The reaction of silver(I) nitrate with the mono-substituted pyrazine carboxamide ligand, N-(pyridin-2-ylmethyl)-pyrazine-2-carboxamide, led to the formation of a metal-organic framework (MOF) structure.
Keywords: crystal structure, metal-organic framework, MOF, silver(I), pyrazine, carboximide, pyridine, nitrate, hydrogen bonding
Abstract
The reaction of silver(I) nitrate with the mono-substituted pyrazine carboxamide ligand, N-(pyridin-2-ylmethyl)pyrazine-2-carboxamide (L), led to the formation of the title compound with a metal–organic framework (MOF) structure, [Ag(C11H10N4O)(NO3)]n, poly[μ-nitrato-[μ-N-(pyridin-2-ylmethyl-κN)pyrazine-2-carboxamide-κN 4]silver(I)]. The silver(I) atom is coordinated by a pyrazine N atom, a pyridine N atom, and two O atoms of two symmetry-related nitrate anions. It has a fourfold N2O2 coordination sphere, which can be described as distorted trigonal–pyramidal. The ligands are bridged by the silver atoms forming –Ag–L–Ag–L– zigzag chains along the a-axis direction. The chains are arranged in pairs related by a twofold screw axis. They are linked via the nitrate anions, which bridge the silver(I) atoms in a μ2 fashion, forming the MOF structure. Within the framework there are N—H⋯O and C—H⋯O hydrogen bonds present.
Chemical context
We have shown recently that by using silver(I) nitrate and various tetrakis-substituted pyrazine ligands, one-, two- and three-dimensional coordination polymers can be formed (Assoumatine & Stoeckli-Evans, 2017 ▸). In the present report, the mono-substituted pyrazine carboxamide ligand, N-(pyridin-2-ylmethyl)pyrazine-2-carboxamide (L), whose crystal structure has been reported (Cati & Stoeckli-Evans, 2014 ▸), was reacted with silver(I) nitrate and led to the formation of a new compound with a metal–organic framework (MOF) structure, (I).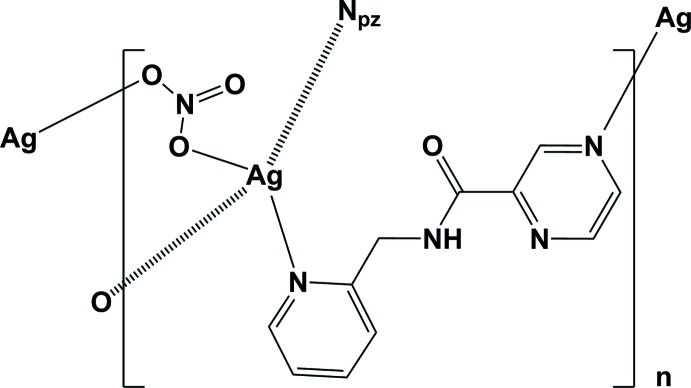
Structural commentary
The molecular structure of the asymmetric unit of compound (I) is illustrated in Fig. 1 ▸. Selected bond lengths and angles involving the Ag1 atom are given in Table 1 ▸. Atom Ag1 is coordinated by a pyrazine N atom, N2, the pyridine N atom, N4, and two O atoms, O11 and O12, of two symmetry-related nitrate anions (Fig. 1 ▸ and Table 1 ▸). Therefore, atom Ag1 has a fourfold N2O2 coordination sphere and a distorted trigonal–pyramidal geometry with a τ4 parameter = 0.72 (τ4 = 1 for a perfect tetrahedral geometry, 0 for a perfect square-planar geometry; for intermediate structures, including trigonal–pyramidal and seesaw, the values of τ4 fall within the range of 0 to 1.0; Yang et al., 2007 ▸). Atom O13 of the nitrate anion lies above atom Ag1 with a distance Ag1⋯O13 of 2.864 (11) Å. The ligands are bridged by the silver atoms, forming –Ag–L–Ag–L– zigzag chains propagating along the a-axis direction (Fig. 2 ▸ and Table 1 ▸). They are arranged in pairs related by a twofold screw axis (Fig. 2 ▸).
Figure 1.
A view of the molecular structure of the asymmetric unit of the title compound (I), with atom labelling. Displacement ellipsoids are drawn at the 50% probability level. For this figure, the symmetry codes are: (i) x −  , −y, z; (ii) −x, −y + 1, z +
, −y, z; (ii) −x, −y + 1, z +  ; (iii) −x, −y + 1, z −
; (iii) −x, −y + 1, z −  ; (iv) x +
; (iv) x +  , −y, z.
, −y, z.
Table 1. Selected geometric parameters (Å, °).
| Ag1—N2i | 2.238 (7) | Ag1—O12ii | 2.520 (9) |
| Ag1—N4 | 2.259 (8) | Ag1—O13 | 2.864 (8) |
| Ag1—O11 | 2.498 (9) | ||
| N2i—Ag1—N4 | 140.8 (3) | N2i—Ag1—O12ii | 115.0 (3) |
| N2i—Ag1—O11 | 117.1 (3) | N4—Ag1—O12ii | 89.9 (4) |
| N4—Ag1—O11 | 98.5 (3) | O11—Ag1—O12ii | 72.6 (3) |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Figure 2.
A view along the c axis of the –Ag–L–Ag–L zigzag chains propagating along the a-axis direction (silver atoms are grey balls and H atoms have been omitted for clarity).
Supramolecular features
In the crystal of (I), the chains are bridged by the nitrate anions, leading to the formation of the three-dimensional framework structure (Figs. 3 ▸ and 4 ▸). The nitrate anions bridge the silver atoms in a μ2 manner (Fig. 4 ▸), one of the many ways in which the nitrate anion interacts with silver atoms (Cambridge Structural Database; Groom et al., 2016 ▸). Its role here is essential in forming the MOF structure.
Figure 3.
A view along the c axis of (I). The H atoms have been omitted for clarity, and the silver atoms and the nitrate anions are shown as balls.
Figure 4.
A view along the a axis of (I). The H atoms have been omitted for clarity, and the silver atoms and the nitrate anions are shown as balls.
Within the framework, there is an N—H⋯O hydrogen bond linking the amine group and carbonyl O atom of twofold-screw-related chains. There is also a C—H⋯O hydrogen bond present involving a pyrazine H atom and the third O atom of the nitrate anion, O13 (Table 2 ▸). There are small voids of ca 68 Å3 in the framework structure, equivalent to 4.8% of the volume of the unit cell.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N3—H3N⋯O1iii | 0.87 (3) | 2.35 (12) | 2.914 (12) | 123 (11) |
| C2—H2⋯O13iv | 0.94 | 2.59 | 3.330 (15) | 136 |
Symmetry codes: (iii)  ; (iv)
; (iv)  .
.
Database survey
A search of the Cambridge Structural Database (Version 5.38, update February 2017; Groom et al., 2016 ▸) for the title ligand (L) gave 15 hits. These include a report of the crystal structure of (L) (Cati & Stoeckli-Evans, 2014 ▸), and that of a silver(I) BF4 − coordination polymer (PORZOM; Hellyer et al., 2009 ▸). Here the ligand bridges the silver(I) atoms, coordinating in a bidentate (via the pyridine N atom and the carbonyl O atom) and monodentate (to a pyrazine N atom) fashion, forming zigzag chains along [010]. The chains are linked by Ag⋯Ag contacts, of ca 3.32 Å, forming slabs (or metal–organic networks) lying parallel to the bc plane. The remainder of the hits in the above search are mainly first row transition metal complexes or coordination polymers.
Synthesis and crystallization
The synthesis of the ligand (L) has been described previously (Cati & Stoeckli-Evans, 2014 ▸). Ligand (L) (27 mg, 0.126 mmol) and AgNO3 (43 mg, 0.252 mmol) were introduced into 15 ml of acetonitrile in a two-necked flask (100 ml), isolated from the light by aluminium foil. The solution was refluxed for 5 h. The resulting limpid solution was filtered and the filtrate allowed to stand at room temperature. Colourless plate-like crystals were obtained in a few days (yield 42 mg, 87%).
Spectroscopic data: IR (KBr disc, cm−1): 3330 (s), 3063 (m), 1670 (vs), 1656 (vs), 1598 (s), 1571 (s), 1538 (vs), 1520 (vs), 1473 (s), 1463 (s), 1386 (b and vs), 1327 (vs), 1289 (vs), 1158 (s), 1101 (m), 1064 (m), 1023 (s), 877 (w), 825 (m), 776 (m), 706 (m), 667 (s), 611 (m), 533 (m), 456 (m). The broad and very strong absorption band at 1386 cm−1 indicates the presence of a coordinating nitrate anion. Elemental Analysis for AgC11H10N5O4 (M r = 384.10 g mol−1): Calculated: C 34.40; H 2.62; N, 18.23%; found: C 34.58; H 2.55; N 18.05%.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. The NH H atom was located in a difference-Fourier map and freely refined. The C-bound H atoms were included in calculated positions and treated as riding: C—H = 0.94–0.98 Å with U iso(H) = 1.2U eq(C).
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | [Ag(C11H10N4O)(NO3)] |
| M r | 384.11 |
| Crystal system, space group | Orthorhombic, P c a21 |
| Temperature (K) | 223 |
| a, b, c (Å) | 17.522 (3), 8.9559 (18), 8.9860 (13) |
| V (Å3) | 1410.1 (4) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 1.45 |
| Crystal size (mm) | 0.68 × 0.61 × 0.08 |
| Data collection | |
| Diffractometer | STOE–Siemens AED2 four-circle |
| Absorption correction | Multi-scan (MULABS; Spek, 2009 ▸) |
| T min, T max | 0.910, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 3655, 2628, 2384 |
| R int | 0.022 |
| (sin θ/λ)max (Å−1) | 0.605 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.047, 0.128, 1.10 |
| No. of reflections | 2628 |
| No. of parameters | 194 |
| No. of restraints | 2 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 1.04, −1.56 |
| Absolute structure | Flack x determined using 1006 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) |
| Absolute structure parameter | −0.06 (2) |
Supplementary Material
Crystal structure: contains datablock(s) I, Global. DOI: 10.1107/S2056989017003930/zl2699sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017003930/zl2699Isup2.hkl
CCDC reference: 1537331
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| [Ag(C11H10N4O)(NO3)] | Dx = 1.809 Mg m−3 |
| Mr = 384.11 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, Pca21 | Cell parameters from 20 reflections |
| a = 17.522 (3) Å | θ = 10.0–13.4° |
| b = 8.9559 (18) Å | µ = 1.45 mm−1 |
| c = 8.9860 (13) Å | T = 223 K |
| V = 1410.1 (4) Å3 | Plate, colourles |
| Z = 4 | 0.68 × 0.61 × 0.08 mm |
| F(000) = 760 |
Data collection
| STOE–Siemens AED2 four-circle diffractometer | 2384 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.022 |
| Plane graphite monochromator | θmax = 25.5°, θmin = 2.3° |
| ω/2θ scans | h = −21→21 |
| Absorption correction: multi-scan (MULABS; Spek, 2009) | k = −10→10 |
| Tmin = 0.910, Tmax = 1.000 | l = −10→10 |
| 3655 measured reflections | 2 standard reflections every 60 min |
| 2628 independent reflections | intensity decay: 3% |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.047 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.128 | w = 1/[σ2(Fo2) + (0.0872P)2 + 0.889P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.10 | (Δ/σ)max < 0.001 |
| 2628 reflections | Δρmax = 1.04 e Å−3 |
| 194 parameters | Δρmin = −1.56 e Å−3 |
| 2 restraints | Absolute structure: Flack x determined using 1006 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| Primary atom site location: structure-invariant direct methods | Absolute structure parameter: −0.06 (2) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ag1 | 0.05137 (3) | 0.36456 (6) | 0.69187 (15) | 0.0402 (3) | |
| N1 | 0.3810 (5) | −0.0242 (8) | 0.9863 (8) | 0.0371 (16) | |
| N2 | 0.4836 (4) | −0.1928 (9) | 0.8154 (8) | 0.0338 (15) | |
| N3 | 0.2698 (5) | 0.1329 (10) | 0.8498 (9) | 0.048 (2) | |
| H3N | 0.279 (8) | 0.131 (14) | 0.945 (5) | 0.08 (5)* | |
| N4 | 0.1730 (4) | 0.4408 (9) | 0.6583 (9) | 0.045 (2) | |
| O1 | 0.3124 (5) | 0.0642 (12) | 0.6234 (8) | 0.048 (2) | |
| C1 | 0.3775 (5) | −0.0301 (10) | 0.8352 (9) | 0.0322 (17) | |
| C2 | 0.4271 (6) | −0.1143 (9) | 0.7518 (11) | 0.0336 (18) | |
| H2 | 0.421457 | −0.116833 | 0.647769 | 0.040* | |
| C3 | 0.4894 (6) | −0.1818 (12) | 0.9640 (11) | 0.039 (2) | |
| H3 | 0.529260 | −0.232096 | 1.013043 | 0.047* | |
| C4 | 0.4380 (6) | −0.0979 (12) | 1.0473 (10) | 0.038 (2) | |
| H4 | 0.444189 | −0.093686 | 1.151129 | 0.045* | |
| C5 | 0.3163 (7) | 0.0593 (13) | 0.7613 (12) | 0.039 (3) | |
| C6 | 0.2071 (5) | 0.2203 (12) | 0.7917 (11) | 0.044 (2) | |
| H6B | 0.171943 | 0.242975 | 0.873408 | 0.053* | |
| H6A | 0.179317 | 0.159729 | 0.718878 | 0.053* | |
| C7 | 0.2304 (5) | 0.3641 (9) | 0.7190 (11) | 0.040 (3) | |
| C8 | 0.3053 (5) | 0.4149 (12) | 0.706 (2) | 0.057 (3) | |
| H8 | 0.345961 | 0.359520 | 0.745841 | 0.068* | |
| C9 | 0.3187 (7) | 0.5463 (16) | 0.6332 (18) | 0.074 (4) | |
| H9 | 0.368904 | 0.582281 | 0.625229 | 0.088* | |
| C10 | 0.2607 (11) | 0.6256 (14) | 0.573 (3) | 0.086 (5) | |
| H10 | 0.269429 | 0.716443 | 0.523371 | 0.103* | |
| C11 | 0.1881 (7) | 0.5672 (14) | 0.5870 (18) | 0.067 (3) | |
| H11 | 0.147229 | 0.619736 | 0.543869 | 0.081* | |
| N10 | −0.0058 (5) | 0.3498 (9) | 0.3740 (9) | 0.0390 (18) | |
| O11 | −0.0039 (7) | 0.4610 (9) | 0.4543 (11) | 0.072 (3) | |
| O12 | −0.0112 (8) | 0.3691 (9) | 0.2369 (8) | 0.074 (3) | |
| O13 | −0.0028 (7) | 0.2247 (10) | 0.4254 (12) | 0.085 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ag1 | 0.0352 (4) | 0.0473 (4) | 0.0380 (4) | −0.0043 (2) | −0.0034 (4) | 0.0055 (5) |
| N1 | 0.048 (4) | 0.039 (4) | 0.023 (3) | 0.006 (3) | 0.003 (3) | 0.001 (3) |
| N2 | 0.036 (4) | 0.037 (4) | 0.028 (4) | 0.006 (3) | −0.004 (3) | −0.001 (3) |
| N3 | 0.043 (5) | 0.073 (6) | 0.027 (4) | 0.021 (4) | 0.004 (4) | 0.010 (4) |
| N4 | 0.037 (4) | 0.046 (4) | 0.051 (6) | 0.001 (3) | −0.004 (3) | 0.010 (4) |
| O1 | 0.047 (5) | 0.074 (6) | 0.024 (5) | 0.017 (5) | 0.001 (3) | 0.006 (4) |
| C1 | 0.031 (4) | 0.039 (4) | 0.027 (4) | 0.002 (3) | 0.000 (3) | 0.003 (3) |
| C2 | 0.037 (4) | 0.036 (4) | 0.028 (4) | 0.000 (4) | −0.006 (4) | −0.001 (3) |
| C3 | 0.047 (6) | 0.042 (5) | 0.029 (5) | −0.004 (4) | −0.003 (4) | 0.006 (4) |
| C4 | 0.043 (5) | 0.047 (5) | 0.024 (4) | 0.002 (4) | −0.003 (3) | 0.000 (4) |
| C5 | 0.040 (6) | 0.043 (6) | 0.034 (7) | 0.003 (5) | 0.001 (5) | 0.007 (5) |
| C6 | 0.033 (5) | 0.061 (6) | 0.039 (5) | 0.011 (4) | 0.004 (4) | 0.006 (4) |
| C7 | 0.036 (5) | 0.049 (5) | 0.035 (8) | 0.000 (3) | −0.001 (4) | −0.004 (4) |
| C8 | 0.036 (4) | 0.057 (5) | 0.077 (8) | 0.005 (4) | 0.001 (7) | −0.011 (8) |
| C9 | 0.039 (6) | 0.067 (8) | 0.115 (13) | −0.007 (6) | 0.004 (6) | −0.015 (7) |
| C10 | 0.079 (10) | 0.055 (8) | 0.125 (15) | −0.016 (7) | 0.014 (10) | 0.029 (8) |
| C11 | 0.051 (7) | 0.053 (7) | 0.098 (10) | 0.001 (5) | 0.005 (6) | 0.031 (7) |
| N10 | 0.040 (4) | 0.048 (5) | 0.029 (4) | 0.009 (3) | −0.001 (3) | 0.002 (3) |
| O11 | 0.106 (7) | 0.061 (6) | 0.048 (4) | 0.025 (6) | −0.024 (4) | −0.021 (5) |
| O12 | 0.135 (10) | 0.064 (6) | 0.022 (4) | 0.005 (5) | 0.012 (4) | 0.004 (3) |
| O13 | 0.147 (11) | 0.046 (5) | 0.062 (6) | 0.012 (6) | −0.008 (7) | 0.011 (5) |
Geometric parameters (Å, º)
| Ag1—N2i | 2.238 (7) | C3—C4 | 1.392 (16) |
| Ag1—N4 | 2.259 (8) | C3—H3 | 0.9400 |
| Ag1—O11 | 2.498 (9) | C4—H4 | 0.9400 |
| Ag1—O12ii | 2.520 (9) | C6—C7 | 1.500 (13) |
| Ag1—O13 | 2.864 (8) | C6—H6B | 0.9800 |
| N1—C4 | 1.317 (12) | C6—H6A | 0.9800 |
| N1—C1 | 1.360 (11) | C7—C8 | 1.395 (14) |
| N2—C2 | 1.342 (12) | C8—C9 | 1.36 (2) |
| N2—C3 | 1.343 (12) | C8—H8 | 0.9400 |
| N3—C5 | 1.316 (14) | C9—C10 | 1.35 (2) |
| N3—C6 | 1.445 (12) | C9—H9 | 0.9400 |
| N3—H3N | 0.87 (3) | C10—C11 | 1.38 (2) |
| N4—C11 | 1.327 (14) | C10—H10 | 0.9400 |
| N4—C7 | 1.334 (12) | C11—H11 | 0.9400 |
| O1—C5 | 1.242 (10) | N10—O13 | 1.212 (12) |
| C1—C2 | 1.373 (13) | N10—O11 | 1.231 (12) |
| C1—C5 | 1.494 (14) | N10—O12 | 1.248 (11) |
| C2—H2 | 0.9400 | ||
| N2i—Ag1—N4 | 140.8 (3) | O1—C5—C1 | 120.1 (11) |
| N2i—Ag1—O11 | 117.1 (3) | N3—C5—C1 | 116.4 (9) |
| N4—Ag1—O11 | 98.5 (3) | N3—C6—C7 | 114.6 (8) |
| N2i—Ag1—O12ii | 115.0 (3) | N3—C6—H6B | 108.6 |
| N4—Ag1—O12ii | 89.9 (4) | C7—C6—H6B | 108.6 |
| O11—Ag1—O12ii | 72.6 (3) | N3—C6—H6A | 108.6 |
| C4—N1—C1 | 115.5 (8) | C7—C6—H6A | 108.6 |
| C2—N2—C3 | 116.2 (8) | H6B—C6—H6A | 107.6 |
| C2—N2—Ag1iii | 122.7 (6) | N4—C7—C8 | 120.4 (9) |
| C3—N2—Ag1iii | 120.2 (7) | N4—C7—C6 | 114.6 (8) |
| C5—N3—C6 | 121.5 (9) | C8—C7—C6 | 125.0 (9) |
| C5—N3—H3N | 118 (9) | C9—C8—C7 | 118.9 (11) |
| C6—N3—H3N | 121 (9) | C9—C8—H8 | 120.5 |
| C11—N4—C7 | 119.2 (9) | C7—C8—H8 | 120.5 |
| C11—N4—Ag1 | 120.6 (7) | C10—C9—C8 | 121.0 (12) |
| C7—N4—Ag1 | 120.0 (6) | C10—C9—H9 | 119.5 |
| N1—C1—C2 | 122.5 (8) | C8—C9—H9 | 119.5 |
| N1—C1—C5 | 117.0 (8) | C9—C10—C11 | 117.2 (12) |
| C2—C1—C5 | 120.4 (8) | C9—C10—H10 | 121.4 |
| N2—C2—C1 | 121.4 (9) | C11—C10—H10 | 121.4 |
| N2—C2—H2 | 119.3 | N4—C11—C10 | 123.4 (12) |
| C1—C2—H2 | 119.3 | N4—C11—H11 | 118.3 |
| N2—C3—C4 | 121.6 (10) | C10—C11—H11 | 118.3 |
| N2—C3—H3 | 119.2 | O13—N10—O11 | 121.5 (10) |
| C4—C3—H3 | 119.2 | O13—N10—O12 | 120.5 (9) |
| N1—C4—C3 | 122.5 (9) | O11—N10—O12 | 118.0 (9) |
| N1—C4—H4 | 118.7 | N10—O11—Ag1 | 103.4 (6) |
| C3—C4—H4 | 118.7 | N10—O12—Ag1iv | 108.1 (6) |
| O1—C5—N3 | 123.5 (12) | ||
| C4—N1—C1—C2 | 3.7 (14) | C11—N4—C7—C8 | −1.0 (16) |
| C4—N1—C1—C5 | −176.6 (9) | Ag1—N4—C7—C8 | −176.5 (9) |
| C3—N2—C2—C1 | −1.3 (14) | C11—N4—C7—C6 | −177.8 (11) |
| Ag1iii—N2—C2—C1 | 167.9 (6) | Ag1—N4—C7—C6 | 6.7 (11) |
| N1—C1—C2—N2 | −1.7 (14) | N3—C6—C7—N4 | 176.6 (9) |
| C5—C1—C2—N2 | 178.5 (9) | N3—C6—C7—C8 | −0.1 (15) |
| C2—N2—C3—C4 | 2.3 (15) | N4—C7—C8—C9 | 2 (2) |
| Ag1iii—N2—C3—C4 | −167.2 (7) | C6—C7—C8—C9 | 178.3 (13) |
| C1—N1—C4—C3 | −2.7 (14) | C7—C8—C9—C10 | −1 (2) |
| N2—C3—C4—N1 | −0.2 (16) | C8—C9—C10—C11 | 0 (3) |
| C6—N3—C5—O1 | 3 (2) | C7—N4—C11—C10 | −1 (2) |
| C6—N3—C5—C1 | −178.4 (9) | Ag1—N4—C11—C10 | 174.9 (14) |
| N1—C1—C5—O1 | 176.6 (13) | C9—C10—C11—N4 | 1 (3) |
| C2—C1—C5—O1 | −3.7 (19) | O13—N10—O11—Ag1 | 19.1 (14) |
| N1—C1—C5—N3 | −1.8 (15) | O12—N10—O11—Ag1 | −160.9 (10) |
| C2—C1—C5—N3 | 178.0 (10) | O13—N10—O12—Ag1iv | 164.7 (9) |
| C5—N3—C6—C7 | −73.6 (14) | O11—N10—O12—Ag1iv | −15.2 (14) |
Symmetry codes: (i) x−1/2, −y, z; (ii) −x, −y+1, z+1/2; (iii) x+1/2, −y, z; (iv) −x, −y+1, z−1/2.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N3—H3N···O1v | 0.87 (3) | 2.35 (12) | 2.914 (12) | 123 (11) |
| C2—H2···O13iii | 0.94 | 2.59 | 3.330 (15) | 136 |
Symmetry codes: (iii) x+1/2, −y, z; (v) −x+1/2, y, z+1/2.
References
- Assoumatine, T. & Stoeckli-Evans, H. (2017). Acta Cryst. E73, 434–440. [DOI] [PMC free article] [PubMed]
- Cati, D. S. & Stoeckli-Evans, H. (2014). Acta Cryst. E70, 18–22. [DOI] [PMC free article] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hellyer, R. M., Larsen, D. S. & Brooker, S. (2009). Eur. J. Inorg. Chem. pp. 1162–1171.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Stoe & Cie (1997). STADI4 and X-RED. Stoe & Cie GmbH, Damstadt, Germany.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Yang, L., Powell, D. R. & Houser, R. P. (2007). Dalton Trans. pp. 955–964. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, Global. DOI: 10.1107/S2056989017003930/zl2699sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017003930/zl2699Isup2.hkl
CCDC reference: 1537331
Additional supporting information: crystallographic information; 3D view; checkCIF report






