Abstract
A biofilter with fungus was developed for efficient degradation of benzene, which can overcome the potential risk of leakage commonly found in such services. Results indicated that the optimum parameter values were temperature 40 °C, pH 6, and 500 mg L−1 of the initial benzene concentration. Besides, the empty bed residence time and inlet load range of biofilter were set to 20 s and 21.23–169.84 g m−3 h−1 respectively. Under these conditions, this biofilter can obtain the maximum removal efficiency of more than 90%, the eliminating capacity could be up to 151.67 g m−3 h−1. Furthermore, scanning electron microscopy was used to investigate three filler materials for packing fungus biofilm. This is the first study introducing an Aspergillus strain for benzene removal and these results highlight that the development of this biofilter has the potential scaling-up application as gas-processing of industrial wastes.
Volatile organic compounds (VOCs) are a class of common industrial pollutants mainly consists of benzene, toluene, ethylbenzene, and xylene (BTEX), among which benzene emission playing an extremely important role, and has been a serious threat to the environment and human health1,2,3,4. Benzene could be absorbed by bodies via the skin and respiratory tract and could result in pernicious effect on human health, including genotoxicity, hematotoxicity, and leukemia5.
For the purpose of metabolizing benzene, catalytic oxidation and intermolecular electron transitions methods have been commonly applied, however, these methods are limited because of their low yields or strict requirement on lights of characteristic wavelengths6,7,8. Recently membrane separation processes have also become restricted due to the high cost9,10,11. Slominska et al. regarded plasma methods and biological methods as future trends in the contaminated air purification technology12. Recent findings demonstrate that plasma purification method always produces byproducts in process of BTEX degradation, which may cause secondary pollution13. Biofilter turns out to be one of the most cost-effective methods to treat the exhaust gas because it has no secondary pollution and especially has superior efficiency in dealing with the low concentration of exhaust gas14,15.
Suitable microorganisms and filler would not only optimize the biodegrability but also shorten the waste air treatment period. Fungal filament is able to contact with waste gas directly and to decrease mass transfer resistance16,17. Therefore, biofilters with fungus can withstand harsher conditions than those with bacteria. In spite of its potential leakage risk, security design has received little attention in bioreactor studies.
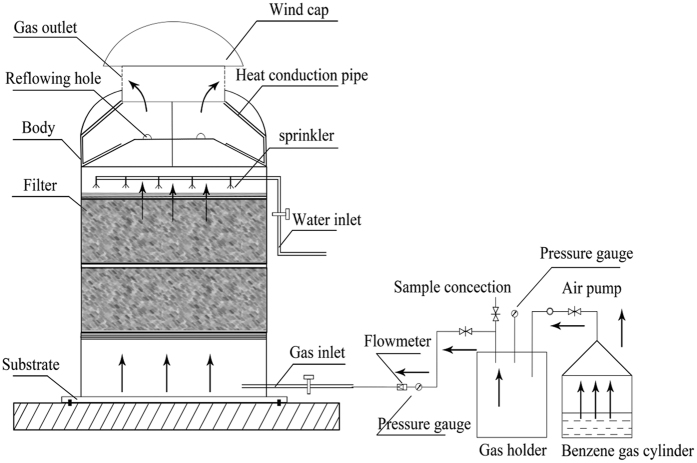
In this study the microbial strain applied to benzene degradation, namely HD-5, was identified with morphological and molecular identification. In addition, we designed and patented a biofilter (Chinese patent No.: 2005100066284) for gas treatment18. Such a biofilter generates enough vacuum through wind cap and solar energy heat-collection plates on top of the biofilter so that gas leakage would be avoided when some cracks appear in the tower body due to the inwards flow of air. The ability to resist impact load and severe environment, and the optimal process conditions of the modified biofilter (Fig. 1) applied with HD-5 strain and fillers were systematically investigated.
Figure 1. Schematic diagram of biofilter for benzene removal.
Materials and Methods
Identification of microorganism
Using benzene (Shenkai gases Technology Co., Shanghai, China) as the sole carbon source, strain HD-5 with effective benzene degrading capability was isolated from activated sludge of sewage treatment plant (Chongqing, China). Strain HD-5 was obtained using centrifugation after being incubated in PDA culture medium for 7 d. A primary morphological observation of HD-5 was performed with an AXIOVERT 35 microscope (Carl Zeiss Jena, Oberkochen, DE). Then the DNA of HD-5 was extracted using the PowerSoil® DNA extraction kit (Mobio Technologies, Carlsbad, CA). A polymerase chain reaction (PCR) reaction was generated with the internal transcribed spacer (ITS) universal primer set ITS1 (5′-TCCGATGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′)19. Reactions contained 1 μL of each primer (10 μmol L−1), 12.5 μL 2 × Taq MasterMix (CWBIO, Beijing, China). It was performed using 30 cycles with the following thermocycle profile: initial denaturation at 95 °C for 5 min followed by (95 °C for 30 s, 60 °C for 30 s and 72 °C for 1 min) followed by a final extension at 72 °C for 10 min. Total PCR reaction volume was 25 μL. PCR products were conducted by electrophoresis in a 1% agarose gel. All tested samples were amplified by PCR with ITS universal primers to determine the presence of possible PCR inhibitors in the sample.
These PCR products in agarose were inserted into a pUC19 cloning vector using a simple TA cloning kit (CWBIO, Beijing, China). Clones picked for each of the PCR products were sent to sequencing company (Biomed Co., Beijing, China). Nucleotide sequences were edited and classified, respectively, using the VecScreen of National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/blast/Blast.cgi) and the Ribosomal Database Project II (RDP; http://rdp.cme.msu.edu/classifier/classifier.jsp)20,21.
Parameters optimization of the degradation reaction
Nitrogen gas was purchased from Shenkai gases Technology Co., Ltd. (Shanghai, China). pH (4,6, and 8) and temperature (TEMP; 30, 40, and 50 °C) were used as single factors to investigate the optimum process. Degradation of benzene was studied in a 250 mL Erlenmeyer shake flask, containing 50 mL basal salt medium (Hope Biol-technology, Qingdao, China). The flask was covered with an aluminum foil seal film (QiYi packaging materials, Shanghai, China) to prevent benzene from escaping. Each experiment was replicated three times, and all of the operations were completed in a SWCJIFD super-clean worktable (Antai Airtech Co., Suzhou, China). Two fungal tablets were transferred with ø10 mm stopper borer from PDA plate culture media and inoculated into the Erlenmeyer shake flask. Then shaking culture (180 r min−1) for 8 days following the conditions provided in Supplementary Material (Table S1), and the benzene concentration was replicated three times daily.
Parameters optimization of biofilter
As shown in Fig. 1, this tower height was 1000 mm with a diameter of 300 mm. The volume of biofilter was 0.071 m3 with a cross-sectional area of 0.071 m2. The biofilter was evenly divided into two spaces, each filling layer was 400 mm in height. 500–1000 mL nutrient solution (Table 1) was sprayed each day at 30 °C. Strains HD-5 was injected into biofilter according to the parameters listed in Table 2 with an empty bed residence time (EBRT) of 20 s. The biofilter experienced three periods: low load, intermediate load, and high load operation. The removal efficiency of benzene (RE) was calculated based on the following equation:
Table 1. Media formulations used in this study.
| Nutrient | K2HPO4 | KH2PO4 | 1NaNO3 | MgSO4 · 7H2O | FeSO4 · 7H2O |
|---|---|---|---|---|---|
| Concentration (mg L−1) | 627 | 191 | 500 | 48 | 7.6 |
Table 2. The options of running parameters of biofilter.
| Run time (d) | Inlet concentration of benzene (mg m−3) | Benzene load of biofilter (gm−3 h−1) | Gas flow rate (m3 h−1) |
|---|---|---|---|
| 1–30 | 1000 | 42.46 | 2.4 |
| 31–60 | 2000 | 84.92 | 2.4 |
| 61–90 | 3000 | 127.42 | 2.4 |
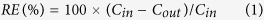 |
Where Cin and Cout are the benzene concentrations for inlet and outlet respectively.
The following steps were taken to limit the inevitable contamination of the biofilter with other organisms introduced through the waste being treated: (i) HD-5 was inoculated to fillers after the sterilization process of fillers and biofilter. (ii) To ensure that HD-5 has a growth advantage, it will be cultured in sealed overnight.
The concentrations of benzene were measured using a HP6890 GC (Agilent Technologies, Palo Alto, US) with a flame ionization detector and a DB-1 chromatographic column (Model 125-103 J; Agilent Technologies, Palo Alto, US) under the options provided in Supplementary Material (Table S2)22.
During biofilm formation (30 d), their surface was observed through a VEGA3SBH scanning electron microscopy (SEM) (Tescan Co., Brno, Czech) at operating voltage of 20 kV. Fillers were picked out after the conclusion of the biofilter run, and then fixed with 2.5% glutaraldehyde. Fillers were then dehydrated with a graded ethanol series of 50%, 75%, 95%, and 100%. The coupons were sputter coated with gold for SEM imaging.
At the end of the treatment, the optimum filler was selected, and the low load period of above-mentioned test was repeated with different EBRTs (10 s,20 s, and 30 s) and inlet loads (21.23, 42.46, 84.92, 169.84, 339.68 and 679.36 g m−3 h−1). Four criteria for fillers are needed in order to provide good growing conditions for microbes and large-scale application23: (1) great surface area; (2) surface hydrophilicity; (3) stable chemical properties; (4) low cost. Thus, coal columnar activated carbon (CCAC), bamboo wood (BW), and biological ceramsite (BC) have been widely applied as fillers of biofilter24.
Quantitative variance of HD-5
Approximately 1 gram each of filler material was milled then put into 9 mL of sterile extraction buffer (0.1% Na4P2O7·10H2O and 2% NaCl) and vortexed at maximum speed for 10 minutes. The suspension isolated from solid composition by sedimentation, serially dilution with 0.9% NaCl solution, incubation in a PDA plate for 3 days at 30 ± 2 °C25,26. The procedure of coating plates was replicated two times.
Results and Discussion
Morphological observation and molecular identification
After incubation at 30 °C for 7 days the HD-5 strain colony showed white color as a thin membrane, which had the round morphology with a diameter of 18 mm at first 5 days (Fig. S1), and later on expanded into layer-by-layer rosette patterns and finally formed greenish cottony hyphae. Microscopic image presented the conidial head and conidiophores in scale of hundreds of micrometers, while the diameters of hemispheric acrocyst are 10–20 μm. All these indicate that strain HD-5 is a member of Moniliales27.
A sequence of ITS region with 567 bp was obtained from sequencing data, the warcup fungal ITS training sets were used to classify fungal ITS sequences which can offer accurate results of classification28. The sequence identified with the classifier programme of RDP indicated that the strain HD-5 is Aspergillus versicolor. The results obtained by both methods reached a similar conclusion.
Effects of the pH and TEMP on the benzene removal
Figure 2 exhibits that the removal efficiency reaches maximum value of 98 ± 1.4% when the pH is 6 and the TEMP is 40 °C. it may be due to the enzyme producing ability of Aspergillus versicolor achieving the top level under these conditions29. As the key enzymes in the biodegradation of benzene series, monooxygenase and dioxygenase appeared in many metabolic pathways of biodegradation30,31,32,33. And the removal efficiency became relatively low departing from the optimum reaction TEMP which accorded with the enzymatic properties. pH plays an important role in the secretion of active metabolite. Under abiotic factors duress, the expression of enzyme genes related to benzene metabolism could change accordingly to permit survival of the affected34,35,36. RE can exceed 80% in most of the experimental range which may benefit from the resilience of HD-5 to severe environment. Previous studies found that fungus as the biofilm for biofilter has an advantage over bacteria in removing organics37,38. This advantage cannot be ignored in the practical industry with a great pH fluctuation of industrial wastes39,40.
Figure 2. The comparison of benzene removal efficiency with different parameters in 8 d.
The selection of fillers
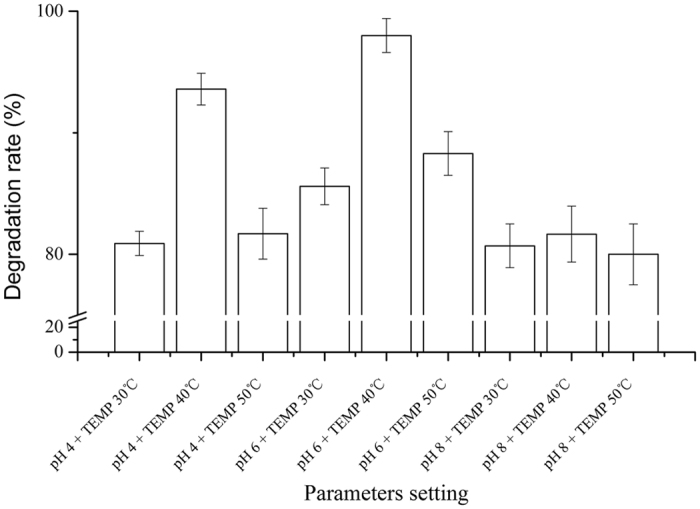
It is evident from Fig. 3 that average RE (REave) of CCAC (93.95%) and BC (92.63%) were comparable and significantly superior to BW (76.24%) in the low load. For intermediate and high load operation, REave of CCAC was still the best which can reach 90.5% and 89.3%. Acclimated with metals of low concentration benzene in the initial 30 days, microbes can form biofilms on the surfaces of fillers (Fig. 4). Figure 4 (a,b) illustrates the successful membrane-hanging with good results of acclimation on the CCAC and BC while the surface of BC had less fungal hyphae. It could be due to that CCAC and BC have the larger specific surface areas41 which have contributed to the increase of conversion efficiency from gas to liquid or biological phase42.
Figure 3.
RE of biofilters with different fillers in three periods: low load (a), intermediate load (b) and high load operation (c).
Figure 4.
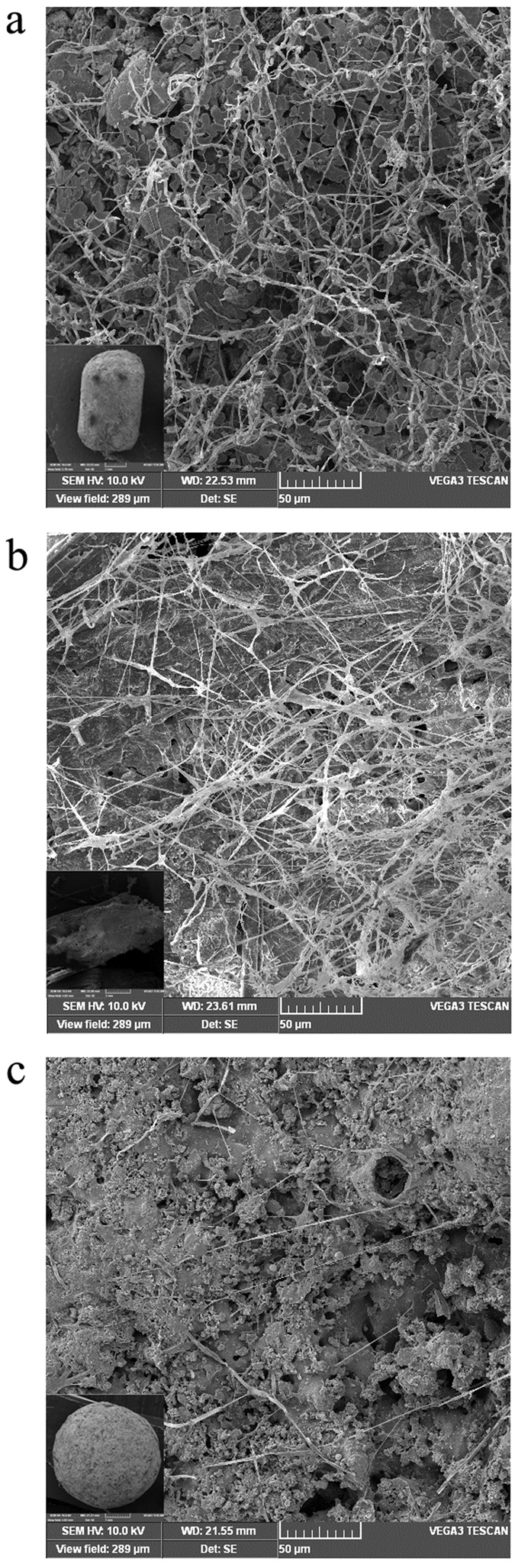
SEM images of biofilm on packing filler: (a) CCAC, (b) BC, (c) BW.
Effect of inlet load, EBRT and biomass on the benzene removal
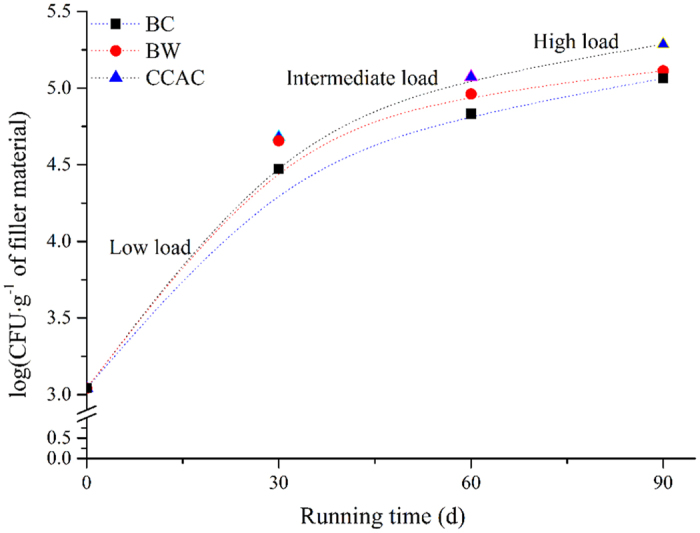
According to the above analysis, biofilter with CCAC was selected as the focus of further investigation. Under the same EBRT (20 s), the effect of biological benzene removal depends on the quantity and activity of HD-5 in CCAC and biofilm. Figure 5 indicates all RE of different EBRT reach the summit when the inlet load was 42.46 g m−3 h−1. It might be attributed to the relatively few of HD-5 when the inlet load was 21.23 g m−3 h−1, and the quantity of HD-5 was increased with the inlet load (Fig. 6). Meanwhile the obstacle of mass transfer within the biofilm was triggered by the low diffusive fluxes of benzene, and the biofilm was not fully utilized43.
Figure 5. Effect of inlet load and EBRT on benzene removal.
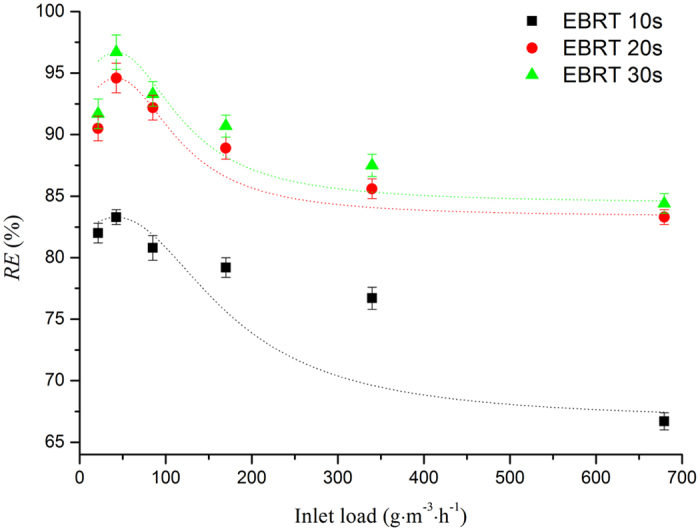
Figure 6. Quantitative variance of HD-5 in the filler materials at the three load periods vs. time.
However, RE decreased when the inlet load increased to 169.84 g m−3 h−1, mainly because the HD-5 growth did not keep pace with benzene load (Fig. 6). Besides, matrix suppression effect could also be a major cause44,45.
Figure 5 Also shows the upside for RE is quite limited with EBRT of 20 s which is related directly with operating efficiency and cost. Combined with actual production, the results showed that the ideal EBRT was less than 20 s and that the ideal inlet load is not higher than 169.84 g m−3 h−1. At last, the results revealed that the performance parameters of biofilter with CCAC and HD-5 have been tremendously strengthened comparing to the biofilters as reported in Table 3.
Table 3. Performance of biofilters for the removal of benzene as a sole-substrate, as reported in selected previous biofiltration studies, and compared to this study.
| Bioreactor/filter media | Inlet concentration (g m−3) | Loading rate (g m−3 h−1) | Removal efficiency (%) | Elimination Capacity (g m−3 h−1) | Reference |
|---|---|---|---|---|---|
| Biofilter/sugarcane bagasse–glass bead mixture | 0.01–0.05 | 6.12 | 43 | 3.8 | 46 |
| Biofilter/sugarcane bagasse | 0.4 | 12 | 53 | 6.4 | 47 |
| Biofilter/peat | 0.4 | 31 | 84 | 26 | 47 |
| Biofilter/granular activated carbon mixture | 0.7 | 21 | 96 | 20.1 | 48 |
| Trickling biofilter/fibrous bed | 0.37–1.7 | 2.6–25.6 | >90 | 11.5 | 49 |
| Trickling biofilter/coal particles | 0.47 | 64 | 90 | 57.6 | 50 |
| Biofilter/CCAC with HD-5 | 2 | 169.84 | >90 | 151.67 | This study |
The present biofilter has simple structure and no need of excessive power source in preventing and eliminating gas leakage effectively. Under windy conditions, the wind cap would be triggered then change the movement of air from original horizontal to vertical direction, thus resulting in a negative pressure. When windless, the heat energy absorbed by solar energy heat-collection plates make a hot-cell occurs between partition and wind cap. The air in the hot-cell rise up so continuously that an airflow from deep to upside happens, thereby the gas leakage could be avoided. In addition, despite efforts to control microbial contamination, there is no guarantee that all contaminations will be complete avoided. Therefore, the future study will include developing qPCR assay or high-throughput sequencing for investigation of the microbial communities’ variation at different operating phases of the biofilter.
Conclusion
Compared to the biofilters with bacteria, the biofilter applied with HD-5 strain exhibited advantages of high removal efficiency (more than 90%), eliminating capacity (151.67 g m−3 h−1), and excellent ability to resist impact load and environment. In addition, the results of SEM were consistent with biomass showing that CCAC was a satisfactory filler material for hanging-membrane of fungus. Based on these properties, this biofilter has a spacious application foreground of the large-scale industrial production.
Additional Information
How to cite this article: Sun, D. et al. Potential application of an Aspergillus strain in a pilot biofilter for benzene biodegradation. Sci. Rep. 7, 46059; doi: 10.1038/srep46059 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was funded by grants from the National Natural Science Foundation of China (11332003, 31370949, 81400329), the National Key Research and Development Program (2016YFC1102305), the Fundamental Research Funds for the Central Universities (106112015CDJZR235504), the Science and Technology Commission of Yuzhong District in Chongqing (20150135), the Chongqing Engineering Laboratory in Vascular Implants, the National “111 Plan” Base (B06023) and the Public Experiment Center of State Bioindustrial Base (Chongqing) of China. We acknowledge Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education, China (CQKLBST-2014-008). Support from the Sino-Danish Centre for Education and Research (SDC) is also fully acknowledged.
Footnotes
The authors declare no competing financial interests.
Author Contributions D.S., K.Z. and C.D. designed the experiments and conducted the data analysis; D.S., K.Z., C.D., J.T., J.C. and J.W. carried out the experiments; D.S., C.D., G.W. and W.W. contributed to the planning and coordination of the project; D.S., K.Z., M.S., W.W., D.D., L.L., Y.C., X.X. and D.Y. wrote and edited the manuscript. All authors participated in discussion about the results and the manuscript.
References
- Miller L., Xu X., Grgicak-Mannion A., Brook J. & Wheeler A. Multi-season, multi-year concentrations and correlations amongst the BTEX group of VOCs in an urbanized industrial city. Atmos. Environ. 61, 305–315 (2012). [Google Scholar]
- An T. et al. Pollution profiles and health risk assessment of VOCs emitted during e-waste dismantling processes associated with different dismantling methods. Environ. Int. 73, 186–194 (2014). [DOI] [PubMed] [Google Scholar]
- Dinh T.-V. et al. Emission characteristics of VOCs emitted from consumer and commercial products and their ozone formation potential. Environ. Sci. Pollut. Res. 22, 9345–9355 (2015). [DOI] [PubMed] [Google Scholar]
- Mitri S. et al. Metabolic polymorphisms and clinical findings related to benzene poisoning detected in exposed Brazilian gas-station workers. Int. J. Environ. Res. Public. Health 12, 8434–47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg E. F. et al. Detection of DNA damage in workers exposed to JP-8 jet fuel. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 747, 218–227 (2012). [DOI] [PubMed] [Google Scholar]
- Hinojosa-Reyes M., Rodriguez-Gonzalez V. & Arriaga S. Enhancing ethylbenzene vapors degradation in a hybrid system based on photocatalytic oxidation UV/TiO2-In and a biofiltration process. J. Hazard. Mater. 209, 365–371 (2012). [DOI] [PubMed] [Google Scholar]
- Joung H.-J. et al. Catalytic oxidation of VOCs over CNT-supported platinum nanoparticles. Appl. Surf. Sci. 290, 267–273 (2014). [Google Scholar]
- Pham T. & Lee B. Novel adsorption and photocatalytic oxidation for removal of gaseous toluene by V-doped TiO/PU under visible light. J. Hazard. Mater. 30, 493–503 (2015). [DOI] [PubMed] [Google Scholar]
- Trusek-Holownia A. & Noworyta A. Advanced treatment of wastewater with BTEX. Desalination Water Treat 50, 440–445 (2012). [Google Scholar]
- Liu Y. & Shi B. Interaction parameter method for investigating BTEX extraction using the hollow-fiber microporous membrane liquid/liquid extraction technique. Chem. Eng. Technol. 32, 926–931 (2009). [Google Scholar]
- Yahaya G. O. Separation of volatile organic compounds (BTEX) from aqueous solutions by a composite organophilic hollow fiber membrane-based pervaporation process. J. Membr. Sci. 319, 82–90 (2008). [Google Scholar]
- Slominska M., Krol S. & Namiesnik J. Removal of BTEX compounds from waste gases; destruction and recovery techniques. Crit. Rev. Environ. Sci. Technol. 43, 1417–1445 (2013). [Google Scholar]
- Kuroki T., Hirai K., Kawabata R., Okubo M. & Yamamoto T. Decomposition of adsorbed xylene on adsorbents using nonthermal plasma with gas circulation. Ieee Trans. Ind. Appl. 46, 672–679 (2010). [Google Scholar]
- Chen X., Qian W., Kong L., Xiong Y. & Tian S. Performance of a suspended biofilter as a new bioreactor for removal of toluene. Biochem. Eng. J. 98, 56–62 (2015). [Google Scholar]
- Rene E. R. et al. Start-up, performance and optimization of a compost biofilter treating gas-phase mixture of benzene and toluene. Bioresour. Technol. 190, 529–535 (2015). [DOI] [PubMed] [Google Scholar]
- Spigno G., Pagella C., Fumi M. D., Molteni R. & De Faveri D. M. VOCs removal from waste gases: gas-phase bioreactor for the abatement of hexane by Aspergillus niger. Chem. Eng. Sci. 58, 739–746 (2003). [Google Scholar]
- van Groenestijn J. W. & Liu J. X. Removal of alpha-pinene from gases using biofilters containing fungi. Atmos. Environ. 36, 5501–5508 (2002). [Google Scholar]
- Sun D., Duan C., Liu A., Huang Y. & Bao J. Patent center of Chongqing University. A biological filter. Chinese patent CN 2,012,200,181,465. 2012 Jan 16.
- WHITE T. Analysis of phylogenetic relationships by amplification and direct sequencing of ribosomal RNA genes. PCR Protoc. Guide Methods Appl. doi: 10.1007/978-94-011-4020-1_3 (1990). [DOI] [Google Scholar]
- Cole J. R. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33, D294–D296 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. et al. Application of Faecalibacterium 16S rDNA genetic marker for accurate identification of duck faeces. Environ. Sci. Pollut. Res. 23, 7639–7647 (2016). [DOI] [PubMed] [Google Scholar]
- Jiang C. et al. Enhanced butanol production through adding organic acids and neutral red by newly isolated butanol-tolerant bacteria. Appl. Biochem. Biotechnol. 180, 1–12 (2016). [DOI] [PubMed] [Google Scholar]
- Álvarez-Hornos F. J. et al. Biofiltration of ethylbenzene vapours: Influence of the packing material. Bioresour. Technol. 99, 269–276 (2008). [DOI] [PubMed] [Google Scholar]
- Wang G. et al. Adsorption of benzene, cyclohexane and hexane on ordered mesoporous carbon. J. Environ. Sci.-China 30, 65–73 (2015). [DOI] [PubMed] [Google Scholar]
- Saravanan V. & Rajamohan N. Treatment of xylene polluted air using press mud-based biofilter. J. Hazard. Mater. 162, 981–988 (2009). [DOI] [PubMed] [Google Scholar]
- Rene E. R., Murthy D. V. S. & Swaminathan T. Steady- and transient-state effects during the biological oxidation of gas-phase benzene in a continuously operated biofilter. Clean Technol. Environ. Policy 12, 525–535 (2010). [Google Scholar]
- Barnett H. L. & Hunter B. B. Illustrated genera of imperfect fungi. (APS Press, 1998). [Google Scholar]
- Wang Q., Garrity G. M., Tiedje J. M. & Cole J. R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade S. D., Polizeli M., Terenzi H. F. & Jorge J. A. Effect of carbon source on the biochemical properties of beta-xylosidases produced by Aspergillus versicolor. Process Biochem. 39, 1931–1938 (2004). [Google Scholar]
- Lovley D., Coates J., Woodward J. & Phillips E. Benzene oxidation coupled to sulfate reduction. Appl. Environ. Microbiol. 61, 953–958 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider J. & Fuchs G. Microbial anaerobic aromatic metabolism. Anaerobe 3, 1–22 (1997). [DOI] [PubMed] [Google Scholar]
- Hendrickx B. et al. PCR-DGGE method to assess the diversity of BTEX mono-oxygenase genes at contaminated sites. Fems Microbiol. Ecol. 55, 262–273 (2006). [DOI] [PubMed] [Google Scholar]
- Hendrickx B. et al. Alternative primer sets for PCR detection of genotypes involved in bacterial aerobic BTEX degradation: Distribution of the genes in BTEX degrading isolates and in subsurface soils of a BTEX contaminated industrial site. J. Microbiol. Methods 64, 250–265 (2006). [DOI] [PubMed] [Google Scholar]
- Lopez-Malo A., Alzamora S. M. & Palou E. Aspergillus flavus growth in the presence of chemical preservatives and naturally occurring antimicrobial compounds. Int. J. Food Microbiol. 99, 119–128 (2005). [DOI] [PubMed] [Google Scholar]
- LopezMalo A., Alzamora S. M. & Argaiz A. Effect of vanillin concentration, pH and incubation temperature on Aspergillus flavus, Aspergillus niger, Aspergillus ochraceus and Aspergillus parasiticus growth. Food Microbiol. 14, 117–124 (1997). [Google Scholar]
- Redkar R. J., Locy R. D. & Singh N. K. Biosynthetic pathways of glycerol accumulation under salt stress in Aspergillus nidulans. Exp. Mycol. 19, 241–246 (1995). [DOI] [PubMed] [Google Scholar]
- Maestre J. P., Gamisans X., Gabriel D. & Lafuente J. Fungal biofilters for toluene biofiltration: Evaluation of the performance with four packing materials under different operating conditions. Chemosphere 67, 684–692 (2007). [DOI] [PubMed] [Google Scholar]
- Garcia-Pena I., Ortiz I., Hernandez S. & Revah S. Biofiltration of BTEX by the fungus Paecilomyces variotii. Int. Biodeterior. Biodegrad. 62, 442–447 (2008). [Google Scholar]
- Valdez-Vazquez I., Rios-Leal E., Carmona-Martinez A., Munoz-Paez K. M. & Poggi-Varaldo H. M. Improvement of biohydrogen production from solid wastes by intermittent venting and gas flushing of batch reactors headspace. Environ. Sci. Technol. 40, 3409–3415 (2006). [DOI] [PubMed] [Google Scholar]
- Baawain M. S., Al-Jabri M. & Choudri B. S. Characterization of industrial wastewater sludge in Oman from three different regions and recommendations for alternate reuse applications. Iran. J. Public Health 44, 1473–1481 (2015). [PMC free article] [PubMed] [Google Scholar]
- Ducman V. & Mirtic B. The applicability of different waste materials for the production of lightweight aggregates. Waste Manag. 29, 2361–2368 (2009). [DOI] [PubMed] [Google Scholar]
- Pedersen L.-F., Pedersen P. B. & Sortkjaer O. Temperature-dependent and surface specific formaldehyde degradation in submerged biofilters. Aquac. Eng. 36, 127–136 (2007). [Google Scholar]
- Singh K., Singh R. S., Rai B. N. & Upadhyay S. N. Biofiltration of toluene using wood charcoal as the biofilter media. Bioresour. Technol. 101, 3947–3951 (2010). [DOI] [PubMed] [Google Scholar]
- Dorado A. D., Baeza J. A., Lafuente J., Gabriel D. & Gamisans X. Biomass accumulation in a biofilter treating toluene at high loads - Part 1: Experimental performance from inoculation to clogging. Chem. Eng. J. 209, 661–669 (2012). [Google Scholar]
- Gutierrez-Acosta O. B., Arriaga S., Escobar-Barrios V. A., Casas-Flores S. & Almendarez-Camarillo A. Performance of innovative PU-foam and natural fiber-based composites for the biofiltration of a mixture of volatile organic compounds by a fungal biofilm. J. Hazard. Mater. 201, 202–208 (2012). [DOI] [PubMed] [Google Scholar]
- Sene L., Converti A., Felipe M. G. A. & Zilli M. Sugarcane bagasse as alternative packing material for biofiltration of benzene polluted gaseous streams: a preliminary study. Bioresour. Technol. 83, 153–157 (2002). [DOI] [PubMed] [Google Scholar]
- Zilli M., Daffonchio D., Di Felice R., Giordani M. & Converti A. Treatment of benzene-contaminated airstreams in laboratory-scale biofilters packed with raw and sieved sugarcane bagasse and with peat. Biodegradation 15, 87–96 (2004). [DOI] [PubMed] [Google Scholar]
- Kim J. O. Degradation of benzene and ethylene in biofilters. Process Biochem. 39, 447–453 (2003). [Google Scholar]
- Zhou Q., Huang Y. L., Tseng D. H., Shim H. & Yang S. T. A trickling fibrous-bed bioreactor for biofiltration of benzene in air. J. Chem. Technol. Biotechnol. 73, 359–368 (1998). [Google Scholar]
- Lu C. Y., Chu W. C. & Lin M. R. Removal of BTEX vapor from waste gases by a trickle bed biofilter. J. Air Waste Manag. Assoc. 50, 411–417 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.