Abstract
We investigated the consequences of colistin use in backyard chicken farms in Vietnam by examining the prevalence of mcr-1 in fecal samples from chickens and humans. Detection of mcr-1–carrying bacteria in chicken samples was associated with colistin use and detection in human samples with exposure to mcr-1–positive chickens.
Keywords: zoonoses, poultry farms, Vietnam, antibiotic resistance, transmission, E. coli, chicken, food production, colistin, fecal colonization, colistin resistance, backyard farm, small-scale farm, antimicrobial resistance, bacteria
Colistin resistance is a gradually emerging problem among gram-negative bacteria in clinical settings in many countries (1). A transferable plasmid-derived colistin resistance gene mcr-1 discovered in China and subsequently found worldwide could be mediating this emergence (2,3). Use of colistin in animal production has been suggested as the most likely factor contributing to the emergence of the mcr-1 gene (2). However, systematic studies applying the One Health approach to investigate the epidemiologic link between the use of colistin in agriculture and colonization with mcr-1–carrying bacteria in the community are lacking (4).
Colistin use in humans is negligible (5), but it is one of the most commonly used antimicrobial drugs in animal production in Vietnam (6). We investigated the consequences of colistin use in chicken farms by assessing chickens, farmers, and nearby persons for the presence of mcr-1–carrying bacteria and performing epidemiologic analyses to assess the risk for subsequent transmission to unexposed human populations in southern Vietnam.
The Study
From March 2012 to April 2013, we conducted a systematic, cross-sectional study examining antimicrobial drug use and colonization with antimicrobial-resistant E. coli in chickens and humans in Tien Giang Province, Vietnam. Fecal samples from 204 chicken farms and rectal swabs from 204 chicken farmers (1 farmer/farm) were collected as described (Technical Appendix 1) (7,8). We additionally collected rectal swabs from age- and sex-matched persons not involved in poultry farming from the same districts (rural persons, n = 204) and from their provincial capitals (urban persons, n = 102) (8).
Samples were cultured on MacConkey plates with and without antimicrobial drugs. A sweep of the full growth on plain MacConkey plates was collected and screened for the presence of mcr-1 by PCR as described previously (2). Logistic regression models were built to investigate the risk factors associated with the presence of mcr-1 on chicken farms and in human participants. Then, we selected (using a random number table) individual E. coli colonies (n = 200) and extended-spectrum β-lactamase (ESBL)–producing E. coli colonies (n = 122) growing on different MacConkey plates and repeated PCR to confirm the presence of mcr-1 in E. coli isolated from chickens and humans. We tested all mcr-1–positive E. coli isolates for colistin susceptibility using Etest (bioMérieux, Marcy l’Etoile, France) and interpreted test results in accordance with the European Committee on Antimicrobial Susceptibility Testing breakpoints (9). In addition, whole-genome sequencing was performed on all mcr-1–positive E. coli isolates as described (Technical Appendix 1).
From a total of 204 chicken and 510 human fecal specimens, 188 and 440 MacConkey sweeps were available for mcr-1 screening by PCR, respectively. The adjusted prevalence of mcr-1 was 59.4% (95% CI 47.9%–71.0%) in chicken and 20.6% (95% CI 15.9%–25.2%) in human fecal samples (Table 1).
Table 1. Prevalence of fecal colonization with mcr-1–carrying bacteria in chickens and humans, Tien Giang Province, Vietnam, 2012–2013.
| Source | Prevalence of fecal colonization with mcr-1–carrying bacteria |
|
|---|---|---|
| No. positive sweeps/total (%) | Adjusted prevalence, % (95% CI) | |
| All chicken farms | 93/188 (49.5) | 59.4 (47.9–71.0) |
| Household chicken farms | 53/94 (56.4) | 59.5 (47.9–71.1) |
| Small-scale chicken farms | 40/94 (42.6) | 47.9 (35.4–60.3) |
| All human participants | 84/440 (19.1) | 20.6 (15.9–25.2) |
| All farmers | 45/179 (25.1) | 25.2 (18.3–32.0) |
| Farmers exposed to mcr-1–negative chickens | 16/91 (17.6) | 15.5 (7.7–23.3) |
| Farmers exposed to mcr-1–positive chickens | 29/88 (33.0) | 34.7 (23.9–45.5) |
| Rural persons | 31/173 (17.9) | 17.6 (11.6–23.7) |
| Urban persons | 8/88 (9.1) | 9.1 (3.1–15.1) |
Among 200 E. coli isolates, mcr-1 was detected in 10/78 (12.8%) isolates from chickens, 2/50 (4.0%) isolates from farmers, and 0/72 isolates from persons who did not farm. Similarly, mcr-1 was detected in 9/38 (23.7%) and 1/44 (2.3%) of ESBL-producing E. coli isolated from chickens and farmers, respectively.
The MIC of colistin for the 22 mcr-1–carrying E. coli isolates ranged 3–4 mg/L. Because the Etest might underestimate the true MIC (10), these results indicate reduced susceptibility. Single-nucleotide polymorphism (SNP)–based phylogenetic analyses of the core genomes showed little genomic similarity between isolates, but the analyses did show many isolates belonged to the same multilocus sequence types (n = 14) (Figure). Analysis of the acquired resistance genes, reflecting the presence of an accessory genome, showed a large variation in resistance gene content, with only the tet(A) gene, encoding for tetracycline resistance, present in all genomes (Technical Appendix 2 Table). De novo bacterial genome assembly was performed, and the contigs carrying mcr-1 were analyzed. A replication origin could be located in 5 isolates, leading to the identification of plasmid incompatibility groups IncHI2 (1 isolate), IncI2 (2 isolates), and combined IncHI2 and IncHI2A (2 isolates). Transposon ISAplI, initially described as carrying the mcr-1 gene (2), was identified in 18 of 22 contigs.
Figure.
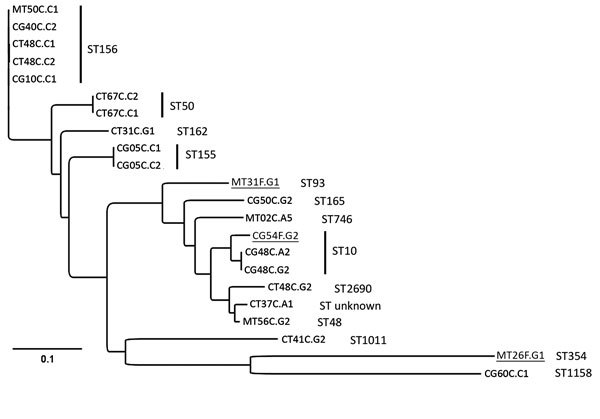
Phylogenetic analyses of mcr-1–positive Escherichia coli isolated from chickens and chicken farmers, Vietnam, 2012–2013. Maximum-likelihood tree of 22 mcr-1–carrying E. coli isolated from 15 chicken fecal samples and 3 human fecal swab samples (underlined), constructed by using CSI Phylogeny 1.4 (https://cge.cbs.dtu.dk//services/CSIPhylogeny/), shows a genome-wide single-nucleotide polymorphism (SNP) comparison. A total of 74,585 SNPs were concatenated for pairwise comparison (difference between pairs 0–32,267 SNPs). The multilocus sequence types (ST) are indicated next to the isolate names. The ST155 isolates CG05C.C1 and CG05C.C2 differ by 1 SNP; the ST10 isolates CG48C.A2 and CG48C.G2 differ by 1 SNP and 1 antimicrobial resistance gene; the ST156 isolates CT48C.C1 and CT48C.C2 differ by 4 SNPs and 3 antimicrobial resistance genes; and the ST50 isolates CT67C.C1 and CT67C.C2 are phenotypically different but have 0 SNP differences and originate from the same sample and are therefore likely to be highly related or identical. Scale bar indicates number of nucleotide substitutions per site.
We investigated risk factors for fecal colonization with mcr-1–carrying bacteria separately for small-scale farms and household farms because a joint model did not converge due to inflated sampling weight assigned to household chicken farms (Technical Appendix 1 Table 1). Multivariate analysis identified the presence of younger chickens (<20.5 weeks old) and the use of colistin as independent risk factors for fecal colonization with mcr-1–carrying bacteria in chickens (odds ratios [ORs] 21.3 and 5.1, respectively) in small-scale farms (Table 2). We were unable to identify potential risk factors associated with fecal colonization with mcr-1-carrying bacteria in chickens in household farms. Among human participants, farmers who were exposed to mcr-1–positive chickens showed a significantly increased risk for colonization with mcr-1–carrying bacteria (OR 5.3; Table 2) in contrast with urban individuals not involved in chicken farming, rural individuals not exposed to chickens, and farmers with mcr-1–negative chickens.
Table 2. Multivariate analysis of risk factors associated with fecal colonization with mcr-1–carrying bacteria in small-scale chicken farms (N = 94) and in humans (N = 440), Vietnam, 2012–2013*.
| Variables |
No. tested |
No. mcr-1–positive |
OR (95% CI) |
p value |
|---|---|---|---|---|
| Small-scale chicken farms | ||||
| Age of chickens | ||||
| Chickens <20.5 weeks old | 47 | 32 | 21.3 (5.8–78.5) | <0.001 |
| Chickens ≥20.5 weeks old | 47 | 8 | Referent | |
| Use of colistin |
21 |
14 |
5.1 (1.4–18.8) |
0.017 |
| Humans | ||||
| Urban persons† | 88 | 8 | Referent | |
| Rural persons† | 173 | 31 | 2.1 (0.9–5.0) | 0.075 |
| Farmers exposed to mcr-1–negative chickens | 91 | 16 | 1.8 (0.7–4.7) | 0.205 |
| Farmers exposed to mcr-1–positive chickens | 88 | 29 | 5.3 (2.2–12.7) | <0.001 |
*OR, odds ratio. †Not involved in poultry farming.
Conclusions
Our study shows that colonization with mcr-1–carrying bacteria in chickens is associated with colistin usage and colonization of humans is associated with exposure to mcr-1–positive chickens. These findings suggest that colistin use is the main driver for the observed high prevalence (59.4%) of mcr-1 in fecal samples from chickens, with zoonotic transmission explaining the high prevalence (34.7%) in farmers. Zoonotic transmission of colistin-resistant E. coli from a domesticated pig (11) and companion animals (12) to humans has been reported.
We found that younger chickens were more likely to be colonized with mcr-1–carrying bacteria than older chickens (>20.5 weeks), probably because of the higher antimicrobial treatment incidence in younger chickens (74.0 [interquartile range 0–278]/1,000 chickens treated daily with 1 defined daily dose) than in older chickens (46.3 [interquartile range 0–124]/1,000 chickens treated daily with 1 defined daily dose) (N.V. Trung, unpub. data). However, our study was insufficiently powered to detect such an association in multivariate analysis. In addition, the gastrointestinal tract of younger chickens might be colonized by antimicrobial-resistant bacteria more readily than older chickens (13).
The spread of the mcr-1 gene on different plasmid types (IncI2, IncHI2, and IncHI2A) might explain its successful spread in different E. coli clones. We also identified the ISApl1 transposon in 81.8% (18/22) of our isolates. Because this genetic element is involved in horizontal gene transfer, it is likely to be a key factor contributing to the widespread dissemination of mcr-1 (14).
Our study is subject to several limitations. First, the cross-sectional study design precludes the demonstration of direct transmission of the mcr-1 gene between chickens and humans. Second, the presence of colistin in chicken feeds could not be verified and thus misclassification of farms in terms of their colistin use was possible. Last, we did not screen for the mcr-2 gene, which is also involved in colistin resistance (15).
In summary, our results show an association between colistin use on farms and the presence of the mcr-1 gene in animals. Given the potentially serious consequences of the spread of the mcr-1 gene from food production animals to humans, prudent use of antimicrobial drugs in animal production should be enforced globally, including in small-scale and household farms.
Detailed materials and methods describing the selection and recruitment of study subjects, data collection, sample analyses, real-time PCR detection of the mcr-1 gene, adjustment of prevalence estimates, whole-genome sequencing analyses, and risk factor analyses. Univariate analyses of the risk factors associated with the presence of mcr-1–carrying bacteria in chickens and humans in Vietnam in 2012−2013, comparison of characteristics of study participants and farms included and excluded from the risk factor analyses, and use of antimicrobial drugs in chickens and humans in southern Vietnam.
Characteristics of mcr-1–positive Escherichia coli from chickens and farmers, Vietnam, 2012–2013.
Acknowledgment
The authors would like to thank the staff at the Preventive Medicine Center and Sub-Department of Animal Health of Tien Giang for their support in sampling and data collection.
This work was supported by The Netherlands Organisation for Health Research and Development, The Netherlands Organisation for Scientific Research (ZoNMW/NWO-WOTRO grant no. 205100012), The Wellcome Trust, UK (grant nos. 089276/Z/09/Z and 110085/Z/15/Z), AXA project (Outlook at University of Oxford for 2014 to N.T.H.), and the European Commission (COMPARE-H2020, grant no. 643476).
Biography
Mr. Trung is a doctoral student at the Academic Medical Center, University of Amsterdam, the Netherlands, and Oxford University Clinical Research Unit in Ho Chi Minh City, Vietnam. His research interests include epidemiology of zoonotic pathogens and dynamics of antimicrobial resistance in bacterial populations.
Footnotes
Suggested citation for this article: Trung NV, Matamoros S, Carrique-Mas JJ, Nghia NH, Nhung NT, Chieu TTB, et al. Zoonotic transmission of mcr-1 colistin resistance gene from small-scale poultry farms, Vietnam. Emerg Infect Dis. 2017 Mar [date cited]. http://dx.doi.org/10.3201/eid2303.161553
These authors contributed equally to this article.
References
- 1.Olaitan AO, Diene SM, Kempf M, Berrazeg M, Bakour S, Gupta SK, et al. Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in Lao PDR, Thailand, Israel, Nigeria and France owing to inactivation of the PhoP/PhoQ regulator mgrB: an epidemiological and molecular study. Int J Antimicrob Agents. 2014;44:500–7. 10.1016/j.ijantimicag.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 2.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–8. 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- 3.Skov RL, Monnet DL. Plasmid-mediated colistin resistance (mcr-1 gene): three months later, the story unfolds. Euro Surveill. 2016;21:30155. 10.2807/1560-7917.ES.2016.21.9.30155 [DOI] [PubMed] [Google Scholar]
- 4.Schwarz S, Johnson AP. Transferable resistance to colistin: a new but old threat. J Antimicrob Chemother. 2016;71:2066–70. 10.1093/jac/dkw274 [DOI] [PubMed] [Google Scholar]
- 5.The GARP-Vietnam National Working Group. Situation analysis. Antibiotic use and resistance in Vietnam. 2010 Oct [cited 2016 Sep 12]. http://www.cddep.org/sites/default/files/vn_report_web_1_8.pdf
- 6.Carrique-Mas JJ, Trung NV, Hoa NT, Mai HH, Thanh TH, Campbell JI, et al. Antimicrobial usage in chicken production in the Mekong Delta of Vietnam. Zoonoses Public Health. 2015;62(Suppl 1):70–8. 10.1111/zph.12165 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen VT, Carrique-Mas JJ, Ngo TH, Ho HM, Ha TT, Campbell JI, et al. Prevalence and risk factors for carriage of antimicrobial-resistant Escherichia coli on household and small-scale chicken farms in the Mekong Delta of Vietnam. J Antimicrob Chemother. 2015;70:2144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trung NV, Carrique-Mas JJ, Nghia NH, Tu LT, Mai HH, Tuyen HT, et al. Non-typhoidal Salmonella colonization in chickens and humans in the Mekong Delta of Vietnam. Zoonoses Public Health. 2016. 10.1111/zph.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0. 2016 Jan 1 [cited 2016 Sep 12]. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf
- 10.European Committee on Antimicrobial Susceptibility Testing. EUCAST warnings concerning antimicrobial susceptibility testing products or procedures. 2016 [cited 2016 Sep 12]. http://www.eucast.org/ast_of_bacteria/warnings/
- 11.Olaitan AO, Chabou S, Okdah L, Morand S, Rolain JM. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis. 2016;16:147. 10.1016/S1473-3099(15)00540-X [DOI] [PubMed] [Google Scholar]
- 12.Zhang XF, Doi Y, Huang X, Li HY, Zhong LL, Zeng KJ, et al. Possible transmission of mcr-1-harboring Escherichia coli between companion animals and human. Emerg Infect Dis. 2016;22:1679–81. 10.3201/eid2209.160464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JL, Drum DJ, Dai Y, Kim JM, Sanchez S, Maurer JJ, et al. Impact of antimicrobial usage on antimicrobial resistance in commensal Escherichia coli strains colonizing broiler chickens. Appl Environ Microbiol. 2007;73:1404–14. 10.1128/AEM.01193-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snesrud E, He S, Chandler M, Dekker JP, Hickman AB, McGann P, et al. A model for transposition of the colistin resistance gene mcr-1 by ISApl1. Antimicrob Agents Chemother. 2016;60:6973–6. 10.1128/AAC.01457-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. 2016;21:30280. 10.2807/1560-7917.ES.2016.21.27.30280 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed materials and methods describing the selection and recruitment of study subjects, data collection, sample analyses, real-time PCR detection of the mcr-1 gene, adjustment of prevalence estimates, whole-genome sequencing analyses, and risk factor analyses. Univariate analyses of the risk factors associated with the presence of mcr-1–carrying bacteria in chickens and humans in Vietnam in 2012−2013, comparison of characteristics of study participants and farms included and excluded from the risk factor analyses, and use of antimicrobial drugs in chickens and humans in southern Vietnam.
Characteristics of mcr-1–positive Escherichia coli from chickens and farmers, Vietnam, 2012–2013.


