Abstract
Sexual dichromatism is a key proxy for the intensity of sexual selection. Studies of dichromatism in birds may, however, have underestimated the intensity and complexity of sexual selection because they used museum specimens alone without taking colour-fading into account or only measured conspicuous visual traits in live animals. We investigated whether the Himalayan black bulbul (Hypsipetes leucocephalus nigerrimus), which is sexually monomorphic to the human eye, exhibits sexual dichromatism distinguishable by a spectrometer. We measured the reflectance (within both the human visual perceptive and the ultraviolet ranges) of two carotenoid-based parts and eight dull and melanin-based parts for each individual live bird or museum skin sampled. According to an avian model of colour discrimination thresholds, we found that males exhibited perceptibly redder beaks, brighter tarsi and darker plumage than did females. This suggests the existence of multiple cryptic sexually dichromatic traits within this species. Moreover, we also observed detectable colour fading in the museum skin specimens compared with the live birds, indicating that sexual dichromatism could be underestimated if analysed using skin specimens alone.
One of the most robust and widely used indices of the intensity of sexual selection in birds is sexual dichromatism, in which males are typically brighter and more colourful or have more distinguishing features than females1,2. Although intersexual differentiation in mating behaviours, habitat preferences and predator avoidance could also have promoted the evolution of colouration, sexual dichromatism is considered to be driven mainly by female preferences or male-male competition3 for sexual recognition, individual quality assessment and sexual attraction4. However, several pitfalls in studies on sexual dichromatism may have led to an overall underestimation of the intensity of sexual selection in birds. Notably, these studies have mainly focused on conspicuous colour differences5,6,7 in the 400–700 nm range, which is perceptible to the human eye8,9. However, birds have a wider visual colour perception range (300–700 nm), and can detect ultraviolet (UV: 300–400 nm) colour differences10. Producing and maintaining UV colouration can be resource-intensive for birds11,12,13; therefore such colours can be used as a signal14,15 or a target16 for mate choice. With the aid of spectrometers, several avian species that were presumed to be monochromatic have been revealed to have dichromatic UV colouration17,18. Additional studies are, however, required for further evaluation of the extent of prevalence of UV dichromatism in birds.
Furthermore, examination of melanin-based colouration, which appears dull to humans but which may still carry signals of individual quality to birds given their superior vision, is underrepresented in avian sexual dichromatism studies19. Melanin-based characteristics are associated with an individual’s qualities, namely social rank, aggressive behaviour and immunocompetence, which are equally important as targets for sexual selection as are carotenoid-based characteristics19,20,21. Although the expression of both melanin- and carotenoid-based traits can be affected by an individual’s status22,23,24, melanin deposition is more significantly controlled by genes than carotenoid deposition19,25. The sexual selection pressures on these traits might differ from those on other types of traits existing in the same organism. In recent years, increasing attention has been paid to the relative contribution of the two pigment-based colouration systems within the same species26.
The use of museum skin specimens for avian colouration studies could also lead to the underestimation of sexual dichromatism because specimens’ feather colours fade over time. This colour degradation is species-dependent and also depends on when the specimen was collected27,28,29. It has been shown that colour fading is significant for museum skin specimens collected more than 50 years previously29, but the level of degradation in the colour of newly collected museum specimens has been controversial27,28,29.
In this study, we used a spectrometer to study sexual dichromatism in a passerine, the Himalayan black bulbul (H. leucocephalus nigerrimus). This species is sexually monomorphic to the human eye: both sexes are entirely covered with a melanin-based black plumage with grey patches on their wings and have a carotenoid-based red beak and tarsus25. We investigate whether intersexual differences in characteristics are perceptible to the bulbul itself according to the Vorobyev-Osorio colour discrimination model30,31, which is based on the avian tetrahedral colour space32. Both live birds and skin specimens were measured to compare colour differences between them. Research skins from two museums were used, so different specimen preservations methods may have been used.
In this paper, we show that sexual dichromatism exists in the Himalayan black bulbul and provide insights into the potential functional roles of melanin- and carotenoid-based characteristics in this species. Meanwhile, considerable colour fading was observed in the museum skin specimens collected less than five years previously, which raises concerns regarding the use of newly collected skin specimens for studies on avian cryptic sexual dichromatism.
Results
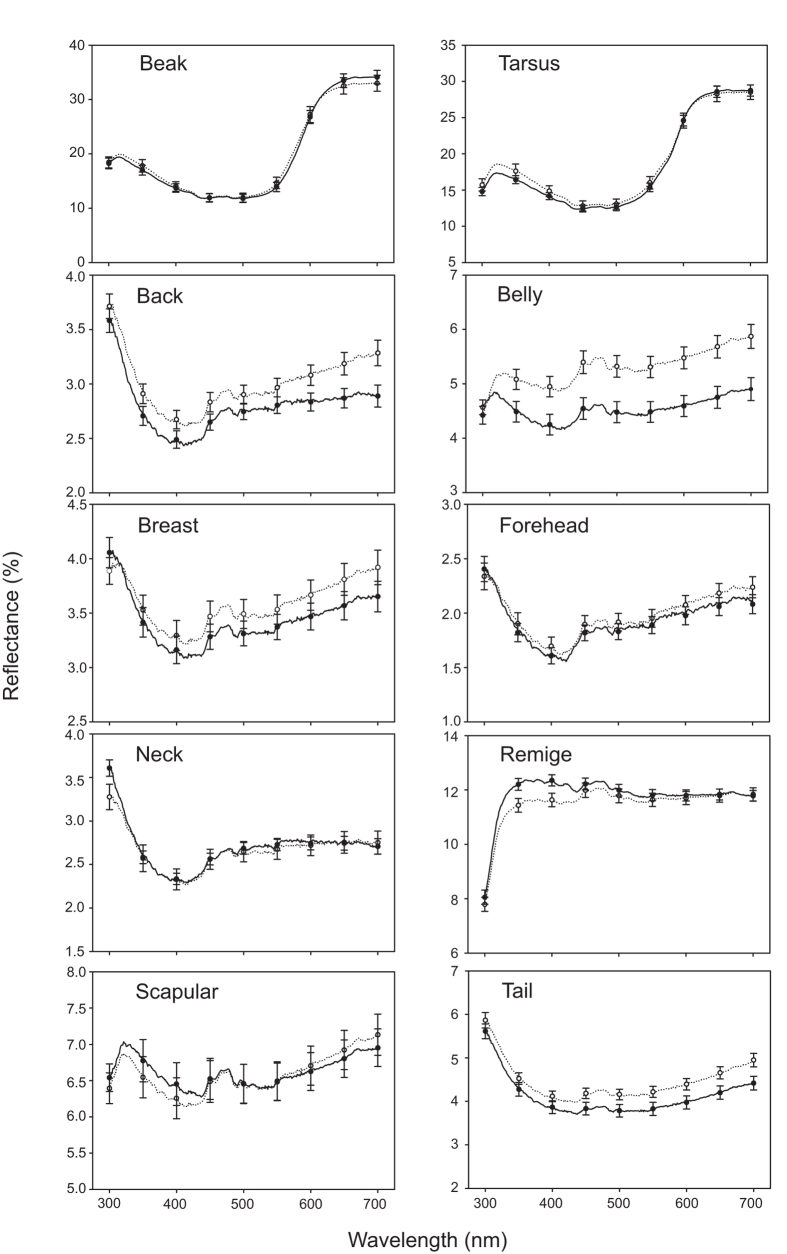
The average spectra of the two sexes were nearly identical in appearance but varied in total reflectance (Fig. 1). The carotenoid-based evaluations for beaks and tarsi showed two peaks at wavelengths of 300–400 nm and 600–700 nm, which are the reflectance ranges of UV light and carotenoid-based feathers respectively. By contrast, the spectra for the melanin-based parts were almost flat but with a moderate rise in the UV section. In the live birds, the carotenoid-based parts differed significantly between the sexes (Table 1; whole model, F = 2.82, p = 0.01), specifically in beak hue, where males exhibited redder beaks than did females (Ls mean: males, 590.2 ± 0.6 nm and females, 587.9 ± 0.7, ANOVA with the covariate of year and the interaction factors of sex and year, F = 5.95, p = 0.016, Supplementary Fig. S1). No significant differences were observed between the sexes when considering the melanin-based parts (Table 1, whole model, F = 1.47, p = 0.136). A significant effect of study year was observed in both carotenoid- and melanin-based parts (Table 1). Melanin-based plumage in the museum specimens did not differ between the sexes (Table 1).
Figure 1. Spectra of ten characteristics in two sexes in live birds.
Dotted lines indicate spectra of females (n = 57) and solid lines indicate females (n = 55).
Table 1. Sexual dichromatism showed in carotenoid-based and melanin-based parts.
| Parts tested | Item | df | F | p |
|---|---|---|---|---|
| Carotenoid-based parts | ||||
| Yeara | 14 | 27.18 | <0.0001* | |
| Sex | 7 | 2.82 | 0.010* | |
| Sex*Year | 14 | 1.29 | 0.216 | |
| Live melanin-based parts | ||||
| Year | 30 | 9.69 | <0.0001* | |
| Sex | 15 | 1.47 | 0.135 | |
| Sex*Year | 30 | 0.80 | 0.758 | |
| Skin specimen melanin-based parts | ||||
| Museumb | 15 | 18.21 | 0.182 | |
| Sex | 15 | 9.05 | 0.256 | |
| Specimen agec | 30 | 7.31 | 0.033* | |
| Season of colloectiond | 15 | 8.33 | 0.266 | |
MANOVA.
aThree different sampling years, including 2008, 2009 and 2011.
bSkin specimens were collected from two museums, including Taiwan’s National Museum of Natural Science and Endemic Species Research Institute.
cSpecimens were classified into three age categories, <5 yrs, 5–10 yrs and 10–15 yrs.
dThe season collection of specimens were classified into two categories, breeding season (from April to July) and non-breeding season (from August to March).
An analysis using the Vorobyev-Osorio colour discrimination model revealed more parts, namely carotenoid-based beak and tarsus and the melanin-based remige and tail, that were considerably dichromatic in live male and female birds (Table 2). In the museum skin specimens, in addition to the breast, the belly was also found to be sexually dichromatic. In summary, different subsets of the selected body parts were discovered to be sexually dichromatic in our live birds and in the museum skin specimens (Table 2).
Table 2. ΔS between the two sexes for live birds and skin specimens in different parts.
| Parts | Live birds | Skin specimens |
|---|---|---|
| Beak | 3.15 | — |
| Tarsus | 2.28 | — |
| Back | 0.28 | 0.88 |
| Belly | 0.64 | 1.49 |
| Nape | 0.08 | 0.61 |
| Breast | 0.09 | 1.14 |
| Forehead | 0.28 | 0.81 |
| Remige | 1.19 | 0.99 |
| Scapular | 0.66 | 1.12 |
| Tail | 3.81 | 0.52 |
ΔS > 1 is in bold and italics.
The beak and tarsus did not measure in skin specimens due to visible colour fading.
Colour comparisons between the live birds and museum skin specimens showed significant colour fading in the latter (MANOVA, df = 15; FSample type = 6.35, p < 0.0001; FSex = 2.13, p = 0.01; FSample type×Sex = 1.57, p = 0.097). Live birds had brighter breasts and scapular feathers but lower brightness in the tail (Table 3a, Supplementary Table S1); they also had higher chromaUV in every part (Table 3b, Supplementary Table S1). Different preservation methods or seasons of collection did not affect the colouration of skin specimens (MANOVA, museum: df = 15, F = 18.21, p = 0.182; season of collection: df = 15, F = 8.33, p = 0.266, Table 1). Although all skin specimens were collected less than 20 years previously, a weak effect of the age of specimens was observed (MANOVA, df = 30, F = 7.31, p = 0.033, Table 1), specifically in the scapular feather where older specimens were significantly less bright than more recent ones (specimens 15–10 years old 4.52 ± 0.99%, specimens 10–5 years old 5.13 ± 0.53%, specimens less than 5 years old 7.21 ± 0.69%; F = 3.70, p = 0.042, Supplementary Table S2).
Table 3. Post-hoc test (Student’s t) of total brightness and chromaUV between live birds and skin specimens after two-way ANOVA test (Supplementary Table S1).
| (a) Total brightness | ||||
|---|---|---|---|---|
| Parts | Item | Mean* ± SE | Lower CL Difference | Upper CL Difference |
| Breast | Live | 3.49 ± 0.08 | 0.24 | 1.00 |
| Skin | 2.87 ± 0.17 | |||
| Scapular | Live | 6.61 ± 0.19 | 0.41 | 2.11 |
| Skin | 5.36 ± 0.38 | |||
| Tail | Live | 4.23 ± 0.10 | −1.44 | −0.55 |
| Skin | 5.22 ± 0.20 | |||
|
(b) ChromaUV | ||||
| Back | Live | 24.13 ± 0.19 | 1.57 | 3.29 |
| Skin | 21.70 ± 0.39 | |||
| Belly | Live | 23.13 ± 0.18 | 0.60 | 2.26 |
| Skin | 21.70 ± 0.38 | |||
| Nape | Live | 24.03 ± 0.23 | 2.19 | 4.20 |
| Skin | 20.83 ± 0.45 | |||
| Breast | Live | 24.10 ± 0.20 | 2.09 | 3.93 |
| Skin | 21.09 ± 0.42 | |||
| Forehead | Live | 23.39 ± 0.24 | 1.71 | 3.81 |
| Skin | 20.63 ± 0.48 | |||
| Remige | Live | 22.99 ± 0.17 | 0.07 | 1.64 |
| Skin | 22.13 ± 0.36 | |||
| Scapula | Live | 23.95 ± 0.16 | 0.95 | 2.36 |
| Skin | 22.30 ± 0.32 | |||
| Tail | Live | 25.79 ± 0.16 | 0.41 | 1.87 |
| Skin | 24.65 ± 0.33 | |||
*Least Square Mean, unit = %.
The Upper CL Difference and Lower CL Difference are the 95% confidence intervals for μ1 (Meanlive) - μ2 (Meanskin).
Variability in colour brightness within females was nearly identical to that among males for all body parts (Table 4). Surprisely, the variability in the brightness of different individuals’ sexually dichromatic parts (carotenoid-based beak, tarsus and melanin-based remige and tail) was similar with those of the sexually monochromatic parts (Table 2, variances of sexually dichromatic traits 25.82 ± 2.52%, variances of non-sexually dichromatic traits 28.06 ± 2.06%, Two-way ANOVA with cofactor sex, F = 0.457, p = 0.500).
Table 4. Variabilities (coefficients of variation, %) of brightness in the each sex among different parts.
| Parts | Female | Male |
|---|---|---|
| Beak | 37.43 | 38.76 |
| Tarsus | 29.53 | 24.50 |
| Back | 22.55 | 22.20 |
| Belly | 26.85 | 31.72 |
| Nape | 33.59 | 19.76 |
| Breast | 28.13 | 27.48 |
| Forehead | 32.55 | 31.79 |
| Remige | 14.84 | 12.12 |
| Scapular feather | 30.18 | 29.95 |
| Tail | 21.47 | 27.90 |
Chi-squared test. P < 0.05 is in bold and italics.
Discussion
We have shown significant sexual dichromatism in both the carotenoid- and melanin-based body parts of the Himalayan black bulbul by considering both their reflectance and spectral shape. Males’ redder bills, brighter tarsi and darker plumage were significantly different enough from females’ for birds to distinguish between them. This provides an insight into this species’ mating behaviour. We also found colour degradation in museum samples, which could lead to different conclusions on sexual dichromatism when skin specimens or live birds are studied.
Feather colourations of the Himalayan black bulbul and most pycnonotids (members of Pycnonotidae), appear dull to humans and they are considered monomorphic33. However, our results suggest that the extent of their sexual dichromatism could be underestimated. The differences are not large but are distinguishable (Table 2; ΔS of Black bird (Turdus merula): 5.56–9.21; ΔS of Black cap (Sylvia atricapilla): 1.48–16.9; ΔS of Greenfinch (Carduelis chloris): 2.26–8.10)34, indicating the potential for moderate sexual selection. Like most pycnonotids, the Himalayan black bulbul is socially monogamous and provides bi-parental care33, [personal observation]. Dunn et al.35 analysed more than 1000 species of birds and observed lower sexual dimorphism in monogamous species than that observed in birds with polygynous or lekking mating systems, where the variance in male mating success is thought to be lower. Nevertheless, other aspects of the Himalayan black bulbul and related species’ reproductive biology may contribute to sexual dichromatism; these include the genetic mating system and the parental investment of each sex, which should be investigated further.
Where males are subject to female mate choice, their sexually selected traits are usually more variable than females3,36. The similar variability that we found in female and male black bulbuls’ sexually dichromatic traits suggests that mate choice might be mutual in this species. Whereas studies of sexual selection have mostly focused on female choice and male–male competition, data increasingly shows that males can be choosy and benefit from mating females whose reproductive potential is high37,38,39,40. Kokko and Johnstone41 suggested that high species-specific and high sex-specific mate-encounter rates, high cost of breeding (parental investment), low cost of mate searching and highly variable quality of the opposite sex could promote the evolution of choosiness and that the primary determinant of sex roles in mate choice is parental investment. According to this hypothesis, the sex with a high breeding cost (mortality during signalling and caring) should evolve to be choosy. The reproductive biology of the Himalayan black bulbuls is unclear, but research on pycnonotids suggests comparable parental care loads between the sexes, and their breeding success is generally low (8.3–15%33,42) while the rate of predation is high. As such, high breeding costs and a comparable load of parental care between the sexes might promote mutual selection in pycnonotids. This is similar to trends also found in mammals43.
In many animal species, sexual dichromatism is strong and almost complete; almost any part of the male and female can be distinguished visually (e.g., peacock, Pavo cristatus; Orchard Orioles, Icterus spurius). However, there are many other species in which sexual dichromatism is much more subtle, including the Himalayan black bulbul. Dichromatism is termed “cryptic” when the sexes appear similar to the human eye, but display subtle, but statistically significant differences that on average separate males and females e.g.6,17,18. Although sexual dichromatism can be functional14,44,45,46,47, and is often the object of female choice, one single subtle sexually dichromatic trait might not provide sufficient information about the carrier to be useful. Species may therefore evolve the use of multiple characteristics to evaluate conspecifics. Studies have shown that females may choose mates based on multiple sexual ornaments48,49; multiple ornaments provide females with diverse information at different stages of mate choice50, or function as redundant signals to improve the accuracy of mate assessment51,52. Our data shows that both carotenoid- and melanin-based characteristics are sexually dichromatic in Himalayan black bulbuls, and both could convey information about an individuals’ physical conditions53, [unpublished]. We here propose that in our species, individuals may use multiple cues in sexual selection.
According to the condition-dependent handicap model, sexually selected traits show larger variability than non-sexually selected traits3,36. However, in our study, brightness did not vary more in the sexually dichromatic parts of the Himalayan black bulbul (beak, tarsus, remige and tail) than in the sexually monochromatic parts, which was consistent with Delhey and Peters’ finding when considering six avian species34. When combined with the findings that males’ colouration was no more variable in males than females’, this suggests that differences in colour between sexes in our species might not be shaped by the forces of sexual selection alone. As well as being the object of female choice, sexually dichromatic traits have been proved to function in quality signaling45,47 and agonistic interactions in several avian species14,44,46.
Study year had a significant effect on both carotenoid- and melanin-based characteristics in live birds (see in Table 1). Sex ratios varied among years (Female/Male ratios are 0.86, 2.18 and 0.61 in 2008, 2009 and 2011 respectively), but as we treated sex as a cofactor in our analysis, it cannot explain the remaining effect. Several factors, such as varying sex ratios, different population or different environment among years, could have caused the effects. Studies have shown that the colour of melanin-based plumage can vary among geographic populations54 or according to the nutrition changes in environment55. Either of these factors, might have caused the colour variation among years in our study.
Different sets of sexually dichromatic parts were detected in the live birds and museum skin specimens, and significant degradation of colour - whether pigment-based or structural - was found in skin specimens, some of which had been preserved for less than 5 years. These results suggest that the use of skin specimens in avian colouration study may be error-prone, contradicting the previous findings indicating that melanin- and carotenoid-based skins colors remain unchanged for at least 50 years after preservation56. Conversely, our results corroborated the conclusion drawn in a study comparing live birds and skin specimens of long-tailed manakins that significant differences in colorimetric variables were attributable to the age of the specimens27. Their results are consistent with another study reporting UV colour degradation in preserved skin specimens of approximately 300 bird species throughout Europe and the USA29. Colour degradation could possibly be caused by the preservation process, preservation agents, specimen preparation, contamination or simply age27. Given that museum skin specimens are widely used in studies of avian colouration57, we suggest that skin specimen colour should be pre-tested against live birds to minimize the possible effects of colour fading; measurements obtained from skin samples should be corrected for age and/or preservation condition, and the results should be interpreted with greater caution than before.
Methods
Study Species
The Himalayan black bulbul (H. leucocephalus nigerrimus) is a subspecies endemic to Taiwan. It is widely distributed, inhabiting broadleaf, evergreen and mixed deciduous forests, groves, clearings and edges. It breeds monogamously from April to July. A total of 112 live individuals were bought from a pet-shop (San Xing Bird Shop, Taipei, Taiwan; 25.034398,121.504444) during the non-breeding seasons (mainly in November and December) in 2008, 2009 and 2011. These birds were all captured from the southern mountainous areas in Taiwan based on information provided by the pet shop owner. A blood sample was taken from each bulbul for molecular sex typing before proceeding to colour quantification. We also examined 37 research specimens from the archives of both Taiwan’s National Museum of Natural Science (five females and 11 males) and Endemic Species Research Institute (seven females and 14 males); all specimens had been collected within the previous 15 years. In both museums, all study skins are kept in the drawers in the archive room at constant temperature and humidity (20–25 °C and 40–45% in Taiwan’s National Museum of Natural Science, 20–22 °C and 50–55% in Endemic Species Research Institute).
Molecular Sex Typing
Genomic DNA was extracted from the blood samples with traditional proteinase K digestion followed by a LiCl extraction58. The detailed program set-up of the polymerase chain reactions (PCRs) for molecular sex typing59 was the same as in Hung and Li53. In total, 55 male and 57 female live bulbuls were identified.
Colour Measurement
For each individual, the reflectance of ten body parts, including two carotenoid-based parts, the beak and tarsus, and eight melanin-based parts, the forehead, nape, back, breast, belly, tail, remige and scapular feathers, were measured using a USB2000 spectrometer (Ocean Optics, Dunedin, FL, USA) with a HL2000 deuterium-halogen light source (Ocean Optics). The procedure for measurement was as described in Hung and Li53. Because of the obvious fading of carotenoid-based colouration, we did not score the colouration of beaks and tarsi in the museum specimens. We measured the colouration after verifying the absence of obvious stains or abrasions on the surface to reduce errors caused by diminished light reflectance. The data on melanin-based parts used in this study were extracted and reanalysed from Hung and Li53.
Colour Quantification
We used a combination of colorimetric variables, namely hue, total brightness and chroma60, to quantify colouration of each characteristic of each individual. Hue was calculated for beaks and tarsi by identifying the wavelength of the mean of maximum and minimum reflectance values within a range 550–700 nm. Total brightness was calculated for all selected parts by averaging the reflectance observed within a range of 300–700 nm. Two kinds of chroma were calculated: ChromaRED is the proportion of the reflectance for beak and tarsus within the range of 550–700 nm in the total brightness. ChromaUV is the proportion of the reflectance for all parts within the range of 300–400 nm in the total brightness.
Colour Discrimination
To distinguish between colorimetric variables for both sexes, we used multivariate analysis of variance (MANOVA) to compare the male and female average measurements while including the year of examination as a cofactor. MANOVA was used for the museum specimens with cofactors of the date of sample collection (in years) and the museums in which the samples were preserved. We also used the same statistical method to compare the same colorimetric variables between skin specimens and the live birds.
We calculated the variability (coefficients of variation) in brightness among individuals of each sex to examine whether the divergence of colour differences among females was different from that among males for the various body parts. We used two-way ANOVA for evaluating whether sexually dichromatic parts exhibited higher variability than others.
In addition, considering the differences in the spectral sensitivity of the four avian cone types, we mapped their spectra onto Goldsmith’s tetrahedral colour space system32 that has recently been recommended for analysing avian colouration6,61,62. We converted the spectral measurements into points within a tetrahedron, where the vertices correspond to exclusive stimulation of the ultraviolet (UV)-, blue (B)-, green (G)- and red (R)- sensitive cones in the avian eye. The quantum catch of each receptor was measured using the following equation:
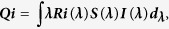 |
where λ denotes wavelength, Ri(λ) is the spectral sensitivity of the cone cell type i (i from 1–4 represent the four cone cells, UVS or VS, SWS, MWS and LWS respectively), S(λ) is the reflectance spectrum of a given feather patch, I (λ) is the irradiance spectrum entering the eye and integration is over the entire avian visual range (300–700 nm). The program Tetracolorspace61 was used for spectrum conversion, and the average spectral sensitivity curves of the UVS-type retinas63 were selected as the candidate avian vision in this study. After calculating the Qi, we calculated discriminability of colours in different body parts of each pair of average males and females in different body patches using the Vorobyev–Osorio colour discrimination model30,31. The model calculates the distance (ΔS) between two sexes, defined as the difference in the quantum catch of each receptor type (cone cell) in the avian retina6 between two sexes, in avian colour space. To calculate ΔS, we used the following formula:
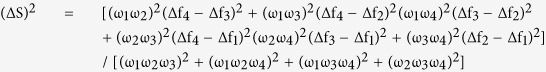 |
where ωi is the constant noise-to-signal ratio (Weber fraction) for receptor type i, which in this study is based on empirical estimates obtained from the Pekin robin (Leiothrix lutea, ω4 = 0.05, following the ratio of the numbers of cones (UV: S: M: L = 1:2:2:4). Furthermore, fi is proportional to the natural logarithm of the respective receptor quantum catches, which are normalized against an adapting background (according to the equations 2 and 3 in a study performed by Vorobyev et. al.31, and Δfi is the difference between the signals in receptor i between two stimuli (two colours). When ΔS is below a threshold value of 1, colours were assumed to be indistinguishable.
Ethics statement
Live birds were housed in the Animal Care House of the National Taiwan Normal University and cared for using procedures approved by the Institutional Animal Care and Use Committee of the Department of Life Science (IACUC Approval No 96026).
Additional Information
How to cite this article: Hung, H.-Y. et al. Himalayan black bulbuls (Hypsipetes leucocephalus niggerimus) exhibit sexual dichromatism under ultraviolet light that is invisible to the human eye. Sci. Rep. 7, 43707; doi: 10.1038/srep43707 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The project was founded by the Population Genetics Lab, Department of Life Science, National Taiwan Normal University. We thank the following for laboratory assistance: C-F Yen, R-W Chu and R-C Lin. We also thank Z-R Hung and Y-Z Yi for helping care for the birds. Specially, we thank A. Watson for editing our English. We finally thank the editorial board member and reviewers of Scientific Reports, who provided valuable comments and helped English editing to our manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions H.Y.H., C.K.L.Y. and S.H.L. conceived and designed the experiments, and H.Y.H. performed the experiments. K.E.O. provided new ideas into the paper. C.T.Y. and C.J.Y. provided skin specimens used in this experiment, and help handle the birds during and after the experiment. All the authors participated in the data analysis and paper writing. All provided the insights of the manuscript and authors read and approved the final version of the manuscript.
References
- Owens I. P. F. & Hartley I. R. Sexual dimorphism in birds: why are there so many different forms of dimorphism? P Roy Soc B 265, 397–407 (1998). [Google Scholar]
- Seddon N. et al. Sexual selection accelerates signal evolution during speciation in birds. P Roy Soc B 280, 20131065 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M. B. Sexual selection (Princeton University Press, 1994). [Google Scholar]
- Dale J. Intraspecific variation in coloration in Bird coloration, function and evolution (eds Hill G. E. & McGraw K. J.) Vol. 2, 36–86 (President and Fellows of Harvard College, 2006)
- Bortolotti G. R., Negro J. J., Tella J. L., Marchant T. A. & Bird D. M. Sexual dichromatism in birds independent of diet, parasites and androgens. P Roy Soc B 263, 1171–1176 (1996). [Google Scholar]
- Eaton M. D. Human vision fails to distinguish widespread sexual dichromatism among sexually monochromatic birds. P Natl Acad Sci USA 102, 10942–10946 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. A. Carotenoids and sexual dichromatism in North American passerine birds. Am Nat 148, 453–480 (1996). [Google Scholar]
- Cuthill I., Bennett A., Partridge J. & Maier E. Plumage reflectance and the objective assessment of avian sexual dichromatism. Am Nat 153, 183–200 (1999). [DOI] [PubMed] [Google Scholar]
- Neitz J. & Jacobs G. H. Polymorphism of the long-wavelength cone in normal human colour vision. Nature 323, 623- 625 (1986). [DOI] [PubMed] [Google Scholar]
- Chen D.-M., Collins J. S. & Goldsmith T. H. The ultraviolet receptor of bird retinas. Science 225, 337–340 (1984). [DOI] [PubMed] [Google Scholar]
- Griggio M., Hoi H. & Pilastro A. Plumage maintenance affects ultraviolet colour and female preference in the budgerigar. Behav Process 84, 739–744 (2010). [DOI] [PubMed] [Google Scholar]
- Griggio M., Serra L., Licheri D., Campomori C. & Pilastro A. Moult speed affects structural feather ornaments in the blue tit. J Evol Biol 22, 782–792 (2009). [DOI] [PubMed] [Google Scholar]
- Griggio M., Zanollo V. & Hoi H. UV plumage color is an honest signal of quality in male budgerigars. Ecol Res 25, 77–82 (2010). [Google Scholar]
- Alonso-Alvarez C., Doutrelant C. & Sorci G. Ultraviolet reflectance affects male-male interactions in the blue tit (Parus caeruleus ultramarinus). Behav Ecol 15, 805–809 (2004). [Google Scholar]
- Siefferman L. & Hill G. E. UV-blue structural coloration and competition for nestboxes in male eastern bluebirds. Anim Behav 69, 67–72 (2005). [Google Scholar]
- Bennett A. T., Cuthill I. C., Partridge J. C. & Lunau K. Ultraviolet plumage colors predict mate preferences in starlings. P Natl Acad Sci USA 94, 8618–8621 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igic B. et al. Size dimorphism and avian perceived sexual dichromatism in a New Zealand endemic bird, the whitehead Mohoua albicilla. J Morphol 271, 697–704 (2010). [DOI] [PubMed] [Google Scholar]
- Mays H. L. Jr et al. Sexual dichromatism in the yellow breasted chat Icteria virens: spectrophotometric analysis and biochemical basis. J Avian Biol 35, 125–134 (2004). [Google Scholar]
- McGraw K. J. Mechanics of melanin-based coloration in Bird Coloration: Function and evolution (eds Hill G. E. & McGray K. J.) Vol. 2 243–295 (Harvard University Press, 2006)
- Kingma S. A. et al. Sexual selection and the function of a melanin-based plumage ornament in polygamous penduline tits Remiz pendulinus. Behav Ecol Sociobiol 62, 1277–1288 (2008). [Google Scholar]
- Tarof S. A., Dunn P. O. & Whittingham L. A. Dual functions of a melanin-based ornament in the common yellowthroat. P Roy Soc B 272, 1121–1127 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. W., Hillgarth N., Leu M. & McClure H. E. High parasite load in house finches (Carpodacus mexicanus) is correlated with reduced expression of a sexually selected trait. Am Nat 149, 270–294 (1997). [Google Scholar]
- Hill G. E. Proximate basis of variation in carotenoid pigmentation in male house finches. Auk 109, U1–12 (1992). [Google Scholar]
- Nolan P. M., Hill G. E. & Stoehr A. M. Sex, size, and plumage redness predict house finch survival in an epidemic. P Roy Soc B 265, 961–965 (1998). [Google Scholar]
- McGraw K. Mechanics of carotenoid-based coloration in Bird Coloration: Function and evolution (eds Hill G. E. & McGray K. J.) Vol. 2 177–242 (Harvard University Press, 2006).
- Henschen A. E., Whittingham L. A. & Dunn P. O. Oxidative stress is related to both melanin-and carotenoid-based ornaments in the common yellowthroat. Func Ecol 30, 749–758 (2015). [Google Scholar]
- Doucet S. M. & Hill G. E. Do museum specimens accurately represent wild birds? A case study of carotenoid, melanin, and structural colours in long-tailed manakins Chiroxiphia linearis. J Avian Biol 40, 146–156 (2009). [Google Scholar]
- McNett G. D., Marchetti K. & Zink R. Ultraviolet degradation in carotenoid patches: live versus museum specimens of wood warblers (Parulidae). Auk 122, 793–802 (2005). [Google Scholar]
- Pohland G. & Mullen P. Preservation agents influence UV-coloration of plumage in museum bird skins. J Ornithol 147, 464–467 (2006). [Google Scholar]
- Vorobyev M. & Osorio D. Receptor noise as a determinant of colour thresholds. P Roy Soc B 265, 351–358 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyev M., Osorio D., Bennett A., Marshall N. & Cuthill I. Tetrachromacy, oil droplets and bird plumage colours. J Comp Phys A 183, 621–633 (1998). [DOI] [PubMed] [Google Scholar]
- Goldsmith T. H. Optimization, constraint, and history in the evolution of eyes. Q Rev Biol 65, 281–322 (1990). [DOI] [PubMed] [Google Scholar]
- Fishpool L. D. C. & Tobias J. A. Family Pycnonotidae (Bulbuls) In Handbook of the Birds of the World, Vol 10 (eds Del Hoyo J., Elliot A. & Sargatal J.) Vol. 10, 124–250 (Lynx Editions, 2005). [Google Scholar]
- Delhey K. & Peters A. Quantifying variability of avian colours: are signalling traits more variable? PLoS ONE 3, e1689 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn P. O., Garvin J. C., Whittingham L. A., Freeman-Gallant C. R. & Hasselquist D. Carotenoid and melanin-based ornaments signal similar aspects of male quality in two populations of the common yellowthroat. Funct Ecol 24, 149–158 (2010). [Google Scholar]
- Darwin C. The origin of species by means of natural selection: Or, the preservation of favoured races in the struggle for life and the descent of man and selection in relation to sex (Modern Library, 1872). [Google Scholar]
- Edward D. A. & Chapman T. The evolution and significance of male mate choice. Trends Ecol Evol 26, 647–654 (2011). [DOI] [PubMed] [Google Scholar]
- Jones K. M., Monaghan P. & Nager R. G. Male mate choice and female fecundity in zebra finches. Anim Behav 62, 1021–1026 (2001). [Google Scholar]
- Amundsen T. & Forsgren E. Male mate choice selects for female coloration in a fish. P Natl Acad Sci USA 98, 13155–13160 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggio M., Valera F., Casas A. & Pilastro A. Males prefer ornamented females: a field experiment of male choice in the rock sparrow. Anim Behav 69, 1243–1250 (2005). [Google Scholar]
- Kokko H. & Johnstone R. A. Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Philost T Roy Soc B 357, 319–330 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan P. Reproductive biology of the square-tailed Black Bulbul Hypsipetes ganeesa in the Western Ghats, India. Indian Birds 5, 134–138 (2010). [Google Scholar]
- Jones C. B. The Evolution of Mammalian Sociality by Sexual Selection In The Evolution of Mammalian Sociality in an Ecological Perspective (eds Vol. 8, 81–96 (Springer, 2014). [Google Scholar]
- Bright A. & Waas J. R. Effects of bill pigmentation and UV reflectance during territory establishment in blackbirds. Anim Behav 64, 207–213 (2002). [Google Scholar]
- Hill G. E. Redness as a measure of the production cost of ornamental coloration. Ethol Ecol Evol 8, 157–175 (1996). [Google Scholar]
- Préault M., Deregnaucourt S., Sorci G. & Faivre B. Does beak coloration of male blackbirds play a role in intra and/or intersexual selection? Behav Process 58, 91–96 (2002). [DOI] [PubMed] [Google Scholar]
- Walker L. K., Stevens M., Karadaş F., Kilner R. M. & Ewen J. G. A window on the past: male ornamental plumage reveals the quality of their early-life environment. P Roy Soc B 280, 2012–2852 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaine A. S. & Lyon B. E. Adaptive plasticity in female mate choice dampens sexual selection on male ornaments in the lark bunting. Science 319, 459–462 (2008). [DOI] [PubMed] [Google Scholar]
- Doucet S. M. & Montgomerie R. Multiple sexual ornaments in satin bowerbirds: ultraviolet plumage and bowers signal different aspects of male quality. Behav Ecol 14, 503–509 (2003). [Google Scholar]
- Borgia G. Complex male display and female choice in the spotted bowerbird: specialized functions for different bower decorations. Anim Behav 49, 1291–1301 (1995). [Google Scholar]
- Johnstone R. A. Honest signalling, perceptual error and the evolution of’all-or-nothing’displays. P Roy Soc B 256, 169–175 (1994). [Google Scholar]
- Moller A. & Pomiankowski A. Why have birds got multiple sexual ornaments? Behav Ecol Sociobio 32, 167–176 (1993). [Google Scholar]
- Hung H.-Y. & Li S.-H. Brightness of melanin-based plumage coloration is a cue to oxidative stress in Himalayan Black Bulbuls (Hypsipetes leucocephalus nigerrimus). Avian Res 6, 1 (2015). [Google Scholar]
- Argüelles-Ticó A. et al. Geographic variation in breeding system and environment predicts melanin-based plumage ornamentation of male and female Kentish plovers. Behav Ecol Sociobiol 70, 49–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe K. L. & Vitousek M. N. Melanin plumage ornaments in both sexes of Northern Flicker are associated with body condition and predict reproductive output independent of age. Auk 132, 507–517 (2015). [Google Scholar]
- Armenta J. K., Dunn P. O. & Whittingham L. A. Effects of specimen age on plumage color. Auk 125, 803–808 (2008). [Google Scholar]
- Bridge E. S., Hylton J., Eaton M. D., Gamble L. & Schoech S. J. Cryptic plumage signaling in Aphelocoma scrub-jays. J Ornithol 149, 123–130 (2008). [Google Scholar]
- Gemmell N. & Akiyama S. An efficient method for the extraction of DNA from vertebrate tissues. Trends Genet 12, 338–339 (1996). [DOI] [PubMed] [Google Scholar]
- Fridolfsson A. K. & Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30, 116–121 (1999). [Google Scholar]
- Montgomerie R. Analyzing colors in Bird coloration (eds Hill G. E. & McGraw K. J.) Vol. 1 90–147 (Harvard University Press, 2006). [Google Scholar]
- Stoddard M. C. & Prum R. O. Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of new world buntings. Am Nat 171, 755–776 (2008). [DOI] [PubMed] [Google Scholar]
- Stoddard M. C. & Prum R. O. How colorful are birds? Evolution of the avian plumage color gamut. Behav Ecol 22, 1042–1052 (2011). [Google Scholar]
- Endler J. & Mielke JR P. Comparing entire colour patterns as birds see them. Biol J Linn Soc 86, 405–431 (2005). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.