Despite slightly increased cases in these areas, human infection with this cattle pathogen remains rare.
Keywords: Mycobacterium bovis, Mycobacterium tuberculosis, tuberculosis and other mycobacteria, zoonoses, zoonotic diseases, epidemiology, England, Wales, Northern Ireland, bacteria
Abstract
Despite control efforts, Mycobacterium bovis incidence among cattle remains high in parts of England, Wales, and Northern Ireland, attracting political and public health interest in potential spread from animals to humans. To determine incidence among humans and to identify associated factors, we conducted a retrospective cohort analysis of human M. bovis cases in England, Wales, and Northern Ireland during 2002–2014. We identified 357 cases and observed increased annual case numbers (from 17 to 35) and rates. Most patients were >65 years of age and born in the United Kingdom. The median age of UK-born patients decreased over time. For 74% of patients, exposure to risk factors accounting for M. bovis acquisition, most frequently consumption of unpasteurized milk, was known. Despite the small increase in case numbers and reduction in patient age, M. bovis infection of humans in England, Wales, and Northern Ireland remains rare.
After the 1960s, the number of human cases of tuberculosis (TB) caused by Mycobacterium bovis decreased significantly in England, Wales, and Northern Ireland, co-inciding with widespread implementation of milk product pasteurization and national bovine TB control programs (1–3). During the past 2 decades in these 3 countries, an average of 30 cases of M. bovis in humans occurred annually; numbers decreased in the early 2000s before again increasing (4–6). During the same period, incidence of M. bovis in cattle herds in parts of England, Wales, and Northern Ireland increased substantially but has now plateaued (4,7–9).
M. bovis control (2,7,10,11) attracts political, public health, and media interest because of potential spread from animals to humans, effects on animal health and trade (1), and the role of wildlife in the transmission cycle (12). Highly visible interventions, including wildlife management to prevent transmission to livestock, are used to attempt to control M. bovis spread (6,9,10), thereby protecting human health.
Compared with other countries in western Europe, the rate of TB among humans in the United Kingdom is high: 9.6 cases/100,000 population (6,240 cases) in 2015 (13). Most TB cases occurred in those born abroad, who probably acquired infection before entering the United Kingdom. Although only 1.1% (42 cases) of culture-confirmed TB cases were caused by M. bovis (6), it remains a public health priority.
The drivers of the epidemiology of M. tuberculosis are well described (13–15). However, there is comparatively less information on the sources of M. bovis in humans, other than the recognized risks of unpasteurized milk consumption and close contact with infected cattle (1,3). We provide an update on the demographic characteristics of humans with M. bovis disease in England, Wales, and Northern Ireland (16). To address the gap in knowledge regarding lesser known sources of acquisition, we describe the demographic and clinical characteristics of humans with TB caused by M. bovis compared with M. tuberculosis. In addition, we describe potential human exposures that may indicate M. bovis acquisition and include a genotyping comparison of the causative organisms.
Materials and Methods
Study Population and Definitions
Our retrospective cohort study included all human M. bovis patients in the descriptive analysis. To describe demographic and clinical characteristics associated with M. bovis disease, we compared all M. bovis notified patients with all M. tuberculosis notified patients. Potential exposures to risk factors associated with M. bovis acquisition were collected through a questionnaire and limited to M. bovis cases identified during 2006–2014, when the questionnaire return rate was high (>80%).
An M. bovis case was defined as a culture-confirmed human case of TB speciated as M. bovis isolated during 2002–2014. A notified M. bovis case was an M. bovis case clinically notified to the Enhanced TB Surveillance system (ETS); a nonnotified M. bovis case was an M. bovis not reported clinically to ETS. An M. tuberculosis notified case was defined as a culture-confirmed human case of TB speciated as M. tuberculosis isolated during 2002–2014 and clinically notified to ETS.
Data Collection
Results from culture-positive laboratory isolates were sent from Mycobacterium reference laboratories in England, Wales, and Northern Ireland to Public Health England. These results were matched with notified TB cases from ETS, used for statutory notification of TB, by use of a probabilistic matching method (17).
Data on demographics (age, sex, ethnicity, country of birth, time since UK entry, address, and occupation); clinical factors (site of disease and previous diagnosis); and social risk factors (current or past imprisonment, homelessness, drug and alcohol misuse) were obtained from ETS notifications. For nonnotified M. bovis cases, the only patient demographic information available was age, sex, and address; the disease site was inferred from specimen site. For analysis, we used the age groups 0–14, 15–44, 45–64, and >65 years and the ethnic groups white, black African, Indian subcontinent (Indian, Pakistani, and Bangladeshi grouped together), and other. After assignment to a geographic area of residence based on address, the place of residence was classified as rural or urban by using 2011 census classifications (18).
After identification of an M. bovis case (based on phenotypic, PCR, and genotypic methods [19,20]), a questionnaire (Technical Appendix) (21) was issued to collect information on potential recognized current or past M. bovis exposures. These exposures were contact with a human TB patient, travel (for >2 weeks) to or residence in a country with high TB incidence (defined as having an estimated rate of >40 cases/100,000 population during 2002–2014), consumption of unpasteurized milk product, occupational contact with animals, physical contact with wild (nondomestic) animals, and physical contact with any animal with TB (including pets).
M. bovis Trend Analysis
We calculated incidence rates per 100,000 population by using mid-year population estimates produced by the UK Office for National Statistics (22). We used Poisson regression to calculate the incidence rate ratio to assess the trend in M. bovis incidence over time. We used a nonparametric test for trend across ordered groups to assess the age trend of M. bovis patients and the χ2 test for trend to assess the proportion of M. bovis among culture-confirmed TB cases.
Factors Associated with M. bovis Disease and M. tuberculosis Disease
Demographic and clinical characteristics for M. bovis notified patients were compared with those of M. tuberculosis notified patients by using univariable and multivariable logistic regression to calculate odds ratios to identify factors associated with M. bovis disease. A forward stepwise multivariable logistic regression model was used, including sex and all variables with a p value <0.2 in univariable analysis; likelihood ratios were assessed after each stepwise addition to the model. In addition, we conducted a stratified analysis based on place of birth (UK-born/non–UK-born). A p value of <0.05 was considered statistically significant. We tested interactions between biologically and statistically plausible variables in the model by using likelihood ratios. All analyses were conducted by using Stata 13.1 (StataCorp LLC, College Station, TX, USA).
Exposures to Risk Factors Associated with M. bovis Disease
To identify frequent exposure to risk factors among the cohort, we used case exposure history, as collected through the questionnaire (Technical Appendix), for descriptive analysis. In addition to obtaining questionnaire information about contact with another human TB patient, for culture-positive isolates identified during 2010–2014, we also obtained 24-loci mycobacterial interspersed repetitive unit–variable tandem repeat (MIRU-VNTR) strain typing results (20) from Mycobacterium reference laboratories. This information enabled us to identify strain type clusters, defined as >2 human TB cases with indistinguishable MIRU-VNTR profiles (or with an indistinguishable profiles but with 1 case only typed to 23 loci), Clustered cases were further investigated to identify possible epidemiologic links, the identification of which suggest recent human-to-human transmission (23).
Results
Demographics of M. bovis Patients
For 2002–2014, we identified 357 culture-confirmed cases of M. bovis disease in humans. During this time, the proportion of all culture-confirmed TB cases speciated as M. bovis increased from 0.4% to 0.9% (p<0.001). Annual case numbers ranged from 17 in 2002 to 35 in 2014, and the incidence rate fluctuated between 0.03 and 0.06 cases/100,000 population (Figure 1); the incidence rate ratio per year was 1.04 (95% CI 1.01–1.07). Overall, 92.2% (329/357) of M. bovis cases were notified to ETS; since 2011, all identified cases have been notified.
Figure 1.
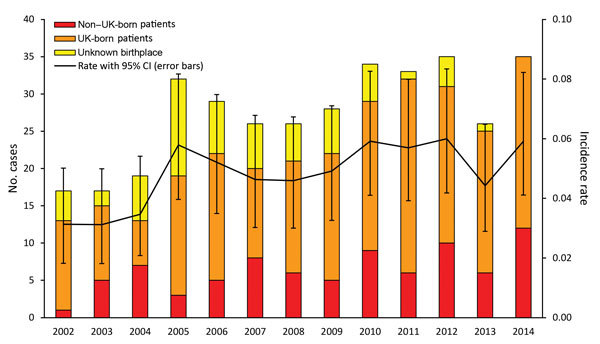
Annual number and incidence rate (no. cases/100,000 population) of notified Mycobacterium bovis cases by patient place of birth, England, Wales, and Northern Ireland, 2002–2014. Unknown place of birth includes notifications with an unknown place of birth and cases that have not been notified.
Among 297 M. bovis patients for whom place of birth was recorded, 214 (72.1%) were born in the United Kingdom. The most frequent countries of birth for the others were Nigeria (18 patients), Morocco (9 patients), and India (8 patients). The age distribution differed significantly between those born and not born in the United Kingdom (p<0.001) (Table 1). The median age of UK-born patients fluctuated over time, from 71 years (interquartile range 60–76) in 2002 to 53 years (interquartile range 35–79) in 2014 (p = 0.099), as did the proportion of cases by age group (Figure 2). Only 6 M. bovis cases in patients <15 years of age were reported (all 11–14 years of age).
Table 1. Characteristics of patients with Mycobacterium bovis disease, England, Wales, and Northern Ireland, 2002–2014*.
| Characteristic† |
All patients, no. (%), n = 357‡ |
UK-born patients, no. (%), n = 214§ |
Non–UK-born patients, no. (%), n = 83¶ |
| Age group, y | |||
| 0–14 | 6 (1.7) | 4 (1.9) | 2 (2.4) |
| 15–44 | 106 (29.7) | 39 (18.2) | 54 (65.1) |
| 45–64 | 70 (19.6) | 45 (21.0) | 12 (14.5) |
|
>65 |
175 (49.0) |
126 (58.9) |
15 (18.1) |
| Male sex |
196 (55.1) |
130 (60.8) |
37 (44.6) |
| Ethnicity | |||
| White | 230 (73.0) | 199 (93.9) | 15 (18.5) |
| Black African | 37 (11.8) | 2 (0.9) | 35 (43.2) |
| Indian subcontinent | 16 (5.1) | 3 (1.4) | 9 (11.1) |
| Other |
32 (10.2) |
8 (3.8) |
22 (27.2) |
| Time since entered United Kingdom, y | |||
| <2 | NA | NA | 10 (14.7) |
| 2–5 | NA | NA | 17 (25.0) |
| 6–10 | NA | NA | 20 (29.4) |
| >10 |
NA |
NA |
21 (30.9) |
| Place of residence | |||
| Rural | 86 (24.9) | 62 (29.0) | 9 (10.8) |
| Urban |
259 (75.1) |
152 (71.0) |
74 (89.2) |
| Pulmonary TB# | |||
| Yes | 199 (56.9) | 131 (61.5) | 38 (45.8) |
| No |
151 (42.3) |
82 (38.5) |
45 (54.2) |
|
>1 social risk factor** |
12 (7.9) |
6 (5.9) |
5 (11.1) |
| Previous TB diagnosis | 17 (6.1) | 13 (6.7) | 5 (5.4) |
*IQR, interquartile range; NA, not applicable; TB, tuberculosis. †Sex, age, and site of disease reported for all cases (excluding breakdowns by birth in or not in the United Kingdom); all other characteristics reported only for notified cases. ‡Median age (IQR) 58 (36–77) y. §Median age (IQR) 70 (52–79) y. ¶Median age (IQR) 35 (28–58) y. #Pulmonary TB with or without extrapulmonary TB, those recorded as “no” had exclusively extrapulmonary TB. **Data only available from 2010 on.
Figure 2.
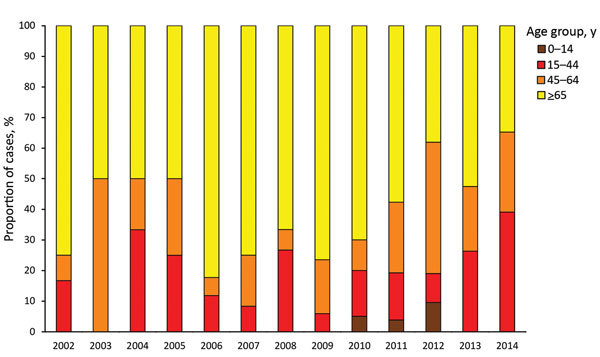
Annual number of notified UK-born Mycobacterium bovis cases, by patient age group, England, Wales, and Northern Ireland, 2002–2014.
For all 3 countries, the highest proportion of M. bovis patients resided in London, England (18.5%; 66/357), followed by the South West (15.4%; 55) and West Midlands regions of England (13.2%; 47) (Figure 3, panel A). However, the incidence rate was highest in Northern Ireland (0.11 cases/100,000 population), followed by the South West (0.08/100,000) and West Midlands (0.07/100,000) regions. The highest proportion (71.9%; 41/57) of M. bovis patients not born in the United Kingdom lived in London. In comparison, 85.1% (40/47) and 82.1% (32/39) of patients from the South West and West Midlands, respectively, were born in the United Kingdom.
Figure 3.
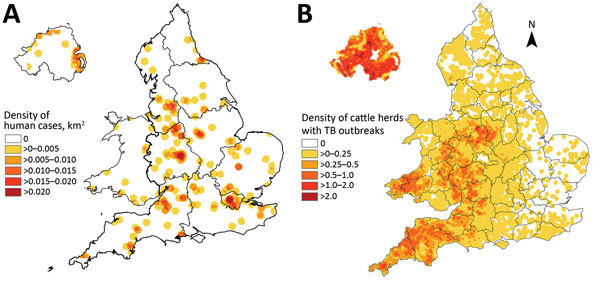
Cases of Mycobacterium bovis disease in England, Wales, and Northern Ireland, 2002–2014. A) Density of human cases. B) Density of cattle herds with TB outbreaks. This material is based on Crown Copyright and is reproduced with the permission of Land & Property Services under delegated authority from the Controller of Her Majesty’s Stationery Office.
Comparison between Notified M. bovis and M. tuberculosis Patients
Univariable analysis showed that, when compared with M. tuberculosis notified patients, M. bovis notified patients were more likely to be >45 years of age, born in the United Kingdom, of an ethnic group other than that of the Indian subcontinent, live in a rural area, and work in agricultural or animal-related occupations. M. bovis patients were less likely than M. tuberculosis patients to have pulmonary disease. Multivariable analysis showed that the same factors, other than age, were independently associated with M. bovis; only those >65 years of age were more likely to have M. bovis disease. The strongest risk factor for M. bovis disease was working in an agricultural or animal-related occupation (adjusted odds ratio 29.5, 95% CI 16.9–51.6; Table 2). The model showed no interactions between explanatory variables. Analysis stratifying by place of birth (UK-born vs. non–UK-born) indicated that the same variables were significant.
Table 2. Demographics and risk factors for patients with Mycobacterium tuberculosis and M. bovis disease, England, Wales, and Northern Ireland, 2002–2014*.
| Characteristic |
M. bovis patients, no. (%), n = 329 |
M. tuberculosis patients, no. (%), n = 58,540 |
Univariable analysis |
|
Multivariable analysis |
||
| OR (95% CI) |
p value |
OR (95% CI) |
p value |
||||
| Age group, y | |||||||
| 0–14 | 6 (1.8) | 1,200 (2.1) | 1.9 (0.8–4.3) | <0.001 | 1.5 (0.6–3.6) | <0.001 | |
| 15–44 | 102 (31.0) | 38,558 (65.9) | Referent | Referent | |||
| 45–64 | 61 (18.5) | 10,953 (18.7) | 2.1 (1.5–2.9) | 1.3 (0.9–2.0) | |||
|
>65 |
160 (48.6) |
7,826 (13.4) |
7.7 (6.0–9.9) |
|
|
3.6 (2.6–5.2) |
|
| Sex | |||||||
| M | 179 (54.4) | 33,715 (57.7) | 0.9 (0.7–1.1) | 0.229 | 0.9 (0.7–1.1) | 0.287 | |
| F |
150 (45.6) |
24,721 (42.3) |
Referent |
|
|
Referent |
|
| UK-born | |||||||
| Yes | 214 (72.1) | 13,576 (24.7) | 7.9 (6.1–10.1) | <0.001 | 2.6 (1.7–4.1) | <0.001 | |
| No |
83 (27.9) |
71,361 (75.3) |
Referent |
|
|
Referent |
|
| Ethnicity | |||||||
| White | 230 (73) | 11,968 (21.1) | 28.0 (16.8–46.4) | <0.001 | 14.6 (7.2–26.9) | <0.001 | |
| Black African | 37 (11.8) | 12,501 (22.1) | 4.3 (2.4–7.7) | 7.4 (3.7–14.9) | |||
| Indian subcontinent† | 16 (5.1) | 23,285 (41.1) | Referent | Referent | |||
| Other |
32 (10.2) |
8,905 (15.7) |
5.2 (2.9–9.5) |
|
|
7.3 (3.6–14.8) |
|
| Occupation | |||||||
| Agricultural/animal contact work | 20 (7.9) | 116 (0.3) | 32.4 (19.8–53.0) | <0.001 | 29.5 (16.9–51.6) | <0.001 | |
| Other |
232 (92.1) |
43,698 (99.7) |
Referent |
|
|
Referent |
|
| Site of disease | |||||||
| Pulmonary | 193 (58.8) | 37,580 (64.2) | 0.8 (0.6–1.0) | 0.048 | 0.4 (0.3–0.5) | <0.001 | |
| Extrapulmonary only |
135 (41.2) |
20,938 (35.8) |
Referent |
|
|
Referent |
|
| Place of residence | |||||||
| Rural | 81 (24.6) | 1,944 (3.3) | 9.5 (7.3–12.2) | <0.001 | 2.8 (2.0–3.9) | <0.001 | |
| Urban | 248 (75.4) | 56,380 (96.7) | Referent | Referent | |||
*Interactions between 1) place of birth (UK-born/non–UK-born) and all of the other variables (age, sex, ethnicity, occupation, site of disease, place of residence [rural/urban]) and 2) age and site of disease or place of residence (rural/urban) were tested. No significant interactions existed in the model. OR, odds ratio. †Indian, Bangladeshi, and Pakistani ethnic groups.
M. bovis Patient Exposure to Risk Factors
Of the 272 M. bovis patients identified during 2006–2014, exposure questionnaires were completed for 241 (88.6%). Of these, 179 (74.3%) reported exposure to at least 1 risk factor for M. bovis acquisition; 78 (43.6%) reported 1 exposure, 57 (31.8%) 2 exposures, 28 (15.6%) 3 exposures, and 16 (8.9%) 4 exposures. For 6 patients, no exposure was known; for the remaining 56 patients, data were missing for >1 risk factor and the patients could not be classified as not having been exposed to a risk factor.
The most frequently reported exposure was consumption of unpasteurized milk products (65.7%, 109/166; Table 3); proportions reporting this factor were similar among those born in the United Kingdom and those born elsewhere. Among those for whom the most recent consumption of unpasteurized milk product was known, most (85.9%, 55/64) had consumed the product >5 years before TB diagnosis; 42.2% (27/64) were >50 years of age before diagnosis. No change in the age distribution of patients consuming unpasteurized milk was identified over time; most (56.0%, 61/109) were >65 years of age.
Table 3. Risk factor exposures reported by patients with Mycobacterium bovis disease, England, Wales, and Northern Ireland, 2006–2014*.
| Exposure |
No. characteristics/no. with information recorded (%) |
||
| All patients |
UK-born patients |
Non–UK-born patients |
|
| Consumption of unpasteurized milk products | 109/166 (65.7) | 74/112 (66.1) | 25/37 (67.6) |
| In United Kingdom |
66/88 (75.0) |
60/64 (93.8) |
1/15 (6.7) |
| Travel or residence in a high incidence country |
77/203 (37.9) |
29/126 (23.0) |
44/56 (78.6) |
| Work related animal exposure | 51/103 (49.5) | 35/71 (49.3) | 7/22 (31.8) |
| In United Kingdom |
33/37 (89.2) |
28/29 (96.6) |
2/4 (50.0) |
| Contact with human patient with TB | 33/181 (18.2) | 21/120 (17.5) | 10/42 (23.8) |
| In United Kingdom |
36/38 (94.7) |
19/19 (100) |
6/8 (75.0) |
| Physical contact with wild animal | 18/126 (14.3) | 15/92 (16.3) | 0/25 (0) |
| In United Kingdom |
10/13 (76.9) |
7/10 (70.0) |
Not applicable |
| Physical contact with animal with TB | 18/99 (18.2) | 14/76 (18.4) | 2/18 (11.1) |
| In United Kingdom |
10/1 (90.9) |
9/10 (90.0) |
1/1 (100) |
| Pet with TB† | 2 | 2 | 0 |
| Farm animal with TB† | 11 | 9 | 1 |
| No exposure† | 6 | 6 | 0 |
*TB, tuberculosis. †Denominator not available.
Contact with a human TB patient was reported by 18.2% (33/181), but for most, recorded information was insufficient to identify the contact, particularly if the contact was not recent. Where known, 80.8% (21/26) of contacts occurred >5 years before TB diagnosis. From 24-loci MIRU-VNTR strain typing data available during 2010–2014, a total of 48.7% (57/117) of patients (of which 46 were born in the United Kingdom and 9 were not) were in 15 M. bovis strain type clusters. One cluster contained exclusively patients not born in the United Kingdom and 7 exclusively born in the United Kingdom; 2 of the latter clusters contained the only epidemiologically linked human patients, each with a pair of household contacts.
Recent acquisition of infection cannot be directly measured, but the rate of M. bovis disease among children, along with their exposures, can provide an indirect indicator of recent acquisition. Exposure information was available for 5/6 M. bovis patients <15 years of age and suggested potential overseas acquisition; 5 had traveled to a country where TB incidence was high, 1 of whom had consumed unpasteurized milk while abroad. For 1 child not born in the United Kingdom, a questionnaire response was not obtained.
Overall, among those for whom location of exposure was known, 59.1% (97/164) of patients were exposed to >1 risk factor in the United Kingdom (Table 3). Among those not born in the United Kingdom, 18.0% (9/50) were known to have been exposed to a risk factor while in the United Kingdom, but 4 of the 9 also were exposed outside the United Kingdom.
Discussion
Our findings confirm that M. bovis disease remains rare among humans in England, Wales, and Northern Ireland. Over the study period, the annual rate of M. bovis disease and the proportion of culture-confirmed TB cases with M. bovis identified as the cause displayed a small but statistically significant increase; annual case numbers for the past 10 years were similar to those for the early 1990s (4). Although speciation has improved from the use of strain typing results (19,20), this improvement is unlikely to account for all of the increase identified. Although the previous study by Jalava et al. (4) and our study overlap by 2 years, our results benefit from improved matching (17) between case notification and culture results from 2002 on, thereby providing improved accuracy for reporting annual case numbers.
We identified, unlike previous studies (4,5), that although the number of M. bovis patients not born in the United Kingdom remained low and fluctuated over time, the annual number of cases in this group increased slightly over time. Our finding may be confounded by better recording of place of birth but is not unexpected given the increase during this period in the overall number of TB patients not born in the United Kingdom (13). Similar to previous findings (5), our findings indicate that most M. bovis patients not born in the United Kingdom lived in urban areas, specifically London. These patients originated mostly from low-income countries where TB incidence is high and therefore are at higher risk for human-to-human transmission and animal-to-human transmission because of limited detection of M. bovis in animals and less frequent milk pasteurization (24). Thus, infection was probably acquired before arrival in the United Kingdom and less likely to be related to exposure to risk factors while in the United Kingdom. Unfortunately, speciation is not routinely conducted in many high TB burden, low-income countries (16), so it is difficult to identify in which countries incidence of M. bovis is high and whether the trends in country of birth for M. bovis patients not born in the United Kingdom reflect the global incidence of the disease (24,25).
We identified a decrease over time in the proportion of UK-born patients >65 years of age and a decrease in the median age, although neither was statistically significant. Previously, most cases in UK-born patients were the result of reactivation of infection acquired before the large rollout of pasteurization (by the 1960s) (4), when M. bovis incidence was higher. Given the length of time that widespread pasteurization has been in place, progressively fewer cases among the older population are expected as the cohort exposed before pasteurization decreases. It was unexpected that, despite this decrease, the number of M. bovis cases occurring in the UK-born population did not reduce over the study period. Instead, the number and proportion of younger UK-born patients increased slightly. Numbers remain small, and it is not possible to yet detect any change in exposures; however, in recent years, the media have reported increased public demand for unpasteurized milk, which, if contaminated, could result in more human infections.
No data are available to quantify unpasteurized milk production or consumption within England, Wales, and Northern Ireland. However, results from a 2012 survey of adult consumer attitudes about unpasteurized milk (26) showed that 33% of respondents had consumed unpasteurized milk but only 3% currently consumed unpasteurized milk. Although the proportion who had ever consumed unpasteurized milk was highest among older age groups (18–24 years, 31%; 25–44 years, 28%; 45–64 years, 38%; >65 years, 40%), the proportion of current consumers was higher among younger age groups (18–24 years, 7%; 25–44 years, 4%; 45–64, 1%; >65, 1%). It is possible that increased consumption of unpasteurized milk, as reported by the media, is contributing to the small increase in M. bovis cases and may contribute to a change in demographics of patients over time. Although we do not have evidence to confirm, this hypothesis could be explored further through a formal observation study. The time between unpasteurized milk consumption and onset of TB disease among the M. bovis patients in our cohort emphasizes that the effects of current unpasteurized milk consumption may not be observed for many years.
The results of combining routine 24-loci MIRU-VNTR typing of M. bovis from humans with epidemiologic data provide evidence of only occasional human-to-human M. bovis transmission; despite extensive follow-up of the 57 clustered cases, only 2 instances of 2 cases being epidemiologically linked were found. Only 1 prior occurrence of MIRU-VNTR–confirmed (using 15-loci typing) human-to-human transmission of M. bovis in the United Kingdom has been documented (5,27); it occurred before the rollout of routine prospective 24-loci MIRU-VNTR typing. There are also few examples of human-to-human M. bovis transmission in countries other than those included in this study (28,29), suggesting that such transmission is rarely identified. Overall, the proportion of clustering among M. bovis cases (49%) was slightly lower than that of the overall proportion among all TB cases (56%) observed in England (13).
This analysis also presents findings consistent with those previously reported (4). Although the proportion of cases among the older UK-born population seems to be decreasing, over the study period this group accounted for most cases. Our comparative analysis confirmed that the demographic profile of M. bovis patients differs from that of M. tuberculosis patients. The consumption of unpasteurized milk remained the most frequently reported exposure, and M. bovis patients were more likely than M. tuberculosis patients to work or have worked in agricultural and animal-related occupations. These findings are reassuring and show that M. bovis disease is still largely limited to those with recognized risk factors for infection. Few incidents involving animal-to-human transmission on farms (1,30,31) and a single incident of M. bovis transmission from a pet to its owners 32,33) have occurred during the study period. Most animal-to-human transmission remains sporadic, and implementation of additional specific interventions beyond those currently in place (1,2) would be difficult.
A high proportion of UK-born patients lived in rural areas, especially across the South West and Midlands of England, where M. bovis incidence among cattle is high (Figure 3, panel B). Most of these patients reported consumption of unpasteurized milk or contact with animals. However, human patients without such exposures and who reside in these areas where M. bovis cattle incidence is high should continue to be monitored and thoroughly investigated to ensure that lesser known exposures are not missed.
Similar to our study, a study in the Netherlands identified that the highest proportion of M. bovis cases occurred in the older native population (50%), followed by the foreign-born population (40%) (34). In comparison with our study, studies from the United States found that being foreign born (in particular, being of Hispanic ethnicity) and younger were independently associated with M. bovis when compared with M. tuberculosis (35,36). The difference in demographic characteristics of M. bovis patients in the United States and in England, Wales, and Northern Ireland may be explained by the fact that M. bovis in cattle or wildlife is not frequently reported in the United States (37,38) but is more common in neighboring Mexico (39,40). Thus, the epidemiology of human M. bovis in England, Wales, and Northern Ireland continues to be driven by the past and, to some extent, present prevalence of disease in cattle. Given advances in molecular techniques, improved understanding of animal-to-human transmission will require linking the genotyping results from animals with M. bovis infection in England, Wales, and Northern Ireland with data from humans.
Globally, zoonotic TB should be tackled, and the needs of those affected by M. bovis disease, namely those in animal-related occupations and those consuming unpasteurized milk from infected animals, should be addressed. The implementation of methods to identify M. bovis where culture is not possible have been highlighted as essential (16,41,42). Although findings from England, Wales, and Northern Ireland cannot be extrapolated even to other high-income countries, much less to high TB burden, low-income countries, our study does illustrate the value of monitoring M. bovis disease and the data required to do so.
Our study does have some limitations. The exposure questionnaires return rate was 89%, and some responses were missing, which could lead to some error in the estimation of exposures; in addition, nonresponders were more likely to be urban dwellers. Our comparison of M. bovis and M. tuberculosis patients was limited because exposure questionnaire information was only collected for M. bovis patients; therefore, animal-related exposures, travel to countries with high TB incidence, and contact with human TB patients could not be included in the analysis. In addition, patients not born in the United Kingdom, most of whom belong to Indian subcontinent ethnic groups, are more likely missed in analysis because a higher proportion have exclusively extrapulmonary disease (43), for which culture confirmation is lower. Approximately 60% of TB cases in England, Wales, and Northern Ireland are culture confirmed; therefore, the estimated M. bovis incidence presented in this article is probably an underestimate. The proportion of TB cases culture confirmed over time has remained relatively stable (44), so underascertainment should not affect changes in the number or proportion of TB cases caused by M. bovis.
In conclusion, we found that M. bovis disease continues to account for a small number and low proportion of total TB cases in England, Wales, and Northern Ireland. The proportion of culture-confirmed TB cases caused by M. bovis has increased slightly, and the age of UK-born patients has decreased. The reasons are not fully understood, and trends should continue to be monitored. For most patients, exposure to risk factors for M. bovis acquisition (e.g., unpasteurized milk consumption, farm work, or contact with a human TB patient) were known. The current control measures in place to prevent animal-to-human spread seem to be effective; such spread occurs in a few isolated incidents and sporadic events. However, to increase understanding of M. bovis transmission in England, Wales, and Northern Ireland, we recommend strengthening collaboration between animal and human health, including linking genotyping results.
Technical Appendix. Mycobacterium bovis questionnaire for England, Wales, and Northern Ireland.
Acknowledgments
We thank Ross Harris for his support and guidance on statistical analysis and Adam Brouwer and Maria O’Hagan for providing data on herds with TB outbreaks, shown in Figure 3, panel B.
Biography
Ms. Davidson is an epidemiologist in the Centre of Infectious Disease Surveillance and Control at Public Health England, based in London. Her main research interests cover various aspects of TB epidemiology within the United Kingdom.
Footnotes
Suggested citation for this article: Davidson JA, Loutet MG, O’Connor C, Kearns C, Smith RMM, Lalor MK, et al. Epidemiology of Mycobacterium bovis disease in humans in England, Wales, and Northern Ireland, 2002–2014. Emerg Infect Dis. 2017 Mar [date cited]. http://dx.doi.org/10.3201/eid2303.161408
References
- 1.Torgerson PR, Torgerson DJ. Public health and bovine tuberculosis: what’s all the fuss about? Trends Microbiol. 2010;18:67–72. 10.1016/j.tim.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 2.Department for Environment Food & Rural Affairs. 2010. to 2015 government policy: bovine tuberculosis (bovine TB) [cited 2016 Jul 6]. https://www.gov.uk/government/publications/2010-to-2015-government-policy-bovine-tuberculosis-bovine-tb/2010-to-2015-government-policy-bovine-tuberculosis-bovine-tb
- 3.Grange JM. Mycobacterium bovis infection in human beings. Tuberculosis (Edinb). 2001;81:71–7. 10.1054/tube.2000.0263 [DOI] [PubMed] [Google Scholar]
- 4.Jalava K, Jones JA, Goodchild T, Clifton-Hadley R, Mitchell A, Story A, et al. No increase in human cases of Mycobacterium bovis disease despite resurgence of infections in cattle in the United Kingdom. Epidemiol Infect. 2007;135:40–5. 10.1017/S0950268806006509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandal S, Bradshaw L, Anderson LF, Brown T, Evans JT, Drobniewski F, et al. Investigating transmission of Mycobacterium bovis in the United Kingdom in 2005 to 2008. J Clin Microbiol. 2011;49:1943–50. 10.1128/JCM.02299-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Public Health England. Mycobacterium bovis TB case notifications by country, UK, 1999–2015 [cited 2016 Nov 3]. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/464843/M.bovis_case_notifications_by_country_UK_1999_to_2014.pdf
- 7.Abernethy DA, Upton P, Higgins IM, McGrath G, Goodchild AV, Rolfe SJ, et al. Bovine tuberculosis trends in the UK and the Republic of Ireland, 1995-2010. Vet Rec. 2013;172:312. 10.1136/vr.100969 [DOI] [PubMed] [Google Scholar]
- 8.Animal & Plant Health Agency. Bovine tuberculosis: infection status in cattle in GB. Annual surveillance report 2014. [cited 2015 Nov 20]. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/457332/GB-surveillance-report14.pdf
- 9.de la Rua-Domenech R. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis (Edinb). 2006;86:77–109. 10.1016/j.tube.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 10.Food Standards Agency. Raw drinking milk and raw cream control requirements in the different countries of the UK [cited 2016 Nov 3]. https://www.food.gov.uk/business-industry/farmingfood/dairy-guidance/rawmilkcream#toc-4
- 11.Public Health England. Bovine tuberculosis: public health management. Guidance on management of the public health consequences of tuberculosis in cattle and other animals (England) [cited 2016 Nov 3]. https://www.gov.uk/government/publications/bovine-tuberculosis-tb-public-health-management 2014
- 12.Corner LAL, Ni Bhuachalla D, Gormley E, More S. The role of badgers in the epidemiology of Mycobacterium bovis infection (tuberculosis) in cattle in the United Kingdom and the Republic of Ireland: current perspectives on control strategies. Vet Med Res Rep. 2014;6:27–38. 10.2147/VMRR.S53643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Public Health England. Tuberculosis in England: annual 2016 report (presenting data to end of 2015). 2015. [cited 2016 Oct 26]. https://www.gov.uk/government/publications/tuberculosis-in-england-annual-report
- 14.Crofts JP, Gelb D, Andrews N, Delpech V, Watson JM, Abubakar I. Investigating tuberculosis trends in England. Public Health. 2008;122:1302–10. 10.1016/j.puhe.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 15.Abubakar I, Lipman M, Anderson C, Davies P, Zumla A. Tuberculosis in the UK—time to regain control. BMJ. 2011;343(jul29 1):d4281. 10.1136/bmj.d4281 [DOI] [PubMed] [Google Scholar]
- 16.Stop TB. The paradigm shift: 2016–2020 [cited 2016 Nov 1]. http://www.stoptb.org/assets/documents/global/plan/GlobalPlanToEndTB_TheParadigmShift_2016-2020_StopTBPartnership.pdf
- 17.Aldridge RW, Shaji K, Hayward AC, Abubakar I. Accuracy of probabilistic linkage using the enhanced matching system for public health and epidemiological studies. PLoS One. 2015;10:e0136179. 10.1371/journal.pone.0136179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UK Department for Environment. Rural urban classification [cited 2016 Jul 4]. https://www.gov.uk/government/collections/rural-urban-definition
- 19.Gibson A, Brown T, Baker L, Drobniewski F. Can 15-locus mycobacterial interspersed repetitive unit-variable-number tandem repeat analysis provide insight into the evolution of Mycobacterium tuberculosis? Appl Environ Microbiol. 2005;71:8207–13. 10.1128/AEM.71.12.8207-8213.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rüsch-Gerdes S, Willery E, et al. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44:4498–510. 10.1128/JCM.01392-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Public Health England. Mycobacterium bovis (M. bovis): enhanced surveillance questionnaire. 2015. [cited 2016 Aug 23]. https://www.gov.uk/government/publications/mycobacterium-bovis-m-bovis-enhanced-surveillance-questionnaire
- 22.UK Office for National Statistics. Population estimates [cited 2016 Jul 26]. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates
- 23.Public Health England. TB strain typing and cluster investigation handbook 3rd ed. 2014. [cited 2016 Jul 26. https://www.gov.uk/government/publications/tb-strain-typing-and-cluster-investigation-handbook
- 24.Cosivi O, Grange JM, Daborn CJ, Raviglione MC, Fujikura T, Cousins D, et al. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg Infect Dis. 1998;4:59–70. 10.3201/eid0401.980108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller B, Dürr S, Alonso S, Hattendorf J, Laisse CJM, Parsons SDC, et al. Zoonotic Mycobacterium bovis–induced tuberculosis in humans. Emerg Infect Dis. 2013;19:899–908. 10.3201/eid1906.120543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Food Standards Agency. Impact assessment on the review of the controls governing the sale and marketing of RDM in England [cited 2016 Jul 26]. http://www.food.gov.uk/sites/default/files/multimedia/pdfs/consultation/rawmilk-pack-england.pdf
- 27.Evans JT, Smith EG, Banerjee A, Smith RM, Dale J, Innes JA, et al. Cluster of human tuberculosis caused by Mycobacterium bovis: evidence for person-to-person transmission in the UK. Lancet. 2007;369:1270–6. 10.1016/S0140-6736(07)60598-4 [DOI] [PubMed] [Google Scholar]
- 28.Etchechoury I, Valencia GE, Morcillo N, Sequeira MD, Imperiale B, López M, et al. Molecular typing of Mycobacterium bovis isolates in Argentina: first description of a person-to-person transmission case. Zoonoses Public Health. 2010;57:375–81. 10.1111/j.1863-2378.2009.01233.x [DOI] [PubMed] [Google Scholar]
- 29.Sunder S, Lanotte P, Godreuil S, Martin C, Boschiroli ML, Besnier JM. Human-to-human transmission of tuberculosis caused by Mycobacterium bovis in immunocompetent patients. J Clin Microbiol. 2009;47:1249–51. 10.1128/JCM.02042-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrikrishna D, de la Rua-Domenech R, Smith NH, Colloff A, Coutts I. Human and canine pulmonary Mycobacterium bovis infection in the same household: re-emergence of an old zoonotic threat? Thorax. 2009;64:89–91. 10.1136/thx.2008.106302 [DOI] [PubMed] [Google Scholar]
- 31.Smith RMM, Drobniewski F, Gibson A, Montague JDE, Logan MN, Hunt D, et al. Mycobacterium bovis infection, United Kingdom. Emerg Infect Dis. 2004;10:539–41. 10.3201/eid1003.020819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibbens N. Mycobacterium bovis infection in cats. Vet Rec. 2014;174:331–2. 10.1136/vr.g2344 [DOI] [PubMed] [Google Scholar]
- 33.Roberts T, O’Connor C, Nuñez-Garcia J, de la Rua-Domenech R, Smith NH. Unusual cluster of Mycobacterium bovis infection in cats. Vet Rec. 2014;174:326. 10.1136/vr.102457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majoor CJ, Magis-Escurra C, van Ingen J, Boeree MJ, van Soolingen D. Epidemiology of Mycobacterium bovis disease in humans, the Netherlands, 1993–2007. Emerg Infect Dis. 2011;17:457–63. 10.3201/eid1703.101111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodwell TC, Moore M, Moser KS, Brodine SK, Strathdee SA. Tuberculosis from Mycobacterium bovis in binational communities, United States. Emerg Infect Dis. 2008;14:909–16. 10.3201/eid1406.071485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott C, Cavanaugh JS, Pratt R, Silk BJ, LoBue P, Moonan PK. Human tuberculosis caused by Mycobacterium bovis in the United States, 2006–2013. Clin Infect Dis. 2016;63:594–601. 10.1093/cid/ciw371 [DOI] [PubMed] [Google Scholar]
- 37.Glaser L, Carstensen M, Shaw S, Robbe-Austerman S, Wunschmann A, Grear D, et al. Descriptive epidemiology and whole genome sequencing analysis for an outbreak of bovine tuberculosis in beef cattle and white-tailed deer in northwestern Minnesota. PLoS One. 2016;11:e0145735. 10.1371/journal.pone.0145735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez LR, Harris B, Black WC IV, Meyer RM, Brennan PJ, Vissa VD, et al. Genotyping North American animal Mycobacterium bovis isolates using multilocus variable number tandem repeat analysis. J Vet Diagn Invest. 2008;20:707–15. 10.1177/104063870802000601 [DOI] [PubMed] [Google Scholar]
- 39.de Kantor IN, Ritacco V. An update on bovine tuberculosis programmes in Latin American and Caribbean countries. Vet Microbiol. 2006;112:111–8. 10.1016/j.vetmic.2005.11.033 [DOI] [PubMed] [Google Scholar]
- 40.Rodwell TC, Kapasi AJ, Moore M, Milian-Suazo F, Harris B, Guerrero LP, et al. Tracing the origins of Mycobacterium bovis tuberculosis in humans in the USA to cattle in Mexico using spoligotyping. Int J Infect Dis. 2010;14(Suppl 3):e129–35. 10.1016/j.ijid.2009.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujiwara PI, Olea-Popelka F. Editorial commentary: why it is important to distinguish Mycobacterium bovis as a causal agent of human tuberculosis. Clin Infect Dis. 2016;63:602–3. 10.1093/cid/ciw374 [DOI] [PubMed] [Google Scholar]
- 42.Olea-Popelka F, Muwonge A, Perera A, Dean AS, Mumford E, Erlacher-Vindel E, et al. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis—a call for action. Lancet Infect Dis. 2016;S1473-3099(16)30139-6. [DOI] [PubMed]
- 43.Nnadi CD, Anderson LF, Armstrong LR, Stagg HR, Pedrazzoli D, Pratt R, et al. Mind the gap: TB trends in the USA and the UK, 2000–2011. Thorax. 2016;71:356–63. 10.1136/thoraxjnl-2015-207915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Public Health England. Reports of cases of tuberculosis to enhanced tuberculosis surveillance systems: tuberculosis cases UK, 2000. to 2015. Official Statistic [cited 2016 Nov 3]. https://www.gov.uk/government/statistics/reports-of-cases-of-tuberculosis-to-enhanced-tuberculosis-surveillance-systems-uk-2000-to-2014
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technical Appendix. Mycobacterium bovis questionnaire for England, Wales, and Northern Ireland.


