Abstract
The genus Camellia (C.) contains many species, including C. sinensis, C. assamica, and C. taliensis, C. gymnogyna and C. tachangensis. The polyphenols of C. sinensis and C. assamica are flavan-3-ols monomers and their dimers and trimmers. However, the biosynthesis of procyanidins in Camellia genus remains unclear. In the present study, a comparative chemical analysis of flavan-3-ols, flavan-3-ols glycoside and procyanidins was conducted by high performance liquid chromatography (HPLC) and liquid chromatography diode array detection coupled with triple-quadrupole mass-spectrometry (LC-DAD-QQQ-MS). The results showed that C. tachangensis had a significant higher contents of (-)-epicatechin (EC) and (-)-epigallocatechin (EGC) compared with C. sinensis (p < 0.001). By contrast, higher levels of galloylated catechins were detected in C. sinensis. LC-DAD-MS/MS indicated that the main secondary metabolites of C. tachangensis were non-galloylated catechins, procyanidin dimers and trimers. Furthermore, (-)-epicatechin glucose (EC-glucose) and (-)-epigallocatechin glucose (EGC-glucose) were also abundant in C. tachangensis. A correlation analysis of EC-glucose and procyanidins dimers was conducted in five Camellia species. The levels of EC-glucose were closely related to the procyanidin dimers content. Thus, it was suggested that EC-glucose might be an important substrate for the biosynthesis of procyanidins.
The genus Camellia (C.) is a large family of plants that originated in South-western China and includes hundreds of species. Two famous Camellia species, Camellia sinensis (L.) O. Ktze and Camellia assamica (Mast.) Chang, are cultivated worldwide for tea production. In addition, C. tachangensis is also used as the raw material for tea in Yunnan province, China1. The main secondary metabolites of C. sinensis are flavan-3-ols and their gallate esters2. These compounds are mainly distributed in the leaves of C. sinensis3,4. (-)-Epigallocatechin gallate (EGCG) is a typical flavan-3-ol in the leaves of C. sinensis, whose biosynthetic pathway has been clarified5. It was reported that galloyl-1-O-β-D-glucosyltransferase and 1-O-galloyl-β-D-glucose O-galloyltransferase were the critical enzymes in the biosynthesis of galloylated catechins5. Furthermore, procyanidins are distributed abundantly in the roots of C. sinensis6. However, the biosynthesis of procyanidins in Camellia species has not been clarified.
In 1980s, C. tachangensis was first identified in Yunnan, China. It was thought that C. tachangensis, C. taliensis and C. gymnogyna are different from C. sinensis by chloroplast genome sequences analysis. During the domestication process of Camellia species, C. sinensis has become the optimal species to produce tea products, such as green tea. To study the relationships of various Camellia species, taxonomic approaches have been applied7. The taxonomy of Camellia species relied on the traditional morphology-based classification. However, recently, some molecular markers have been tentatively used in taxonomic studies8. Moreover, chemotaxonomy is also a potent tool to study the relationships among Camellia species9. To understand the value of Camellia species, more attention should be paid to the taste and flavour chemistry of their leaves. The taste and bioactivities of green tea were attributed to a complicated combination of L-theanine, catechins, and caffeine10,11. For example, condensed tannins have an intensely astringent taste. Therefore, they are usually artificially decreased in tea plantations12,13. The chemical units of the procyanidins are usually EC and EGC13. Thus, it was deduced that the biosynthesis of procyanidins derives from the auto-polymerisation of flavan-3-ols monomers14. However, the biosynthesis of procyanidins in Camellia plants remains unclear.
Liquid chromatography coupled mass spectroscopy (LC-MS) is a high throughput and unbiased tool that has been used to identify trace secondary metabolites of plants15. To study the chemical characteristics among various Camellia species, metabolomics analysis combined with LC-MS has been applied to identify the differences between wild-types and cultivars of Camellia species (unpublished data). These metabolomics analyses suggested that procyanidins might be the significant different metabolites of different Camellia species. To study the chemical differences of various Camellia species, we collected the mature leaves of C. sinensis, C. assamica, C. taliensis, C. gymnogyna and C. tachangensis in the same place at the same time, and performed a comprehensive quantitative analysis of the major polyphenols, procyanidins and their probable substrates.
In the present study, the contents of catechins in the mature leaves of C. sinensis, C. assamica, C. taliensis, C. gymnogyna and C. tachangensis were determined by HPLC. The unique secondary metabolites of C. tachangensis were identified by LC-DAD-MS16. The critical substrates involved in the biosynthesis of galloylated catechins and procyanidins were compared17. A proposed biosynthetic pathway for procyanidins was deduced using a correlation analysis.
Results
Differences in the compounds of C. assamica, C. sinensis, C. taliensis, C. gymnogyna and C. tachangensis by LC-DAD-MS/MS
Figure 1 shows that the total ion chromatography (TIC) profiles of C. sinensis, C. assamica and C. taliensis were similar, but the profiles of C. gymnogyna and C. tachangensis showed significant difference compared with other three Camellia samples. In the TIC of C. tachangensis, four unique compounds with m/z at 577, 593, 729 and 865 were detected. In the TICs of C. assamica, C. sinensis and C. taliensis, the catechins including EC, EGC, (-)-epicatechin gallate (ECG) and EGCG were also identified.
Figure 1. The TICs of C. assamica, C. sinensis, C. taliensis, C. gymnogyna and C. tachangensis.
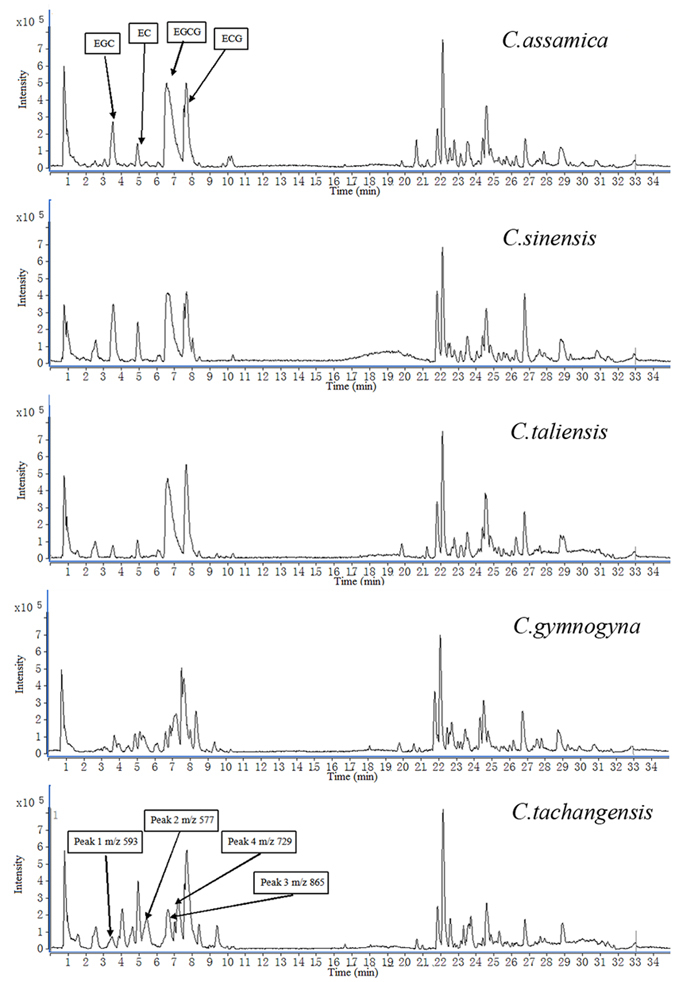
EGC, (-)-epigallocatechin; EC, (-)-epicatechin; EGCG, (-)-epigallocatechin gallate; ECG, (-)-epicatechin gallate.
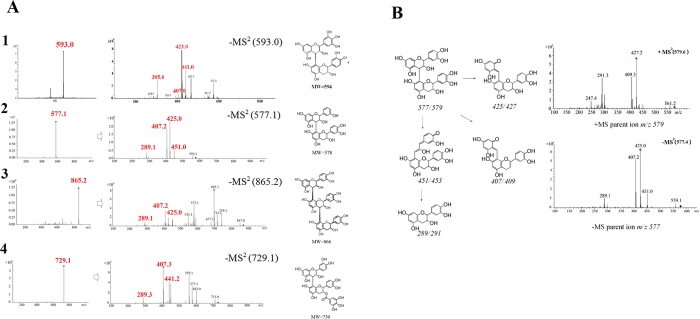
To explore the chemical structures of these four unique compounds in C. tachangensis, the ultraviolet (UV) spectrum and MS/MS spectrum of individual compound was analyzed. Compounds 1–4 showed strong absorbance intensity at the detection wavelength of 278 nm. The UV spectrum of these compounds was similar, having maximum absorption wavelengths at 230 and 278 nm (Supplementary Figure 1). These compounds might contain the flavan-3-ol unit, the maximum absorption wavelengths of which are also about 230 and 278 nm.
Compound 2 (peak 2) had a molecular weight of 578.1, which was deduced by the quasi-molecular ion peak of [M − H]− at m/z 577.1. The molecular formula was calculated as C30H26O12. Under the MS/MS mode, peak 2 showed that [M − H]− at m/z 577.1 produced five major secondary fragments (MS2) at m/z 559.1, 451.0, 425.0, 407.2, and 289.1 (Fig. 2). Compound 2 was identified as a procyanidins dimer (EC-EC). Furthermore, compounds 1, 3 and 4 gave [M + H]+ and [M − H]− parent ions at m/z at 595/593, 867/865 and 731/729, respectively. The molecular weights of compounds 1, 3 and 4 were deduced to be 594, 866 and 730. With reference to the published mass spectra of procyanidins in tea plants, compounds 1, 3 and 4 were identified as procyanidin trimers and dimers, constructing by EGC-EC, EC-EC-EC and ECG-EC respectively18,19.
Figure 2. The MS/MS fragment ions of peak 1–4.
(A), the parent ion and fragment ions of compound 1–4; (B), the fragmentation pathway of compound 2.
Loss of cleavage analysis provided structural information. The loss of a 126 Da fragment was the cleavage of the A-ring of flavan-3-ols. The loss of a 152 Da fragment was from the retro-Diels-Alder (RDA) fragmentation of the B-ring of flavan-3-ols. The loss of a 170 Da fragment might be derived from the continuous loss of OH after the RDA cleavage. Therefore, the neutral loss of 126, 152, and 170 could be used as the main fragmentation markers of procyanidins to analyse their chemical structures.
The contents of catechins, gallic acid and caffeine in C. assamica, C. sinensis, C. taliensis, C. gymnogyna and C. tachangensis by HPLC
Catechins and galloylated catechins are the main secondary metabolites of C. sinensis and C. assamica2. According to the published method16, the levels of the main compounds in the five Camellia samples were determined. Table 1 showed that the levels of galloylated catechins, including and ECG and EGCG in C. sinensis and C. assamica were significantly higher than those in C. tachangensis (p < 0.001). Furthermore, the contents of caffeine in C. tachangensis were also significantly lower than those of C. sinensis and C. tachangensis (p < 0.001).
Table 1. The contents of major compounds of C. sinensis, C. assamica, C. taliensis, C. gymnogyna and C. tachangensis (mg/g) (Mean ± S.d.).
| Compounda | GA | GC | EGC | EC | CAF | EGCG | GCG | ECG |
|---|---|---|---|---|---|---|---|---|
| C. assamica | 0.47 ± 0.01 | 0.37 ± 0.01 | 3.74 ± 0.08 | 1.99 ± 0.04 | 7.84 ± 0.24 | 34.73 ± 0.83 | 0.37 ± 0.01 | 4.39 ± 0.11 |
| C. sinensis | 0.44 ± 0.01 | 3.19 ± 0.16 | 8.79 ± 0.07 | 4.16 ± 0.02 | 6.41 ± 0.06 | 23.91 ± 0.19 | 0.19 ± 0.01 | 4.38 ± 0.04 |
| C. taliensis | 0.58 ± 0.01 | 1.43 ± 0.03 | ND | ND | 0.65 ± 0.16 | 15.37 ± 0.23 | 0.14 ± 0.00 | 3.39 ± 0.04 |
| C. gymnogyna | 0.10 ± 0.01 | ND | 1.20 ± 0.09 | 0.58 ± 0.02 | 2.34 ± 0.25 | 0.64 ± 0.06 | 0.17 ± 0.01 | 3.99 ± 0.13 |
| C. tachangensis | 0.82 ± 0.01*** | ND | 12.01 ± 0.14*** | 9.50 ± 0.10*** | 0.13 ± 0.03*** | 1.48 ± 0.03*** | 0.32 ± 0.01 | 0.09 ± 0.01*** |
ND, not detected, ***p < 0.001 compared with C. sinensis. GA, gallic acid; GC, (-)-gallocatechin; (-)-EGC, (-)-epigallocatechin; EC, (-)-epicatechin; CAF, caffeine; EGCG, (-)-epigallocatechin gallate; GCG, (-)-gallocatechin gallate; ECG, (-)-epicatechin gallate.
An important finding was that the contents of EC and EGC in C. tachangensis were significantly higher than those of C. sinensis and C. assamica. Usually, EGCG is the major polyphenol in the fresh leaves of C. sinensis and C. assamica20. In the present study, the EC and EGC content of C. tachangensis was about two-fold higher than that in C. sinensis. Even so, the amount of galloylated (-)-epicatechin in C. tachangensis was not increased in proportion, which means that the biosynthesis of galloylated catechins was decreased in the leaves of C. tachangensis21,22. These results suggested that the main polyphenols compounds in C. tachangensis were different from C. sinensis and C. assamica.
The quantitative analysis of procyanidins, EC-glucose, EGC-glucose by LC-QQQ-MS
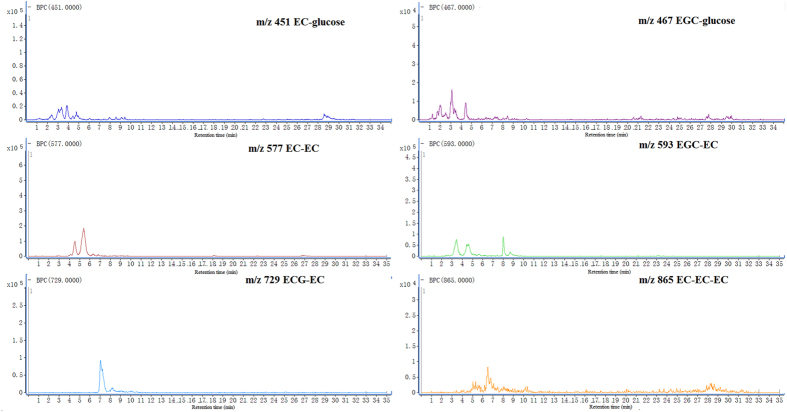
As shown in Fig. 3, the m/z at 577, 593, 729 and 865 were targeted in the TIC of mature leaves extract of C. tachangensis. Furthermore, the m/z at 451 and 467 were also identified as the EC-glucose and EGC-glucose by MS/MS spectrum (Supplementary Figure 2). The fragment ions of m/z at 451 and 467 showed main fragment ions of 289.0 ([M − H]− of catechin) and 305.0 ([M − H]− of gallocatechin) by losing the neutral loss 162 Da [glucose-H2O].
Figure 3. The targeted parent ions of EC-glucose, EGC-glucose, EC-EC, EGC-EC, ECG-EC and EC-EC-EC in C. tachangensis.
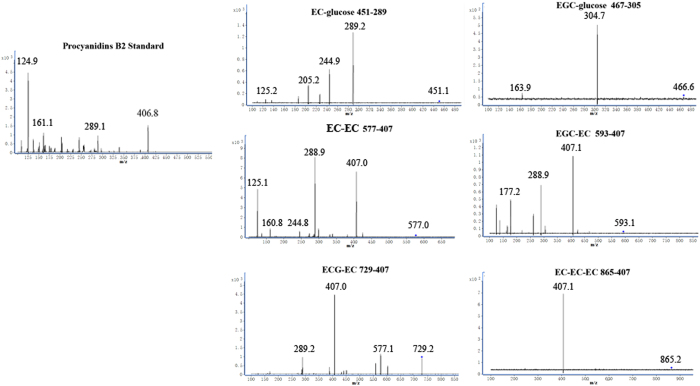
Through the LC-QQQ-MS, the product ions of procyanidins, EC-glucose and EGC-glucose were obtained. All of the four procyanidins compounds showed a same product ion m/z at 407, which is a critical fragment of procyanidins. On the other side, the EC-glucose and EGC-glucose showed the main product ions m/z at 289 and 305, which are the [M − H]− ions of EC and EGC, respectively. The collision energies (CE) were selected at 20 V for EC-glucose, EGC-glucose, procyanidins EC-EC, procyanidins EGC-EC, procyanidins ECG-EC and procyanidins EC-EC-EC. As shown in Fig. 4 multiple reaction monitoring (MRM) mode was employed to detect the target compounds by selected product ions from the parent ions (EC-glucose, 451 → 289; EGC-glucose, 467 → 305; EC-EC, 577 → 407; EGC-EC, 593 → 407; ECG-EC, 729 → 407 and EC-EC-EC, 865 → 407).
Figure 4. The parent ions and product ions of mass spectra (MS/MS) of EC-glucose, EGC-glucose, EC-EC, EGC-EC, ECG-EC and EC-EC-EC at the 20 V collision energy of LC-QQQ-MS.
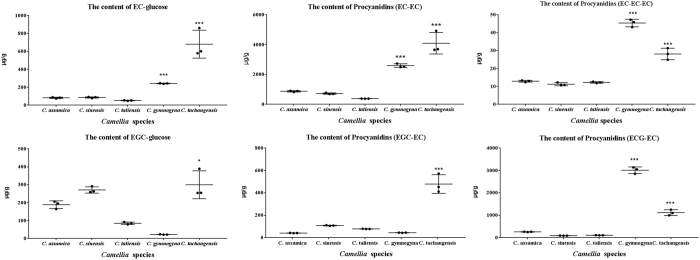
Firstly, the calibration curve of procyanidins B2 was established (Supplementary Figure 3). The regression coefficient (r) of linear equation indicated that the LC-QQQ-MS was with good linearity for the determination of analytes. The levels of procyandins, EC-glucose and EGC-glucose were caculated. As shown in Fig. 5, the content of EC-glucose in C. tachangensis was significantly higher than other Camellia species (p < 0.001). The content of EC-glucose was 681.19 ± 156.35 μg/g in C. tachangnesis, compared with the 242.18 ± 3.75 μg/g, 52.03 ± 3.57 μg/g, 86.45 ± 7.50 μg/g and 82.32 ± 7.50 μg/g in C. gymnogyna, C. taliensis, C. sinensis and C. assamica. Correspondingly, the main procyanidins B2 in C. tachangensis was 4090.69 ± 719.86 μg/g, which was significantly higher than those in C. gymnogyna (2589.89 ± 124.03 μg/g), C. taliensis (380.08 ± 5.94 μg/g), C. sinensis (722.57 ± 47.51 μg/g) and C. assamica (872.29 ± 43.45 μg/g) (p < 0.001).
Figure 5. The contents of EC-glucose, EGC-glucose, EC-EC, EGC-EC, ECG-EC and EC-EC-EC in C. assamica, C. sinensis, C. taliensis, C. gymnogyna and C. tachangensis.
*p < 0.05, ***p < 0.001 compared with C. sinensis.
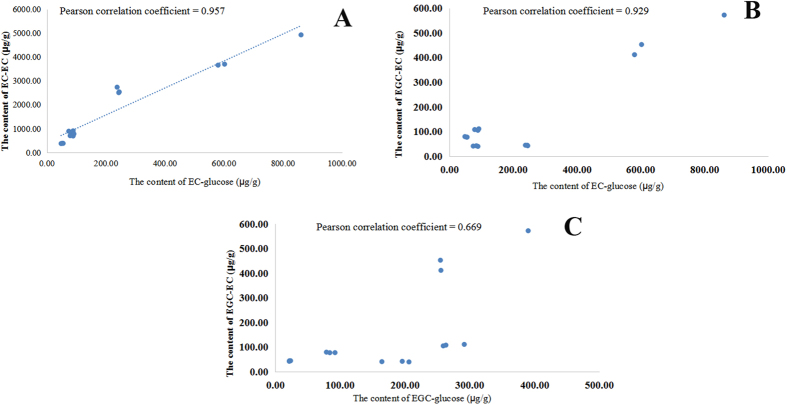
To calculate the pearson’s correlation coefficient of the contents of EC-glucose and procyanidins B2, the contents of EC-glucose and EC-EC were profiled as shown in Fig. 6. With the increasing amounts of EC-glucose, the contents of procyanidins dimers (EC-EC) increased correspondingly.
Figure 6.
The correlation coefficient of the contents of EC-glucose and procyanidin EC-EC (A), EGC-glucose and procyanidin EGC-EC (B), EC-glucose and procyanidin EGC-EC (C) in five Camellia species.
Except for the procyanidins B2 (EC-EC), a procyanidins dimer consisting of EGC and EC unit was also identified and compared in five Camellia species. The EGC-glucose was also simultaneously determined with the contents of procyanidins EGC-EC. The mean value of the contents of EGC-glucose in five Camellia species were listed in order as C. tachangensis > C. sinensis > C. assamica > C. taliensis > C. gymnogyna, while the contents of EGC-EC were C. tachangensis > C. sinensis > C. taliensis > C. gymnogyna > C. assamica. These results suggested that the correlation of EGC-glucose and EGC-EC was weak, which was also confirmed by the pearson correlation coefficient = 0.669. Based on the procyanidins biosynthesis hypothesis, the results supposed that the contents of EC-glucose were the limiting factor for the biosynthesis of EGC-EC.
Quantificational Real-time Polymerase Chain Reaction (qPCR) Analysis
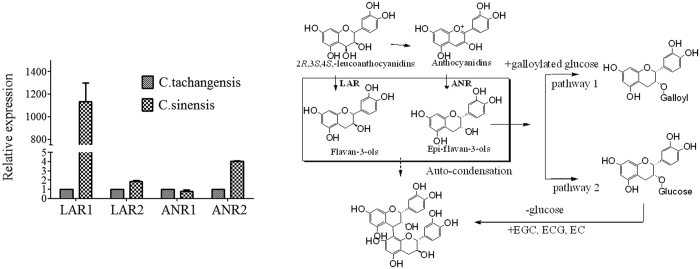
By homology analysis, we found LAR and ANR genes in C. sinensis shared a high homology (>98%) with that in C. tachangensis (Supplementary Figure 4). And this result showed that the primers designed for C. sinensis is available for C. tachangensis. The amplification efficiency of these primers were added in Supplementary Figure 5. Designed primers of each gene were specific, and amplification efficiency were between 90 and 110%, so these primers were considered acceptable for qPCR reactions. Leucocyanidin reductase (LAR) (EC 1.17.1.3) and anthocyanidin reductase (ANR) (EC 1.3.1.77) are the main enzymes responsible for the biosynthesis of catechin and epicatechin, respectively23,24. Traditionally, flavan-3-ol has been considered the product of LAR, which converts leucocyanidin to catechins, after which an epimerase converts catechin to epicatechin25. During the biosynthesis of flavan-3-ols, anthocyanidin synthase and ANR catalyse the conversion of leucodelphinidin to (-)-epigallocatechin via delphinidin and/or leucocyanidin to (-)-epicatechin via cyanidin26. Therefore, high expressions of ANR and LAR are closely related to the high synthesis of epicatechin and catechin. In the present study, two genes in the multi-gene family of LAR (LAR1 and LAR2) and ANR (ANR1 and ANR2) were analysed by qPCR, respectively. The results showed that the relative expressions of LAR1 and LAR2 were higher in cultivars of C. sinensis compared with those in C. tachangensis. The relative expression of LAR1 in C. sinensis was one thousand times higher than that in C. tachangensis. The higher gene expression level might result in higher contents of the main flavan-3-ols (GC, gallocatechin) in C. sinensis compared with those in C. tachangensis.
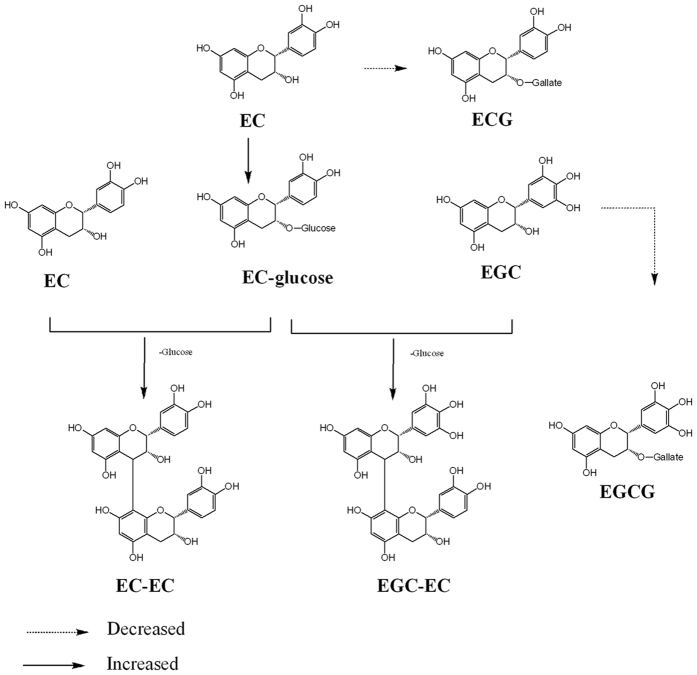
By contrast, the ANR1 and ANR2 expression didn’t show significant differences between C. sinensis and C. tachangensis. This suggested that the biosynthesis of epi-flavan-3-ols was similar in these species. Similarly high contents of epi-flavan-3-ols in C. sinensis and C. tachangensis confirmed this result. However, the main epi-flavan-3-ol of C. tachangensis was EC and EGC, while that in C. sinensis was EGCG and ECG. The LC-TOF-MS analysis suggested that the down-stream metabolism of (-)-epicatechin has two pathways (Fig. 7). One pathway is the classical gallic esterification, which produces galloylated (-)-epicatechin. The other is the glycosylation of (-)-epicatechin, which would contribute to the biosynthesis of procyanidins.
Figure 7. Expression of representative flavonoid biosynthetic genes, as analysed by qPCR.
Discussion
The chemical characteristics and secondary metabolism of Camellia species
Liquid chromatography time-of-flight mass spectrometry (LC-TOF-MS) and LC-DAD-MS/MS are suitable to study the chemical compounds of different Camellia species, whose secondary metabolites are structurally similar. Some metabolomics analyses have used LC-TOF-MS in comparative chemical study of green tea or tea leaves under different processing conditions27. They provided much useful information on the trace secondary metabolites. In a preliminary study, we found that the differences between C. tachangensis and C. sinensis were obvious, and we would not need to use metabolomics analysis.
The flavonoids pathway is a classical metabolic pathway in plant secondary metabolism. Traditionally, the clarification of plant secondary metabolism has relied on interpreting critical catalysing enzymes and molecular biological verification. In this process, the first step is to separate and purify the enzymes from the plant tissue, and then verify the exclusive functional reactions using related substances. This is a basic tool to study plant secondary metabolism5. In the present study, we attempted to elucidate the relationships of secondary metabolites using LC- MS.
In the present study, three types of secondary metabolites (phenolic acids, flavan-3-ols and flavonoids glycosides) were monitored and quantitatively analyzed using LC-QQQ-MS (Supplementary Figure 4). Firstly, the main secondary metabolites of phenylpropanoid pathway were compared in five Camellia species. Galloylated glucose and galloylated quinic acid were assessed28. These compounds are the main galloylated derivatives for the transportation and storage of gallic acid. The content of galloylated quinic acid in C. tachangensis were significantly higher than those in C. assamica and C. sinensis, and the level of galloylated glucose of C. tachangensis was also significantly higher than C. sinensis. In a recently published article, it was verified that galloylated glucose is the main substance for the biosynthesis of galloylated catechins5. Furthermore, the results suggested that galloylated quinic acid might also be involved in this process.
Secondly, the flavonoids glycosides are also major secondary metabolites of flavonoids pathways. They are distributed widely in many angiosperm plants29. In tea plants, over one hundred flavonoid glycosides have been identified30. In the present study, six typical kaempferol and quercetin glycosides were determined. As shown in Supplementary Figure 6, the contents of two falvonoids mono-glycosides (kaempferol glucose-rhamnose and quercetin glucose-rhamnose) in C. tachangensis were significantly higher than C. sinensis, while the flavonoids di-glycosides and tri-gylcosides didn’t show significant differences.
Finally, our quantitative analysis showed that the contents of four procyanidins in C. tachangensis were higher than those in C. sinensis. We tried to explain the proposed mechanism of increased biosynthesis of procyanidins using chemical information from the LC-MS data. In brief, we proposed the biosynthesis pathway and procyanidins of Camellia species, as shown in Fig. 8.
Figure 8. The proposed biosynthesis of procyanidins in the Camellia species.
As shown in Table 1, the contents of EC and EGC in C. tachangensis were significantly higher compared with that in C. sinensis. Correspondingly, the higher level of EC and EGC led to increased contents of procyanidins EC-EC, EGC-EC, ECG-EC and EC-EC-EC in C. tachangensis. These results suggested that EC and EGC might be the critical substrates for the biosynthesis of procyanidins in Camellia plants. The physicochemical properties of EC and EGC are similar to other flavonoid aglycones. It has poor solubility in water; therefore, it might be transported in the form of their glucoside31. The flavan-3-ols are mainly bio-synthesized in the young shoots and leaves of tea plants, while the procyanidins are mostly distributed in the roots6. Therefore, it was suggested that (-)-epicatechin glucoside might be an important carrier for transporting (-)-epicatechin from the young shoots of tea.
Consequently, we hypothesised that EC-glucose might be an important substrate for the biosynthesis of procyanidins in Camellia species. To test this hypothesis, we performed a correlation analysis of EC-glucose and procyanidins dimers. The correlation of EC-glucose and procyanidins (EC-EC) exactly matched the biosynthesis hypothesis of procyanidins which we supposed. Although the main secondary metabolites of C. tachangensis were different from those of C. sinensis, EC and EGC were detected in both species. The accurate determination of flavan-3-ols (catechins) also confirmed that the most remarkable difference between C. tachangensis and C. sinensis was their contents. To study the secondary metabolism of C. tachangensis, the upstream and downstream metabolites of EC were determined quantitatively. The results showed that the biosynthesis of galloylated catechins was decreased in C. tachangensis, because the proposed competitive biosynthesis of procyanidins were enhanced in C. tachangensis.
Furthermore, the contents of galloylated glucose and galloylated quninc acid of C. tachangensis were significantly higher than those of C. sinensis (p < 0.001) in Supplementary Figure 6, but galloylated catechins were not increased. It was supposed that the glycosylation of EC and EGC competed with the galloylation of EC and EGC, and then the procyanidins were increased but galloylated catechins were decreased.
Methods
Plant Materials
Five Camellia species were used in this experiment: C. sinensis, C. gymnogyna, C. taliensis, C. tachangensis, and C. assamica. The mature leaves of Camellia species were collected in Theacease plant garden in Jinghua city, Zhejiang province, China. All of these fresh tea samples were transported in the liquid nitrogen and dried by freezing dry at the temperature of −40°C. Before analysis, these samples were stored at −80 °C.
Chemicals and Reagents
Gallic acid (GA), caffeine (CAF), EC, (-)-gallocatechin (GC), EGC, (-)-gallocatechin gallate (GCG), EGCG, and ECG standards were purchased from Shanghai Tongtian Biotechnology company and with the purity more than 98%. Procyanidins B2 standard was purchased from Shanghai Yuanye Biotechnology company, and with the purity more than 98%. HPLC-grade acetonitrile (CH3CN) and distilled water was used as the mobile phase of HPLC. Other reagents were of analytical grade.
Preparation of Samples for LC-MS Analysis
Dried tea leaves were ground with a mortar. 200 mg of tea leaves were extracted with 10 mL of a mixed solvent comprising MeOH and H2O at a ratio of 4:1 (v/v). The mixture was extracted for 30 min and then centrifuged at 2600 g for 3 min. Subsequently, 100 μL of the supernatant was transferred to a 1.5 mL Eppendorf tube. After adding 900 μL of methanol, the extracts were filtered through a 0.22-μm poly-tetrafluoroethylene filter for LC-MS analysis.
The determination of EC-glucose, EGC-glucose and procyanidins by LC-QQQ-MS
To determine the contents of EC-glucose, EGC-glucose and procyanidins dimers and trimer, the fresh mature tea leaves were collected and dried by freezing-dry at −40°C and reduced pressure for 24 hours. The dried tea leaves were milled in the liquid nitrogen. To extract the secondary metabolites, 200 mg of each tea sample was extracted with 10 mL of a mixed solvent comprising MeOH and H2O at a ratio of 4:1 (v/v). The mixture was extracted for 30 min by ultrasonic treatment at room temperature and then centrifuged at 2600 g for 3 min. The extract was filtered through a 0.22-μm poly-tetrafluoroethylene filter for LC-QQQ-MS analysis, and 3 μL of extract was injected to LC-QQQ-MS for analysis.
Procyanidins B2 was used as the reference standard in establishing the calibration curve. The different concentrations of procyanidins B2 solution were prepared by continuous diluting the procyanidins B2 stock solution with methanol. 3 μL of procyanidins B2 standard solutions with different concentration (0.0001, 0.001, 0.01, 0.02 mg/mL) were injected to the LC-QQQ-MS. The chromatographic and mass parameters were listed as below.
LC-QQQ-MS analysis was performed on a UHPLC-ESI-MS system consisting of a Agilent 6460 triple-quadrupolemass spectrometer (Agilent 6460, San Jose, CA, USA) coupled to a Agilent 1260 series HPLC system (Agilent Technologies, Palo Alto, CA, USA) equipped with auto-injector, a quaternary solvent delivery system. Chromatographic separation of black tea extract was conducted using a Acquity UPLC shield RP-18 column (50 mm × 2.1 mm, 1.7 μm) equipped with an Acquity UPLC C18 guard column (Waters, Milford, MA, USA) at a flow rate of 0.3 mL/min, and the column was thermostated at 30 °C. The mobile phase consisted of 0.1% formic acid in water (v/v) (A) and acetonitrile (B), and with the gradient elution at 0–5 min: 5–15% B, 5–8 min: 15–30% B, 8–13 min: 30% B, 13–23 min: 30–88% B, 23–28 min: 88–93% B, 28–30 min: 93–93% B, 30–33 min: 93–5% B, 33–35 min: 5% B. The injection volume was 3.0 μL. The entire eluant was sprayed into the mass spectrometer at −3500 kV with nebulizer, sheath and sweep gases set at 70, 20 and 5 arbitrary units, respectively, and desolvation of the solvent droplets was further aided by setting the heated capillary temperature at 350 °C.
RNA Extraction and qPCR analysis
Total RNA was extracted separately from leaves using the modified cetyl trimethylammonium bromide (CTAB) method32. RNA integrity was measured using gel electrophoresis and spectrophotometry (Nanodrop), and the single-stranded cDNAs used for qPCR analysis were synthesized from the RNAs using a Prime-Script™ 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). The primers for LAR1, LAR2, ANR1, ANR2 were the same as those referenced in a previous publication6. Primers were ordered from Sangon Biotech (Shanghai) Co., Ltd. qPCR was performed using the SYBR Green qPCR mastermix (Takara, SYBR Premix Ex TaqII™) at an annealing temperature of 60 °C on a Bio-Rad CFX 96™ real-time PCR system (Bio-Rad), according to the manufacturer’s instructions. The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference gene, and the relative expression was calculated using the 2−ΔΔCT method33.
Molecular cloning of LAR and ANR in C. tachangensis and C. sinensis
Total RNA was reverse transcribed using the PrimeScript™ Reagent Kit with the gDNA Eraser (TaKaRa). The obtained cDNA was used as the template for subsequent PCR reactions. PCR reaction was performed with F and R primer sets (Supplementary Table 1) with cDNA as the template to amplify these four fragments. The PCR mixture (25 mL) consisted of 100 ng cDNA, 0.5 μl KOD, 2.5 μl dNTP Mixture, 1.5 μl MgSO4, F and R primers and ddH2O. A total of 4 μl of PCR products were visualized by electrophoresis on a 1% (w/v) agarose gel. The target DNA band in agarose was cut under UV and fragment was extracted using Gel Extraction Kit. Then these genes were cloned into vector and sequenced. Genes sequencing were performed by Shanghai RuiDi Biological Technology Co. Ltd.
Statistical analysis
Results were expressed as mean ± SD, with the number of determinations (n = 3) representing separate experiments. Data were evaluated at a 0.05 level of significance with one-way ANOVA with post-hoc testing by Fisher’s protected least significant differences procedure. The correlation coefficient was calculated by SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
Additional Information
How to cite this article: Zhang, L. et al. The proposed biosynthesis of procyanidins by the comparative chemical analysis of five Camellia species using LC-MS. Sci. Rep. 7, 46131; doi: 10.1038/srep46131 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by grants from the Earmarked Fund for Modern Agro-industry Technology Research System in Tea Industry of Chinese Ministry of Agriculture (nycytx-26), the Chang-Jiang Scholars and the Innovative Research Team in University (IRT1101), the National Natural Science Foundation of China (31201335), and the Anhui Provincial Natural Science Foundation (1708085MC73).
Footnotes
The authors declare no competing financial interests.
Author Contributions X.C. Wan designed the study; L. Zhang and Y.L. Tai performed the most of the experiments; Y.J. Wang, Q.L. Meng and Y.Q. Yang assisted with the chemical analysis; S.H. Zhang did the mass data processing; H. Yang provided the raw materials; Z.Z. Zhang and D.X. Li guided the analysis and revised the manuscript. All authors have read and approved the final version of the manuscript.
References
- Yang J. B., Yang J., Li H. T., Zhao Y. & Yang S. X. Isolation and characterization of 15 microsatellite markers from wild tea plant (Camellia taliensis) using FIASCO method. Conserv. Genet. 10, 1621–1623 (2009). [Google Scholar]
- Yang X. R., Ye C. X., Xu J. K. & Jiang Y. M. Simultaneous analysis of purine alkaloids and catechins in Camellia sinensis, Camellia ptilophylla and Camellia assamica var. kucha by HPLC. Food Chem. 100, 1132–1136 (2007). [Google Scholar]
- Ashihara H., Deng W. W., Mullen W. & Crozier A. Distribution and biosynthesis of flavan-3-ols in Camellia sinensis seedlings and expression of genes encoding biosynthetic enzymes. Phytochemistry 71, 559–566 (2010). [DOI] [PubMed] [Google Scholar]
- Chen C. N. et al. Capillary electrophoretic determination of theanine, caffeine, and catechins in fresh tea leaves and Oolong tea and their effects on rat neurosphere adhesion and migration. J. Agr. Food Chem. 51, 7495–7503 (2003). [DOI] [PubMed] [Google Scholar]
- Liu Y. J. et al. Purification and characterization of a novel galloyltransferase involved in catechin galloylation in the tea plant (Camellia sinensis). J. Biol. Chem. 287, 44406–44417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X. et al. Tissue-specific, development-dependent phenolic compounds accumulation profile and gene expression pattern in tea plant [Camellia sinensis]. PLoS One 8, e62315 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan K., Zhang W. J. & Tsou C. H. Molecular taxonomy of Camellia (Theaceae) inferred from nrITS sequences. Am. J. Bot. 96, 1348–1360 (2009). [DOI] [PubMed] [Google Scholar]
- Huang H., Shi C., Liu Y., Mao S. Y. & Gao L. Z. Thirteen Camellia chloroplast genome sequences determined by high-throughput sequencing: genome structure and phylogenetic relationships. BMC Evol. Biol. 14, 4302–4315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-B., Hashimoto F., Shimizu K. & Sakata Y. Chemical taxonomy of red-flowered wild Camellia species based on floral anthocyanins. Phytochemistry 85, 99–106 (2013). [DOI] [PubMed] [Google Scholar]
- Yu P., Yeo A. S. L., Low M. Y. & Zhou W. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chem. 155, 9–16 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang S. et al. TBC2health: a database of experimentally validated health-beneficial effects of tea bioactive compounds. Briefings Bioinf., doi: 10.1093/bib/bbw055 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. et al. Influence of shade on flavonoid biosynthesis in tea (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 141, 7–16 (2012). [Google Scholar]
- Pascal C., Poncet-Legrand C. L., Cabane B. & Vernhet A. Aggregation of a proline-rich protein induced by epigallocatechin gallate and condensed tannins: effect of protein glycosylation. J. Agr. Food Chem. 56, 6724–6732 (2008). [DOI] [PubMed] [Google Scholar]
- Jiang X. et al. Analysis of accumulation patterns and preliminary study on the condensation mechanism of proanthocyanidins in the tea plant [Camellia sinensis]. Sci. Rep. 5, 8742 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongsuwan W. et al. High-throughput technique for comprehensive analysis of Japanese green tea quality assessment using ultra-performance liquid chromatography with time-of-flight mass spectrometry (UPLC/TOF MS). J. Agr. Food Chem. 56, 10705–10708 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang L., Li N., Ma Z. Z. & Tu P. F. Comparison of the Chemical Constituents of Aged Pu-erh Tea, Ripened Pu-erh Tea, and Other Teas Using HPLC-DAD-ESI-MSn. J. Agr. Food Chem. 59, 8754–8760 (2011). [DOI] [PubMed] [Google Scholar]
- Punyasiria P. A. N. et al. Flavonoid biosynthesis in the tea plant Camellia sinensis: properties of enzymes of the prominent epicatechin and catechin pathways. Arch. Biochem. Biophys. 431, 22–30 (2004). [DOI] [PubMed] [Google Scholar]
- Del Rio D. et al. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J. Agr. Food Chem. 52, 2807–2815 (2004). [DOI] [PubMed] [Google Scholar]
- Ridder L. et al. Automatic chemical structure annotation of an LC–MS n based metabolic profile from green tea. Anal. Chem. 85, 6033–6040 (2013). [DOI] [PubMed] [Google Scholar]
- Wei K. et al. Catechin contents in tea (Camellia sinensis) as affected by cultivar and environment and their relation to chlorophyll contents. Food Chem. 125, 44–48 (2011). [Google Scholar]
- Saijo R. Pathway of gallic acid biosynthesis and its esterification with catechins in young tea shoots. Agric. Biol. Chem. 47, 455–460 (1983). [Google Scholar]
- Niemetz R. & Gross G. G. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry 66, 2001–2011 (2005). [DOI] [PubMed] [Google Scholar]
- Tanner G. J. et al. Proanthocyanidin biosynthesis in plants. Purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J. Biol. Chem. 278, 31647–31656 (2003). [DOI] [PubMed] [Google Scholar]
- Xie D. Y., Sharma S. B., Paiva N. L., Ferreira D. & Dixon R. A. Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 299, 396–399 (2003). [DOI] [PubMed] [Google Scholar]
- Park J. S. et al. EST analysis of genes involved in secondary metabolism in Camellia sinensis (tea), using suppression subtractive hybridization. Plant Sci. 166, 953–961 (2004). [Google Scholar]
- Zhang X. L. et al. Characterisation of anthocyanidin reductase from Shuchazao green tea. J. Sci. Food Agric. 92, 1533–1539 (2012). [DOI] [PubMed] [Google Scholar]
- Ku K. M. et al. Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J. Agr. Food Chem. 58, 418–426 (2009). [DOI] [PubMed] [Google Scholar]
- Gross G. G. Partial purification and properties of UDP-glucose: vanillate 1-O-glucosyl transferase from oak leaves. Phytochemistry 22, 2179–2182 (1983). [Google Scholar]
- Harborne J. B. & Williams C. A. Advances in flavonoid research since 1992. Phytochemistry 55, 481–504 (2000). [DOI] [PubMed] [Google Scholar]
- Yue Y. et al. Tmdb: a literature-curated database for small molecular compounds found from tea. BMC Plant Biol. 14, 243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y., Peel G. J., Sharma S. B., Tang Y. & Dixon R. A. A transcript profiling approach reveals an epicatechin-specific glucosyltransferase expressed in the seed coat of Medicago truncatula. Proc. Natl. Acad. Sci. 105, 14210–14215 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai Y. et al. Transcriptomic and phytochemical analysis of the biosynthesis of characteristic constituents in tea (Camellia sinensis) compared with oil tea (Camellia oleifera). BMC Plant Biol. 15, 1–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.