Abstract
We tested 3 ild Asian elephants (Elephas maximus) in southern India and confirmed infection in 3 animals with Mycobacterium tuberculosis, an obligate human pathogen, by PCR and genetic sequencing. Our results indicate that tuberculosis may be spilling over from humans (reverse zoonosis) and emerging in wild elephants.
Keywords: tuberculosis, Mycobacterium tuberculosis, elephant, Elephas maximus, India, zoonoses, tuberculosis and other mycobacteria, PCR, genetic sequencing, bacteria
Infection with Mycobacterium tuberculosis in domestic and wild animals of various species living in close contact with humans has been reported (1). Elephants in captivity are known to be susceptible to infection with M. tuberculosis, and there is a potential for transmission of M. tuberculosis between humans and elephants (2–4). In 2013, a case of tuberculosis (TB) in a wild elephant in Africa, which had been under human care, was reported (5), after which another case in a wild Asian elephant in Sri Lanka was reported (6). Habitat encroachment and competition for resources brings wild elephants into closer contact with humans, providing opportunities for zoonoses and reverse zoonoses to occur and for a previously unknown pathogen to emerge in captive free-ranging and wild elephant populations.
The Study
In March 2007, an emaciated wild bull elephant, estimated to be 20 years of age, died shortly after it was found recumbent in the Muthanga range of the Wayanad Wildlife Sanctuary in southern India (case 1). Postmortem examination revealed purulent exudates throughout the lungs, an enlarged liver, enlarged mesenteric lymph nodes, surface nodules containing caseated yellowish-white material (Figure 1). We found serosanguinous fluid in the pericardial sac and slightly hypertrophied heart ventricles. We saw focal areas of necrosis in the renal cortices but noted no other gross lesions. Ziehl-Neelsen staining of lung, liver, kidney, and mesenteric lymph node impression smears revealed numerous acid-fast bacilli. We confirmed the presence of M. tuberculosis by using PCR amplification of the targeted bacterial genome, gel documentation of the amplified products, and sequencing.
Figure 1.
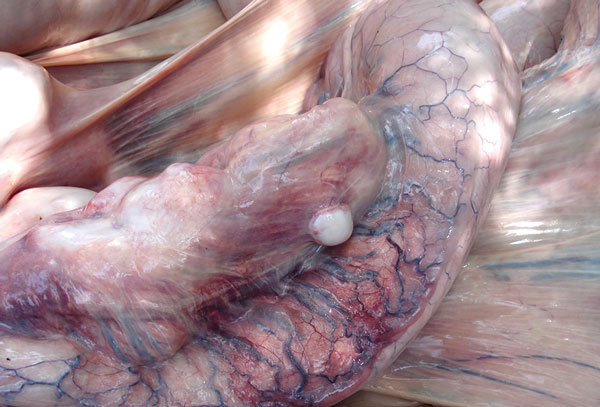
Intestine from a wild bull elephant, estimated at 20 years of age, Wayanad Wildlife Sanctuary, India, 2007. Multiple white-to-tan discrete nodules (granulomas) are protruding from the serosal surface, and less well-defined areas of pale discoloration are visible within the intestinal wall. Serosal blood vessels are markedly dilated, tortuous, and congested.
Subsequently, a surveillance program was initiated (until March 2014), and all fresh elephant carcasses in the study area were examined for evidence of TB (n = 88). In May 2010, a bull elephant, ≈30 years of age, was found dead in the Kurichiyat range (case 2). Postmortem examination revealed extensive caseated lesions in the lungs (Figure 2) and mild mesenteric lymph node hypertrophy. In May 2013, TB infection was diagnosed in a bull ≈40 years of age that was found in the same forest range and had extensive caseated lung lesions (case 3). Both bulls were emaciated.
Figure 2.
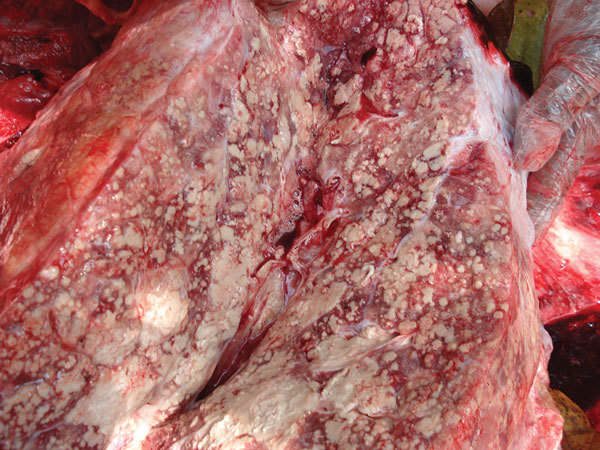
Lung from a bull elephant, estimated at 30 years of age, Kurichiyat Range, India, 2010. Note the multifocal to coalescing pale tan-to-white firm nodules (granulomas) effacing much of the lung parenchyma. Some areas of white chalky mineralization are also present.
We fixed samples for histopathological studies in 10% formol saline and embedded them in paraffin. We found numerous acid-fast organisms in lung impression smears and tissue sections. Granulomatous lesions encapsulated by connective tissue with aggregated macrophages and central areas of necrosis were seen during histopathologic examination of the lungs for all 3 cases and of the kidney and liver in case 1. Langerhans-type giant cells were observed in cases 2 and 3 but not in case 1.
Tissues for molecular studies were collected in absolute alcohol. We extracted total DNA from tissues by using DNeasy Blood & Tissue Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s protocol. DNA was subjected to a tetraplex PCR to differentiate between M. tuberculosis complex and nontuberculous mycobacteria. DNA was subjected to amplification and sequencing of the 3 target regions separately, 16S–23S internal transcribed spacer region, hsp65, and rpoB separately (7). M. tuberculosis H37Rv and M. bovis bacilli Calmette-Guérin genomic DNA was used as control DNA for the PCR studies.
We observed the expected 4-band pattern after tetraplex PCR. As the MTP40 fragment was amplified, M. bovis was ruled out because the plcA gene (mtp40), one of the members of the plc family of genes that code for the phospholipase C enzyme, is deleted in the M. bovis and M. bovis bacilli Calmette-Guérin RD5 region (8). Sequences that were generated were assembled and edited by using the alignment software Seqscape (http://www.seqscape.software.informer.com). BLAST (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi) analysis of the edited sequences revealed that the elephant sequences showed 100% similarity with the M. tuberculosis genome fragment. We also used DNA for large sequence polymorphism analysis to determine the lineage of M. tuberculosis using RD239 and RD750 primers (9,10). The genomic deletion analysis revealed a deletion in RD239, which is characteristic of the Indo-Oceanic lineage (10), also referred to as the East African–Indian lineage (11).
Conclusions
There are reports of mycobacterial infections in captive elephants in India from as early as 1925 (12). We report M. tuberculosis infection in wild elephants in India. In this study, 3 (3.4%) of 88 elephants undergoing postmortem examination were confirmed to be infected with M. tuberculosis. All 3 animals were emaciated, and we considered TB to be the cause of death.
The close interaction between humans and captive elephants is presumed to be a key risk factor for the interspecies transmission of TB. The epidemiology of TB among wild elephants, now documented in 3 countries, has yet to be elucidated. In our study, there were no known captive elephant releases or reintroductions into the study area, and the interaction between captive and wild elephants is considered negligible. However, native tribes do live within the park; many tribal members are employed by the forest department for protection and ecotourism activities. Tourists may visit specified areas only under supervision; there are no overnight facilities. Human–elephant conflict is a problem; most conflicts are caused by resident bulls. All 3 TB cases reported here were in bulls. Exposure of bulls to humans infected with TB during conflict activities is a possible explanation.
More than 3,000 native cattle reside within the sanctuary, cared for by the Animal Husbandry Department, Kerala State. No cases of TB among cattle have been reported. Cattle would be more likely to be infected with M. bovis than with M. tuberculosis, but comprehensive testing would be informative. Cattle living in close proximity to TB-infected humans can become infected with M. tuberculosis (13). Whether such infected cattle could then transmit M. tuberculosis to elephants through contamination of shared grazing lands is yet another research question.
The M. tuberculosis complex is thought to have emerged as a human pathogen in Africa rather than arising from an animal source (14). Although the epidemiology has not been defined, our study and previous reports indicate that M. tuberculosis appears to be spilling over into elephants (reverse zoonosis) and emerging among wild elephant populations. Although these cases may have resulted from individual introductions, if M. tuberculosis becomes established, wild elephants and other susceptible species will be at risk.
Ecologic, environmental, or demographic factors that place animals or humans at increased contact can contribute to disease emergence. Certainly, the increased human–elephant conflict in India and other Asian elephant range countries attests to the narrowing interface between humans and elephants. This study suggests that M. tuberculosis is emerging in the largest single population of Asian elephants in India. Continued surveillance in India and other Asian elephant range countries is warranted.
Acknowledgments
We acknowledge permission from Kerala State Forest Department for conducting postmortem examinations in elephants in the Wayanad Wildlife Sanctuary.
This work was funded through a grant from US Fish and Wildlife Services, Asian Elephant Conservation Fund grant no. ASE 0491, and partial funding from the Scigenom Research Foundation.
The work was done at the Centre for Wildlife Studies, Kerala Veterinary and Animal Sciences University.
Biography
Dr. Zachariah is the assistant forest veterinary officer in the Department of Forests and Wildlife, Government of Kerala, India. His research interests are wildlife disease ecology, host–pathogen coevolution, and disease spillover among wildlife, humans, and domestic livestock.
Footnotes
Suggested citation for this article: Zachariah A, Pandiyan J, Madhavilatha GK, Mundayoor S, Chandramohan B, Sajesh PK, et al. Mycobacterium tuberculosis in wild Asian elephants, southern India. Emerg Infect Dis. 2017 Mar [date cited]. http://dx.doi.org/10.3201/eid2303.161741
References
- 1.Montali RJ, Mikota SK, Cheng LI. Mycobacterium tuberculosis in zoo and wildlife species. Rev Sci Tech. 2001;20:291–303. 10.20506/rst.20.1.1268 [DOI] [PubMed] [Google Scholar]
- 2.Michalak K, Austin C, Diesel S, Bacon MJ, Zimmerman P, Maslow JN. Mycobacterium tuberculosis infection as a zoonotic disease: transmission between humans and elephants. Emerg Infect Dis. 1998;4:283–7. 10.3201/eid0402.980217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikota SK, Maslow JN. Tuberculosis at the human-animal interface: an emerging disease of elephants. Tuberculosis (Edinb). 2011;91:208–11. 10.1016/j.tube.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 4.Murphree R, Warkentin JV, Dunn JR, Schaffner W, Jones TF. Elephant-to-human transmission of tuberculosis, 2009. Emerg Infect Dis. 2011;17:366–71. 10.3201/eid1703.101668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obanda V, Poghon J, Yongo M, Mulei I, Ngotho M, Waititu K, et al. First reported case of fatal tuberculosis in a wild African elephant with past human-wildlife contact. Epidemiol Infect. 2013;141:1476–80. 10.1017/S0950268813000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perera BVP, Salgadu MA, Gunawardena GSPdeS, Smith NH, Jinadasa HRN. First confirmed case of fatal tuberculosis in a wild Sri Lankan elephant. Gajah. 2013;41:28–31. [Google Scholar]
- 7.Anilkumar AK, Madhavilatha GK, Paul LK, Radhakrishnan I, Kumar RA, Mundayoor S. Standardization and evaluation of a tetraplex polymerase chain reaction to detect and differentiate Mycobacterium tuberculosis complex and nontuberculous Mycobacteria—a retrospective study on pulmonary TB patients. Diagn Microbiol Infect Dis. 2012;72:239–47. 10.1016/j.diagmicrobio.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 8.Gordon SV, Brosch R, Billault A, Garnier T, Eiglmeier K, Cole ST. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol Microbiol. 1999;32:643–55. 10.1046/j.1365-2958.1999.01383.x [DOI] [PubMed] [Google Scholar]
- 9.Tsolaki AG, Hirsh AE, DeRiemer K, Enciso JA, Wong MZ, Hannan M, et al. Functional and evolutionary genomics of Mycobacterium tuberculosis: insights from genomic deletions in 100 strains. Proc Natl Acad Sci U S A. 2004;101:4865–70. 10.1073/pnas.0305634101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, et al. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2006;103:2869–73. 10.1073/pnas.0511240103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brudey K, Driscoll JR, Rigouts L, Prodinger WM, Gori A, Al-Hajoj SA, et al. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 2006;6:23. 10.1186/1471-2180-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narayanan RS. A case of tuberculosis in an elephant. J Comp Pathol. 1925;38:96–7. 10.1016/S0368-1742(25)80016-X [DOI] [Google Scholar]
- 13.Ocepek M, Pate M, Zolnir-Dovc M, Poljak M. Transmission of Mycobacterium tuberculosis from human to cattle. J Clin Microbiol. 2005;43:3555–7. 10.1128/JCM.43.7.3555-3557.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. 2013;45:1176–82. 10.1038/ng.2744 [DOI] [PMC free article] [PubMed] [Google Scholar]


