Abstract
The thymus in teleost fishes plays an important role in producing functionally competent T‐lymphocytes. However, the thymus in tilapia is not well known, which greatly hampers investigations into the immune responses of tilapia infected by aquatic pathogens. The histological structure and ultrastructure of the thymus in Oreochromis niloticus, including embryos and larvae at different developmental stages, juveniles, and adult fish, were systematically investigated using whole mount in situ hybridization (WISH), and light and transmission electron microscopy (TEM). The position of the thymus primordium was first labeled in the embryo at 2 days post‐fertilization (dpf) with the thymus marker gene recombination activating gene 1 (Rag1), when the water temperature was 27 °C. Obvious structures of the thymus were easily observed in 4‐dpf embryos. At this stage, the thymus was filled with stem cells. At 6 dpf, the thymus differentiated into the cortex and medulla. The shape of the thymus was ‘broad bean’‐like during the early stages from 4 to 10 dpf, and became wedge‐shaped in fish larvae at 20 dpf. At 6 months post‐fertilization (mpf), the thymus differentiated into the peripheral zone, central zone, and inner zone. During this stage, myoid cells and adipocytes appeared in the inner zone following thymus degeneration. Then, the thymus displayed more advanced degeneration by 1 year post‐fertilization (ypf), and the separation of cortex and medulla was not observed at this stage. The thymic trabecula and lobule were absent during the entire course of development. However, the typical Hassall's corpuscle was present and underwent degeneration. Additionally, TEM showed that the thymic tissues contained a wide variety of cell types, namely lymphocytes, macrophages, epithelial cells, fibroblasts, and mastocytes.
Keywords: embryonic development, thymus, tilapia, ultrastructure
Introduction
The immune system is divided into the innate immune system and adaptive immune system, and has a series of physical barriers, as well as cellular and humoral components to defend the body against pathogens and foreign substances. Both internal and external factors can stimulate this system (Uribe et al. 2011; Biller‐Takahashi & Urbinati, 2014). The innate immune system is the main defense in invertebrates and a fundamental defense mechanism in vertebrates (Benhamed et al. 2014). Unlike vertebrates that have both innate and adaptive immune systems, invertebrates have only an innate immune system and cannot produce cellular or humoral immune responses. However, recent studies indicate that there are many molecular configurations of immune recognition, signal transduction, and effector synthesis involved in cellular and humoral immunity in scallops, shrimps, and other invertebrates (Arala‐Chaves & Sequeira, 2000; Kurtz & Armitage, 2006; Song et al. 2015). This suggests that adaptive immune responses occur in some invertebrates. As the largest group of vertebrates, fishes are a heterogeneous group that includes the jawless fishes (lampreys and myxines), cartilaginous fishes (sharks and rays), and teleosts (bony fishes; Magadan et al. 2015).
The epithelial and mucosal barrier of the skin, gills, and alimentary tract constitutes the physical barrier of the innate immune system in fishes, playing an important role in combating pathogens, with other innate immune components, including humoral factors, cells, and tissues, and antimicrobial peptides and complementary factors (Magnadottir, 2006; Benhamed et al. 2014). Jawless fishes (Cyclostomata) lack the immunoglobulin‐based adaptive immune system of jawed vertebrates; however, they have evolved a different adaptive immune system with lymphocyte‐like cells and thymoids, which is a candidate thymus in lampreys (Bajoghli et al. 2011).
Although cartilaginous and teleost fishes belong to the lower order of vertebrates, they have evolved a complete immune system. Cartilaginous fishes were the first vertebrate group to evolve a thymus and distinguishable T and B cells (Dooley & Flajnik, 2006; Boehm & Bleul, 2007). Immune system organs or tissues in teleost fishes include the thymus, head kidney, spleen, and the main mucosa‐associated lymphoid tissues (MALT; Zapata et al. 2006; Salinas, 2015). The MALT in fishes, composed of the skin‐associated lymphoid tissue (SALT), gut‐associated lymphoid tissue (GALT), gill‐associated lymphoid tissue (GIALT), and nasopharynx‐associated lymphoid tissue (NALT), is the major lymphoid structure in the body and plays a crucial role in responses to infection (Benhamed et al. 2014; Salinas, 2015). All MALT in fishes are known as diffuse MALT, except for the interbranchial lymphoid tissue (ILT) of gills, which is known as the organized MALT. The ILT is a specialized lymphoid tissue and was first described in Atlantic salmon (Salmo salar); it plays a role in the immune responses of salmon against viruses (Haugarvoll et al. 2008; Koppang et al. 2010; Aas et al. 2014).
The thymus in freshwater teleosts was the first organ to become lymphoid (Romano et al. 1999b; Zapata et al. 2006). Similar to mammals, most modern bony fishes have a thymus, which is the main site for T‐lymphocyte proliferation, differentiation, and maturation, and plays a key role in the development of the immune system and immune responses (dos Santos et al. 2000). Although T‐lymphocytes have been studied in birds and mammals for 50 years, T‐lymphocytes in fishes were only recently described in Atlantic salmon in 2010 (Koppang et al. 2010). In addition, T‐lymphocytes participate in humoral immunity mediated by B‐lymphocytes through helper T‐cells, and thus are involved in protection via vaccination.
Tilapia (Oreochromis sp.) production is the most widespread type of aquaculture in the world (FAO, 2014). Since 2004, China has been the biggest tilapia producer in the world, with production of about 1.2 million tons annually (Ye et al. 2011). However, tilapia production in major areas of China is hampered by disease outbreaks, particularly from streptococcosis, with the cumulative mortality reaching between 30 and 80% from 2009 to 2011 (Mian et al. 2009; Chen et al. 2012a,b). Traditionally, streptococcosis has been controlled by antibiotics, but their overuse has resulted in the evolution of antibiotic resistance (Defoirdt et al. 2011).
Recent research demonstrates that inactive vaccines, or subunit vaccines of Streptococcus agalactiae, can significantly enhance protection against streptococcosis (Yi et al. 2014; Li et al. 2015; Zhang et al. 2016), and vaccination is considered one of the most effective means of streptococcosis prevention in tilapia culture at present. However, the application of vaccines to fish is dependent on an overall understanding of their specific immune systems. In higher vertebrates, stimulation of the immune system at an early stage before the immune system is fully developed has been shown to induce tolerance, rather than protection (Brown et al. 1996; Petrie‐Hanson & Ainsworth, 1999; Rombout et al. 2005); therefore, it is important to investigate the stages of development and differentiation of specific immune organs. To fully understand the immune system of Nile tilapia and to improve the effectiveness of vaccination against streptococcosis, we conducted a systematic study of the development and histological structure of the thymus in embryos, larvae at different development stages, juveniles, and adults of Nile tilapia, O. niloticus, using whole mount in situ hybridization (WISH), tissue biopsies, and transmission electron microscopy (TEM).
Materials and methods
Animals and sample collection
Adult specimens of Nile tilapia, O. niloticus, were maintained in a 50‐L tank with one fish per tank. Tilapia eggs and sperm were collected and used in artificial insemination following standard procedures as described previously (Fujimura & Okada, 2007). Embryos were hatched in an artificial incubator at 27 ± 0.5 °C, and the developmental stage was determined according to Fujimura & Okada (2007). Larvae were fed artemia and weaned fish were fed commercial artificial prawn feed at approximately 20 days post‐fertilization (dpf). Samples for WISH and paraffin sections of 20 embryos were collected every day from 2 to 15 dpf, and fixed in 4% paraformaldehyde (PFA) in phosphate‐buffered saline (PBS) overnight at 4 °C. The thymus of 10‐dpf embryos were separated and fixed in 0.25% PBS buffered glutaraldehyde for electron microscopy.
To prepare paraffin and electron microscopy sections, the thymus of larvae at 1, 3, and 6 months post‐fertilization (mpf), and 1 year post‐fertilization (ypf) were separated and fixed in 4% PFA and 0.25% PBS buffered glutaraldehyde, respectively.
Whole mount in situ hybridization (WISH)
Whole mount in situ hybridization (WISH) of tilapia embryos was carried out as previously described (Lyon et al. 2013), with some modifications (Cao et al. 2014). The template for RNA probe synthesis was prepared by inserting recombination activating gene 1 (Rag1) cDNA (518 bp; forward primer: tRag1F 5′‐TGGTCTTCGTCGGTGGAT‐3′; reverse primer: tRag1R 5′‐TTCTCATCATATCCCGTGCCCCTGA‐3′; GenBank accession numbers: XM_019361952.1) into pEASY‐T3 vector (TransGen Biotech, China). The template plasmid was linearized by Apa I and Nde I restriction digestion for sense and anti‐sense probe preparation, respectively. Thereafter, digoxigenin (DIG)‐labeled antisense and sense riboprobes were synthesized according to the manufacturer instructions (Roche) using SP6/T7 polymerase as reported previously (Cao et al. 2014).
Histology
Samples fixed in 4% PFA were washed to remove the PFA with three 10‐min washes in PBS, dehydrated through a graded ethanol series, and embedded in paraffin. Sections were cut to 8 μm, and mounted on glass slides (Leica) and left overnight in an incubator at 37 °C. They were then de‐waxed in xylene and rehydrated in a graded series of ethanol baths, and stained with hematoxylin and eosin (HE), and Masson's trichrome stain for histological investigation.
Transmission electron microscopy (TEM)
For ultrastructural studies, specimens were fixed in 0.25% PBS‐buffered glutaraldehyde, washed three times in the same buffer, fixed with 1% osmium tetroxide in phosphate buffer (pH 7.0) for 2 h, and washed three times in the phosphate buffer. After dehydration in an increasing ethanol series and acetone, the specimens were placed in a 1 : 1 mixture of absolute acetone and the final Spurr resin mixture for 1 h at room temperature, transferred to a 1 : 3 mixture of absolute acetone and the final resin mixture for 3 h, then to final Spurr resin mixture, and left overnight. Specimens were placed in capsules that contained embedding medium and heated at 60 °C for 48 h. After samples were embedded in resin, the ultra‐thin sections were prepared using a Leica UC7 ultra microtome, stained with uranyl acetate and alkaline lead citrate for 15 min, and observed using a TEM, Model Tecnai G2 20 TWIN. The cells and historical structures in the TEM images were named and described according to the previous study in cichlid fishes and other fish (Alvarez et al. 1995; Fishelson, 1995; Romano et al. 1999a; Mohammad et al. 2007).
Results
Anatomical observation of the thymus in Nile tilapia
The thymus primordium of tilapia was formed in the embryo at 2 dpf, when the water temperature was 27 °C. Though its position was not distinguished from its appearance, the thymus primordium was first identified by the thymus marker gene recombination activating gene 1 (Rag1) and WISH (Iwanami et al. 2008). WISH of the Rag1 gene in Nile tilapia showed that the thymus primordium in 2‐dpf embryos only had a few cells, located on the surface of the section behind the metencephalon (Fig. 1A). At 5 dpf, Rag1 was expressed above the pectoral fin and the top junction of the branchial arch (Fig. 1B).
Figure 1.
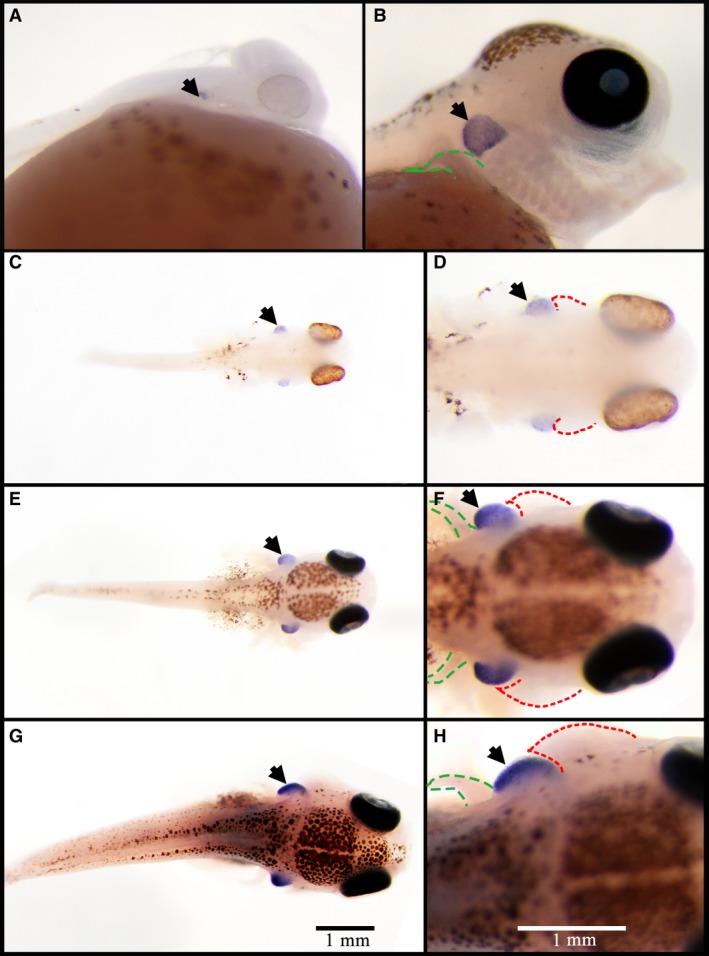
Position of the thymus marked by whole mount in situ hybridization of Rag1 gene in Nile tilapia. (A) Embryos at 3 days post‐fertilization (dpf); (B) embryos at 5 dpf. Embryos (A and B) were mounted with anterior sides to the right and lateral sides toward the reader. (C,D) Embryos at 4 dpf; (E,F) embryos at 7 dpf; (G,H) embryos at 10 dpf; embryos (C–H) were mounted with anterior sides to the right and top sides toward the reader. Expression positions of Rag1 are indicated by black arrows. Gill cover primordium is indicated by red dotted line and pectoral fin is indicated by green dotted line.
Following cell proliferation and further development, the thymus increased in size. Subsequently, the thymus became a ‘broad bean’‐like structure protruding from the skin (Fig. 1C–H). WISH results of Rag1 in 4‐ to 10‐dpf embryos showed that the thymus was located between the gill cover primordium and the pectoral fin, and distributed symmetrically on both sides of the head (Fig. 1F,H). The thymus and gill cover primordium were raised above the external surface in 4‐dpf embryos, and the thickness of each was almost equal with an inconspicuous demarcation (Fig. 1D). As the embryo developed, the gill cover primordium extended backwards and formed the shape of the gill cover primordium that covers the thymus (Fig. 1F,H).
The thymus of Nile tilapia in histologic sections was confirmed by comparison with the WISH Rag1 results and sections stained by HE (Fig. 2). The color of the thymus in histologic sections with HE staining was darker than other structures, thus the position of the thymus was easily depicted (Fig. 2B,C,E,F,H,I). In spite of the different shapes of the three‐dimensional histologic sections of thymus in 10‐dpf embryos, the profile of the thymus in histologic sections was consistent with the region labeled by WISH (Fig. 2).
Figure 2.
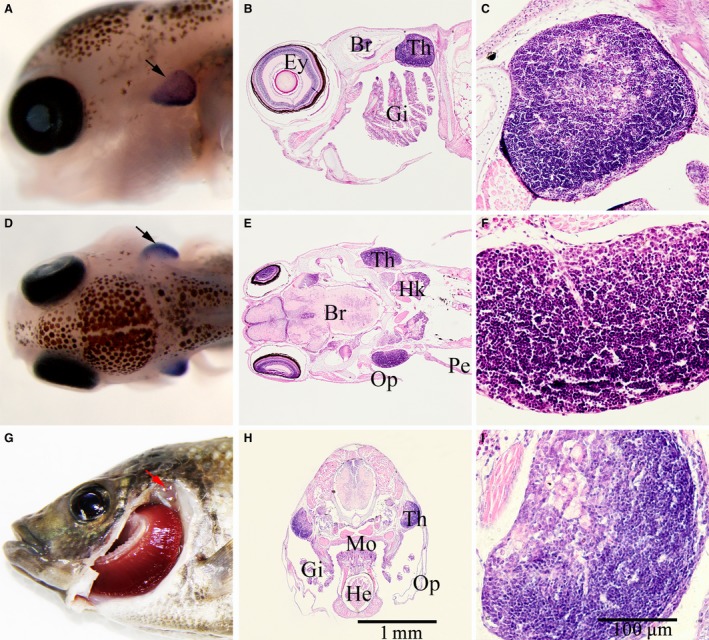
Comparison between whole mount in situ hybridization of Rag1 and tissue slices of Nile tilapia. (A) Embryo of whole mount in situ hybridization (WISH) on Rag1, mounted with anterior side to the left and lateral side toward the reader. (B) Tissue slice of Nile tilapia, mounted with anterior side to the left and lateral side toward the reader. (D) Embryo of WISH on Rag1, mounted with anterior side to the left and top side toward the reader. (E) Tissue slice of Nile tilapia, mounted with anterior side to the left and top side toward the reader. (G) Position of the thymus in adult tilapia, indicated by red arrow. (H) Transverse section of Nile tilapia. (C,F,I) Thymus of Nile tilapia. All embryos in WISH and sections were approximately 10 days post‐fertilization, black arrow indicates the thymus. B, Brain; E, eye; G, gill; H, heart; HK, head kidney; M, mouth; O, operculum; P, pectoral; T, thymus. Scale bars: (A,B,D,E,H) as shown in image (H); (C,F,I) as shown in image (I).
When Nile tilapia developed into adults, the distribution of the thymus was similar to other fish. They were located on the edge of the gill cover, close to the opercular cavity, and distributed symmetrically on both sides of the body (Fig. 2G, red arrow). The thymus was semitransparent and covered by a layer of connective tissue, with a few melanophores scattered on the surface.
The histological development of thymus in Nile tilapia
To investigate the process of genesis, development, and degeneration of thymus in Nile tilapia, we analyzed the tissue slices of embryos, larvae at different developmental stages, and juveniles and adult fish. Five to eight embryos or tissues were observed in each group, but there was no variation in the histological structures between different embryos or thymus at the same developmental stage. Results of histological development of tilapia thymus are described below.
4 dpf
At this stage, the thymus was filled with thymic cells, a form of stem cell, and coated with one to two layers of epithelial cells. Thymic cells had a large volume compared with the cells around the thymus and an irregular polygon shape, with a big nucleus that occupied almost the whole cell. There was a distinct boundary between the thymus and other tissues (Fig. 3A,A′).
Figure 3.
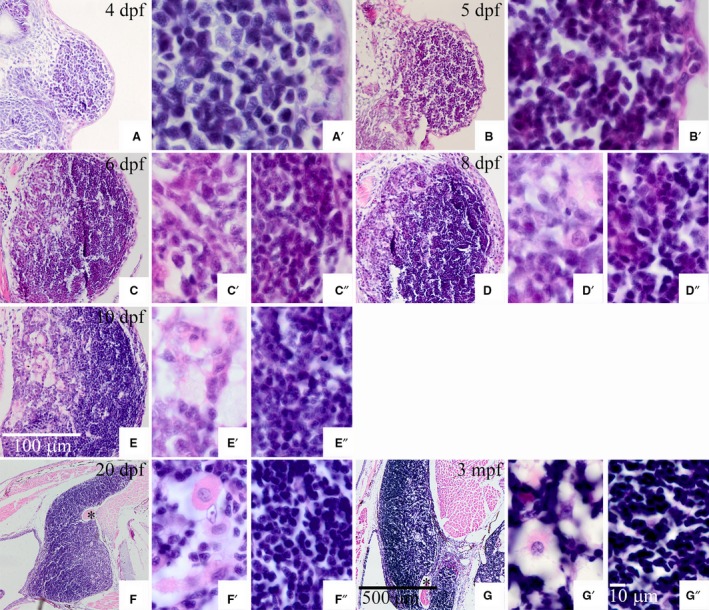
Tissue section of the thymus in Nile tilapia at 4 days post‐fertilization to 3 months post‐fertilization. (C′,D′,E′,F′,G′) Thymic medulla; (C″,D″,E″,F″,and G″) thymic cortex. Numbers in the top right corner of images indicate the developmental stage. Black asterisk indicates the muscle enveloped in the thymus. Scale bars: (A,B,C,D,E) as shown in image (E); (F,G) as shown in image (G); (A′,B′,C′,C″,D′,D″,E′,E″,F′,F″,G′,G″) as shown in image (G″).
5 dpf
The volume of the thymus increased with the numbers of thymic cells. However, the volume of thymic cells was reduced compared with those observed at 4 dpf (Fig. 3B,B′).
6 dpf
A primary differentiation occurred at this stage. Two histological regions, the cortex and medulla, were distinguished with HE staining. The cortex was close to the outside and stained more heavily. Conversely, the medulla region was near the inside and stained lightly. Although the cortex and medulla were obviously distinct, there was no evident boundary between them (Fig. 3C). The cells in the cortex were smaller in volume and stained more heavily than cells in the medulla. Additionally, the density of cortex cells was higher than that in the medulla (Fig. 3C′,C″).
8–10 dpf
The volume of the thymus continued increasing, and differentiation became more distinct. Epithelial cells coated the whole thymus and increased to approximately six layers, defined as the external cortex (Fig. 3D–E″). Except for undifferentiated thymic cells, some differentiated cells, such as macrophages and reticular cells, were found in the medulla (Fig. 3D′). At 10 dpf, there were several bubbly structures in the medulla (Fig. 3E′).
20 dpf
Concomitant with increasing thymus volume, the differentiation of the cortex and medulla was complete in fish larvae by 20 dpf. Meanwhile, the shape of the thymus changed prodigiously, turning from a broad bean appearance to a wedge‐shape, with the anterior end becoming thinner and inserting into the area between the gill cover and the head (Fig. 3F). Along with the forward migration of thymus cells, a portion of muscle was surrounded by thymus cells, yet was still close to the muscle under the gill cover (Fig. 3F, black asterisk).
3 mpf
The volume of the thymus continued increasing. The muscle surrounded by thymus cells was separated from the muscle under the gill cover, due to the forward migration of thymus cells (Fig. 3G, black asterisk). Concurrently, the thymus and head kidney were joined by the connective tissue. Further cell differentiation of the thymus was evident. With heavy staining and tight arrangement, cells in the cortex had the typical characters of thymic cells (Fig. 3G″). Cells in the medulla, located on the inner side of the thymus, were arranged sparsely (Fig. 3G′). In addition, more than 20% of non‐thymic cells with more cytoplasm were in the medulla, so that this region was stained pink with easily observed non‐thymic cells (Fig. 3G′).
6 mpf
There were three histological regions, including outer zone (peripheral zone), center zone, and inner zone. The outer zone was defined as outer cortex, and had three strata (Fig. 4E; L.a, L.b, and L.c). Stratum L.a had a stratified epithelium structure, comprising mucous cells (or myxocytes) and reticular epithelial cells. Strata L.b and L.c were composed of loose and dense connective tissue, respectively. The central zone was composed of thymic parenchymal tissue, which mostly resembled cortex, so there were no differences between the cortex and medulla (Fig. 4C,E; L.d). At this stage, degeneration of the tilapia thymus was evident, and this led to the generation of the inner zone. The inner zone was composed of adipocytes and myoid cells that originated from the degeneration of the medulla (Fig. 4C,G). There was a stratum of dense collagenous fibers between strata L.a and L.b, defined as the basement membrane of stratified epithelium, in the sections examined with Masson's stain (Fig. 5A; indicated by red arrow). Moreover, the myxocytes were dyed blue (Fig. 5A, indicated by black arrow). A stratum of dense collagenous fibers existed between strata L.c and L.d (Fig. 5B, indicated by red arrow), which had invaded the L.c stratum (Fig. 5B, indicated by red triangle).
Figure 4.
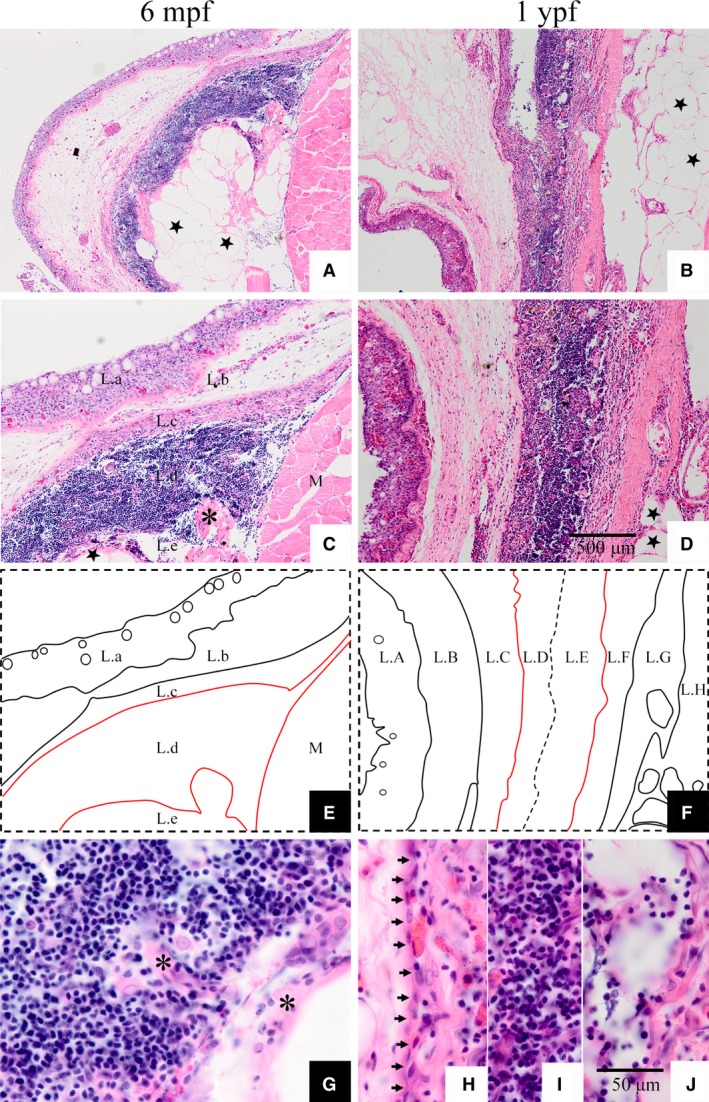
Hematoxylin and eosin staining of thymus sections at 6 months post‐fertilization and 1 year post‐fertilization in Nile tilapia. (E) Diagrammatic drawing of 6 mpf thymus. (F) Diagrammatic drawing of 1 ypf thymus. ★, Adipocyte; black asterisk, myoid cell; M, muscle in images (C, G); black arrow in (H) indicates the simple epithelium between L.C and L.D strata. Scale bars: (A‐D) as shown in image (D); (G‐J) as shown in image (J).
Figure 5.
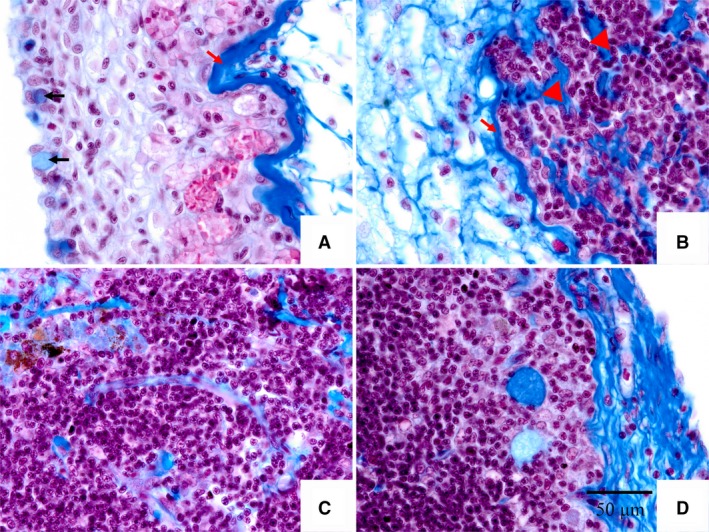
Masson's staining of thymus section at 6 months post‐fertilization. Image A shows the stratum L.a in Fig. 4. Image B shows the boundary between stratum L.c and L.d. Images (C, D) show the centre zone of the tilapia thymus. Black arrow in image A indicates myxocyte. Red arrow in images (A, B) indicates dense connective tissue. Red triangle in image B indicates collagenous fibers invading the thymus. Scale bar: all images as shown in (D).
1 ypf
The thymus of 1‐ypf tilapia contained an outer zone, center zone, and inner zone, which were subdivided into L.A to L.H 8 strata (Fig. 4D,F). Strata L.A, L.B, and L.C formed the outer zone. There was no difference between the L.A stratum and the homologous structure in 6‐mpf thymus, which was composed of stratified pavement epithelium. The L.B stratum was dense connective tissue, consisting of numerous fibroblasts, whereas the L.C stratum was composed of loose connective tissue and had many eosinophilic granulocytes. The parenchyma of 1‐ypf thymus was composed of the strata L.D, L.E, and L.F (Fig. 4D,F). With a large number of fibroblasts and few lymphocytes, the character of the L.D stratum was different from the cortex, despite being outside of the thymus. A monolayer of epithelial cells separated stratum L.C from L.D (Fig. 4H; short black arrows). The most obvious difference between strata L.D and L.C was that fewer cells were observed in L.C, as most had been replaced by collagenous fibers. Stratum L.E was composed of canonical thymic tissue, with abundant thymic cells, few macrophages or reticular cells, and typical Hassall's corpuscles; however, there was no partition of the cortex and medulla, and this stratum was more medulla‐like when compared with the parenchyma of 6‐mpf thymus. Stratum L.F was similar to stratum L.D, but had fewer thymic cells and more fibroblasts. The inside L.G stratum differed from the L.B and L.C strata outside of the thymus, and was composed of dense connective tissue with more collagenous fibers and fewer cells. Furthermore, the L.H stratum was adipose tissue with a large quantity and volume of adipocytes, which appeared as vacuoles in paraffin sections (Fig. 4B‐D; black stars). There was no obvious demarcation between the strata L.D, L.E, L.F, L.G, and L.H tissue layers; however, they were distinguished by their cell composition and tissue characteristics.
The microstructure and ultrastructure of tilapia thymus
Vasculature of tilapia thymus
There were several blood vessels in the tilapia thymus, which were typical capillaries and were formed by a single layer of epithelial cells. Two types of capillary were observed in tilapia thymus sections stained with HE (Fig. 6A,B). One type was the continuous capillary, rising from the embranchment of the arteriole, and it was distributed in the thymus cortex. The epithelial cells of the continuous capillary, and the erythrocytes within it, were stretched to different lengths (Fig. 6A, black arrow indicates the nucleus of vascular endothelial cell, and the red arrow indicates erythrocytes). The second type was the fenestrated capillary, characterized by embranchments and distributed in the thymus medulla. Mature thymocytes were observed in the fenestrated capillary, as this is the main route for mature thymocytes entering the blood (Fig. 6B, indicated by red triangle).
Figure 6.
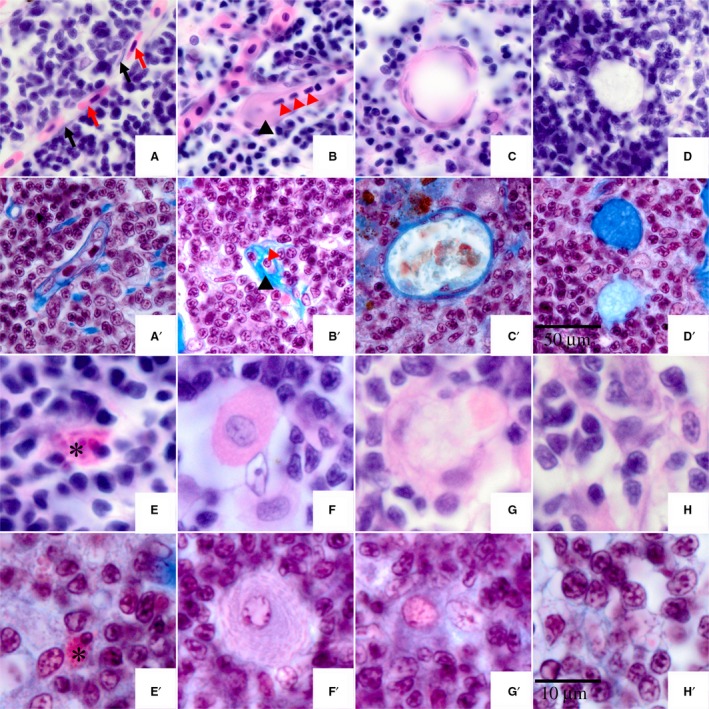
Circulation system structures and cell types in thymus of Nile tilapia. (A,A′) Capillary vessel of thymus; black arrow indicates the nucleus of vascular endothelial cell, and the red arrow indicates erythrocyte. (B,B′) Suspected lymphatic capillary of thymus; black triangle indicates the position of the suspected lymphatic capillary, the red triangle indicates a lymphocyte. (C,C′) Hassall's corpuscle. (D,D′) Degraded Hassall's corpuscle. (E,E′) Asterisk indicates mastocyte. (F,F′) Plasma cells. (G,G′) Macrophage. (H,H′) Reticular cells. (A‐H) Images were stained with hematoxylin and eosin. (A′‐H′) Images were stained with Masson's stain. Scale bars: (A‐D) and (A′‐D’) as shown in (D′); (E‐H) and (E′‐H’) as shown in (H′).
Although it was not easy to observe the walls of thymic capillaries, the position of the capillary wall was identified by the erythrocytes. Moreover, the vascular endothelial cells were dyed blue with Masson's stain (Fig. 6A′), which identified the position of the capillary wall. Hence, the vasculature of tilapia thymus was identified clearly by HE and Masson's stain.
Suspected lymphatic vessels of tilapia thymus
There is another specialized circulatory system, known as the lymphatic circulation. The lymphatic vessel is an important part of the lymphatic circulation. In tilapia thymus sections dyed with HE, the suspected lymphatic vessels were easily identified by thick walls and small cells (Fig. 6B,B′). The cells in the suspected lymphatic vessels were different from the erythrocytes in the capillary, as the erythrocytes had pink cytoplasm stained by eosin, whereas the cells in the suspected lymphatic vessels did not. Masson's stain confirmed that the cells in the suspected lymphatic vessels were lymphocytes or mature thymocytes which had the same phenotype as the thymic cells outside of the suspected lymphatic vessels (Fig. 6B′).
Hassall's corpuscle
Hassall's corpuscle, also known as thymic corpuscle, was the feature structure of the medulla. In Nile tilapia, Hassall's corpuscle first appeared in the medulla at 20 dpf (Fig. 3F). The structure of Hassall's corpuscle in Nile tilapia was similar to other animals, with a concentric ring structure formed by multilayered epithelial cells (Fig. 6C,C′). The number of epithelial cell layers showed the developmental status, i.e. the more layers indicated younger Hassall's corpuscles. Hassall's corpuscle finished degeneration after the apoptosis of all epithelial cells was complete. The degenerated Hassall's corpuscle was filled with compositions of collagenous fibers, which were dyed slightly pink by HE stain and water blue by Masson's stain (Fig. 6D,D′).
Lymphocytes
The lymphocytes, also called thymic cells or thymocytes, are the most numerous cells in the thymus. Lymphocytes in Nile tilapia are structurally similar to that of higher vertebrate animals. Tilapia lymphocytes were dyed violet by HE stain, with no distinction between the cytoplasm and the nucleus. Under the TEM, lymphocytes were observed to have cytoplasm that was distributed around the nucleus in a narrow ring (Fig. 7; Ly). The cloddy heterochromatin was scattered in the nucleus with a dark color under the TEM, due to its higher electron‐density. Additionally, there was another ring signal of high electron‐density distributed in the nucleus membrane. The shape of the lymphocytes in the tilapia thymus was different at different developmental stages. The volume of lymphocytes in the tilapia thymus in 10‐dpf embryos was relatively large, which was classified as undifferentiated lymphoblasts (Fig. 7A,B). However, the number of differentiated, small lymphocytes exceeded that of the undifferentiated lymphoblasts in 1‐ypf thymus (Fig. 7E,F). The differentiated lymphocytes entered the circulatory system via migration into the capillaries or lymphatic capillaries (Fig. 7E,F; indicated by white asterisks).
Figure 7.
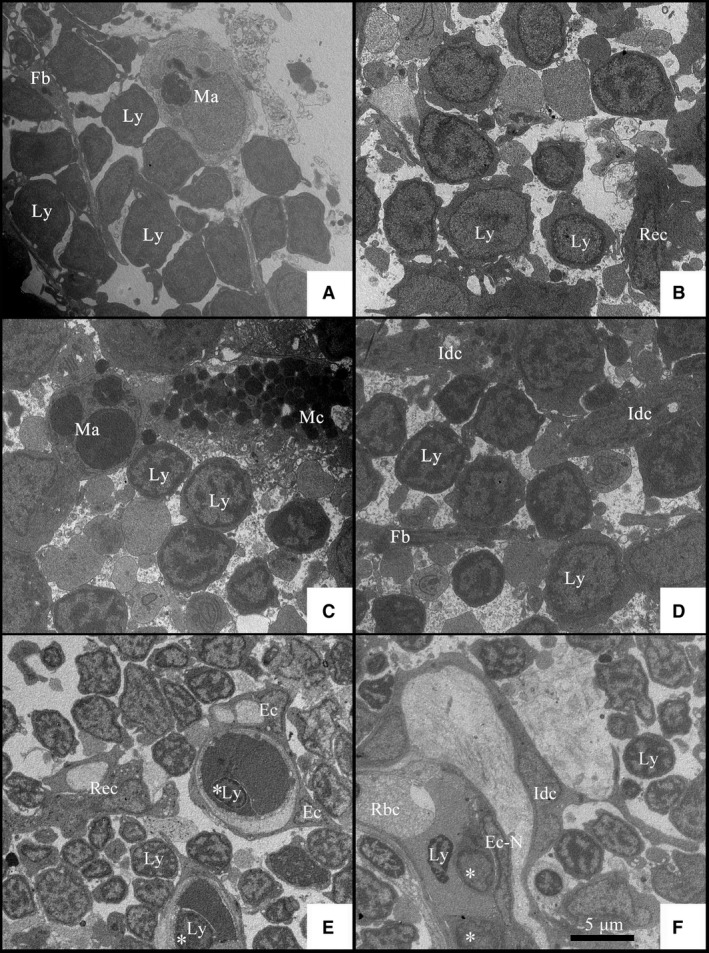
Transmission electron micrographs of Nile tilapia in different developmental stages. (A,B) Transmission electron micrographs of thymus in 10 days post‐fertilization embryos. (C,D) Transmission electron micrographs of thymus in 1 month post‐fertilization juveniles. (E,F) Transmission electron micrographs of thymus in 6 months post‐fertilization adult fish. Ec, Endotheliocyte; Ec‐N, nucleus of endotheliocyte; Fb, fibroblast; Idc, interdigitating cell; Ly, lymphocyte; Ma, macrophage; Mc, mastocyte; RBC, red blood cell; Rec, reticular epithelial cells. Asterisk indicates the lymphocyte in the capillary vessel or the lymphatic capillary of thymus.
Mast cells
There were few mast cells, also known as the mastocyte or labrocyte, in tilapia thymus. A lot of granules were stained red by eosin in the cytoplasm so that the shape of the mast cells was anomalous (Fig. 6E,E′). Under the TEM, the granules in the mast cells were not of uniform size, and characterized by high electron‐density (Fig. 7C; Mc).
Macrophages
The macrophages were observed in the tilapia thymus, characterized by large volume, unfixed shape, and presence of pseudopods. The cytoplasm of the macrophages was colored pink by HE stain with heavily stained pseudopods around the cells (Fig. 6G). It was not easy to identify the macrophages after Masson's staining, although they were verified by the characteristics of a large, pale nucleus and intricate cytoskeleton in the cytoplasm (Fig. 6G′). However, it was easy to distinguish the macrophages in the TEM slices by the numerous organelles including ribosomes, lysosomes, and large mitochondria. In addition, there were some membrane vesicles with high electron‐density and cell debris that were phagocytosed by the macrophage (Fig. 7A,C; Ma).
Constructional cells in the tilapia thymus
The basic structure of the tilapia thymus was similar to the thymus of other fishes; they all have mesh scaffolds constituted by reticular cells and fibrocytes, to which the thymic cells, macrophages, and mast cells are all attached. The endothelial cells formed the functional structures of the thymus including the capillaries and Hassall's corpuscle (Fig. 6A–D, A′–D′). Compared with the endothelial cells, the reticular cells had large volume and irregular morphology.
In the sections of tilapia thymus stained by HE, the medulla had many reticular epithelial cells that presented an aggregate distribution (Fig. 6H). Mesh scaffolds formed by interlacing of reticular epithelial cells were clearly observed after Masson's staining (Fig. 6H′). Long, digitate protrusions were observed in the reticular epithelial cells under TEM, thus these cells were called interdigitating cells (Idc). Phagocytic vacuoles and cell debris were observed in some of the reticular epithelial cells (Fig. 7E; Rec). Fibroblasts were not easily observed in the tilapia sections after HE staining. Although collagenous fibers in the thymus were easily identified after Masson's staining, it was difficult to distinguish the fibroblasts. Fibroblasts were more obvious under TEM, as their long cell bodies, as well as parallel and spaced distribution, were interspersed with other cell types (Fig. 7A,D; Fb).
Immature plasma cells
Another type of lymphocyte, immature plasma cells, was observed in the thymus of Nile tilapia. This cell type had a low cell count and was only distributed in the medulla of the thymus, which was generally round or oval. The cell nucleus was either large and oval or round. The plasma cells had high cytoplasmic content, which was colored red by HE stain in sections (Fig. 6F). Endoplasmic reticulum structure of the cells showed a layered parallel or circular arrangement, which was easily observed after Masson's staining (Fig. 6F′). The immature plasma cells of Nile tilapia were easily confused with macrophages; however, the main differences between the two cell types were that the former had a more regular cellular morphology and did not have pseudopods.
Discussion
Both Chondrichthyes and Osteichthyes have a thymus. Although there are numerous studies on the histology of the tilapia thymus, only a few studies have focused on its generation and development. Sailendri & Muthukkaruppan (1975) systematically studied the structure of the thymus in adult Oreochromis mossambicus. Fishelson (1995) studied the thymus structure in several Cichlidae species, including bottom‐spawning Tilapia zillii, Tilapia tholloni, as well as mouth‐brooding Oreochromis aureus, O. niloticus, O. mossambicus, and Sarotherodon galilaeus. Although the thymus structure is apparent in the early developmental stages in different types of tilapia, the research on thymus development in different species remains unsystematic. Morrison et al. (2001) conducted a systematic investigation of the histology of Nile tilapia embryos but did not perform further observations on the thymus. The present study is the first to track the developmental process of the tilapia thymus. The histology and anatomy of thymus development, and cellular ultrastructure at different stages were also analyzed using molecular and histological methods.
Previous studies have shown that recombination‐activating genes are highly conserved in different types of fishes (Brinkmann et al. 2004; Hunt & Rice, 2008) and are specifically expressed in the early stage of thymus development (Hunt & Rice, 2008; Iwanami et al. 2008, 2011). Therefore, in the present study, the position of the thymus in the early developmental stages was confirmed using the position of Rag1 in WISH of tilapia embryos. By comparing the results of tissue slices, the position of the tilapia thymus was confirmed through specific molecular markers. This method can also be extended to the development of other organs in tilapia.
The position of the thymus primordium in tilapia was similar to that in other species, such as zebrafish (Danio rerio; Alvarez et al. 1995), medaka (Oryzias latipes; Iwanami et al. 2004), and carp (Cyprinus carpio; Bowden et al. 2005). Our results showed a prominent tilapia thymus at 4 dpf, similar to the results of Morrison et al. (2001), which showed the presence of a prominent thymus gland at 100 hpf. The thymus cells started to differentiate into the medulla and cortex at 6 dpf, whereas differentiation occurred in medaka thymus cells at 20 days post‐hatching (Ghoneum & Egami, 1982), and were just formed in the carp thymus primordium at 12 dpf (Bowden et al. 2005). This indicates that the tilapia thymus develops faster than that of the other two species. Romano et al. (1999a) showed that thymus primordium in sharp‐snout seabream, Diplodus puntazzo, began to form at 20 dpf and to differentiate into the medulla and cortex at 66 dpf. However, the thymus structure in Harpagifer antarcticus from Signy Island, appeared at 4 weeks post‐hatching in the colder environments of natural water bodies (O'Neill, 1989). These studies indicate that the rate of thymus development varies across species and environments. Nile tilapia is a freshwater fish with a faster rate of thymus development and earlier differentiation.
Our findings indicate that the thymus in tilapia younger than 3 months can be divided into two histological regions, the cortex and medulla, whereas after 6 months of age, they can be divided into the outer zone (or peripheral zone), center zone, and inner zone. The outer (peripheral) zone is equivalent to the outer cortex in other studies, the center zone includes the inner cortex and medulla, and the inner zone is where medulla degeneration occurs. The thymus of adult cling fish Sicyases sanguineus and O. mossambicus were also divided into outer cortex, inner cortex, and medulla (Sailendri & Muthukkaruppan, 1975; Gorgollon, 1983). However, Fishelson (1995) did not observe the outer cortex of the thymus in O. mossambicus. This may have been due to the experimental materials used in that study. Furthermore, adult O. mossambicus were studied by Sailendri & Muthukkaruppan (1975), whereas larvae of 40–60 mm in length were studied by Fishelson (1995). Our results confirm that during the development of the tilapia thymus, differentiation into the cortex and medulla zones occurred first, followed by the formation of the outer cortex as individuals matured.
The outer cortex contained stratified epithelium and connective tissues. The stratified epithelium was next to the gill cavities with scattered abundant mucous cells. Mucous cells are also known as cystic cells in some studies and are able to secrete neutral mucus to lubricate glands, protect tissues from external infections, and support and protect against exogenous foreign bodies entering the thymus directly through the gills (Padros et al. 2011). Mucous cells exist in the thymus of several fish species (Hansen & Zapata, 1998; Mohammad et al. 2007). Different fishes have different boundary characteristics between the outer and center zones. The stratified epithelium and connective tissues in the outer cortex are divided by compact collagenous fibers. A boundary layer of epithelial cells separating the outer cortex and inner cortex also exists in the tilapia thymus at 1 ypf. The connective tissues and epithelial cells in the thymus became thicker as it developed, which is consistent with the research on H. antarcticus (O'Neill, 1989).
The tilapia thymus started to degenerate at 6 mpf, accompanied by the thickening of connective tissues in the outer cortex, the replacement of the inner zone by connective and adipose tissues, and volumetric reduction of the thymus. Similar thymus degeneration was also observed in medaka (Ghoneum & Egami, 1982), and occurred as well in fish infected by pathogens (Alvarez et al. 1995). In addition, the number of non‐lymphoid cells, especially fibroblasts, increased in the degenerated tilapia thymus. After 6 months, the degenerated inner zone appeared to have reticulate‐cavity structures. These structures were indicative of fatty degeneration, and were formed after lipid droplets were stained by paraffin in fat cells.
Lobular structures were not found in the tilapia thymus, and trabeculae were not observed after Masson's straining. Trabecular structures started to appear in sharp‐snout seabream at 91 dpf, with more obvious lobular structures by 8 mpf (Romano et al. 1999a). Lobulation began in 3‐month‐old medaka but was absent prior to this point (Ghoneum & Egami, 1982). Furthermore, incomplete lobules existed by 30 weeks post‐fertilization in Cyprinus carpio (Romano et al. 1999b), and typical lobules divided by trabeculae existed in the thymus of adult Psetta maxima (Vigliano et al. 2011). Based on these findings, we speculate that lobules may be related to the developmental process or ages of the individuals. The first signs of sexual maturity in tilapia appear at approximately 4–6 months of age. However, no lobules were found in the thymus in this study, which tracked individuals until they were 1 year old. In addition, no lobules were found in 12‐year‐old O. aureus, according to Fishelson (1995), suggesting that lobules do not exist in the tilapia thymus.
There are two major circulatory systems in mammals, the blood and lymphatic systems. The lymphatic system in mammals, composed of blind‐ending lymphatic vessels, lymph nodes, and other tissues, plays an essential role in fat absorption, immune responses, and fluid homeostasis, and is also involved in many pathological processes, including tumor metastasis, chronic inflammation, and lymphedema (Deguchi et al. 2012; Rummer et al. 2014). In 1969, Kampmeier described circulatory vessels containing clear fluid as lymphatic vessels in teleost fishes (Rummer et al. 2014). There are many studies on lymphatic vessels of zebrafish and medaka using transgenic technology, which enables the lymphatic vessels to be observed using enhanced green fluorescent protein (EGFP) or other reporter genes (Yaniv et al. 2007; Geudens et al. 2010; Deguchi et al. 2012; Okuda et al. 2012; Kim & Jin, 2014). Nevertheless, it is debatable whether there are lymphatic vessels in fishes or whether these vessels belong to the secondary vascular system (SVS). Many investigators claim that there are no lymphatic vessels in teleost fishes and the so‐called lymphatic vessels belong to the SVS (Dahl Ejby Jensen et al. 2009; Vogel, 2010; Rasmussen et al. 2013; Rummer et al. 2014). In this study, a suspected lymphatic vessel was identified in the tilapia thymus. Although this may be controversial, this kind of vessel was much more likely to be a lymphatic vessel, rather than a capillary, as there were plenty of thymic cells present, whereas the capillary was filled with erythrocytes. Furthermore, the capillaries of the SVS are situated exclusively on the body surfaces of fish, such as the gills, skin, pharyngeal, and oral cavity (Vogel, 2010; Rasmussen et al. 2013); therefore, the suspected lymphatic vessels in the tilapia thymus were less likely to be the capillary of the SVS.
Hassall's corpuscles in different fish species also have different distribution characteristics. Hassall's corpuscles were observed in the thymus of 3‐month‐old tilapia, and in many other species of fish, such as O. mossambicus (Sailendri & Muthukkaruppan, 1975), D. puntazzo (sharp‐snout seabream; Romano et al. 1999a), Neoceratodus forsteri (Queensland lungfish; Mohammad et al. 2007), C. carpio (Romano et al. 1999b), S. sanguineus (Gorgollon, 1983), P. maxima (Vigliano et al. 2011), and Hippoglossus hippoglossus (Atlantic halibut; Bowden et al. 2005), but not in H. antarcticus (O'Neill, 1989). Hassall's corpuscle did not exist in the early stage of tilapia thymus development, and was only formed through differentiation as development of the thymus progressed. Along with the development and differentiation of the thymus, the number of Hassall's corpuscles increased. At the same time, the structure of Hassall's corpuscles showed the phenomena of development and degeneration.
TEM showed that tilapia had abundant thymus cell types, similar to other types of Osteichthyes. As the central lymphoid organ of immunity, the thymus is the primary center of development and differentiation of T‐lymphocytes, playing an important role in cellular and humoral immunities. In this study, the formation and development of the tilapia thymus was systematically investigated using histological and molecular methods, thus providing fundamental data for subsequent studies on tilapia immunity.
Author contributions
Cao Jianmeng and Lu Maixin conceived and designed the study. Cao Jianmeng, Chen Qiong, and Hu Xinxin carried out the experiments. Cao Jianmeng drafted the manuscript. Cao Jianmeng, Lu Maixin, and Wang Miao revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to express thanks to Dr. Ye Xing from Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, for providing the tilapia embryos and experimental suggestions. This work was supported by the National Natural Science Foundation of China (31502205), China Agriculture Research System (CARS‐49), and Guangdong Natural Science Foundation (2014A030310337).
References
- Aas IB, Austbo L, Konig M, et al. (2014) Transcriptional characterization of the T cell population within the salmonid interbranchial lymphoid tissue. J Immunol 193, 3463–3469. [DOI] [PubMed] [Google Scholar]
- Alvarez F, Villena A, Zapata A, et al. (1995) Histopathology of the thymus in Saprolegnia‐infected wild brown trout, Salmo trutta L. Vet Immunol Immunopathol 47, 163–172. [DOI] [PubMed] [Google Scholar]
- Arala‐Chaves M, Sequeira T (2000) Is there any kind of adaptive immunity in invertebrates? Aquaculture 191, 247–258. [Google Scholar]
- Bajoghli B, Guo P, Aghaallaei N, et al. (2011) A thymus candidate in lampreys. Nature 470, 90–94. [DOI] [PubMed] [Google Scholar]
- Benhamed S, Guardiola FA, Mars M, et al. (2014) Pathogen bacteria adhesion to skin mucus of fishes. Vet Microbiol 171, 1–12. [DOI] [PubMed] [Google Scholar]
- Biller‐Takahashi JD, Urbinati EC (2014) Fish immunology. The modification and manipulation of the innate immune system: Brazilian studies. An Acad Bras Cienc 86, 1484–1506. [DOI] [PubMed] [Google Scholar]
- Boehm T, Bleul CC (2007) The evolutionary history of lymphoid organs. Nat Immunol 8, 131–135. [DOI] [PubMed] [Google Scholar]
- Bowden TJ, Cook P, Rombout JHWM (2005) Development and function of the thymus in teleosts. Fish Shellfish Immunol 19, 413–427. [DOI] [PubMed] [Google Scholar]
- Brinkmann H, Venkatesh B, Brenner S, et al. (2004) Nuclear protein‐coding genes support lungfish and not the coelacanth as the closest living relatives of land vertebrates. Proc Natl Acad Sci U S A 101, 4900–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Iwawa GK, Evelyn TPT (1996) The effect of early exposure of Coho salmon (Oncorhynchus kisutch) eggs to the p57 protein of Renibacterium salmoninarum on the development of immunity to the pathogen. Fish Shellfish Immunol 6, 149–165. [Google Scholar]
- Cao JM, Lu MX, Ye X, et al. (2014) A novel protocol of whole mount in situ hybridization (WISH) and its primary application in tilapia. J Fish China 11, 1847–1854 (in Chinese with English abstract). [Google Scholar]
- Chen M, Li LP, Wang R, et al. (2012a) PCR detection and PFGE genotype analyses of streptococcal clinical isolates from tilapia in China. Vet Microbiol 159, 526–530. [DOI] [PubMed] [Google Scholar]
- Chen M, Wang R, Li LP, et al. (2012b) Screening vaccine candidate strains against Streptococcus agalactiae of tilapia based on PFGE genotype. Vaccine 30, 6088–6092. [DOI] [PubMed] [Google Scholar]
- Dahl Ejby Jensen L, Cao R, Hedlund EM, et al. (2009) Nitric oxide permits hypoxia‐induced lymphatic perfusion by controlling arterial‐lymphatic conduits in zebrafish and glass catfish. Proc Natl Acad Sci U S A, 106, 18408–18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14, 251–258. [DOI] [PubMed] [Google Scholar]
- Deguchi T, Fujimori KE, Kawasaki T, et al. (2012) In vivo visualization of the lymphatic vessels in pFLT4‐EGFP transgenic medaka. Genesis 50, 625–634. [DOI] [PubMed] [Google Scholar]
- Dooley H, Flajnik MF (2006) Antibody repertoire development in cartilaginous fish. Dev Comp Immunol 30, 43–56. [DOI] [PubMed] [Google Scholar]
- FAO (2014) The State of World Fisheries and Aquaculture 2014. Rome: Food Agricultural Organization United Nations. [Google Scholar]
- Fishelson L (1995) Cytological and morphological ontogenesis and involution of the thymus in cichild fishes (cichildae, teleostei). J Morphol 223, 175–190. [DOI] [PubMed] [Google Scholar]
- Fujimura K, Okada N (2007) Development of the embryo, larva and early juvenile of Nile tilapia Oreochromis niloticus (Pisces: Cichlidae). Developmental staging system. Dev Growth Differ 49, 301–324. [DOI] [PubMed] [Google Scholar]
- Geudens I, Herpers R, Hermans K, et al. (2010) Role of delta‐like‐4/Notch in the formation and wiring of the lymphatic network in zebrafish. Arterioscler Thromb Vasc Biol 30, 1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoneum MM, Egami N (1982) Age related changes in morphology of the thymus of the fish, Oryzias latipes . Exp Gerontol 17, 33–40. [DOI] [PubMed] [Google Scholar]
- Gorgollon P (1983) Fine structure of the thymus in the adult cling fish Sicyases sanguineus (Pisces, Gobiesocidae). J Morphol 177, 25–40. [DOI] [PubMed] [Google Scholar]
- Hansen JD, Zapata AG (1998) Lymphocyte development in fish and amphibians. Immunol Rev 166, 199–220. [DOI] [PubMed] [Google Scholar]
- Haugarvoll E, Bjerkas I, Nowak BF, et al. (2008) Identification and characterization of a novel intraepithelial lymphoid tissue in the gills of Atlantic salmon. J Anat 213, 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LR, Rice CD (2008) Lymphoid tissue ontogeny in the mummichog, Fundulus heteroclitus . Anat Rec (Hoboken) 291, 1236–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanami N, Takahama Y, Kunimatsu S, et al. (2004) Mutations affecting thymus organogenesis in Medaka, Oryzias latipes . Mech Dev 121, 779–789. [DOI] [PubMed] [Google Scholar]
- Iwanami N, Higuchi T, Sasano Y, et al. (2008) WDR55 is a nucleolar modulator of ribosomal RNA synthesis, cell cycle progression, and teleost organ development. PLoS Genet 4, e1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanami N, Mateos F, Hess I, et al. (2011) Genetic evidence for an evolutionarily conserved role of IL‐7 signaling in T cell development of zebrafish. J Immunol 186, 7060–7066. [DOI] [PubMed] [Google Scholar]
- Kim JD, Jin SW (2014) A tale of two models: mouse and zebrafish as complementary models for lymphatic studies. Mol Cells 37, 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppang EO, Fischer U, Moore L, et al. (2010) Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue. J Anat 217, 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J, Armitage SA (2006) Alternative adaptive immunity in invertebrates. Trends Immunol 27, 493–496. [DOI] [PubMed] [Google Scholar]
- Li LP, Wang R, Liang WW, et al. (2015) Development of live attenuated Streptococcus agalactiae vaccine for tilapia via continuous passage in vitro. Fish Shellfish Immunol 45, 955–963. [DOI] [PubMed] [Google Scholar]
- Lyon RS, Davis A, Scemama JL (2013) Spatio‐temporal expression patterns of anterior Hox genes during Nile tilapia (Oreochromis niloticus) embryonic development. Gene Expr Patterns 13, 104–108. [DOI] [PubMed] [Google Scholar]
- Magadan S, Sunyer OJ, Boudinot P (2015) Unique features of fish immune repertoires: particularities of adaptive immunity within the largest group of vertebrates. Results Probl Cell Differ 57, 235–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnadottir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20, 137–151. [DOI] [PubMed] [Google Scholar]
- Mian GF, Godoy DT, Leal CA, et al. (2009) Aspects of the natural history and virulence of S. agalactiae infection in Nile tilapia. Vet Microbiol 136, 180–183. [DOI] [PubMed] [Google Scholar]
- Mohammad MG, Chilmonczyk S, Birch D, et al. (2007) Anatomy and cytology of the thymus in juvenile Australian lungfish, Neoceratodus forsteri . J Anat 211, 784–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CM, Miyake T, Wright JR Jr (2001) Histological study of the development of the embryo and early larva of Oreochromis niloticus (Pisces: Cichlidae). J Morphol 247, 172–195. [DOI] [PubMed] [Google Scholar]
- Okuda KS, Astin JW, Misa JP, et al. (2012) lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 139, 2381–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill JG (1989) Ontogeny of the lymphoid organs in an Antarctic teleost, Harpagifer antarcticus (Notothenioidei: Perciformes). Dev Comp Immunol 13, 25–33. [DOI] [PubMed] [Google Scholar]
- Padros F, Villalta M, Gisbert E, et al. (2011) Morphological and histological study of larval development of the Senegal sole Solea senegalensis: an integrative study. J Fish Biol 79, 3–32. [DOI] [PubMed] [Google Scholar]
- Petrie‐Hanson L, Ainsworth AJ (1999) Humoral immune responses of channel catfish (Ictalurus punctatus) fry and fingerlings exposed to Edwardsiella ictaluri . Fish Shellfish Immunol 9, 579–589. [Google Scholar]
- Rasmussen KJ, Steffensen JF, Buchmann K (2013) Differential occurrence of immune cells in the primary and secondary vascular systems in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 36, 675–679. [DOI] [PubMed] [Google Scholar]
- Romano N, Fanelli M, Maria Del Papa G, et al. (1999a) Histological and cytological studies on the developing thymus of sharpsnout seabream, Diplodus puntazzo . J Anat 194(Pt 1), 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano N, Taverne‐Thiele AJ, Fanelli M, et al. (1999b) Ontogeny of the thymus in a teleost fish, Cyprinus carpio L.: developing thymocytes in the epithelial microenvironment. Dev Comp Immunol 23, 123–137. [DOI] [PubMed] [Google Scholar]
- Rombout JH, Huttenhuis HB, Picchietti S, et al. (2005) Phylogeny and ontogeny of fish leucocytes. Fish Shellfish Immunol 19, 441–455. [DOI] [PubMed] [Google Scholar]
- Rummer JL, Wang S, Steffensen JF, et al. (2014) Function and control of the fish secondary vascular system, a contrast to mammalian lymphatic systems. J Exp Biol 217, 751–757. [DOI] [PubMed] [Google Scholar]
- Sailendri K, Muthukkaruppan V (1975) Morphology of lymphoid organs in a cichlid teleost, Tilapia mossambica (Peters). J Morphol 147, 109–121. [DOI] [PubMed] [Google Scholar]
- Salinas I (2015) The mucosal immune system of teleost fish. Biology (Basel) 4, 525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos NM, Romano N, de Sousa M, et al. (2000) Ontogeny of B and T cells in sea bass (Dicentrarchus labrax, L.). Fish Shellfish Immunol 10, 583–596. [DOI] [PubMed] [Google Scholar]
- Song L, Wang L, Zhang H, et al. (2015) The immune system and its modulation mechanism in scallop. Fish Shellfish Immunol 46, 65–78. [DOI] [PubMed] [Google Scholar]
- Uribe C, Folch H, Enriquez R, et al. (2011) Innate and adaptive immunity in teleost fish: a review. Vet Med 56, 486–503. [Google Scholar]
- Vigliano FA, Losada AP, Castello M, et al. (2011) Morphological and immunohistochemical characterisation of the thymus in juvenile turbot (Psetta maxima, L.). Cell Tissue Res 346, 407–416. [DOI] [PubMed] [Google Scholar]
- Vogel WO (2010) Zebrafish and lymphangiogenesis: a reply. Anat Sci Int 85, 118–119. [DOI] [PubMed] [Google Scholar]
- Yaniv K, Isogai S, Castranova D, et al. (2007) Imaging the developing lymphatic system using the zebrafish. Novartis Found Symp 283, 139–148; discussion 148–151, 238–241. [DOI] [PubMed] [Google Scholar]
- Ye X, Li J, Lu M, et al. (2011) Identification and molecular typing of Streptococcus agalactiae isolated from pond‐cultured tilapia in China. Fish Sci 77, 623–632. [Google Scholar]
- Yi T, Li Y, Liu L, et al. (2014) Protection of Nile tilapia (Oreochromis niloticus L.) against Streptococcus agalactiae following immunization with recombinant FbsA and α‐enolase. Aquaculture 428–429, 35–40. [Google Scholar]
- Zapata A, Diez B, Cejalvo T, et al. (2006) Ontogeny of the immune system of fish. Fish Shellfish Immunol 20, 126–136. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zeng Z, Hu C, et al. (2016) Controlled and targeted release of antigens by intelligent shell for improving applicability of oral vaccines. Biomaterials 77, 307–319. [DOI] [PubMed] [Google Scholar]


