29:510–514. doi: 10.1016/j.tips.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DuSell CD, Nelson ER, Wang X, Abdo J, Modder UI, Umetani M, Gesty-Palmer D, Javitt NB, Khosla S, McDonnell DP. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology. 2010;151:3675–3685. doi: 10.1210/en.2010-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA, Umetani M, Euhus DM, Xie Y, Shaul PW. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5:637–645. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 31.Umetani M, Shaul PW. 27-Hydroxycholesterol: The first identified endogenous SERM. Trends Endocrinol Metab. 2011;22:130–135. doi: 10.1016/j.tem.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umetani M, Ghosh P, Ishikawa T, Umetani J, Ahmed M, Mineo C, Shaul PW. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab. 2014;20:172–182. doi: 10.1016/j.cmet.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 34.Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 35.Ahonen L, Maire FBR, Savolainen M, Kopra J, Vreeken RJ, Hankemeier T, Myohanen T, Kylli P, Kostiainen R. Analysis of oxysterols and vitamin D metabolites in mouse brain and cell line samples by ultra-high-performance liquid chromatography-atmospheric pressure photoionization-mass spectrometry. J Chromatogr A. 2014;1364:214–222. doi: 10.1016/j.chroma.2014.08.088. [DOI] [PubMed] [Google Scholar]
- 36.O’Neill RA, Bhamidipati A, Bi X, Deb-Basu D, Cahill L, Ferrante J, Gentalen E, Glazer M, Gossett J, Hacker K, Kirby C, Knittle J, Loder R, Mastroieni C, Maclaren M, Mills T, Nguyen U, Parker N, Rice A, Roach D, Suich D, Voehringer D, Voss K, Yang J, Yang T, Vander Horn PB. Isoelectric focusing technology quantifies protein signaling in 25 cells. Proc Natl Acad Sci U S A. 2006;103:16153–16158. doi: 10.1073/pnas.0607973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beccano-Kelly DA, Kuhlmann N, Tatarnikov I, Volta M, Munsie LN, Chou P, Cao LP, Han H, Tapia L, Farrer MJ, Milnerwood AJ. Synaptic function is modulated by LRRK2 and glutamate release is increased in cortical neurons of G2019S LRRK2 knock-in mice. Front Cell Neurosci. 2014;8:301–312. doi: 10.3389/fncel.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartz AM, Zhong Y, Wolf A, LeVine H, III, Miller DS, Bauer B. Amyloid-beta 40 reduces p-glycoprotein at the blood-brain barrier through the ubiquitin-proteasome pathway. J Neurosci. 2016;36:1930–1941. doi: 10.1523/JNEUROSCI.0350-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasanthi JR, Dasari B, Marwarha G, Larson T, Chen X, Geiger JD, Ghribi O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic Biol Med. 2010;49:1212–1220. doi: 10.1016/j.freeradbiomed.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deci S, Lemieux SK, Smith-Bell CA, Sparks DL, Schreurs BG. Cholesterol increases ventricular volume in a rabbit model of Alzheimer’s disease. J Alzheimers Dis. 2012;29:283–292. doi: 10.3233/JAD-2011-111415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richner M, Bach G, West MJ. Over expression of amyloid beta-protein reduces the number of neurons in the striatum of APPswe/PS1DeltaE9. Brain Res. 2009;1266:87–92. doi: 10.1016/j.brainres.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 42.West MJ, Bach G, Soderman A, Jensen JL. Synaptic contact number and size in stratum radiatum CA1 of APP/PS1DeltaE9 transgenic mice. Neurobiol Aging. 2009;30:1756–1776. doi: 10.1016/j.neurobiolaging.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Craw-ford F, Fidani L, Giuffra L, Haynes A, Irving N, James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 44.St George-Hyslop PH, Tanzi RE, Polinsky RJ, Haines JL, Nee L, Watkins PC, Myers RH, Feldman RG, Pollen D, Drachman D. The genetic defect causing familial Alzheimer’s disease maps on chromosome 21. Science. 1987;235:885–890. doi: 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- 45.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HA, Haines JL, Perkicak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 46.St George-Hyslop P, Haines J, Rogaev E, Mortilla M, Vaula G, Pericak-Vance M, Foncin JF, Montesi M, Bruni A, Sorbi S, Rainero I, Pinessi L, Pollen D, Polinsky R, Nee L, Kennedy J, Macciardi F, Rogaeva E, Liang Y, Alexandrova N, Lukiw W, Schlumpf K, Tanzi R, Tsuda T, Farrer L, Cantu JM, Duara R, Amaducci L, Bergamini L, Gusella J, Roses A, Crapper MD. Genetic evidence for a novel familial Alzheimer’s disease locus on chromosome 14. Nat Genet. 1992;2:330–334. doi: 10.1038/ng1292-330. [DOI] [PubMed] [Google Scholar]
- 47.Van BC, Backhovens H, Cruts M, De WG, Bruyland M, Cras P, Martin JJ. Mapping of a gene predisposing to early-onset Alzheimer’s disease to chromosome 14q24.3. Nat Genet. 1992;2:335–339. doi: 10.1038/ng1292-335. [DOI] [PubMed] [Google Scholar]
- 48.Sherrington R, Froelich S, Sorbi S, Campion D, Chi H, Rogaeva EA, Levesque G, Rogaev EI, Lin C, Liang Y, Ikeda M, Mar L, Brice A, Agid Y, Percy ME, Clerget-Darpoux F, Piacentini S, Marcon G, Nacmias B, Amaducci L, Frebourg T, Lannfelt L, Rommens JM, St George-Hyslop PH. Alzheimer’s disease associated with mutations in presenilin 2 is rare and variably penetrant. Hum Mol Genet. 1996;5:985–988. doi: 10.1093/hmg/5.7.985. [DOI] [PubMed] [Google Scholar]
- 49.Benilova I, Karran E, De SB. The toxic Abeta oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 50.Castello MA, Soriano S. On the origin of Alzheimer’s disease. Trials and tribulations of the amyloid hypothesis. Ageing Res Rev. 2014;13:10–12. doi: 10.1016/j.arr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Lutjohann D, Papassotiropoulos A, Bjorkhem I, Locatelli S, Bagli M, Oehring RD, Schlegel U, Jessen F, Rao ML, von BK, Heun R. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41:195–198. [PubMed] [Google Scholar]
- 52.Mateos L, Ismail MA, Gil-Bea FJ, Leoni V, Winblad B, Bjorkhem I, Cedazo-Minguez A. Upregulation of brain renin angiotensin system by 27-hydroxycholesterol in Alzheimer’s disease. J Alzheimers Dis. 2011;24:669–679. doi: 10.3233/JAD-2011-101512. [DOI] [PubMed] [Google Scholar]
- 53.Hughes TM, Rosano C, Evans RW, Kuller LH. Brain cholesterol metabolism, oxysterols, and dementia. J Alzheimers Dis. 2013;33:891–911. doi: 10.3233/JAD-2012-121585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes TM, Kuller LH, Lopez OL, Becker JT, Evans RW, Sutton-Tyrrell K, Rosano C. Markers of cholesterol metabolism in the brain show stronger associations with cerebrovascular disease than Alzheimer’s disease. J Alzheimers Dis. 2012;30:53–61. doi: 10.3233/JAD-2012-111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuliani G, Donnorso MP, Bosi C, Passaro A, Dalla NE, Zurlo A, Bonetti F, Mozzi AF, Cortese C. Plasma 24S-hydroxycholesterol levels in elderly subjects with late onset Alzheimer’s disease or vascular dementia: A case-control study. BMC Neurol. 2011;11:121–129. doi: 10.1186/1471-2377-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beach TG, Maarouf CL, Brooks RG, Shirohi S, Daugs ID, Sue LI, Sabbagh MN, Walker DG, Lue L, Roher AE. Reduced clinical and postmortem measures of cardiac pathology in subjects with advanced Alzheimer’s Disease. BMC Geriatr. 2011;11:1–7. doi: 10.1186/1471-2318-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sparks DL, Hunsaker JC, III, Scheff SW, Kryscio RJ, Henson JL, Markesbery WR. Cortical senile plaques in coronary artery disease, aging and Alzheimer’s disease. Neurobiol Aging. 1990;11:601–607. doi: 10.1016/0197-4580(90)90024-t. [DOI] [PubMed] [Google Scholar]
- 58.Nelson ER, DuSell CD, Wang X, Howe MK, Evans G, Michalek RD, Umetani M, Rathmell JC, Khosla S, Gesty-Palmer D, McDonnell DP. The oxysterol, 27-hydroxycholesterol, links cholesterol metabolism to bone homeostasis through its actions on the estrogen and liver X receptors. Endocrinology. 2011;152:4691–4705. doi: 10.1210/en.2011-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sparks DL. The early and ongoing experience with the cholesterol-fed rabbit as a model of Alzheimer’s disease: The old, the new and the pilot. J Alzheimers Dis. 2008;15:641–656. doi: 10.3233/jad-2008-15410. [DOI] [PubMed] [Google Scholar]
- 60.McEwen BS, Gould E, Orchinik M, Weiland NG, Woolley CS. Oestrogens and the structural and functional plasticity of neurons: Implications for memory, ageing and neurodegenerative processes. Ciba Found Symp. 1995;191:52–66. doi: 10.1002/9780470514757.ch4. [DOI] [PubMed] [Google Scholar]
- 61.Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol Rev. 2015;95:785–807. doi: 10.1152/physrev.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han X, Aenlle KK, Bean LA, Rani A, Semple-Rowland SL, Kumar A, Foster TC. Role of estrogen receptor alpha and beta in preserving hippocampal function during aging. J Neurosci. 2013;33:2671–2683. doi: 10.1523/JNEUROSCI.4937-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brookmeyer R, Evans DA, Hebert L, Langa KM, Heeringa SG, Plassman BL, Kukull WA. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011;7:61–73. doi: 10.1016/j.jalz.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chapman RM, Mapstone M, Gardner MN, Sandoval TC, McCrary JW, Guillily MD, Reilly LA, DeGrush E. Women have farther to fall: Gender differences between normal elderly and Alzheimer’s disease in verbal memory engender better detection of Alzheimer’s disease in women. J Int Neuropsychol Soc. 2011;17:654–662. doi: 10.1017/S1355617711000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer’s disease: A meta analysis. J Clin Exp Neuropsychol. 2012;34:989–998. doi: 10.1080/13803395.2012.712676. [DOI] [PubMed] [Google Scholar]
- 68.Zhao L, Mao Z, Woody SK, Brinton RD. Sex differences in metabolic aging of the brain: Insights into female susceptibility to Alzheimer’s disease. Neurobiol Aging. 2016;42:69–79. doi: 10.1016/j.neurobiolaging.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qu J, Liu W, Huang C, Xu C, Du G, Gu A, Wang X. Estrogen receptors are involved in polychlorinated biphenyl-induced apoptosis on mouse spermatocyte GC-2 cell line. Toxicol In Vitro. 2014;28:373–380. doi: 10.1016/j.tiv.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 70.Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, Brann DW. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci U S A. 2011;108:E617–E624. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Srivastava DP, Woolfrey KM, Penzes P. Insights into rapid modulation of neuroplasticity by brain estrogens. Pharmacol Rev. 2013;65:1318–1350. doi: 10.1124/pr.111.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor alpha and beta selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- 74.Szymczak S, Kalita K, Jaworski J, Mioduszewska B, Savonenko A, Markowska A, Merchenthaler I, Kaczmarek L. Increased estrogen receptor beta expression correlates with decreased spine formation in the rat hippocampus. Hippocampus. 2006;16:453–463. doi: 10.1002/hipo.20172. [DOI] [PubMed] [Google Scholar]
- 75.Merlo S, Spampinato SF, Capani F, Sortino MA. Early beta-amyloid-induced synaptic dysfunction is counteracted by estrogen in organotypic hippocampal cultures. Curr Alzheimer Res. 2016;13:631–640. doi: 10.2174/1567205013666160125113509. [DOI] [PubMed] [Google Scholar]
- 76.Fester L, Labitzke J, Hinz R, Behem C, Horling K, Bernhard T, Bader MI, Vollmer G, Rune GM. Estradiol responsiveness of synaptopodin in hippocampal neurons is mediated by estrogen receptor beta. J Steroid Biochem Mol Biol. 2013;138:455–461. doi: 10.1016/j.jsbmb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Yang SH, Sarkar SN, Liu R, Perez EJ, Wang X, Wen Y, Yan LJ, Simpkins JW. Estrogen receptor beta as a mitochondrial vulnerability factor. J Biol Chem. 2009;284:9540–9548. doi: 10.1074/jbc.M808246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mateos L, Ismail MA, Gil-Bea FJ, Schule R, Schols L, Heverin M, Folkesson R, Bjorkhem I, Cedazo-Minguez A. Side chain-oxidized oxysterols regulate the brain renin-angiotensin system through a liver X receptor-dependent mechanism. J Biol Chem. 2011;286:25574–25585. doi: 10.1074/jbc.M111.236877. [DOI] [PMC free article] [PubMed] [Google Scholar]