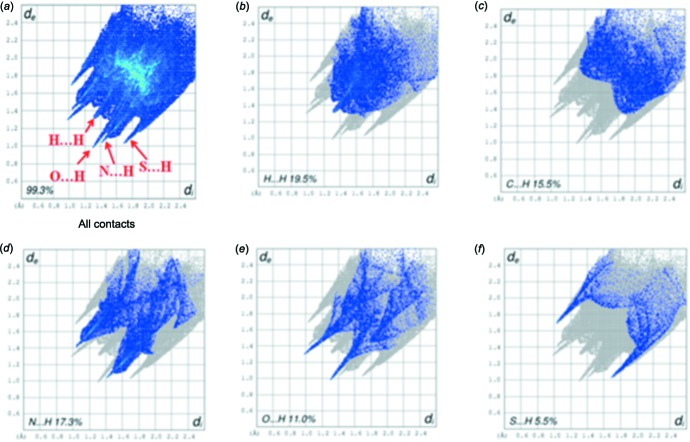
In the crystal, the molecules are linked into [100] chains by way of C—H⋯O, C—H⋯N, C—H⋯S hydrogen bonds. The Hirshfeld surface analysis indicates that the most important contributions to the packing are H⋯H (19.5%), N⋯H (17.3%), C⋯H (15.5%), Br⋯H (11.7%), and O⋯H (11.0%) interactions.
Keywords: oxadizole, bromophenyl, X-ray structure, Hirshfeld surface analysis, crystal structure
Abstract
In the title compound, C15H10BrN3O2S, the dihedral angles between the 1,3,4-oxadiazole ring and the 3-pyridinyl and bromobenzene rings are 12.17 (15) and 18.74 (15)°, respectively. In the crystal, the molecules are linked into [100] chains by way of C—H⋯O, C—H⋯N, C—H⋯S hydrogen bonds. The Hirshfeld surface analysis indicates that the most important contributions to the packing are H⋯H (19.5%), N⋯H (17.3%), C⋯H (15.5%), Br⋯H (11.7%), and O⋯H (11.0%) interactions.
Chemical context
Substituted 1,3,4-oxadiazoles exhibit numerous biological activities such as antibacterial and antifungal (Prakash et al., 2010 ▸, Chandrakantha et al., 2010 ▸), anticancer (Abu-Zaied et al., 2011 ▸), anti-inflammatory, analgesic (Husain et al., 2009 ▸, Omar et al., 1996 ▸), anticonvulsant and neurotoxic activities (Rajak et al., 2010 ▸, Zarghi et al., 2005 ▸). Chemical compounds having a 1,3,4-oxadiazole moiety are also important contributors towards the synthesis of biologically active heterocyclic compounds having antibacterial activity against resistant strains (Bharti et al., 2010 ▸). As part of our studies in this area, we now describe the synthesis and structure of the title compound (I), a product of the condensation reaction between alcoholic solutions of 5-(3-pyridyl)-1,3,4-oxadiazole-2-thiol and 2,4-dibromoacetophenone in the presence triethyl amine (Kashtoh et al., 2014 ▸).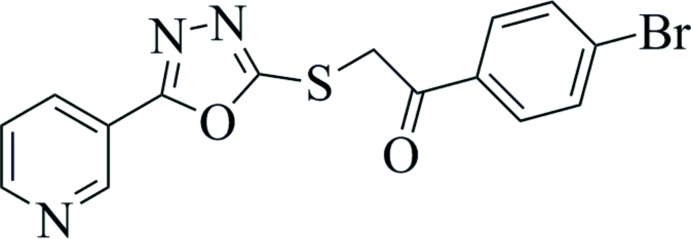
Structural commentary
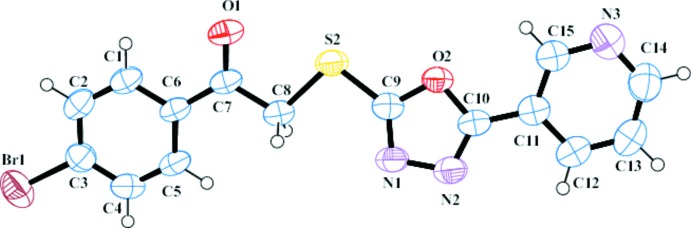
The structure of (I) (Fig. 1 ▸) is composed of three near-planar aromatic rings [bromophenyl (A), 3-pyridinyl (B) and 1,3,4-oxadiazol (C)]. The inter-ring dihedral angles are A/B = 6.93 (15), A/C = 18.74 (15) and B/C = 12.17 (15)°. The C7—C8—S2—C9 torsion angle of 172.56 (17)° indicates approximate coplanarity of these atoms. Otherwise, geometrical data for (I) are similar to those found in structurally related compounds (Xia et al., 2011 ▸; Xu et al., 2005 ▸).
Figure 1.
The molecular structure of (I) with displacement ellipsoids drawn at the 30% probability level.
Hydrogen bonding and Hirshfeld surface analysis
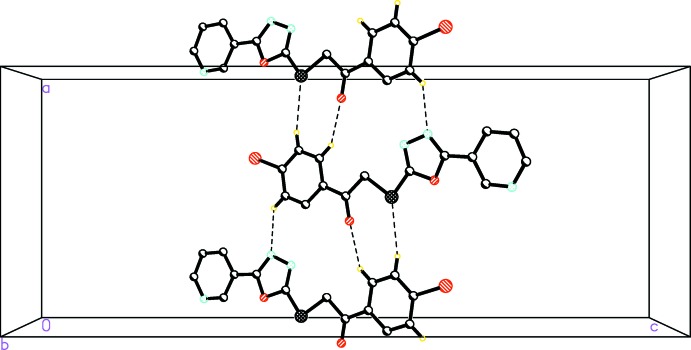
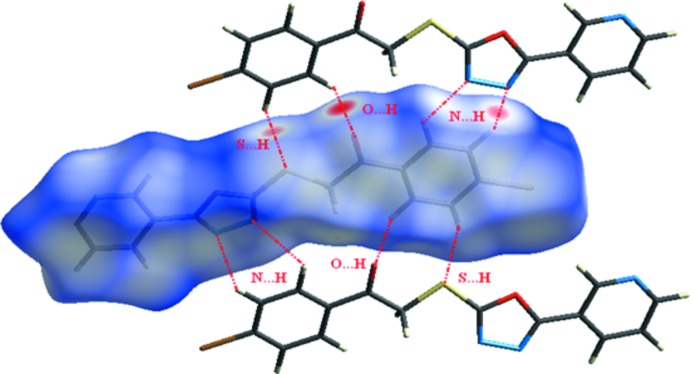
The packing of (I) is consolidated by C1—H1B⋯O1, C1—H2B⋯S2 and C4—H4A⋯N2 hydrogen bonds, which form chains running along a-axis direction (Fig. 2 ▸, Table 1 ▸). The Hirshfeld surface analysis (Hirshfeld, 1977 ▸) of the crystal structure indicates that the contribution of the H⋯H intermolecular interactions to the crystal packing amounts to 19.5%, N⋯H = 17.3%, Br⋯H = 11.7% and O⋯H = 11.0%. Minor intermolecular contacts for the cohesion of the structure are: C⋯O = 4.7%, C⋯C = 3.6% and others (Br⋯C, C⋯S, C⋯N, Br⋯S, N⋯N, Br⋯N, O⋯N)= 10.4%. These contacts are represented by conventional mapping of d norm on the molecular Hirshfeld surface, as shown in Fig. 3 ▸. The H⋯H contribution to the crystal packing is shown as a Hirshfeld surface two-dimensional fingerprint plot with red dots (Wolff et al., 2012 ▸). The d e (y axis) and d i (x axis) values are the closest external and internal distances (Å) from given points on the Hirshfeld surface (Fig. 4 ▸).
Figure 2.
The crystal packing of the title compound (I). Only hydrogen atoms involved in hydrogen bonding (dashed lines) are shown.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C1—H1B⋯O1i | 0.93 | 2.42 | 3.260 (3) | 150 |
| C2—H2B⋯S2i | 0.93 | 2.86 | 3.716 (3) | 153 |
| C4—H4A⋯N2ii | 0.93 | 2.58 | 3.372 (4) | 144 |
Symmetry codes: (i) 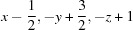 ; (ii)
; (ii) 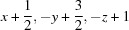 .
.
Figure 3.
d norm mapped on the Hirshfeld surface illustrating the intermolecular contacts of the title compound. Dotted lines indicate hydrogen bonds.
Figure 4.
Fingerprint plots of the title compound, for (a) all, (b) H⋯H, (c) C⋯H, (d) N⋯H, (e) O⋯H and (f) S⋯H contacts. The outline of the full fingerprint plot is shown in grey. d i is the closet internal distance from a given point on the Hirshfeld surface and d e is the closest external contact.
Comparison with reported literature
A database search disclosed a long list of compounds containing the 1,3,4-oxadiazole moiety; however, only two examples of sulfanylethanone-substituted 1,3,4-oxadiazole derivatives were found, viz. 1,3-bis{[5-(pyridin-2-yl)-1,3,4-oxadiazol-2-yl]sulfanyl}propan-2-one (II) (Xia et al., 2011 ▸) and 2-{5-[(1H-1,2,4-triazol-1-yl)-methyl]-1,3,4-oxadiazol-2-ylthio}-1-(2,4-dichlorophenyl)ethanone (III) (Xu et al., 2005 ▸). H⋯N interactions were found to be the most relevant intermolecular interactions to form hydrogen bonds with neighboring molecules. Therefore, D—H⋯N interactions were considered in a comparison with reported structures. In the crystal of (II), the molecules are linked into a three-dimensional network via weak C—H⋯N hydrogen bonds (H⋯N distances = 2.51 and 2.54 Å) In (III), the C—H⋯N hydrogen bonds are found to be slightly weaker in comparison with the first structure (H⋯N distances = 2.41 Å). The change in substituents also changes the packing pattern towards zigzag chains extending along the b-axis direction. In addition, both (II) and (III) feature aromatic π–π stacking interactions, which are not observed in (I).
Synthesis and crystallization
5-(3-Pyridyl)-1,3,4-oxadiazole-2-thiol (179 mg, 1 mmol) and triethyl amine (0.1 ml) were added in ethanol (10 ml) and stirred for 10 min in a round-bottomed flask. After 10 min, to the reaction mixture was slowly added 2 4-dibromacetophenone (278 mg, 1 mmol). The mixture was refluxed until complete consumption of starting materials, the progress of reaction being monitored by TLC. After 2 h, the precipitate that had formed was separated, washed with ethanol and recrystallized from methanol solution to afford colourless blocks (346 mg, 92% yield).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. H atoms were positioned geometrically with C—H = 0.93 Å (CH) or 0.97 Å (CH2) and constrained to ride on their parent atoms with U iso(H)= 1.2U eq(C).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C15H10BrN3O2S |
| M r | 376.23 |
| Crystal system, space group | Orthorhombic, P b c a |
| Temperature (K) | 273 |
| a, b, c (Å) | 11.9144 (16), 8.3755 (12), 30.382 (4) |
| V (Å3) | 3031.8 (7) |
| Z | 8 |
| Radiation type | Mo Kα |
| μ (mm−1) | 2.86 |
| Crystal size (mm) | 0.47 × 0.39 × 0.11 |
| Data collection | |
| Diffractometer | Bruker SMART APEX CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2000 ▸) |
| T min, T max | 0.347, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 16806, 2765, 2106 |
| R int | 0.038 |
| (sin θ/λ)max (Å−1) | 0.606 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.035, 0.114, 1.13 |
| No. of reflections | 2765 |
| No. of parameters | 199 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.40, −0.25 |
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989017004819/hb7660sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017004819/hb7660Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989017004819/hb7660Isup3.cml
CCDC reference: 1540579
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors are thankful to the financial support of Higher Education Commission (HEC) Pakistan through research projects No. 20–1910 and 20–2830.
supplementary crystallographic information
Crystal data
| C15H10BrN3O2S | Dx = 1.648 Mg m−3 |
| Mr = 376.23 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, Pbca | Cell parameters from 3887 reflections |
| a = 11.9144 (16) Å | θ = 2.7–22.9° |
| b = 8.3755 (12) Å | µ = 2.86 mm−1 |
| c = 30.382 (4) Å | T = 273 K |
| V = 3031.8 (7) Å3 | Block, colorless |
| Z = 8 | 0.47 × 0.39 × 0.11 mm |
| F(000) = 1504 |
Data collection
| Bruker SMART APEX CCD diffractometer | 2765 independent reflections |
| Radiation source: fine-focus sealed tube | 2106 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.038 |
| ω scan | θmax = 25.5°, θmin = 1.3° |
| Absorption correction: multi-scan (SADABS; Bruker, 2000) | h = −14→13 |
| Tmin = 0.347, Tmax = 0.746 | k = −10→10 |
| 16806 measured reflections | l = −36→36 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.035 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.114 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.13 | w = 1/[σ2(Fo2) + (0.0639P)2 + 0.238P] where P = (Fo2 + 2Fc2)/3 |
| 2765 reflections | (Δ/σ)max = 0.002 |
| 199 parameters | Δρmax = 0.40 e Å−3 |
| 0 restraints | Δρmin = −0.25 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.32966 (4) | 1.17413 (5) | 0.651655 (12) | 0.0855 (2) | |
| S2 | 0.48889 (6) | 0.57198 (9) | 0.42780 (3) | 0.0573 (2) | |
| O1 | 0.58756 (16) | 0.7313 (2) | 0.49352 (7) | 0.0627 (6) | |
| O2 | 0.42221 (15) | 0.3878 (2) | 0.36429 (6) | 0.0519 (5) | |
| N1 | 0.2823 (2) | 0.4393 (3) | 0.40960 (8) | 0.0593 (6) | |
| N2 | 0.2436 (2) | 0.3373 (3) | 0.37529 (9) | 0.0632 (7) | |
| N3 | 0.4463 (3) | 0.0865 (4) | 0.25578 (10) | 0.0847 (9) | |
| C1 | 0.3327 (2) | 0.8827 (4) | 0.54232 (11) | 0.0561 (7) | |
| H1B | 0.2798 | 0.8391 | 0.5233 | 0.067* | |
| C2 | 0.2986 (3) | 0.9787 (4) | 0.57656 (11) | 0.0642 (8) | |
| H2B | 0.2227 | 1.0005 | 0.5806 | 0.077* | |
| C3 | 0.3762 (3) | 1.0418 (3) | 0.60463 (9) | 0.0561 (7) | |
| C4 | 0.4894 (2) | 1.0117 (3) | 0.59921 (10) | 0.0572 (8) | |
| H4A | 0.5416 | 1.0550 | 0.6186 | 0.069* | |
| C5 | 0.5238 (2) | 0.9178 (3) | 0.56513 (10) | 0.0528 (7) | |
| H5A | 0.5999 | 0.8979 | 0.5612 | 0.063* | |
| C6 | 0.4458 (2) | 0.8510 (3) | 0.53610 (9) | 0.0450 (6) | |
| C7 | 0.4875 (2) | 0.7497 (3) | 0.49968 (9) | 0.0468 (6) | |
| C8 | 0.4052 (2) | 0.6676 (3) | 0.46970 (10) | 0.0495 (7) | |
| H8A | 0.3543 | 0.7445 | 0.4566 | 0.059* | |
| H8B | 0.3617 | 0.5892 | 0.4858 | 0.059* | |
| C9 | 0.3863 (2) | 0.4642 (3) | 0.40109 (9) | 0.0501 (7) | |
| C10 | 0.3272 (2) | 0.3106 (3) | 0.35029 (10) | 0.0514 (7) | |
| C11 | 0.3335 (2) | 0.2139 (3) | 0.31076 (11) | 0.0541 (7) | |
| C12 | 0.2370 (3) | 0.1585 (3) | 0.29051 (11) | 0.0621 (8) | |
| H12A | 0.1665 | 0.1820 | 0.3020 | 0.075* | |
| C13 | 0.2476 (3) | 0.0681 (4) | 0.25307 (11) | 0.0717 (10) | |
| H13A | 0.1842 | 0.0287 | 0.2389 | 0.086* | |
| C14 | 0.3520 (3) | 0.0365 (4) | 0.23684 (12) | 0.0779 (10) | |
| H14A | 0.3575 | −0.0232 | 0.2111 | 0.093* | |
| C15 | 0.4355 (3) | 0.1747 (4) | 0.29175 (12) | 0.0702 (9) | |
| H15A | 0.5005 | 0.2127 | 0.3051 | 0.084* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.1083 (4) | 0.0886 (3) | 0.0597 (3) | −0.00727 (19) | 0.02208 (18) | −0.00144 (17) |
| S2 | 0.0409 (4) | 0.0600 (4) | 0.0711 (5) | −0.0033 (3) | 0.0054 (3) | −0.0031 (4) |
| O1 | 0.0357 (14) | 0.0716 (13) | 0.0808 (15) | 0.0013 (10) | −0.0025 (10) | 0.0025 (11) |
| O2 | 0.0407 (11) | 0.0572 (10) | 0.0578 (12) | −0.0024 (9) | 0.0065 (9) | 0.0010 (9) |
| N1 | 0.0405 (14) | 0.0658 (15) | 0.0716 (17) | −0.0039 (11) | 0.0083 (12) | −0.0052 (12) |
| N2 | 0.0454 (16) | 0.0682 (16) | 0.0759 (18) | −0.0064 (11) | 0.0074 (14) | −0.0062 (13) |
| N3 | 0.075 (2) | 0.103 (2) | 0.076 (2) | 0.0034 (17) | 0.0039 (16) | −0.0197 (17) |
| C1 | 0.0354 (17) | 0.0653 (17) | 0.068 (2) | −0.0096 (13) | −0.0067 (13) | 0.0019 (15) |
| C2 | 0.0432 (18) | 0.075 (2) | 0.074 (2) | −0.0041 (15) | 0.0102 (15) | 0.0014 (17) |
| C3 | 0.059 (2) | 0.0566 (16) | 0.0527 (17) | −0.0062 (14) | 0.0036 (14) | 0.0095 (13) |
| C4 | 0.055 (2) | 0.0570 (17) | 0.0592 (19) | −0.0102 (13) | −0.0151 (14) | 0.0116 (14) |
| C5 | 0.0409 (16) | 0.0536 (16) | 0.0638 (18) | −0.0013 (12) | −0.0102 (13) | 0.0114 (14) |
| C6 | 0.0343 (15) | 0.0450 (13) | 0.0558 (16) | −0.0031 (11) | −0.0059 (12) | 0.0128 (12) |
| C7 | 0.0350 (18) | 0.0455 (14) | 0.0600 (17) | −0.0014 (11) | −0.0046 (12) | 0.0130 (12) |
| C8 | 0.0381 (16) | 0.0504 (15) | 0.0601 (17) | −0.0002 (11) | 0.0001 (12) | 0.0039 (12) |
| C9 | 0.0451 (18) | 0.0450 (14) | 0.0600 (18) | 0.0018 (12) | 0.0039 (13) | 0.0057 (13) |
| C10 | 0.0409 (18) | 0.0515 (16) | 0.0619 (19) | −0.0025 (12) | 0.0015 (13) | 0.0096 (13) |
| C11 | 0.053 (2) | 0.0515 (15) | 0.0580 (18) | 0.0001 (12) | −0.0009 (13) | 0.0075 (13) |
| C12 | 0.053 (2) | 0.0605 (18) | 0.073 (2) | −0.0053 (14) | −0.0073 (16) | 0.0071 (15) |
| C13 | 0.074 (3) | 0.069 (2) | 0.072 (2) | −0.0099 (18) | −0.0188 (18) | −0.0001 (17) |
| C14 | 0.085 (3) | 0.079 (2) | 0.070 (2) | 0.002 (2) | −0.010 (2) | −0.0093 (18) |
| C15 | 0.055 (2) | 0.087 (2) | 0.069 (2) | −0.0046 (16) | 0.0017 (16) | −0.0092 (17) |
Geometric parameters (Å, º)
| Br1—C3 | 1.891 (3) | C4—C5 | 1.363 (4) |
| S2—C9 | 1.722 (3) | C4—H4A | 0.9300 |
| S2—C8 | 1.804 (3) | C5—C6 | 1.398 (4) |
| O1—C7 | 1.217 (3) | C5—H5A | 0.9300 |
| O2—C9 | 1.357 (3) | C6—C7 | 1.480 (4) |
| O2—C10 | 1.372 (3) | C7—C8 | 1.504 (4) |
| N1—C9 | 1.283 (4) | C8—H8A | 0.9700 |
| N1—N2 | 1.424 (3) | C8—H8B | 0.9700 |
| N2—C10 | 1.272 (4) | C10—C11 | 1.451 (4) |
| N3—C15 | 1.326 (4) | C11—C12 | 1.384 (4) |
| N3—C14 | 1.330 (5) | C11—C15 | 1.385 (4) |
| C1—C2 | 1.376 (4) | C12—C13 | 1.372 (4) |
| C1—C6 | 1.387 (4) | C12—H12A | 0.9300 |
| C1—H1B | 0.9300 | C13—C14 | 1.364 (4) |
| C2—C3 | 1.365 (4) | C13—H13A | 0.9300 |
| C2—H2B | 0.9300 | C14—H14A | 0.9300 |
| C3—C4 | 1.382 (4) | C15—H15A | 0.9300 |
| C9—S2—C8 | 99.97 (13) | C7—C8—H8A | 110.6 |
| C9—O2—C10 | 102.6 (2) | S2—C8—H8A | 110.6 |
| C9—N1—N2 | 105.2 (2) | C7—C8—H8B | 110.6 |
| C10—N2—N1 | 106.8 (2) | S2—C8—H8B | 110.6 |
| C15—N3—C14 | 116.7 (3) | H8A—C8—H8B | 108.7 |
| C2—C1—C6 | 120.1 (3) | N1—C9—O2 | 113.2 (2) |
| C2—C1—H1B | 119.9 | N1—C9—S2 | 132.5 (2) |
| C6—C1—H1B | 119.9 | O2—C9—S2 | 114.32 (19) |
| C3—C2—C1 | 119.9 (3) | N2—C10—O2 | 112.2 (3) |
| C3—C2—H2B | 120.0 | N2—C10—C11 | 129.3 (3) |
| C1—C2—H2B | 120.0 | O2—C10—C11 | 118.5 (2) |
| C2—C3—C4 | 121.1 (3) | C12—C11—C15 | 117.7 (3) |
| C2—C3—Br1 | 120.0 (2) | C12—C11—C10 | 120.8 (3) |
| C4—C3—Br1 | 118.9 (2) | C15—C11—C10 | 121.5 (3) |
| C5—C4—C3 | 119.2 (3) | C13—C12—C11 | 118.5 (3) |
| C5—C4—H4A | 120.4 | C13—C12—H12A | 120.8 |
| C3—C4—H4A | 120.4 | C11—C12—H12A | 120.8 |
| C4—C5—C6 | 120.7 (3) | C14—C13—C12 | 119.4 (3) |
| C4—C5—H5A | 119.6 | C14—C13—H13A | 120.3 |
| C6—C5—H5A | 119.6 | C12—C13—H13A | 120.3 |
| C1—C6—C5 | 118.9 (3) | N3—C14—C13 | 123.6 (4) |
| C1—C6—C7 | 122.5 (3) | N3—C14—H14A | 118.2 |
| C5—C6—C7 | 118.6 (2) | C13—C14—H14A | 118.2 |
| O1—C7—C6 | 121.1 (2) | N3—C15—C11 | 124.1 (3) |
| O1—C7—C8 | 119.2 (3) | N3—C15—H15A | 117.9 |
| C6—C7—C8 | 119.7 (2) | C11—C15—H15A | 117.9 |
| C7—C8—S2 | 105.69 (19) | ||
| C9—N1—N2—C10 | 0.6 (3) | C10—O2—C9—N1 | 0.1 (3) |
| C6—C1—C2—C3 | −0.5 (5) | C10—O2—C9—S2 | 178.86 (18) |
| C1—C2—C3—C4 | 0.3 (5) | C8—S2—C9—N1 | −7.1 (3) |
| C1—C2—C3—Br1 | 179.8 (2) | C8—S2—C9—O2 | 174.34 (19) |
| C2—C3—C4—C5 | 0.2 (4) | N1—N2—C10—O2 | −0.6 (3) |
| Br1—C3—C4—C5 | −179.3 (2) | N1—N2—C10—C11 | 179.7 (3) |
| C3—C4—C5—C6 | −0.6 (4) | C9—O2—C10—N2 | 0.3 (3) |
| C2—C1—C6—C5 | 0.1 (4) | C9—O2—C10—C11 | −179.9 (2) |
| C2—C1—C6—C7 | −179.5 (3) | N2—C10—C11—C12 | 12.1 (5) |
| C4—C5—C6—C1 | 0.5 (4) | O2—C10—C11—C12 | −167.6 (2) |
| C4—C5—C6—C7 | −179.9 (2) | N2—C10—C11—C15 | −168.4 (3) |
| C1—C6—C7—O1 | 175.7 (3) | O2—C10—C11—C15 | 11.9 (4) |
| C5—C6—C7—O1 | −3.8 (4) | C15—C11—C12—C13 | 0.2 (4) |
| C1—C6—C7—C8 | −4.4 (4) | C10—C11—C12—C13 | 179.7 (3) |
| C5—C6—C7—C8 | 176.0 (2) | C11—C12—C13—C14 | −0.4 (5) |
| O1—C7—C8—S2 | −4.8 (3) | C15—N3—C14—C13 | −1.6 (6) |
| C6—C7—C8—S2 | 175.32 (19) | C12—C13—C14—N3 | 1.2 (6) |
| C9—S2—C8—C7 | 172.56 (17) | C14—N3—C15—C11 | 1.4 (5) |
| N2—N1—C9—O2 | −0.4 (3) | C12—C11—C15—N3 | −0.7 (5) |
| N2—N1—C9—S2 | −178.9 (2) | C10—C11—C15—N3 | 179.8 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C1—H1B···O1i | 0.93 | 2.42 | 3.260 (3) | 150 |
| C2—H2B···S2i | 0.93 | 2.86 | 3.716 (3) | 153 |
| C4—H4A···N2ii | 0.93 | 2.58 | 3.372 (4) | 144 |
Symmetry codes: (i) x−1/2, −y+3/2, −z+1; (ii) x+1/2, −y+3/2, −z+1.
References
- Abu-Zaied, M., El-Telbani, E. M., Elgemeie, G. H. & Nawwar, G. A. (2011). Eur. J. Med. Chem. 46, 229–235. [DOI] [PubMed]
- Bharti, S. K., Nath, G., Tilak, R. & Singh, S. K. (2010). Eur. J. Med. Chem. 45, 651–660. [DOI] [PubMed]
- Bruker (2000). SADABS, SMART and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Chandrakantha, B., Shetty, P., Nambiyar, V., Isloor, N. & Isloor, A. M. (2010). Eur. J. Med. Chem. 45, 1206–1210. [DOI] [PubMed]
- Hirshfeld, H. L. (1977). Theor. Chim. Acta, 44, 129–138.
- Husain, A., Ahmad, A., Alam, M. M., Ajmal, M. & Ahuja, P. (2009). Eur. J. Med. Chem. 44, 3798–3804. [DOI] [PubMed]
- Kashtoh, H., Hussain, S., Khan, A., Saad, S. M., Khan, J. A. J., Khan, K. M., Perveen, S. & Choudhary, M. I. (2014). Bioorg. Med. Chem. 22, 5454–5465. [DOI] [PubMed]
- Nardelli, M. (1995). J. Appl. Cryst. 28, 659.
- Omar, F. A., Mahfouz, N. M. & Rahman, M. A. (1996). Eur. J. Med. Chem. 31, 819–825. [DOI] [PubMed]
- Prakash, O., Kumar, M., Kumar, R., Sharma, C. & Aneja, K. R. (2010). Eur. J. Med. Chem. 45, 4252–4257. [DOI] [PubMed]
- Rajak, H., Deshmukh, R., Veerasamy, R., Sharma, A. K., Mishra, P. & Kharya, M. D. (2010). Bioorg. Med. Chem. Lett. 20, 4168–4172. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Wolff, S. K., Grimwood, D. J., McKinnon, J. J., Turner, M. J., Jayatilaka, D. & Spackman, M. A. (2012). Crystal Explorer. University of Western Australia.
- Xia, C.-H., Mao, C.-B. & Wu, B.-L. (2011). Acta Cryst. E67, o413. [DOI] [PMC free article] [PubMed]
- Xu, L.-Z., Yu, G.-P., Yin, S.-M., Zhou, K. & Yang, S.-H. (2005). Acta Cryst. E61, o3375–o3376.
- Zarghi, A., Tabatabai, S. A., Faizi, M., Ahadian, A., Navabi, P., Zanganeh, V. & Shafiee, A. (2005). Bioorg. Med. Chem. Lett. 15, 1863–1865. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989017004819/hb7660sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017004819/hb7660Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989017004819/hb7660Isup3.cml
CCDC reference: 1540579
Additional supporting information: crystallographic information; 3D view; checkCIF report