Abstract
Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency overlaps with malaria endemicity although it predisposes carriers to hemolysis. This fact supports the protection hypothesis against malaria. The aim of this systematic review is to assess the presence and the extent of protective association between G6PD deficiency and malaria. Thirteen databases were searched for papers reporting any G6PD alteration in malaria patients. Twenty-eight of the included 30 studies were eligible for the meta-analysis. Results showed absence of negative association between G6PD deficiency and uncomplicated falciparum malaria (odds ratio (OR), 0.77; 95% confidence interval (CI), 0.59–1.02; p = 0.07). However, this negative association happened in Africa (OR, 0.59; 95% CI, 0.40–0.86; p = 0.007) but not in Asia (OR, 1.24; 95% CI, 0.96–1.61; p = 0.10), and in the heterozygotes (OR, 0.70; 95% CI, 0.57–0.87; p = 0.001) but not the homo/hemizygous (OR, 0.70; 95% CI, 0.46–1.07; p = 0.10). There was no association between G6PD deficiency and total severe malaria (OR, 0.82; 95% CI, 0.61–1.11; p = 0.20). Similarly, there was no association with other malaria species. G6PD deficiency can potentially protect against uncomplicated malaria in African countries, but not severe malaria. Interestingly, this protection was mainly in heterozygous, being x-linked thus related to gender.
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an incomplete hereditary X-linked hemolytic disease. This enzymopathy is widespread in the tropics and subtropics, synchronizing with endemic or formerly endemic malaria1; which suggests that G6PD deficiency may have arisen, spread, or maintained in frequency through natural selection by malaria2,3. Although it is hard to detect G6PD deficient patients as most affected people are asymptomatic until they are exposed to triggers4, more than 400 million people are thought to be G6PD deficient2.
G6PD catalyzes the reaction in the pentose phosphate pathway that generates reduced form of NADPH, which is in turn responsible for glutathione (GSH) homeostasis5. GSH is an antioxidant, and together, these processes make cells more able to resist and control oxidative stress2. Inability of the erythrocytes to maintain GSH homeostasis results in oxidative stress and affects the integrity of the RBCs, giving rise to hemolysis. Optimum RBC redox status is required by malaria parasites for their survival, replication, and development6. This factor is diminished in G6PD deficient RBCs, supporting the protection hypothesis.
Although circumstantial evidence accumulated to support the hypothesis that G6PD deficiency is protective against severe fatal malaria1,7,8,9; there have been several arguments for7,10,11,12 and against13,14,15. Some researchers also argued that perhaps malaria may not be the only factor affecting the deficiency gene locus16. It remains to be clarified whether a direct association exists between G6PD deficiency and protection from malaria11,17. This systematic review and meta-analysis was undertaken to integrate all the informative studies focusing on the association between malaria and genetically determined G6PD deficiency to assess the presence and the extent of this association.
Results
Study selection
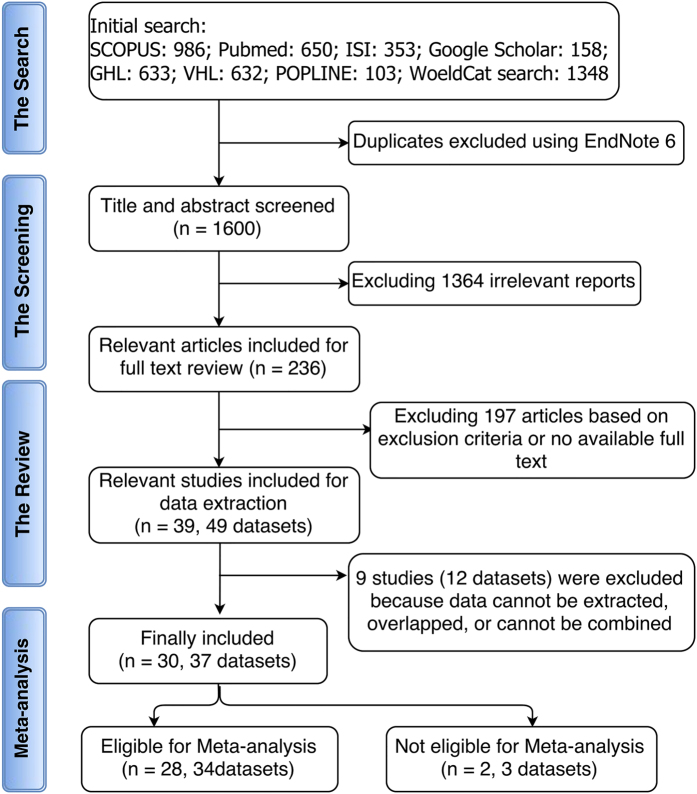
The initial search on public databases yielded 1600 study reports after excluding duplicates. Titles and abstracts screening resulted in exclusion of 1364 studies based on the exclusion criteria. Only 236 studies were considered eligible for inclusion for full-text review. Further 197 studies were excluded after full-text reading. A total of 39 studies were included, which comprises 49 data sets due to multi-center study reports. However, nine studies whose data either cannot be extracted, overlapped, or combined with the rest of the data, and thus were excluded. Among the 30 included studies, the species of Plasmodium are only Plasmodium falciparum in 17 studies3,5,7,10,11,18,19,20,21,22,23,24,25,26,27,28,29, only Plasmodium vivax in one study30, and combined species (P. falciparum with P. vivax, P. falciparum with P. malariae, P. vivax with P. malariae, and P. falciparum with P. vivax and P. malariae) in eight studies14,15,31,32,33,34,35,36. Jalloh 200432, Tantular 199936, and Kruatrachue 196215 reported separate data for P. vivax which meta-analyzed with that of Leslie 201030. The species cannot be determined in four studies37,38,39,40. Finally, a total of 28 studies containing 34 datasets were included in the quantitative data synthesis and meta-analysis (Fig. 1).
Figure 1. Flow diagram showing the method for the search, abstract screening, systematic review, and meta-analysis.
The database search using the search strategy was cleaned up to exclude duplicates. Titles and abstracts were initially screened to include all studies (published before January 2014) describing any association between G6PD deficiency and malaria. In addition to not meeting the inclusion criteria, reviews, case studies, editor correspondences, and studies whose data could not be retrieved were excluded during the full-text review.
Characteristics of included studies
The characteristics of the 30 included studies are described in details in Supplementary Table S1. The extracted items are described in the methodology section. Seventeen (17) studies with 21 datasets were on patients in Africa, 11 studies containing 14 datasets were performed in Asia while one study each was reported from Brazil and Papua New Guinea. All included studies used prospective method for data collection. Seven (7) studies were case-control studies; one was randomized double-blinded clinical trial while the rest were cross-sectional studies. Sixteen (16) studies were performed on children and infants only, while the other studies included children and adults. Only three studies used random sampling, the other adopted consecutive method for patient recruitment.
Association of G6PD deficiency with protection from uncomplicated P. falciparum malaria
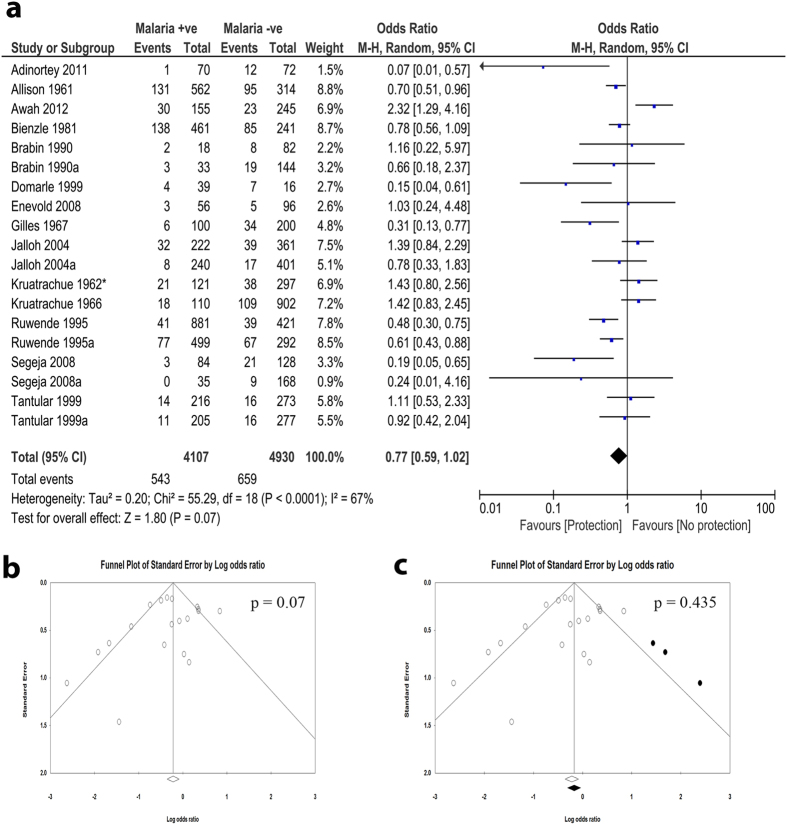
A meta-analysis was performed to assess the frequencies of G6PD deficiency between uncomplicated P. falciparum malaria and malaria negative individuals. The odds ratios (OR) from 19 datasets obtained from 14 studies3,5,7,10,14,15,18,20,22,24,29,31,32,36 were pooled for this meta-analysis. There was significant heterogeneity among the studies; thus, random effect model was applied for the meta-analysis. The combined OR revealed absence of negative association between G6PD deficiency and uncomplicated malaria (OR, 0.77; 95% confidence interval (CI), 0.59–1.02; n, 19; p = 0.07) (Fig. 2a). There was publication bias in favor of studies with negative association (Fig. 2b). When the cut and fill method as proposed by Duval et al.41 was applied, the condition negative association was further lost (OR, 0.88; 95% CI, 0.66–1.17; n, 22; p = 0.435).
Figure 2. Association of G6PD deficiency with protection from uncomplicated falciparum malaria.
(a) The forest plot of meta-analysis assessing the association between G6PD deficiency and prevalence of P. falciparum malaria. The comparators were malaria negative individuals. The observed results revealed absence of negative association between G6PD deficiency and P. falciparum malaria prevalence. (b) Funnel plot revealed the presence of publication bias towards negative association. (c) Trim and fill analysis led to further tilting away from the negative association, confirming the presence of publication bias among the included studies.
Sensitivity analysis was performed to investigate the effect of a single study on the outcome measures. We observed significant effects of single studies on the meta-analysis result. Interestingly, the exclusion of any of four studies10,14,15,32 led to appearance of significant negative association in the meta-analysis (Supplementary Table S2). The cumulative meta-analysis also showed that the inclusion of single studies, especially four studies10,14,18,31, affected the outcome effect measures (Supplementary Table S3).
Subgroup analysis shows that protection from uncomplicated P. falciparum malaria is significantly observed in heterozygotes
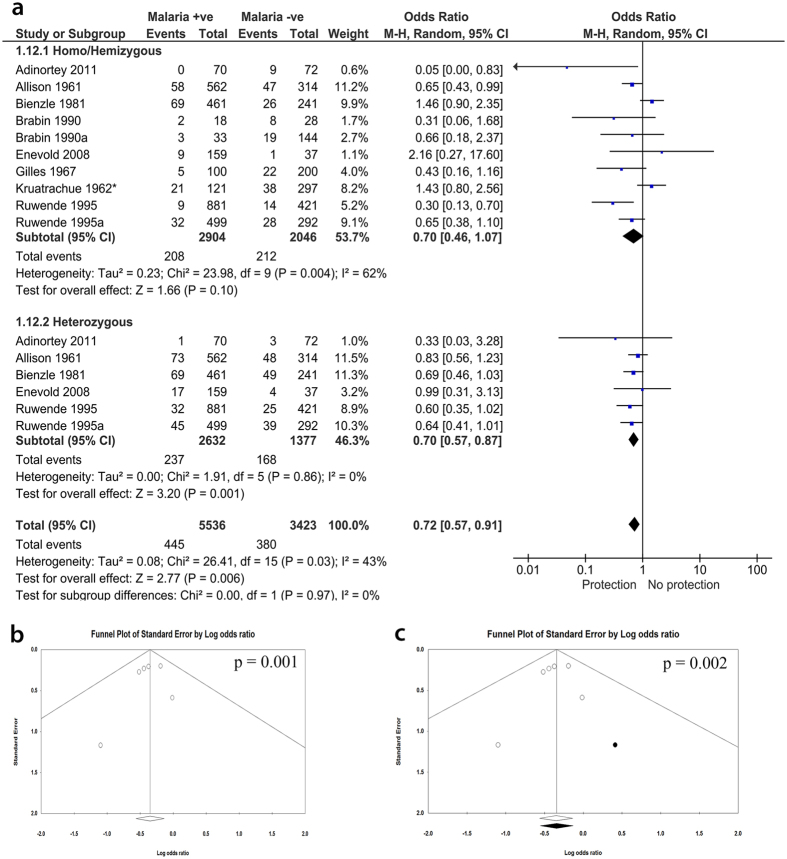
Subgroup analysis was performed to identify the effect of population subgroups on the meta-analysis. We grouped data into genotypes by separately analyzing studies that reported different genotypes. When homo/hemizygous or heterozygous were analyzed separately, the outcome effect measures were significant for heterozygous (OR, 0.70; 95% CI, 0.57–0.87; n, 6; p = 0.001), but not for homo/hemizygous (OR, 0.70; 95% CI, 0.46–1.07; n, 10; P = 0.10) indicating that the association is only present in heterozygous subgroup (Fig. 3a). However, there was a publication bias observed by funnel plot in favor of the protection (Fig. 3b). Trim and fill methods of Duval indicated that this publication bias is so minimal to affect the significant association in the heterozygous subgroup (OR, 0.71; 95% CI, 0.57–0.88; n, 7; P = 0.002) (Fig. 3c). Also, sensitivity analysis showed there was no single study effect on this association (Supplementary Table S4). Subgroup analysis by age revealed negative association with children only (OR, 0.72; 95% CI, 0.53–0.98; n, 9; P = 0.04) but not with children and adult subgroup (Supplementary Fig. S1). When the genders were analyzed separately, the significant association was only present with female subgroup (OR, 0.70; 95% CI, 0.50–0.98; n, 7; P = 0.04) (Supplementary Fig. S2). This corroborates the significant association for heterozygous subgroup since G6PD enzymopathy is sex-linked.
Figure 3. Meta-analysis forest plot for the association of G6PD with falciparum malaria.
(Subgroup analysis by genotypes): homo/hemi or heterozygous). (a) Present the meta-analysis forest plot of the association of G6PD deficiency and falciparum malaria, grouped by genotypes. The heterozygous females were compared to a combined group comprising hemizygous males and homozygous female. There was a strong negative association between G6PD deficiency and malaria for the heterozygous. (b) Funnel plot of the heterozygous subgroup represents presence of publication bias towards negative association. (c) Trim and fill analysis indicate that the publication bias is too minimal to affect the significant negative association.
Subgroup analysis by the location of the participants
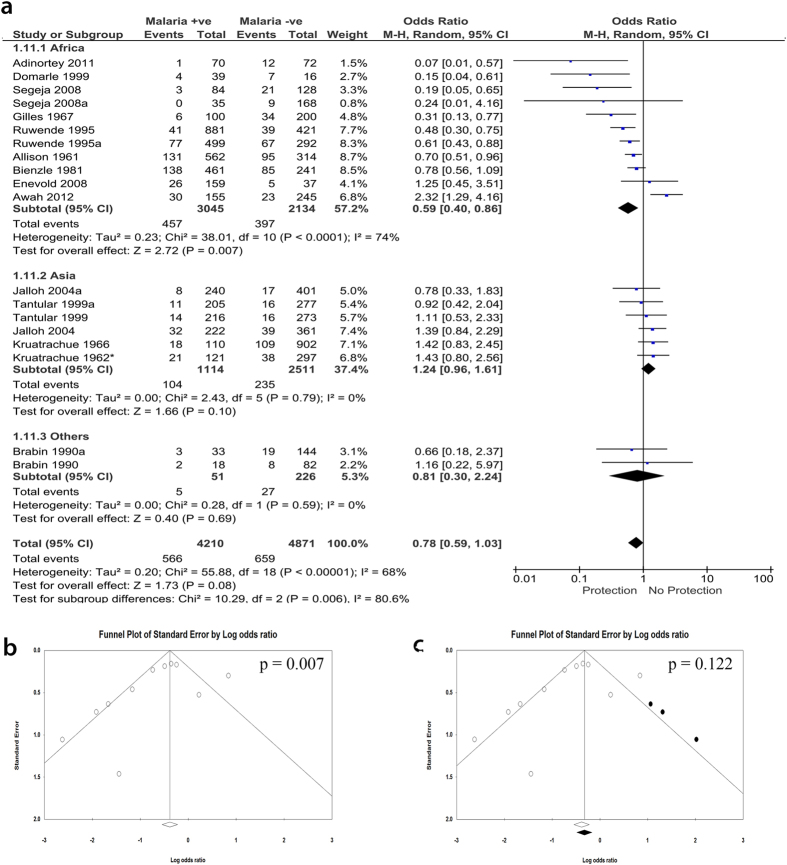
Interestingly, when the studies were grouped according to the continents where the studies were performed, we observed that the negative association between G6PD and malaria was only observed for studies performed in Africa (OR, 0.59; 95% CI, 0.40–0.86; n, 11; P = 0.007) (Fig. 4a). The studies performed in Asia showed no significant association (OR, 1.24; 95% CI, 0.96–1.61; n, 6; P = 0.1) (Fig. 4a). However, although sensitivity analysis did not show any single study or small study effect on the observed negative association, funnel plot showed evidence of publication bias in favor of studies with negative association. When the cut and fill method as proposed by Duval et al.41 was applied, the statistical significance of the association was lost (OR, 0.74; 95% CI, 0.49–1.10; n, 14; P = 0.122) (Fig. 4b and c).
Figure 4. Meta-analysis forest plot for the association of G6PD with falciparum malaria.
(Subgroup analysis by continents where each study was performed (Africa, Asia, or others (Papua New Guinea)). (a) The forest plot of the subgroup analysis showing an association of G6PD deficiency and falciparum malaria, sub-grouped into the continents where study site is located. A statistically significant negative association was observed from the meta-analysis of studies on African subjects. No association was observed for studies performed in Asia. Also, the two data sets of a study from Papua New Guinea showed no association. (b) Funnel plot of studies performed in African. (c) Trim and fill analysis showing presence of publication bias in studies performed in Africa.
Association of G6PD deficiency with severe falciparum malaria
Having identified association between G6PD deficiency and mild P. falciparum malaria, we assessed whether G6PD deficiency is also associated with protection from severe P. falciparum malaria. For this analysis, the comparator was uncomplicated falciparum malaria cases. Different severe falciparum malaria symptoms were considered together and then separately in relation to uncomplicated malaria. There was no association between G6PD deficiency and severe falciparum malaria, in relation to uncomplicated malaria (OR, 0.82, 95% CI, 0.61 to 1.11; n, 114; P = 0.20) (Supplementary Fig. S3). Even when the types of severe falciparum malaria were considered separately, we observed significant negative association between G6PD deficiency and hyperparasitemia (OR, 0.73; 95% CI, 0.56–0.95; n, 8; P = 0.02), but not between G6PD deficiency and severe anemia or cerebral malaria (Supplementary Fig. S3). When doing sensitivity analysis to investigate the effect of one study removal on the observed negative association between G6PD and hyperparasitemia, there was a significant effect of single studies on the observed results. Exclusion of one of two studies, Gilles 196724 and Allison 19617, led to loss of the observed significant association (Supplementary Table S5). The analysis in this section did not show evidence of publication bias as evident in the good symmetry of the funnel plots, except for the hyper-parasitemia, with which, we observed a minimal publication bias that did not affect the results (Supplementary Fig. S3).
G6PD deficiency was not associated with malaria due to other species of Plasmodium
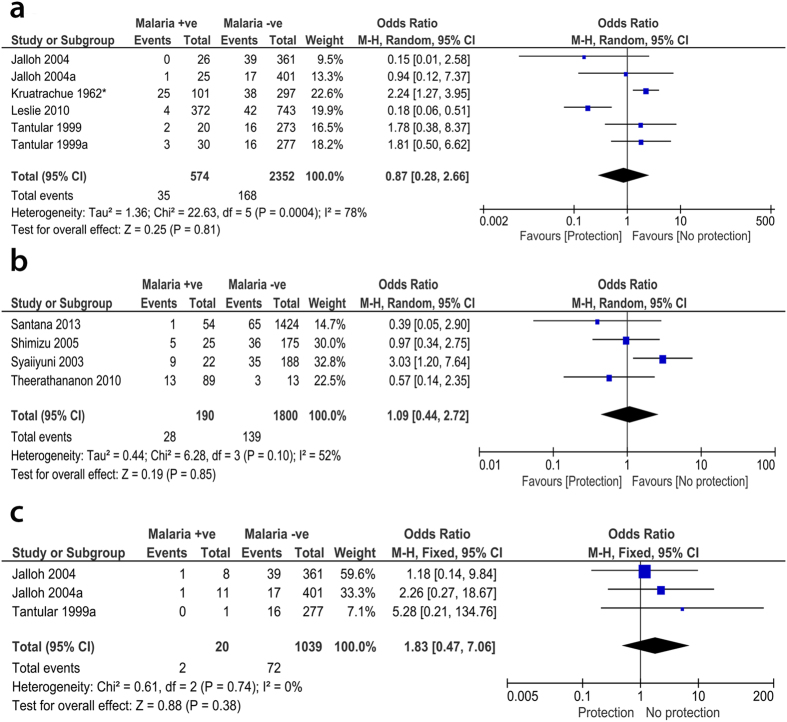
We also investigated the association of G6PD with other species of Plasmodium including P. vivax, P. malariae or combined co-infections. For P. vivax malaria, the OR from six datasets obtained from four papers15,30,32,36 showed that there was no association between G6PD and vivax malaria (OR, 0.87; 95% CI, 0.28–2.66; n, 6; P = 0.81), however, the two non-analyzable papers, Louicharoen 200934 and Khim 201333, reported significant association of G6PD with P. vivax. Similarly, there was no negative association between G6PD and malaria in patients infected with P. malariae, as reported by three datasets from two papers32,36 (OR, 1.83; 95% CI, 0.47–7.06; n, 3; P = 0.38) (Fig. 5a and c).
Figure 5. Meta-analysis forest plot for the association of G6PD with other species apart from P. falciparum malaria.
There was no negative association between G6PD and P. vivax (a), P. malariae (c), or even nondetermined species (b).
When combined co-infection with both P. falciparum and P. vivax was considered, the OR from five datasets derived from three papers14,32,36 also showed no negative association (OR, 0.95; 95% CI, 0.50–1.80; n, 5; P = 0.87). Besides the previous co-infection, the OR from five datasets of three papers32,35,36 also indicated no association with co-infection by P. falciparum and P. malariae (OR, 1.56; 95% CI, 0.29–8.34; n, 5; P = 0.60). Similarly, the pooled OR from four datasets reported in Jalloh 200432 and Tantular 199936 showed no association when combined P. vivax and P. malariae (OR, 1.17; 95% CI, 0.22–6.35; n, 3; P = 0.85) or combined P. falciparum, P. vivax, and P. malariae (OR, 1.90; 95% CI, 0.42–8.51; n, 4; P = 0.40) co-infections were considered (Supplementary Fig. S4). We could not determine the species of Plasmodium reported in four analyzable studies37,38,39,40. Nevertheless, the pooled OR from these studies did not show significant association (OR, 1.09; 95% CI, 0.44–2.72; n, 2; P = 0.85) (Fig. 5b).
Discussion
We observed absence of negative association between G6PD deficiency not only with uncomplicated falciparum malaria but also with severe malaria (Fig. 2). However, single studies may have dramatically affected this analysis and inference. For instance, the exclusion of any of four studies10,14,15,32 led to appearance of negative association in the meta-analysis. There was also evidence of publication bias in favor of negative association (protection). Only one study by Adinortey et al.5 was published in a non-peer-reviewed journal among the included studies, however, it does not affect the meta-analysis as shown in the sensitivity analysis. Similarly, there was no significant association between G6PD deficiency and other species of malaria, including P. vivax and P. malariae or combinations of any two or all the three species. Due to the insufficient number of reports on these species, we could not make any evident conclusion on the exact relationship especially after considering the results of the two non-analyzable papers. Therefore, more studies investigating the association between these species and G6PD deficiency are highly recommended. A negative association was found in studies performed in Africa as compared to Asia and other continents, albeit with presence of publication bias. These results may provide evidence in support of the G6PD-malaria protection hypothesis in Africa. G6PD deficiency is endemic in Africa and the African G6PD deficient patients have relatively higher enzyme activity and milder consequences than Mediterranean or Asian patients3,42. This may be one possible explanation the observed G6PD associated protect from falciparum malaria in Africa. The population history, archeology, and human genetics indicate that the age of this selection by malaria is more than 5,000 years old in Africa3,43. Moreover, the frequencies of deficiency alleles in most G6PD deficient populations in endemic areas do not exceed 40%3,44. Interestingly, the pooled data from Asian countries show no significant association. There may be a confounding relationship between location and the observed negative association in the children subgroup because of the skewing effect of African studies in favor of negative association (Supplementary Fig. S1)14,15,39,40. This suggests that other than endemicity, ethnicity could modify the effect of G6PD deficiency in the study. While the female homozygous and male hemizygous genotypes showed no association with uncomplicated malaria protection, the female heterozygous genotype showed highly significant association with protection from malaria. Unlike in homo/hemizygous subgroup, the association observed for heterozygous subgroup had no indication of heterogeneity or publication bias. This association with heterozygous females is highly supported by the study of Uyoga et al.45 which also indicated that hemizygous males are not only unprotected from malaria, but may be at high risk of sever malaria. This is due to the fact that G6PD-deficient red blood cells are prone to early destruction by oxygen free radicals46. Usanga and Luzzatto hypothesized a prestigious mechanism that explains the association with heterozygous females47. They observed that the parasite undergoes habitual changes when passing through successive G6PD-deficient red cells in order to be more adaptive. These changes resulted in production of the parasite’s own enzyme which in turn resulted in survivability and replication of the parasite48. As the heterozygous females are genetically mosaic, the parasite failed to survive47. Supportingly, when subgrouping the patients according to the enzymatic activity, our analysis revealed that the activity of the enzyme has no effect on the association, although only two analyzable papers reported the enzymatic activity for P. falciparum.
Studies have demonstrated the presence of G6PD variants in geographically diverse locations, endemic or formerly endemic for malaria; suggesting the likelihood of selection by malaria2. However, if malaria were the sole selecting factor on the G6PD gene with no other deleterious factor, an increase in the proportion of the genotype conferring G6PD enzymopathy would be expected to replace the normal allele1,49. The absence of clear evidence of this selection supports the hypothesis that the net protection observed is dependent on the balance between the malaria selection and other deleterious effects of G6PD enzymopathy16. It further confirms that there may be more than one selection factors on this gene, with the effect of the advantageous factors (e.g. malaria) countering the effects of factors that confer net disadvantage (e.g. hemolysis)7,8,16. As a matter of fact, homozygous females and hemizygous males are more severely affected by the hemolytic effects of G6PD deficiency45,49. This may, therefore, explain the better protection from malaria in the heterozygous females.
Conversely, G6PD deficiency was not associated with protection from severe falciparum malaria. Severe malaria is characterized by severe anemia, hyperparasitemia, and cerebral malaria. The net negative odds ratios observed for severe anemia and cerebral malaria were not statistically significant. Peculiarly, the hyperparasitemia showed significant negative association with G6PD deficiency in relation to low parasitemia. There was minimal publication bias in favor of protection that did not affect the significant association. Association with hyperparasitemia may however not only indicate severity of infection but also reinfection rate. These severity factors are associated with or arguably exacerbated by hemolysis, a characteristic feature of G6PD deficiency. However, the hemolytic effect of G6PD deficiency is self-limiting, affecting mainly older erythrocytes than the newly formed ones; thereby establishing a balance between the factors. Thus, the concept of protection from severe malaria or absence thereof remains conflicting10,17,50.
The inherent disadvantage of G6PD deficiency due to the associated hemolysis could be one of the factors that account for the absence of consensus on the G6PD-malaria protection hypothesis. Also, the presence of other factors that could protect from malaria, especially hemoglobinopathies like sickle cell anemia (HbS) which also overlaps with G6PD and malaria endemicity, is considered one of the major confounders of our analysis and limitations of this study.
To recapitulate, we inferred from this analysis that G6PD deficiency may not generally protect against uncomplicated falciparum malaria, P. vivax, or P. malariae. However, the analysis was sensitive to the effect of individual studies exclusion of which resulted in negative association with uncomplicated falciparum malaria, albeit with evidence of publication bias in favor of malaria protection. The protection was found only in African countries but not in Asian. Comparing the genotypes, the negative association was found only in heterozygous females, but not in a combined group comprising homozygous females and hemizygous males. Similarly, the negative association was present in studies including only children but not with combined group comprising children and adults. With the exception of hyperparasitemia, G6PD deficiency showed no association with severe malaria. Further studies are needed to investigate the mechanism of association with heterozygous females.
Methods
Registration of study protocol
This study was performed according to the recommendations of the PRISMA statement51 during all steps of the study. The protocol for this study was prepared prior to the start of the study and was registered in PROSPERO with identification number CRD42014007282.
Study eligibility criteria
Only original articles that report the association of any G6PD alteration in enzymatic activity and genetic variant and malaria were included. There was no restriction made with respect to language, publication year and study design. Case reports, reviews, theses, conference proceedings were excluded. Two reviewers independently extracted the data and subsequently verified by two other reviewers. Any disagreements were resolved by discussion and consensus between the reviewers and a third party, if necessary.
Search strategy
Thirteen electronic databases were searched including; PubMed, Scopus, Google Scholar, ISI, WHO Global Health Library (GHL), Cochrane Review Library, IBECS, POPline, Virtual Health Library (VHL), WorldCat, New York Academy of Medicine Grey Literature Report, System for Information on Grey Literature in Europe (SIGLE), and African Journals Online databases. Searches were conducted to find relevant studies published before January 2014. The initial search strings were done using PubMed and Scopus databases using the broad search term: “((Glucose-6-phosphate dehydrogenase OR G6PD) AND (Malaria[Mesh] OR Antimalarial[Mesh]) and (INDEXTERMS (glucose-6-phosphate dehydrogenase OR G6PD) AND (malaria OR antimalarial))”. Subsequently, this search string was modified to contain search terms suitable for each database. The details of search strategy and terms used in each database are presented in Supplementary File S1.
Study inclusion criteria and study selection
All original studies (published before January 2014) describing association between G6PD deficiency and malaria were included in this systematic review. All eligible studies irrespective of publication type, study design, language and publication date were considered in the qualitative systematic review. There was no restriction on certain population, age, race, ethnicity, or geographic area. Only variables reported by two or more studies and whose primary data can be extracted were included in the meta-analysis. We limited included studies to those performed on human subjects. Reviews, case studies, case series, editor correspondences, news, letters, book chapters and studies whose data could not be reliably retrieved or extracted were excluded. We omitted studies whose data is overlapped with another included study. Two reviewers independently performed initial screening and study selection. Preliminary assessment of the title and abstracts was performed to identify relevant articles. Thereafter, full texts of eligible articles were downloaded and reviewed for qualitative analysis and potential inclusion in the data synthesis. Inclusion of a study by both reviewers was conclusive while discrepancies and disagreements as regards study eligibility were resolved by discussion and/or consensus with a third reviewer.
Data extraction
The data extracted from the included reports include: the first author, year of publication year of data, study design, data collection (prospective or retrospective), country and city of origin, characteristics of participant population (gender, age, and the type of malaria), method for genetic variants detection, number of included individuals (case and control groups), outcomes and times of evaluation. When there was no data available or if there were obvious errors (such as typographical errors, incorrect calculation, or incorrect factors designations) in the original publication, two successive emails were sent to corresponding authors for clarification. When no clarification or genotype information was obtained after at least two emails, we will report such studies as “no data available”. Identification and elimination of potentially overlapped datasets were done under extensive care, especially for multiple reports by the same group reporting the same factors on the same subjects.
Meta-analyses
Meta-analysis to pool data from eligible studies was performed on both RevMan v5.252 and Comprehensive Meta-Analysis v2 software. 2 × 2 contingency tables were generated and the odds ratio with the corresponding 95% CI were calculated for dichotomous outcomes. For outcomes with continuous variables, the input data were mean and standard deviation (SD) with the standardized mean difference (SMD) as the effect measure. When standard deviation was not available, it was computed with the calculator function in RevMan v5.2 by inputting other supplied data (e.g. mean, standard error of mean, p-value etc.), if available. Forest plots showing the respective OR or SMD with their corresponding 95% CI for each dataset and for the pooled data were generated. The overall effect was measured by Z-statistics with statistical significance set at p < 0.05. More clarifications and in-depth analysis were achieved through subgroup analysis.
Test of heterogeneity between studies
The Cochrane Q (Chi2 test) and I2 statistics53 were used to assess inconsistency among studies. For Chi2 test of heterogeneity, statistical significance was set as p < 0.10. Fixed-effects model was adopted when there was a lack of significant heterogeneity (in Chi2, p > 0.10 or I2 values < 50%), otherwise heterogeneity among studies is assumed and random-effects model with weighting of the studies was applied54.
Sensitivity analysis and assessment of publication bias among studies
For sensitivity analysis, each individual study in each meta-analysis was sequentially excluded in the analysis to examine the effect of a single study on the outcome of the meta-analysis. Begg’s funnel plots were used to initially assess publication bias across the reviewed studies55,56. The funnel plots were created by plotting the OR against the standard error of the logarithm of OR. When there was a publication bias, cut and fill method as proposed by Duval and Tweedie41 was conducted to indicate the effect of this publication bias on the estimated effect size.
Additional Information
How to cite this article: Chibunna Mbanefo, E. et al. Association of glucose-6-phosphate dehydrogenase deficiency and malaria: a systematic review and meta-analysis. Sci. Rep. 7, 45963; doi: 10.1038/srep45963 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported in part by a “Grant-in-Aid for Scientific Research (B)” (16H05844, 2016–2019 for Nguyen Tien Huy) from Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) for Kenji Hirayama. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank the reviewers for their instructive comments which helped us to improve our study.
Footnotes
The authors declare no competing financial interests.
Author Contributions E.C.M., A.M.A. and A.T. contributed in all steps including study screening, data extraction, analysis, and manuscript preparation; A.E. participated in data extraction and data analysis, manuscript writing, and made the supplemental tables; N.T.H.T., N.P.L., and N.H.A. wrote the protocol also participated in title and abstract screening, full-text screening and data extraction; T.D.N. and P.T.H. searched databases, participated in title and abstract screening, full-text screening and data extraction; M.V.H. and N.K.A. reviewed the protocol, participated in title and abstract screening and data extraction and N.T.H. proposed the search terms, manage the work, and reviewed data extraction, data analysis and manuscript; K.H. provided general supervision, reviewed data extraction, data analysis and manuscript.
References
- Allison A. C. Malaria and glucose-6-phosphate dehydrogenase deficiency. Nature 197, 609–609 (1963). [DOI] [PubMed] [Google Scholar]
- Ruwende C. & Hill A. Glucose-6-phosphate dehydrogenase deficiency and malaria. Journal of molecular medicine 76, 581–588 (1998). [DOI] [PubMed] [Google Scholar]
- Ruwende C., Khoo S. C., Snow R. W. & Yates S. N. R. Natural selection of hemi-and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature 376, 246 (1995). [DOI] [PubMed] [Google Scholar]
- Brandt O., Rieger A., Geusau A. & Stingl G. Peas, beans, and the Pythagorean theorem–the relevance of glucose‐6‐phosphate dehydrogenase deficiency in dermatology. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 6, 534–539 (2008). [DOI] [PubMed] [Google Scholar]
- Adinortey M. B. et al. The significance of glucose-6-phosphate dehydrogenase deficiency in plasmodium falciparum malaria: A case study of patients visiting the Central Regional Hospital, Cape Coast, Ghana. World Journal of Medical Sciences 6, 126–130 (2011). [Google Scholar]
- Vega-Rodríguez J. et al. The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission. PLoS Pathog 5, e1000302 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C. & Clyde D. F. Malaria in African children with deficient erythrocyte glucose-6-phosphate dehydrogenase. British Medical Journal 1, 1346 (1961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C. & Clyde D. F. Malaria and glucose-6-phosphate dehydrogenase. British medical journal 2, 521 (1961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini E., Gloria-Bottini F. & Maggioni G. On the relation between malaria and G-6-PD deficiency. Journal of medical genetics 15, 363–365 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awah F. M., Chukwuemeka G., Olalekan S. I., Azeke A. E. & Nneka M. A possible protective role of glucose-6-phosphate dehydrogenase deficiency and sickle haemoglobin genes against severe malaria in Madonna University, Elele Community. Journal of Medicine and Medical Sciences 3, 375–381 (2012). [Google Scholar]
- Awah F. M. & Uzoegwu P. N. Malaria Protection In Glucose-6-Phosphate Dehydrogenase Deficient Individuals In Bamenda Population Of Cameroon. Global Journal of Pure and Applied Sciences 14, 343–347 (2008). [Google Scholar]
- Harris R. & Gilles H. M. Glucose‐6‐phosphate dehydrogenase deficiency in the peoples of the Niger Delta. Annals of human genetics 25, 199–206 (1962). [DOI] [PubMed] [Google Scholar]
- Kidson C. & Gorman J. G. A challenge to the concept of selection by malaria in glucose-6-phosphate dehydrogenase deficiency. Nature 196, 49–51 (1962). [DOI] [PubMed] [Google Scholar]
- Kruatrachue M., Bhaibulaya M., Clongkamnaukorn K., Harinasuta C. & Organization, W. H. Re-examination of the relationship of erythrocyte Glucose-6-Phosphate Dehydrogenase and malaria in Thailand(1966). [Google Scholar]
- Kruatrachue M., Charoenlarp P., Chongsuphajaisiddhi T. & Harinasuta C. Erythrocyte glucose-6-phosphate dehydrogenase and malaria in Thailand. The Lancet 280, 1183–1186 (1962). [DOI] [PubMed] [Google Scholar]
- Bernstein S. & Bowman J. G6PD/Malaria hypothesis: a balanced or transient polymorphism. The Lancet 315, 485 (1980). [DOI] [PubMed] [Google Scholar]
- Gilles H. M. & Taylor B. G. The existence of the glucose-6-phosphate dehydrogenase deficiency trait in Nigeria and its clinical implications. Annals of Tropical Medicine & Parasitology 55, 64–69 (1961). [DOI] [PubMed] [Google Scholar]
- Bienzle U. Glucose-6-phosphate dehydrogenase deficiency. Part 1: Tropical Africa. Clinics in haematology 10, 785–799 (1981). [PubMed] [Google Scholar]
- Bienzle U., Guggenmoos-Holzmann I. & Luzzatto L. Malaria and erythrocyte glucose-6-phosphate dehydrogenase variants in West Africa. The American journal of tropical medicine and hygiene 28, 619–621 (1979). [PubMed] [Google Scholar]
- Domarle O. et al. Factors influencing resistance to reinfection with Plasmodium falciparum. The American journal of tropical medicine and hygiene 61, 926–931 (1999). [DOI] [PubMed] [Google Scholar]
- Dunyo S. et al. Randomized trial of safety and effectiveness of chlorproguanil-dapsone and lumefantrine-artemether for uncomplicated malaria in children in the Gambia. PLoS One 6, e17371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enevold A. et al. Reduced risk of uncomplicated malaria episodes in children with alpha+-thalassemia in northeastern Tanzania. The American journal of tropical medicine and hygiene 78, 714–720 (2008). [PubMed] [Google Scholar]
- Fanello C. I. et al. High risk of severe anaemia after chlorproguanil-dapsone+artesunate antimalarial treatment in patients with G6PD (A-) deficiency. PLoS One 3, e4031 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles H. M. et al. Glucose-6-phosphate-dehydrogenase deficiency, sickling, and malaria in African children in South Western Nigeria. The lancet 289, 138–140 (1967). [DOI] [PubMed] [Google Scholar]
- Guindo A., Fairhurst R. M., Doumbo O. K., Wellems T. E. & Diallo D. A. X-linked G6PD deficiency protects hemizygous males but not heterozygous females against severe malaria. PLoS Med 4, e66 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lell B. et al. The role of red blood cell polymorphisms in resistance and susceptibility to malaria. Clinical Infectious Diseases 28, 794–799 (1999). [DOI] [PubMed] [Google Scholar]
- Oo M., Tin-Shwe M.-T. & O’Sullivan W. J. Genetic red cell disorders and severity of falciparum malaria in Myanmar. Bulletin of the World Health Organization 73, 659 (1995). [PMC free article] [PubMed] [Google Scholar]
- Orimadegun A. E. & Sodeinde O. Glucose-6-phosphate dehydrogenase status and severity of malarial anaemia in Nigerian children. The Journal of Infection in Developing Countries 5, 792–798 (2011). [DOI] [PubMed] [Google Scholar]
- Segeja M. D. et al. Prevalence of glucose-6-phosphate dehydrogenase deficiency and haemoglobin S in high and moderate malaria transmission areas of Muheza, north-eastern Tanzania. Tanzania journal of health research 10, 9–13 (2008). [DOI] [PubMed] [Google Scholar]
- Leslie T. et al. The impact of phenotypic and genotypic G6PD deficiency on risk of Plasmodium vivax infection: a case-control study amongst Afghan refugees in Pakistan. PLoS Med 7, e1000283 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabin L. & Brabin B. J. Malaria and glucose 6-phosphate dehydrogenase deficiency in populations with high and low spleen rates in Madang, Papua New Guinea. Human heredity 40, 15–21 (1990). [DOI] [PubMed] [Google Scholar]
- Jalloh A. et al. Rapid epidemiologic assessment of glucose‐6‐phosphate dehydrogenase deficiency in malaria‐endemic areas in Southeast Asia using a novel diagnostic kit. Tropical Medicine & International Health 9, 615–623 (2004). [DOI] [PubMed] [Google Scholar]
- Khim N. et al. G6PD deficiency in Plasmodium falciparum and Plasmodium vivax malaria-infected Cambodian patients. Malaria journal 12, 171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louicharoen C. et al. Positively selected G6PD-Mahidol mutation reduces Plasmodium vivax density in Southeast Asians. Science 326, 1546–1549 (2009). [DOI] [PubMed] [Google Scholar]
- Nkuo-Akenji T. K., Wepngong P. & Akoachere J.-F. Effects of ABO/Rh blood groups, G-6-PD enzyme activity and haemoglobin. African journal of health sciences 11, 93–97 (2004). [PubMed] [Google Scholar]
- Tantular I. S. et al. Field trials of a rapid test for G6PD deficiency in combination with a rapid diagnosis of malaria. Tropical Medicine & International Health 4, 245–250 (1999). [DOI] [PubMed] [Google Scholar]
- Onuma W. T. J. J. F., Tepanata Z., Napaporn P. N. H. A. S., Harnyuttanakorn S. P. & Kanchanakhan N. Prevalence of G6PD Deficiency in malaria endemic area: case study in bongti sub-district, sai yok district, kanchanaburi province, Thailand. J Health Res 24, 55–62 (2010). [Google Scholar]
- Santana M. S. et al. Glucose-6-phosphate dehydrogenase deficient variants are associated with reduced susceptibility to malaria in the Brazilian Amazon. Transactions of the Royal Society of Tropical Medicine and Hygiene 107, 301–306 (2013). [DOI] [PubMed] [Google Scholar]
- Shimizu H., Tamam M., Soemantri A. & Ishida T. Glucose-6-phosphate dehydrogenase deficiency and Southeast Asian ovalocytosis in asymptomatic Plasmodium carriers in Sumba island, Indonesia. Journal of human genetics 50, 420–424 (2005). [DOI] [PubMed] [Google Scholar]
- Syaiiyuni R. Hubungan defisiensi Glucose-6-phosphate dehydrogenase (G-6-PD) dengan kepadatan parasit malaria pada anak usia sekolah di daerah endemis malaria, Program Pendidikan Pasca sarjana Universitas Diponegoro, (2003). [Google Scholar]
- Duval S. & Tweedie R. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 56, 455–463 (2000). [DOI] [PubMed] [Google Scholar]
- Scriver C. R. et al. The Metabolic and Molecular Bases of Inherited Disease. 8th edit McGraw-Hill. New York (2001). [Google Scholar]
- Wernsdorfer W. H. & McGregor I. Malaria: principles and practice of malariology. Vol. 1 (Churchill Livingstone Edinburgh, 1988). [Google Scholar]
- Livingstone F. B. Frequencies of hemoglobin variants: thalassemia, the glucose-6-phosphate dehydrogenase deficiency, G6PD variants, and ovalocytosis in human populations. (Oxford University Press, USA, 1985). [Google Scholar]
- Uyoga S. et al. Glucose-6-phosphate dehydrogenase deficiency and the risk of malaria and other diseases in children in Kenya: a case-control and a cohort study. The Lancet Haematology 2, e437–e444 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L. G6PD deficiency: a polymorphism balanced by heterozygote advantage against malaria. The Lancet Haematology 2, e400–e401 (2015). [DOI] [PubMed] [Google Scholar]
- Usanga E. A. & Luzzatto L. Adaptation of Plasmodium falciparum to glucose 6-phosphate dehydrogenase-deficient host red cells by production of parasite-encoded enzyme, (1985). [DOI] [PubMed] [Google Scholar]
- Yoshida A. & Roth E. F. Jr. Glucose-6-phosphate dehydrogenase of malaria parasite Plasmodium falciparum. Blood 69, 1528–1530 (1987). [PubMed] [Google Scholar]
- Bienzle U., Lucas A., Ayeni O. & Luzzatto L. Glucose-6-phosphate dehydrogenase and malaria: greater resistance of females heterozygous for enzyme deficiency and of males with non-deficient variant. The Lancet 299, 107–110 (1972). [DOI] [PubMed] [Google Scholar]
- Ruwende C., Khoo S. C., Snow R. W., Green L. S. & Danubio M. E. Protection against severe malaria for glucose-6-phosphate dehydrogenase deficiency hemizygotes and heterozygotes in Africa. Adaptation to Malaria: The Interaction of Biology and Culture, 73–86 (1997). [Google Scholar]
- Liberati A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS medicine 6, e1000100, doi: 10.1371/journal.pmed.1000100 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks J. J., Altman D. G. & Bradburn M. J. In Systematic Reviews in Health Care: Meta-Analysis in Context(eds Egger M., Smith G. D. & Altman D. G.) (BMJ Publishing Group, 2008). [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. Bmj 327, 557–560, doi: 10.1136/bmj.327.7414.557 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses [journal article as teaching resource, deposited by John Flynn]. British medical journal 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics, 1088–1101 (1994). [PubMed] [Google Scholar]
- Egger M., Smith G. D., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.