Abstract
The pelvic floor guards the passage of the pelvic organs to the exterior. The near‐epidemic prevalence of incontinence in women continues to generate interest in the functional anatomy of the pelvic floor. However, due to its complex architecture and poor accessibility, the classical ‘dissectional’ approach has been unable to come up with a satisfactory description, so that many aspects of its anatomy continue to raise debate. For this reason, we opted for a ‘sectional’ approach, using the Chinese Visible Human project (four females, 21–35 years) and the Visible Human Project (USA; one female, 59 years) datasets to investigate age‐related changes in the architecture of the anterior and middle compartments of the pelvic floor. The puborectal component of the levator ani muscle defined the levator hiatus boundary. The urethral sphincter complex consisted of a circular proximal portion (urethral sphincter proper), a sling that passed on the vaginal wall laterally to attach to the puborectal muscle (urethral compressor), and a circular portion that surrounded the distal urethra and vagina (urethrovaginal sphincter). The exclusive attachment of the urethral sphincter to soft tissues implies dependence on pelvic‐floor integrity for optimal function. The vagina was circular at the introitus and gradually flattened between bladder and rectum. Well‐developed fibrous tissue connected the inferior vaginal wall with urethra, rectum and pelvic floor. With eight‐muscle insertions, the perineal body was a strong, irregular fibrous node that guarded the levator hiatus. Only loose areolar tissue comprising a remarkably well developed venous plexus connecting the middle and superior parts of the vagina with the lateral pelvic wall. The posterolateral boundary of the putative cardinal and sacrouterine ligaments coincided with the adventitia surrounding the mesorectum. The major difference between the young‐adult and postmenopausal pelvic floor was the expansion of fat in between the components of the pelvic floor. We hypothesize that accumulation of pelvic fat compromises pelvic‐floor cohesion, because the pre‐pubertal pelvis contains very little fibrous and adipose tissue, and fat is an excellent lubricant.
Keywords: adiposity, aging, levator ani muscle, perineal body, urethral sphincter complex
Introduction
Pelvic floor dysfunction is a common problem among women, with vaginal deliveries, aging, and increasing body mass index (BMI) as acknowledged risk factors (Jelovsek et al. 2007). An accurate appreciation of the anatomy of the pelvic floor is a prerequisite for a proper interpretation of data generated by imaging modalities (Stein & DeLancey, 2008). The architecture of the levator and sphincter ani complexes, the urethral sphincter, and the perineal body and its extensions have been topics of contention for over a century and still attract attention (Dickinson, 1889; Oelrich, 1983; DeLancey, 1986; Woodman & Graney, 2002; Kearney et al. 2004; Yucel & Baskin, 2004; Wallner et al. 2009), implying that their anatomy is still incompletely understood. The connections of these entities with neighboring structures in particular remain topics of contention (Uhlenhuth & Day, 1948; Milley & Nichols, 1969; DeLancey & Starr, 1990; DeLancey, 1992; Fritsch et al. 2004; Kraima et al. 2015). These disagreements probably originate in the deep position and, hence, limited accessibility of the pelvic floor within the bony pelvis. Another reason may well be that our models are almost entirely based on dissection. Although dissection is clinically highly relevant, if it only as the tool of surgeons, the identification of structures by dissection relies almost entirely on their instantaneous recognition, with irreversibility and artifacts as major drawbacks. Testing the outcome of the dissectional approach by a different method is, therefore, necessary to confirm or improve the quality of anatomic descriptions.
Sectional anatomy is an obvious approach to address the above problems, because it does not suffer from limited accessibility and (stored) sections can be studied repeatedly. Drawbacks of ‘real’ sectioning – deformation and loss of alignment of the sections – do not apply to a sectional approach that is based on serial ‘shaving’ of thin layers from a frozen specimen and photographic registration of the ‘newly revealed’ surface. This technique, pioneered by Kathrein et al. (1996) and used to produce the Visible Human Project (Spitzer et al. 1996) and the Chinese Visible Human project (Zhang et al. 2003), allows the unambiguous identification and segmentation of most structures by their natural color and surrounding connective‐tissue sheaths.
In our earlier study, we used the ‘Visible‐Human’ approach to investigate the topography of the posterior compartment of the pelvic floor (Wu et al. 2015). The levator ani muscle (LAM) had a common anterior attachment to the pubic bone, but was separated posteriorly into the puborectal and ‘pubovisceral’ muscles (for a summary of our nomenclature, see Table 1). The anal‐sphincter complex comprised the subcutaneous external anal sphincter (EAS) and the U‐shaped puborectal muscles, which define the levator hiatus. In our present study, we investigated the topographic anatomy of the anterior and middle compartments of the young‐adult female pelvic floor. Because human anatomical structures change with age (Collas & Malone‐Lee, 1996; Reay Jones et al. 2003), we compared the findings with a similarly prepared postmenopausal specimen. The data were used to generate detailed interactive 3D topographic models of the pelvic floor.
Table 1.
Terminology of muscles in present study compared with that in International Anatomical Terminology (TA)
| Present study | LAM (TA) | EAS (TA) |
|---|---|---|
| Pubovisceral | Pubococcygeal + iliococcygeal | – |
| LAM | (Pubo‐ &iliococcygeal) + puborectal | – |
| Puborectal (deep portion) | Puborectal | Deep portion |
| Puborectal (superficial portion) | – | Superficial portion |
| EAS | – | Subcutaneous portion |
Note that the LAM has a single anterior attachment to the pubic bone, but separates posteriorly into the pubovisceral and puborectal muscles.
TA: Terminologia Anatomica (International Anatomical Terminology), Whitmore et al. (eds), Stuttgart: Thieme, 1998, 292 pp.
*International Anatomical Terminology, Whitmore et al. (eds), Stuttgart: Thieme, 1998, 292 pp.
Material and methods
Specimens
Four female specimens of the Chinese Visible Human project [CVH‐2 (22y), ‐4 (25y), ‐5 (25y), and CVO (35y) (Wu et al. 2015)] and the female of the Visible Human Project USA [VHF (59y) (Spitzer et al. 1996)] were studied. The preparation of the Visible Human images followed established protocols (Kathrein et al. 1996), as described in detail elsewhere (Spitzer et al. 1996; Zhang et al. 2003). The study was approved by the Ethics Committee of the Third Military Medical University (Chongqing, China). Written informed consent was obtained from the donors or their family members. The available biometric details are given in Table 2. Unfortunately, little is known about the medical backgrounds, including parity, but no significant pelvic pathologies were found in the specimens (see also Bajka et al. 2004). We use superior, inferior, anterior and posterior for topographical description.
Table 2.
Basic biometric data of the female CVH and VHF bodies, and details of the sections used
| CVH‐2 | CVH‐4 | CVH‐5 | CVO | VHF | |
|---|---|---|---|---|---|
| Age, years | 22 | 25 | 25 | 35 | 59 |
| Height (mm) | 1620 | 1620 | 1700 | a | 1700b |
| Weight (kg) | 54 | 57.5 | 59 | a | 83b |
| BMI | 20.5 | 21.9 | 20.4 | 28.7 | |
| Sectioning direction | Transverse | Transverse | Transverse | Sagittal | Transverse |
| Section thickness (μm) | 500 | 500 | 200 | 200 | 330 |
| Image Resolution | 3072 × 2048 | 4064 × 2704 | 4064 × 2704 | 4064 × 2704 | 2048 × 1216 |
Only lower abdomen and pelvis were sectioned.
Image segmentation
The segmentation protocol is described elsewhere (Wu et al. 2015). Criteria for segmentation were (thin) fibrous tissue fascia, and differences in tissue architecture and color. Furthermore, we always inspected the corresponding sections in all other CVH specimens before proceeding to segmentation. When segmenting, we always identified and delineated the unambiguous (parts of) structures first and then proceeded to the remaining portions. Using this approach iteratively, we distinguished 47 structures in the female true pelvis (Wu et al. 2015).
Three‐dimensional reconstruction
The identified structures were reconstructed using the 3D surface‐rendering and ‐smoothing tools of amira software (http://www.amiravis.com). The 3D data were exported to Adobe acrobat 9 pro extended (http://www.adobe.com) to generate an interactive 3D‐pdf file (de Boer et al. 2011). All structures identified are represented in these 3D reconstructions in an interactive format [CVH‐5: Supplemental Figure 3 in Wu et al. (2015); VHF: Supporting Information Fig. S1]. The 3D‐pdf format allows the reader to visualize all structures separately or in self‐chosen combinations to reveal their topographical relations and can be displayed on almost all personal computers. Furthermore, we provide evenly spaced serial sections of the transversely sectioned CVH‐5 and the sagittally sectioned CVO specimens for inspection in Supporting Information Figs S3 and S4, respectively.
Figure 3.

The topographic relations of the perineal body. (I) Position of the transverse sections in (A–C) and the sagittal sections in (D–F). (A–C) Intertwining of the perineal body (whitish) and deep perineal muscle (brown contour), with the venous plexus (black) mostly occupying the periphery of the perineal complex. (D–F) Topographic relation of the perineal body to anterior, superior, and posterior muscles. (G,H) Slightly left oblique view of perineal body as seen from inferior prior to (G) and after removal (H) of the perineal body and deep perineal muscle. The contours in (G,H) delimit both structures and demonstrate the role of the perineal body and muscles as guards of the pelvic floor between the anal canal and vagina. AL, anococcygeal ligament; B, bladder; Bar, Bartholin's gland; DP, deep perineal muscle; EAS, external anal sphincter; PB, perineal body; PR‐D and PR‐S, deep and superficial portions of puborectal muscle; R, rectum; RC, rectococcygeal muscle; U, urethra; UC, urethral compressor; Ute, uterus; UVS, urethrovaginal sphincter; V, vagina; Vv, veins. The color code of the (outlined) structures is shown in Figs S1 and S2.
Results
We encourage readers to inspect and compare shape, relative size, and topographical relations in the CVH‐5 specimen in our previous publication (Wu et al. 2015) with the VHF specimen (Fig. S1). We first describe the topographic anatomy of the anterior and middle compartments of the pelvic floor in the young‐adult CVH specimens and then compare the findings with those in the postmenopausal VHF specimen.
Physical differences among the CVH specimens
Bladders in CVH‐2 and ‐5 had lumens of ~57 and ~72 mL, whereas those in CVH‐4 and CVO were virtually empty. The uteri of CVH‐2 and CVH‐5 were anteverted, whereas those of CVH‐4 and CVO were retroverted, as is common among Orientals. Possibly as a result of their partially filled bladders, the uteri of CVH‐2 and ‐5 both deviated to the right, which caused a markedly more tortuous course of the right than left uterine tube. The volume of the CVH uteri was 57 ± 12 mL and that of both ovaries together 13 ± 6 mL. The proximal part of uterine tube was typically narrow and its wall thin, whereas the distal part was wider and thicker. Biometric data on the position of the pelvic floor are presented in Table 3. As reported (Wu et al. 2015), the levator hiatus in CVH specimens was ~17 mm longer, ~12 mm narrower, and had descended 3–5 mm more than in live Chinese females of the same age (Cai et al. 2013).
Table 3.
Biometric data of the pelvic floor in CVH and VHF specimens
| CVH females | VHF female | |
|---|---|---|
| Pubococcygeal line (PCL) | 94 ± 3 | 96 |
| Bladder neck‐PCL distance | 9 ± 2 | 21 |
| ‘H’‐line | 61 ± 4 | 79 |
| ‘M’‐line | 15 ± 3 | 20 |
| ‘WLH’‐line | 23 ± 3 | 19 |
Pubococcygeal line: pubic arch to coccyx. ‘H’‐line: pubic arch to posterior anorectal junction (~anteroposterior length of levator hiatus). ‘M’‐line: posterior anorectal junction to pubococcygeal line (~descent of levator hiatus). ‘WLH’‐line: width of levator hiatus. Length is given in mm.
The urethral sphincter complex
The urethra was 41 ± 3 mm long in the four specimens. The thickness of the mucosal layer was ~ 0.7 mm and that of the submucosal layer ~ 2.5 mm. The distance from the bladder neck to the pubococcygeal line was 9 ± 2 mm. The biometric data of the urethra is reported in Table 4. The urethral sphincter complex covered the upper ~80% of the urethra, but not its most inferior portion between the vestibular bulbs. It was not possible to delineate the boundary between the inner smooth‐ and the outer striated‐muscle layers. Three components made up the striated urethral sphincter complex: the urethral sphincter proper, the urethral compressor, and the urethrovaginal sphincter. The sphincter proper encircled the urethra and contained a tendinous portion in the posterior midline (Fig. 1A,I). The U‐shaped compressor muscle surrounded the urethra and the urethral sphincter anteriorly and laterally at the transition of the upper two‐third into the lower one‐third (Fig. 1). Posteriorly, the compressor muscle passed on the vaginal wall to insert into the anteroinferior border of the puborectal muscle, just anterior to the attachment of that muscle to the perineal body (Fig. 1E,H). In none of the specimens, did we observe any connection to the pubic bone. The urethrovaginal sphincter surrounded the urethra anteriorly and laterally just inferior to the sphincter proper and surrounded the inferior end of the vaginal wall laterally and posteriorly, where it attached to the anteroinferior part of the perineal body (Fig. 1B–I). The urethrovaginal sphincter was only identifiable in the CVH‐5 and CVO specimens.
Table 4.
Biometry of pelvic structures in CVH and VHF specimens
| CVH | VHF | |
|---|---|---|
| Obturator internus | 94.7 ± 14.9 mL | 98.8 mL |
| Levator ani muscle | 29.2 ± 4.1 mL | 35.0 mL |
| External anal sphincter | 9.4 ± 2.6 mL | 7.9 mL |
| Internal anal sphincter | 5.0 ± 2.2 mL | 5.3 mL |
| Urethral length | 41 ± 3 mm | 41 mm |
| Urethral mucosa thickness | 0.7 mm | 0.7 mm |
| Urethral submucosa thickness | 2.5 mm | 1.7 mm |
| Urethral sphincter complex | 4.2 ± 1.0 mL | 2.9 mL |
| Ovaries | 12.9 ± 6.3 mL | 2.9 mL |
| Uterus | 56.7 ± 12.4 mL | 43.3 mL |
| Vagina (diameter introitus) | 19 ± 3 mm | 7 mm |
| Vagina (anterior width figure H) | 28 ± 1 mm | 28 mm |
| Vagina (subcervical width) | 40 ± 2 mm | 48 mm |
| Vaginal length (anterior) | 57 ± 5 mm | 53 mm |
| Vaginal length (posterior) | 74 ± 4 mm | 76 mm |
| Bulbospongious muscle | 3.1 ± 1.2 mL | 3.1 mL |
| Ischiocavernous muscle | 3.4 ± 0.9 mL | 1.8 mL |
| Cavernous body of clitoris | 1.7 ± 0.4 mL | 2.7 mL |
| Superficial transverse perineal muscle | 1.0 ± 0.4 mL | 3.5 mL |
| Rectovaginal muscle | 1.1 ± 0.4 mL | 0.6 mL |
| Rectococcygeal muscle | 2.1 ± 1.0 mL | 3.3 mL |
| Perineal body | 2.7 ± 1.7 mL | 0.6 mL |
| Perineum | 21 ± 3 mm | 35 mm |
| Mesorectal fat tissue | 5.6 mLa | 28.1 mL |
| Paravesical fat tissue | 20.4 mLa | 94.5 mL |
Data are given as average volume (mL) and standard deviation, except for urethra, vagina, and perineum (given in mm).
Only reconstructed and measured in CVH‐4.
Figure 1.
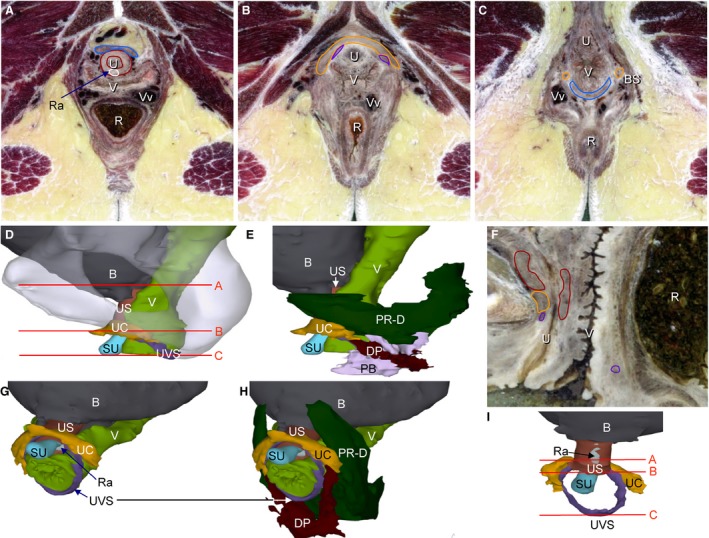
The architecture of the urethral sphincter complex. (D,I) The position of the transverse sections in (A–C) (CVH‐5). (F) Section in the mid‐sagittal plane (CVO). (D,G) Position of the urethral sphincter complex relative to the vagina (left lateral and left‐anterior views, respectively). (E,H) Deep portions of puborectal and deep perineal muscles were added to reveal their relation to the urethral compressor. (E) The perineal body, if well developed, has a superolateral extension above the deep perineal muscle. (F) Position of the three components of the urethral sphincter relative to urethra and vagina. (I) Muscles of the urethral sphincter complex, posterior view. B, bladder; DP, deep perineal muscle; PB, perineal body; PR‐D, deep portion of puborectal muscle; R, rectum; Ra, tendinous raphe of urethral sphincter; SU, submucous portion of urethra; U, urethra; UC, urethral compressor; US, urethral sphincter; UVS, urethrovaginal sphincter; V, vagina; Vv, veins. The color code of the (outlined) structures is shown in Figs S1 and S2.
The vagina and its supports
The biometric data of the vagina is reported in Table 4. The outer shape of the vagina near its junction with the vestibule was circular on cross‐section (diameter: 19 ± 3 mm; Fig. 2A). At this position, we identified the urethrovaginal sphincter on its lateral surface. More superiorly, the anterior wall of the vagina widened to cover the posterolateral wall of the urethra (Fig. 2B). The posterior wall of the vagina also widened, albeit to a lesser extent, to accommodate the rectoperineal muscle. The cross‐sectional shape of the vaginal lumen, therefore, resembled the letter ‘H’ and had a trapezoid outline, with its widest side anteriorly. Superiorly, from the level of bladder neck and anorectal flexure, the folds in the vaginal wall flattened and the vagina widened to 40 ± 2 mm (Fig. 2C,D). The perimeter of the vaginal lumen, therefore, did not differ between its inferior and superior portions. At its superior end, the vagina (fornix) formed an inverted cone around the cervix (Fig. 2F). The distance from the vaginal orifice to the tip of the anterior vaginal fornix was 57 ± 5 mm and ~ 25% shorter than that to the posterior fornix (74 ± 4 mm).
Figure 2.

The shape and topographical relations of the vagina. (J) Position of the transverse sections in panels (A–E). (A) Section through the introitus of the vagina, (B) where the vagina covers the urethra posteriorly and the rectoperineal muscle anteriorly, (C,D) where the vagina is sandwiched between the rectum and urethra (C) and bladder (D), and (E) through the cervix of the uterus. (F,H) Left‐anterior and antero‐inferior views of the changes in shape of the vaginal lumen between the introitus and transition into the uterine cervix. The entrance to the vagina is more or less circular in outline, adopts an H‐shaped figure behind the urethra and a flat, anteriorly concave shape behind the bladder. (G,I) Muscular wall of the vagina from the same point of view as (F,H). (I) Lower part of the vagina has close topographical relations with the urethral sphincter anteriorly and laterally. (J) Venous plexus at the lateral side of the uterine cervix and vagina. The puborectal muscles are shown to avoid overprojection of veins, and the contour of the bladder wall is shown (interrupted orange line). Note the extensive venous plexus near the uterine artery. B, bladder; Cx, cervix; PR‐D, deep puborectal muscle; R, rectum; U, urethra; UC, urethral compressor; US, urethral sphincter; Ute, uterus; V, vagina; Vv, veins. The color code of the (outlined) structures is shown in Figs S1 and S2.
The bulbospongious muscles guard the entrance to the vagina just below the pelvic floor and surround the vestibular bulbs anteriorly and Bartholin's glands posteriorly. Medial to Bartholin's gland, the urethrovaginal sphincter surrounded the vaginal introitus. Well‐developed fibrous tissue connected the vaginal wall to the urethra anteriorly, to the perineal and puborectal muscles laterally, and the perineal body with its attached muscles posteriorly (Fig. 2A,B). More superiorly, however, only loose areolar tissue containing many veins was present between the vaginal wall and the medial layer of the pubovisceral muscle (Fig. 2C–F). Posteriorly, Denonvilliers’ fascia formed the connection between the vaginal and rectal walls (Fig. 2C). The vaginal wall was, thus, surrounded and fixed by dense connective tissue inferiorly, whereas it was only supported by loose connective tissue and a rich venous plexus above the urethral compressor and Denonvilliers’ fascia.
The perineal body
The perineal body was a highly irregular fibrous structure in the wedge‐shaped space between the vagina anteriorly, the anal canal posteriorly, and the anorectal bend superiorly (Fig. 3). Irregular fibrous extensions of the perineal body form the tendinous attachments of eight muscles: the rectoperineal and deep perineal muscles posteriorly, the medial layer of the pubovisceral muscle and the superficial and deep portions of the puborectal muscle laterally, and the urethrovaginal sphincter, superficial transverse perineal and bulbospongious anteriorly. The size of the perineal bodies depended on fibrous tissue development in the specimens and was smallest in the CVH‐4 specimen (1.0 mL) and largest in the CVH‐5 specimen (4.4 mL). The relatively large perineal body in CVH‐5 had superficial and deep parts. The superficial (inferior) part was ‘V’‐shaped, with wings extending anterolaterally towards the lateral sides of the vagina (Fig. 3). The deep (superior) part was formed by a tendinous plate on the inferior surface of the puborectal muscle (Fig. 3). In specimens with a smaller perineal body, the superior part was less developed.
The deep perineal muscle occupied the space between the incompletely separated superficial and deep portions of the perineal body, and extended from the posteroinferior side of the perineal body to the junction of the urethral compressor muscle with the puborectal muscle antero‐superiorly (Figs 2 and 3). The deep perineal muscle co‐localized with the ‘puboperineal’ muscle identified in MRI images (Lien et al. 2004) but did not reach the pubic bone in any of the specimens studied. Several large veins were present in and lateral to the perineal body and deep perineal muscle.
Muscles of the urogenital triangle
The ischiocavernous, bulbospongious and superficial transverse perineal muscles form the ‘urogenital triangle’. The ischiocavernous muscle is attached to the ischial tuberosity posteriorly and to the medial side of the posterior end of the cavernous body of the clitoris anteriorly. The bulbospongious muscles, which covered the vestibular bulbs superficially, attached to the inferior side of the cavernous bodies anteriorly, while its left and right sides connected to each other posterior to the perineal body. The superficial transverse perineal muscles extended laterally as slender ‘twigs’ from the posterior side of the perineal body and the junction of both bulbospongious muscles. The number, size, and length of these twigs differed markedly in the four specimens, but in none of the specimens did the muscle fibers of the superficial transverse perineal muscle reach the ischial tuberosity as is generally shown in atlases. Instead, they ended on fibrous septa in the fat of the ischioanal fossa (Fig. 3).
Vessels
Arteries followed well established courses. An extensive venous plexus was present between the vagina and cervix medially, and the LAM laterally. This plexus was particularly dense around the uterine artery where it approached the uterine cervix, but rapidly attenuated further superiorly in the broad ligament. Inferiorly, where it passed the puborectal muscle medially, the venous plexus separated, relative to the vaginal wall, into antero‐ and posterolateral parts. The anterolateral part descended into the areolar tissue surrounding the bladder neck and urethra above its compressor muscle, whereas the posterolateral part passed, via numerous perforations, through the perineal muscle, perineal body, and between the puborectal muscle and the pelvic bones to the anterior part of the ischioanal fossa. The pelvic venous plexus had hardly any communication with the veins of the lower limbs (Fig. 2).
The effects of aging
Shape of the pelvic floor
The volume of the anteverted VHF uterus was 43 mL (~ 75% of that in the CVH specimens) and that of both ovaries together 3 mL (~ 20% of that in the CVH specimens; Table 4). The contracted state of the upper rectum and large volume of the rectal ampulla (~ twofold larger than in CVH specimens) were other striking features of the VHF. The position of the pelvic floor (Hoyte et al. 2001; Fielding, 2002) was different, too (Table 3). The ‘pubococcygeal line’ (96 mm) was, in agreement with their similar size, not different from that of CVH specimens, but the ‘H‐line’ (length of levator hiatus; 79 mm) and ‘M‐line’ (descent of levator hiatus; 20 mm) were ~ 30% longer than in CVH women. The ‘WLH‐line’ (width of levator hiatus; 19 mm) was ~ 83% of that in CVH females. These biometric data indicate that the postmortem levator hiatus of VHF was 24 mm longer and 6 mm narrower, and had descended 6 mm more than that of asymptomatic live American females of the same age and body mass index (Fielding, 2002). We ascribe these differences to the relaxation of muscles, including those of the pelvic floor, in cadavers (Fig. 4).
Figure 4.

The comparison of young (CVH‐4) and old (VHF) specimens. (M) Level of sections (A–D) (VHF) and (E–H) (CVH‐4). The VHF specimen is compared with the CVH‐4 specimen because transverse sections through these specimens have the same inclination. (A,E) A section through the lower part of the uterus, (B,F) through the cervix, (C,G) through the middle part of the vagina, and (D,H) through the introitus of the vagina. (I–L) Magnifications of the boxed areas in (C,D,G,H). Note the increase of the fat volume of the mesorectum and the paravesical space in panels (A,B) compared with (E,F), the more anterior extension of the ischio‐anal space in (B,C) compared with (F,G), and the atrophic and anteriorly bulging flattened appearance of the vagina in C (I), D (K) compared with (G (J), H(L)). Also note the more atrophic external anal sphincter in (D) compared with (H). The color code of the (outlined) structures is shown in Figs S1 and S2.
Volume of pelvic‐floor muscles
The volume of striated muscles in the pelvic floor was similar in CVH and VHF females (Table 4). The VHF vagina had similar width, but thinner walls and fewer mucosal folds than CVH vaginas. Additionally, the posterior vaginal wall protruded into the vaginal lumen. The volume of the perineal body was ~ fourfold smaller and the length of the perineum ~ 1.5 longer in VHF as compared with CVH specimens (Table 4). The stretch resulting from the large rectal ampulla made the surrounding pelvic‐floor muscles thinner, but their volume was similar to that of CVH specimens.
Differences in volume and distribution of fat
The higher BMI predicted the ~ fivefold higher pelvic fat volume in VHF than in CVH specimens. The abdominal subcutaneous panniculus was ~ twofold thicker in VHF than in CVH specimens. The ischioanal fossa is delimited anteriorly by the attachment of the levator ani muscle to the internal obturator via its tendinous arch. In CVH specimens, the tendinous arch of the LAM was an inconspicuous structure (Fig. 4E–H), but in VHF this arch was virtually absent and fatty tissue was present further anteriorly between internal obturator and the levator ani muscles (Fig. 4A–D). Fat above the levator ani muscle surrounded the base of the bladder down to the urethral sphincter and was very abundant in the adnexa of the vagina and cervix. As a result, the anterior part of the levator ani muscle was sandwiched between abundant layers of fat, and the bladder and vagina appeared to ‘float’ on this sandwich. The venous plexus between bladder and vagina on the one hand and the levator ani on the other was less well developed in VHF than in CVH females.
Summary
Using this approach we found that the levator ani muscles have a different architecture than usually described (Wu et al. 2015), that the urethral sphincter attaches only to the fascia of the LAM, that no direct muscular attachment of the LAM to the vaginal wall exists, and that the eight muscle pairs that guard the levator hiatus are all attached to the perineal body. Though one can object that these findings are based only on four young‐adult and one postmenopausal female cadavers, they were present in every specimen. The most marked difference among the specimens was in the development of connective tissue, which was most prominent in the CVH‐5 and CVO specimens. Furthermore, a sizable fat mass had accumulated at both sides of the LAM in the postmenopausal VHF specimen.
Discussion
A precise knowledge of the uncompromised topography of the pelvic floor is important to recognize degenerative changes due to deliveries or aging (Collas & Malone‐Lee, 1996; Reay Jones et al. 2003). Pelvic‐floor topography as deduced from serial sections that were processed according to the Visible Human protocol (Kathrein et al. 1996; Spitzer et al. 1996; Zhang et al. 2003) does not suffer from topographic and dissection artifacts and allows the use of an iterative approach to delineate structures carefully and properly.
The urethral sphincter complex
Topographic anatomy of the female urethral sphincter is well studied (e.g. Oelrich, 1983; Kokoua et al. 1993; Colleselli et al. 1998; Ludwikowski et al. 2001; Yucel & Baskin, 2004; Sebe et al. 2005). The external urethral sphincter is a complex structure with proximal (superior) and distal (inferior) parts (Oelrich, 1983). The urethral sphincter complex did not cover the portion of the urethra between the vestibular bulbs. The most distal part of the sphincter complex, the thin urethrovaginal sphincter, encircles both urethra and vagina, whereas the more proximal portions were posteriorly open loops with the vaginal wall (urethral compressor) or the dorsal fibrous raphe (urethral sphincter) interposed. The dorsal raphe allows the urethral sphincter to function as a real sphincter and fixes the urethra to the ventral wall of the vagina (DeLancey, 1992; Fritsch et al. 2006). The well developed urethral compressor inserts on the perimysia of the puborectal and deep perineal muscles, as we described previously in fetuses (Wallner et al. 2009). In none of our specimens did the urethral compressor insert, as is usually described (Oelrich, 1983; Fritsch & Frohlich, 1994; Frohlich et al. 1997; Colleselli et al. 1998; Fritsch et al. 2006), on the inferior branch of the pubic bone. The position of the urethral compressor colocalizes with the maximal intra‐urethral pressure during pelvic‐floor contraction in females (Westby et al. 1982; Constantinou, 2009). In fact, its contraction can even be registered in the vagina (Shishido et al. 2008). The sole attachment to soft tissues implies that the function of the urethral compressor, which compresses and bends the urethra in a posterior direction, depends on the functionality of the pelvic‐floor muscles. The alternation of sphincteric and looped portions that, by bending and narrowing the lumen, mediate continence is strikingly similar to the sphincter mechanism of the anorectal canal. We hypothesize that the longer term success of midurethral sling procedures for stress incontinence (Ridgeway & Barber, 2012) depends on accentuation of this mechanism.
The urogenital diaphragm and the deep perineal muscle
The urethral sphincter complex is often linked to the concept of a ‘urogenital diaphragm’, i.e. a ‘deep transverse perineal muscle’ with a thick inferior perimysium, the ‘perineal membrane’ (for review, see Mirilas & Skandalakis, 2004). Some studies found no evidence of a urogenital diaphragm (Dorschner et al. 1999), but others maintain that the deep transverse perineal muscle is present as a smooth muscle in females (Oelrich, 1983; Kokoua et al. 1993; Fritsch et al. 2004; Nakajima et al. 2007). We observed the ‘perineal’ muscle inferior to the puborectal muscles between the superior and inferior wings of the perineal body. The close association with the perineal body and its extensions explains the association with the perineal membrane (Stein & DeLancey, 2008). At this very location, DeLancey cum suis (Lien et al. 2004) observed the ‘puboperineal’ muscle in MRI images. Because of its > threefold stretch during simulated vaginal delivery, it has to be a smooth muscle (Seow & Solway, 2011). However, the perineal muscle we observed did not attach itself to the pubic bone but is contiguous anteriorly with the urethral compressor at the origin of that muscle from the puborectal muscle.
Perineal body
The perineal body is located in the space between the vagina, anal canal, and the perineal skin. On dissection from the perineal surface, it presents as a pyramidal fibromuscular node with superficial, middle, and deep layers that connect to the subcutaneous EAS, and the superficial and deep portions of the puborectal muscle, respectively (Woodman & Graney, 2002; Shafik et al. 2007). However, such dissection is difficult and generates artifacts. In sections, the perineal body is a highly irregular fibrous node that varies in size and extent depending on the degree of connective‐tissue development. It is the true central tendon of the perineum, guarding the levator hiatus, serving as insertion for two unpaired (the urethrovaginal sphincter anterosuperiorly and the rectoperineal muscle posterosuperiorly) and six paired muscles (the medial portion of the pubovisceral muscle and the deep portion of the puborectal muscles laterosuperiorly; the superficial portion of the puborectal muscles posterolaterally; and the superficial transverse perineal, bulbospongious, and perineal muscles posteriorly). These eight muscles exert forces in anterosuperior, posterosuperior, laterosuperior, and posterolateral directions. There exists controversy concerning whether the bulbospongious, superficial transverse perineal muscles, and EAS insert into the perineal body or just pass this structure posteriorly (Shafik et al. 2007; Larson et al. 2010). Our results indicate that fibers of the bulbospongious and superficial transverse perineal muscles are indistinguishable in the midline, where they form a muscle bundle that lies deeper than the EAS and is attached to the posterior part of the perineal body.
The supports of the vagina and uterus
The vagina is often depicted as a tubular structure, but its lumen changes from narrow, with radially oriented epithelial folds at the introitus, to an ‘H’ shape, where it covers the urethra on its posterolateral sides, and then to a flat, anteriorly slightly concave structure on the posterior side of the bladder. This description concurs with that of in vivo vaginal casts (Pendergrass et al. 1996). Despite these regional differences of shape, the perimeter of the vaginal lumen is similar at all levels. The smooth‐muscle wall of the vagina followed the contour of the lumen.
The supports of the vaginal wall that guard against pelvic organ prolapse are still contentious. In DeLancey's concept (DeLancey, 1992), the upper third of the vagina (level I) is suspended from the pelvic walls by the vertical and posterior fibers of the cardinal and uterosacral ligaments, respectively (Ramanah et al. 2012a). The inclusion of tissue surrounding the proximal, more vertical part of the uterine artery in the cardinal or transverse cervical ligament (Ramanah et al. 2012b; Samaan et al. 2014) has made this putative ligament a more vertical structure that would account for ~ 60% of the tension keeping the uterus in place (Chen et al. 2013). However, the sacrouterine ligament is more rigid (Rivaux et al. 2013) and stronger (Buller et al. 2001). Nevertheless, the biomechanical properties of both ligaments vary widely among individuals, with standard deviations being similar to the mean (Rivaux et al. 2013). In agreement with many other studies (Goff, 1931; Koster, 1933; Ricci et al. 1947; Campbell, 1950; Berglas & Rubin, 1953; Range & Woodburne, 1964; Frohlich et al. 1997; De Caro et al. 1998; Vu et al. 2010; Hockel et al. 2011), we (Wu et al. 2015) found no histological basis for either ligament, although sacrouterine folds (Frohlich et al. 1997; Buller et al. 2001) were identifiable. We have not, therefore, tried to reconstruct these ligaments. Unexpectedly, we found that the neurovascular bundle and its associated connective tissue in the deeper part of the cardinal and sacrouterine ligaments (Umek et al. 2004; Hsu et al. 2008; Ramanah et al. 2012a,b) co‐localize with the rectal adventitia that covers the mesorectum, implying that these surgically important structures are one and the same. The discrepancy between the identification of the sacrouterine and cardinal ligaments by surgeons and the absence of histological evidence for these structures can probably be ascribed to their composition of areolar connective tissue fibers: such fibers are not prominent on sections, but the mesh, which also connects to vascular and nervous bundles, transforms into a tense, and hence visible, strand under acute local traction (Range & Woodburne, 1964; De Caro et al. 1998).
In its middle third (‘level II’, DeLancey, 1992), the vagina attaches via its paracolpium to the tendinous arch of the pelvic fascia (DeLancey, 1992). This arch is already identifiable in fetuses (Fritsch et al. 2006; Wallner et al. 2009) and is well documented in adult specimens (Occelli et al. 2001; Pit et al. 2003). However, like the cardinal and sacrouterine ligaments (Rivaux et al. 2013), its strength varies widely among individuals (Pit et al. 2003). The variation in strength of the pelvic ligaments may well reflect the pronounced differences in connective tissue development that we observed among the CVH specimens. However, we did not observe (well developed) fibrous connections between the vagina and the lateral pelvic wall, including the tendinous arch of the pelvic fascia and therefore conclude that level‐II support was virtually non‐existent in our specimens. Other histological studies (Niikura et al. 2007; Zhai et al. 2009), which show ‘fascial bridges’ close to the pelvic organs that rarify laterally, seem to underscore this view. Instead, a very extensive network of mostly very large veins intervened between the vaginal wall and the endopelvic fascia. These veins contain no valves (Batson, 1940), indicating that the paracolpium can rapidly reduce its volume upon a small increase in external pressure. These findings appear to explain the ‘slack cord’ paradigm of DeLancey cum suis, who observed that the force necessary to displace the uterus over a distance as observed upon a Valsalva maneuver was unexpectedly small (Smith et al. 2013; Swenson et al. 2016). In fact, these observations strongly suggest that the large veins in the paracolpium are responsible for the absorbance of acute increases in intra‐abdominal pressure, such as occur during coughing. Dysfunction of this pressure absorbance mechanism due, for example, increased stiffness of the pelvic soft tissues as occurs with aging (Chantereau et al. 2014) is probably one of the causes of stress urinary incontinence.
The wall of the vagina lower third (‘level III’, DeLancey, 1992) contacts the perineal membrane, perineal body, and pelvic floor muscles (Figs 1A,B and 2A–C). Like other studies (Frohlich et al. 1997; Yucel & Baskin, 2004), we did not observe any direct muscular attachment of the puborectal muscle to the vaginal or urethral wall. Instead, we found well developed fibrous tissue at this location that may correspond to the white area just inside the hymenal ring (DeLancey, 1988; DeLancey & Starr, 1990).
The question, therefore, arises as to which structure or structures support the upper vagina and uterus. Seen from below, the fibro‐muscular reinforcement of the posterior compartment of the pelvic floor is striking, whereas the vaginal opening, although laterally firmly attached to the pelvic floor by dense connective tissue, appears vulnerable. One should, however, realize that the pelvic viscera extend laterally and posteriorly relative to the levator hiatus and that in vivo tracing revealed that increased intra‐abdominal pressure pushes the viscera backward and laterally against the levator plate and not towards the hiatus, irrespective of whether the uterus is ante‐ or retroverted (Berglas & Rubin, 1953; Bastian & Lassau, 1982). Reactive contraction of the pelvic floor (Constantinou, 2009) further contributes to this support, because the puborectal muscles move the anorectal junction forward and elevate the perineal body, while the pubovisceral muscles flatten the posterolateral parts of the levator plate. The attachment of the puborectal muscles to the perineal body, therefore, represents a crucial structural feature, without which contraction of the deep portion of the puborectal muscle, because of its curvilinear course (Wu et al. 2015), would be ineffective. Failure of this supporting function of the pelvic floor is thus associated with a more vertical position of the pelvic floor and a downward movement of the pelvic organs during increased intra‐abdominal pressure (Berglas & Rubin, 1953).
Pelvic connective tissue is often divided into presacral, perirectal, and paravisceral compartments (Fritsch & Hotzinger, 1995; Fritsch et al. 2004b). Compared with the young female pelvis, much more adipose tissue was located in the paravisceral and perirectal compartments of the postmenopausal pelvis, which broadened the space between bladder, uterus, cervix, rectum, and obturator internus. The menopausal transition is known to be associated with a relative and absolute increase in visceral fat (for a recent review, see Karvonen‐Gutierrez & Kim, 2016). Since the pre‐pubertal pelvis contains very little fibrous and adipose tissue, and since fat is an excellent lubricant, we hypothesize that pelvic fat compromises cohesion between the components of the pelvic floor, increasing its structural instability, just like a modern car frame becomes less stable as the glue between its components dissipates.
Limitations of the study
Although the number of specimens studied may appear small, only seven adult female ‘Visible Human’ specimens have been reported thus far (Dai et al. 2012). Of these, five were 19–26 years old, one was in her mid‐30s (CVO), and only one was postmenopausal (VHF). Except for the position of the uterus, we have not observed anatomical differences between the Chinese and American specimens.
Conclusion
Our study indicates that the large venous plexuses flanking the vagina serve to cushion rapid increases in intra‐abdominal pressure. Biomechanical modeling of the pelvic floor additionally suggests that all known ‘ligaments’ are necessary to ensure the normal mobility of pelvic organs upon thrusting (Cosson et al. 2013). This finding supports our hypothesis that tissue adhesion is essential and integral to the construction of the female pelvic floor and its proper function. Age‐related changes that diminish tissue adhesion, e.g. accumulation of adipose tissue, may negatively affect the robust construction of the pelvic floor. The progressive deposition of fibrous connective tissue, of which the size of the perineal body appears to be a good marker, may well reflect wear and tear from the stresses and strains (deliveries, erect bipedal status, etc.) to which the female body is subjected during life. Such tissue remodeling attempts may support pelvic‐floor function to some extent, but also probably diminish the cushioning function of the pelvic veins. Our hypotheses are based on a limited number of specimens and need to be tested, but, if correct, imply that interventions to treat pelvic‐floor dysfunction should not increase pelvic‐floor rigidity.
Conflict of interest
None of the authors declare a conflict of interest.
Supporting information
Fig. S1. Interactive 3D rendering of the topographic anatomy of the VHF pelvic floor.
Fig. S2. The color code used to label structures in Figs 1–4 and Fig. S1.
Fig. S3. Serial transverse sections of the lesser pelvis of specimen CVH‐5.
Fig. S4. Serial sagittal sections of the lesser pelvis of specimen CVO.
Acknowledgements
This work was supported by the John L. Emmett Foundation for Urology, The Netherlands, and the National Science Foundation of China (No. 81100480).
Contributor Information
Shao‐Xiang Zhang, Email: zhangsx1021@yahoo.com.
Wouter H. Lamers, Email: w.h.lamers@amc.uva.nl
References
- Bajka M, Manestar M, Hug J, et al. (2004) Detailed anatomy of the abdomen and pelvis of the visible human female. Clin Anat 17, 252–260. [DOI] [PubMed] [Google Scholar]
- Bastian D, Lassau JP (1982) The suspensory mechanism of the uterus. Anat Clin 4, 147–160. [Google Scholar]
- Batson OV (1940) The function of the vertebral veins and their role in the spread of metastases. Ann Surg 112, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglas B, Rubin IC (1953) Histologic study of the pelvic connective tissue. Surg Gynecol Obstet 97, 677–692. [PubMed] [Google Scholar]
- de Boer BA, Soufan AT, Hagoort J, et al. (2011) The interactive presentation of 3D information obtained from reconstructed datasets and 3D placement of single histological sections with the 3D portable document format. Development 138, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller JL, Thompson JR, Cundiff GW, et al. (2001) Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol 97, 873–879. [DOI] [PubMed] [Google Scholar]
- Cai XR, Qiu L, Wu HJ, et al. (2013) Assessment of levator ani morphology and function in asymptomatic nulliparous women via static and dynamic magnetic resonance imaging. Int J Gynaecol Obstet 121, 233–239. [DOI] [PubMed] [Google Scholar]
- Campbell RM (1950) The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol 59, 1–12. [DOI] [PubMed] [Google Scholar]
- Chantereau P, Brieu M, Kammal M, et al. (2014) Mechanical properties of pelvic soft tissue of young women and impact of aging. Int Urogynecol J 25, 1547–1553. [DOI] [PubMed] [Google Scholar]
- Chen L, Ramanah R, Hsu Y, et al. (2013) Cardinal and deep uterosacral ligament lines of action: MRI based 3D technique development and preliminary findings in normal women. Int Urogynecol J 24, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collas DM, Malone‐Lee JG (1996) Age‐associated changes in detrusor sensory function in women with lower urinary tract symptoms. Int Urogynecol J Pelvic Floor Dysfunct 7, 24–29. [DOI] [PubMed] [Google Scholar]
- Colleselli K, Stenzl A, Eder R, et al. (1998) The female urethral sphincter: a morphological and topographical study. J Urol 160, 49–54. [DOI] [PubMed] [Google Scholar]
- Constantinou CE (2009) Dynamics of female pelvic floor function using urodynamics, ultrasound and Magnetic Resonance Imaging (MRI). Eur J Obstet Gynecol Reprod Biol 144(Suppl 1), S159–S165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson M, Rubod C, Vallet A, et al. (2013) Simulation of normal pelvic mobilities in building an MRI‐validated biomechanical model. Int Urogynecol J 24, 105–112. [DOI] [PubMed] [Google Scholar]
- Dai JX, Chung MS, Qu RM, et al. (2012) The Visible Human Projects in Korea and China with improved images and diverse applications. Surg Radiol Anat 34, 527–534. [DOI] [PubMed] [Google Scholar]
- De Caro R, Aragona F, Herms A, et al. (1998) Morphometric analysis of the fibroadipose tissue of the female pelvis. J Urol 160, 707–713. [DOI] [PubMed] [Google Scholar]
- DeLancey JO (1986) Correlative study of paraurethral anatomy. Obstet Gynecol 68, 91–97. [PubMed] [Google Scholar]
- DeLancey JO (1988) Structural aspects of the extrinsic continence mechanism. Obstet Gynecol 72, 296–301. [PubMed] [Google Scholar]
- DeLancey JO (1992) Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 166, 1717–1724; discussion 1724–1728. [DOI] [PubMed] [Google Scholar]
- DeLancey JO, Starr RA (1990) Histology of the connection between the vagina and levator ani muscles. Implications for urinary tract function. J Reprod Med 35, 765–771. [PubMed] [Google Scholar]
- Dickinson RL (1889) Studies of the levator ani muscle. Am J Obstet Gynecol 9, 898–917. [Google Scholar]
- Dorschner W, Biesold M, Schmidt F, et al. (1999) The dispute about the external sphincter and the urogenital diaphragm. J Urol 162, 1942–1945. [DOI] [PubMed] [Google Scholar]
- Fielding JR (2002) Practical MR imaging of female pelvic floor weakness. Radiographics 22, 295–304. [DOI] [PubMed] [Google Scholar]
- Fritsch H, Frohlich B (1994) Development of the levator ani muscle in human fetuses. Early Hum Dev 37, 15–25. [DOI] [PubMed] [Google Scholar]
- Fritsch H, Hotzinger H (1995) Tomographical anatomy of the pelvis, visceral pelvic connective tissue, and its compartments. Clin Anat 8, 17–24. [DOI] [PubMed] [Google Scholar]
- Fritsch H, Lienemann A, Brenner E, et al. (2004) Clinical anatomy of the pelvic floor. Adv Anat Embryol Cell Biol 175, 1–64. [DOI] [PubMed] [Google Scholar]
- Fritsch H, Pinggera GM, Lienemann A, et al. (2006) What are the supportive structures of the female urethra? Neurourol Urodyn 25, 128–134. [DOI] [PubMed] [Google Scholar]
- Frohlich B, Hotzinger H, Fritsch H (1997) Tomographical anatomy of the pelvis, pelvic floor, and related structures. Clin Anat 10, 223–230. [DOI] [PubMed] [Google Scholar]
- Goff BH (1931) A histological study of the perivaginal fascia in a nullipara. Surg Gynecol Obstet 52, 32–42. [Google Scholar]
- Hockel M, Horn LC, Illig R, et al. (2011) Ontogenetic anatomy of the distal vagina: relevance for local tumor spread and implications for cancer surgery. Gynecol Oncol 122, 313–318. [DOI] [PubMed] [Google Scholar]
- Hoyte L, Schierlitz L, Zou K, et al. (2001) Two‐ and 3‐dimensional MRI comparison of levator ani structure, volume, and integrity in women with stress incontinence and prolapse. Am J Obstet Gynecol 185, 11–19. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Lewicky‐Gaupp C, DeLancey JO (2008) Posterior compartment anatomy as seen in magnetic resonance imaging and 3‐dimensional reconstruction from asymptomatic nulliparas. Am J Obstet Gynecol 198, 651 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelovsek JE, Maher C, Barber MD (2007) Pelvic organ prolapse. Lancet 369, 1027–1038. [DOI] [PubMed] [Google Scholar]
- Karvonen‐Gutierrez C, Kim C (2016) Association of mid‐life changes in body size, body composition and obesity status with the menopausal transition. Healthcare (Basel) 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathrein A, Klestil T, Birbamer G, et al. (1996) Rotation cryotomy: medical and scientific value of a new serial sectioning procedure. Clin Anat 9, 227–231. [DOI] [PubMed] [Google Scholar]
- Kearney R, Sawhney R, DeLancey JO (2004) Levator ani muscle anatomy evaluated by origin‐insertion pairs. Obstet Gynecol 104, 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoua A, Homsy Y, Lavigne JF, et al. (1993) Maturation of the external urinary sphincter: a comparative histotopographic study in humans. J Urol 150, 617–622. [DOI] [PubMed] [Google Scholar]
- Koster H (1933) On the supports of the uterus. Am J Obstet Gynecol 25, 67–74. [Google Scholar]
- Kraima AC, West NP, Treanor D, et al. (2015) Whole mount microscopic sections reveal that Denonvilliers’ fascia is one entity and adherent to the mesorectal fascia; implications for the anterior plane in total mesorectal excision? Eur J Surg Oncol 41, 738–745. [DOI] [PubMed] [Google Scholar]
- Larson KA, Yousuf A, Lewicky‐Gaupp C, et al. (2010) Perineal body anatomy in living women: 3‐dimensional analysis using thin‐slice magnetic resonance imaging. Am J Obstet Gynecol 203, 494 e15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean ME, Han TS, Deurenberg P (1996) Predicting body composition by densitometry from simple anthropometric measurements. Am J Clin Nutr 63, 4–14. [DOI] [PubMed] [Google Scholar]
- Lien KC, Mooney B, DeLancey JO, et al. (2004) Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol 103, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwikowski B, Oesch Hayward I, Brenner E, et al. (2001) The development of the external urethral sphincter in humans. BJU Int 87, 565–568. [DOI] [PubMed] [Google Scholar]
- Milley PS, Nichols DH (1969) A correlative investigation of the human rectovaginal septum. Anat Rec 163, 443–451. [DOI] [PubMed] [Google Scholar]
- Mirilas P, Skandalakis JE (2004) Urogenital diaphragm: an erroneous concept casting its shadow over the sphincter urethrae and deep perineal space. J Am Coll Surg 198, 279–290. [DOI] [PubMed] [Google Scholar]
- Nakajima F, Takenaka A, Uchiyama E, et al. (2007) Macroscopic and histotopographic study of the deep transverse perineal muscle (musculus transversus perinei profundus) in elderly Japanese. Ann Anat 189, 65–74. [DOI] [PubMed] [Google Scholar]
- Niikura H, Katahira A, Utsunomiya H, et al. (2007) Surgical anatomy of intrapelvic fasciae and vesico‐uterine ligament in nerve‐sparing radical hysterectomy with fresh cadaver dissections. Tohoku J Exp Med 212, 403–413. [DOI] [PubMed] [Google Scholar]
- Occelli B, Narducci F, Hautefeuille J, et al. (2001) Anatomic study of arcus tendineus fasciae pelvis. Eur J Obstet Gynecol Reprod Biol 97, 213–219. [DOI] [PubMed] [Google Scholar]
- Oelrich TM (1983) The striated urogenital sphincter muscle in the female. Anat Rec 205, 223–232. [DOI] [PubMed] [Google Scholar]
- Pendergrass PB, Reeves CA, Belovicz MW, et al. (1996) The shape and dimensions of the human vagina as seen in three‐dimensional vinyl polysiloxane casts. Gynecol Obstet Invest 42, 178–182. [DOI] [PubMed] [Google Scholar]
- Pit MJ, De Ruiter MC, Nijeholt LA, et al. (2003) Anatomy of the arcus tendineus fasciae pelvis in females. Clin Anat 16, 131–137. [DOI] [PubMed] [Google Scholar]
- Ramanah R, Berger MB, Parratte BM, et al. (2012a) Anatomy and histology of apical support: a literature review concerning cardinal and uterosacral ligaments. Int Urogynecol J 23, 1483–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanah R, Berger MB, Chen L, et al. (2012b) See it in 3D!: researchers examined structural links between the cardinal and uterosacral ligaments. Am J Obstet Gynecol 207, 437 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Range RL, Woodburne RT (1964) The gross and microscopic anatomy of the transverse cervical ligament. Am J Obstet Gynecol 90, 460–467. [DOI] [PubMed] [Google Scholar]
- Reay Jones NH, Healy JC, King LJ, et al. (2003) Pelvic connective tissue resilience decreases with vaginal delivery, menopause and uterine prolapse. Br J Surg 90, 466–472. [DOI] [PubMed] [Google Scholar]
- Ricci JV, Lisa JR, Thom CH, et al. (1947) The relationship of the vagina to adjacent organs in reconstructive surgery; a histologic study. Am J Surg 74, 387–410. [DOI] [PubMed] [Google Scholar]
- Ridgeway B, Barber MD (2012) Midurethral slings for stress urinary incontinence: a urogynecology perspective. Urol Clin North Am 39, 289–297. [DOI] [PubMed] [Google Scholar]
- Rivaux G, Rubod C, Dedet B, et al. (2013) Comparative analysis of pelvic ligaments: a biomechanics study. Int Urogynecol J 24, 135–139. [DOI] [PubMed] [Google Scholar]
- Robinson RJ, Russo J, Doolittle RL (2009) 3D airway reconstruction using visible human data set and human casts with comparison to morphometric data. Anat Rec 292, 1028–1044. [DOI] [PubMed] [Google Scholar]
- Samaan A, Vu D, Haylen BT, et al. (2014) Cardinal ligament surgical anatomy: cardinal points at hysterectomy. Int Urogynecol J 25, 189–195. [DOI] [PubMed] [Google Scholar]
- Sebe P, Fritsch H, Oswald J, et al. (2005) Fetal development of the female external urinary sphincter complex: an anatomical and histological study. J Urol 173, 1738–1742. [DOI] [PubMed] [Google Scholar]
- Seow CY, Solway J (2011) Mechanical and structural plasticity. Compr Physiol 1, 283–293. [DOI] [PubMed] [Google Scholar]
- Shafik A, Sibai OE, Shafik AA, et al. (2007) A novel concept for the surgical anatomy of the perineal body. Dis Colon Rectum 50, 2120–2125. [DOI] [PubMed] [Google Scholar]
- Shen W, Wang ZM, Tang H, et al. (2003) Volume estimates by imaging methods: model comparisons with visible woman as the reference. Obes Res 11, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido K, Peng Q, Jones R, et al. (2008) Influence of pelvic floor muscle contraction on the profile of vaginal closure pressure in continent and stress urinary incontinent women. J Urol 179, 1917–1922. [DOI] [PubMed] [Google Scholar]
- Smith TM, Luo J, Hsu Y, et al. (2013) A novel technique to measure in vivo uterine suspensory ligament stiffness. Am J Obstet Gynecol 209, 484 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer V, Ackerman MJ, Scherzinger AL, et al. (1996) The visible human male: a technical report. J Am Med Inform Assoc 3, 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TA, DeLancey JO (2008) Structure of the perineal membrane in females: gross and microscopic anatomy. Obstet Gynecol 111, 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson CW, Luo J, Chen L, et al. (2016) Traction force needed to reproduce physiologically observed uterine movement: technique development, feasibility assessment, and preliminary findings. Int Urogynecol J 27, 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenhuth E, Day EC (1948) The visceral endopelvic fascia and the hypogastric sheath. Surg Gynecol Obstet 86, 9–28. [PubMed] [Google Scholar]
- Umek WH, Morgan DM, Ashton‐Miller JA, et al. (2004) Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol 103, 447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D, Haylen BT, Tse K, et al. (2010) Surgical anatomy of the uterosacral ligament. Int Urogynecol J 21, 1123–1128. [DOI] [PubMed] [Google Scholar]
- Wallner C, Dabhoiwala NF, DeRuiter MC, et al. (2009) The anatomical components of urinary continence. Eur Urol 55, 932–943. [DOI] [PubMed] [Google Scholar]
- Westby M, Asmussen M, Ulmsten U (1982) Location of maximum intraurethral pressure related to urogenital diaphragm in the female subject as studied by simultaneous urethrocystometry and voiding urethrocystography. Am J Obstet Gynecol 144, 408–412. [DOI] [PubMed] [Google Scholar]
- Woodman PJ, Graney DO (2002) Anatomy and physiology of the female perineal body with relevance to obstetrical injury and repair. Clin Anat 15, 321–334. [DOI] [PubMed] [Google Scholar]
- Wu Y, Dabhoiwala NF, Hagoort J, et al. (2015) 3D Topography of the young adult anal sphincter complex reconstructed from undeformed serial anatomical sections. PLoS One 10, e0132226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel S, Baskin LS (2004) An anatomical description of the male and female urethral sphincter complex. J Urol 171, 1890–1897. [DOI] [PubMed] [Google Scholar]
- Zhai L‐D, Liu J, Li Y‐S, et al. (2009) Denonvilliers’ fascia in women and its relationship with the fascia propria of the rectum examined by successive slices of celloidin‐embedded pelvic viscera. Dis Colon Rectum 52, 1564–1571. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Heng PA, Liu ZJ, et al. (2003) Creation of the Chinese visible human data set. Anat Rec B New Anat 275, 190–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Interactive 3D rendering of the topographic anatomy of the VHF pelvic floor.
Fig. S2. The color code used to label structures in Figs 1–4 and Fig. S1.
Fig. S3. Serial transverse sections of the lesser pelvis of specimen CVH‐5.
Fig. S4. Serial sagittal sections of the lesser pelvis of specimen CVO.


