Summary
Alumina- and zirconia-based ceramic dental restorations are designed to repair functionality as well as esthetics of the failed teeth. However, these materials exhibited several performance deficiencies such as fracture, poor esthetic properties of ceramic cores (particularly zirconia cores), and difficulty in accomplishing a strong ceramic–resin-based cement bond. Therefore, improving the mechanical properties of these ceramic materials is of great interest in a wide range of disciplines. Consequently, spatial gradients in surface composition and structure can improve the mechanical integrity of ceramic dental restorations. Thus, this article reviews the current status of the functionally graded dental prostheses inspired by the dentino-enamel junction (DEJ) structures and the linear gradation in Young's modulus of the DEJ, as a new material design approach, to improve the performance compared to traditional dental prostheses. This is a remarkable example of nature's ability to engineer functionally graded dental prostheses. The current article opens a new avenue for recent researches aimed at the further development of new ceramic dental restorations for improving their clinical durability.
Keywords: Alumina, Zirconia, Dental ceramics, Dentino-enamel junction, Dental multilayer, Functionally graded materials
1. Introduction
Teeth play a critically important role in our lives. Loss of function diminishes our capability to eat a stable diet, which has undesirable consequences for general health. Loss of esthetics can negatively influence social function. Both function and esthetics can be reconstructed with dental prostheses.
Material selection for dental prostheses has turned out to be a sizable field for researchers. Ceramics are frequently used in load-bearing biomedical applications due to their excellent biocompatibility, wear resistance and esthetics [1], [2], [3]. Ceramics are utilized as total hip and knee replacements [4], [5], [6], [7], [8] and adopted for dental restorations [9], [10], [11]. Ceramic dental restorations are designed to repair functionality as well as esthetics of the failed teeth. However these materials showed somewhat poor flexural strength, particularly when exposed to fatigue loading in wet environments [1], [2], [3]. Subsequently, it can cause extensively discomfort to patients and can reduce the durability for ceramic prostheses due to their flexural fracture [12], [13], [14], [15].
The failures of dental restorative systems are due to incorrect selection of materials, incorrect design of the component, the incorrect processing of materials, and presence of defects (e.g. cracks and pores) in the prostheses [16], [17], [18], [19]. Additionally, in metal–ceramic restorations there are mismatches in the mechanical properties between the veneering porcelain and metal core. The Young's modulus of the veneering porcelain is 60–80 GPa, while that of the metal core is in the range of 80–230 GPa [20]. Furthermore, there are mismatches in the thermal properties between the veneering porcelain and metal core, where coefficient thermal expansion for metal core is usually higher than veneering porcelain. The significant mismatch between both material properties concentrate stresses at the interfaces that may cause cracks at the metal–ceramic interface and consequently to the failure of the restoration [21], [22]. Lastly, metal core is more susceptible to corrosion in which its effect ranges from degradation of appearance to loss of mechanical strength [23], [24]. The corrosion products can produce a bluish-gray pigmentation of gingiva and oral mucosa. Furthermore, these products, particularly in immunologically susceptible individuals, can cause local and systemic hypersensitivities [25], [26], [27], [28].
Despite a continuous improvement in the dental prostheses such as using a strong zirconia or alumina core to support the esthetic porcelain veneer, ceramic prostheses are still vulnerable to failure at a rate of approximately 1–3% each year [9]. Additionally, ceramics prostheses have a dense, high purity crystalline structure at the cementation surface that cannot be adhesively bonded to tooth dentin support [29], [30]. Even though some authors recommended particle abrasion for surface roughening treatment to enhance the ceramic-resin-based cements bond using mechanical retention, particle abrasion also introduces surface flaws or microcracks that can cause deterioration in the long-term flexural strength of ceramic prostheses [31], [32], [33], [34], [35], [36], [37]. Further, zirconia cores have a white opaque appearance which needs a thick porcelain veneer with gradual change in translucency to mask the zirconia and to achieve a better esthetic outcome [38]. Further, the dental crowns generate over $2 billion in revenues each year, with 20% of crowns being all ceramic units. Also, aging populations will drive the demand for all types of dental restorations even higher [39]. Moreover, occlusal contact induces the deformation and cracking of dental crowns, which can lead to the failure of the structure [40]. Therefore, it is highly desirable to develop ceramic prostheses that are more resistant to cracking under occlusal contact in recent decade [17], [18].
Composite ceramics have been designed in an effort to improve strength and toughness while expanding functionality. Simple laminate materials have been developed for many years, in which a number of materials with different properties are bonded into a layered structure [41]. Though these composites do combine varying properties, the abrupt interfaces between the two materials often hold residual stresses [42], [43] and perhaps delaminate under load [44].
Recently, bioinspired functionally graded enamel structures in the design of dental multi-layers have been proposed, as alternative technique, aiming the enhancement of the overall performance of metal–ceramic and all-ceramic dental restorative systems. This technique allows the production of a material with very different characteristics within the same material at various interfaces. Bioinspired functionally graded approach is an innovative material technology, which has rapidly progressed both in terms of materials processing and computational modeling in recent years. Bioinspired functionally graded structure allows the integration of dissimilar materials without formation of severe internal stress and combines diverse properties into a single material system [45], [46], [47]. This innovative technology has been applied in medical and dental fields [48], [49], [50], [51], [52], [53], [54], [55], [56].
The graded structure eliminates the sharp interface resulting from traditional core-veneer fabrication, eliminating the potential for delamination between the layers [57]. Graded transitions can also reduce stress concentrations at the intersection between an interface and a free surface [58], [59]. Similarly, the local driving force for crack growth across an interface can be increased or reduced by altering the gradients in elastic and plastic properties across the interface [60], [61].
The bioinspired functionally graded structure can be seen as the precursor to recent studies. Thus, this article reviews the current status of the functionally graded dental prostheses inspired by the dentino-enamel junction (DEJ) structures and the linear gradation in Young's modulus of the DEJ.
2. Natural human enamel
2.1. Microstructure and function of enamel
A human tooth consists of pulp, enamel and dentin. The natural tooth has superior overall properties to artificial crowns [47]. Therefore, the knowledge of the structure of the human tooth is very important for the design of artificial dental crowns.
Human enamel contains on average 95% inorganic substance, 4% water and 1% organic substance by weight or 87% inorganic, 11% water and 2% organic component by volume [63]. Hydroxyapatite substituted with carbonate and hydrogen phosphate ions are the largest mineral constituent, 90–92% by volume. The remaining constituent is organic substance matter and water.
Both enamel protein [64] and water [65] are more abundant in inner enamel close to the dentino-enamel junction. Water in permanent enamel is in the form of free and bound water [66]. Free water refers to those components located in small spaces of enamel, while bound water means those combined with peptide chains or crystal lattices. A study with hydroxyapatite suggested that some of the water in enamel will be more firmly bound to the mineral [67]. Although it is only a minor part of enamel, water plays an important role in enamel's function, because dehydration changes the mechanical properties of enamel significantly [68]. Water forms hydrogen-bond bridges across adjacent peptide chains and maintains functional conformation of protein remnants and collagen fibers in mature enamel [69]. Fox [70] proposed that the water fluid is essential in explaining load-bearing behavior of enamel as, for instance, the “stiff sponge” model, in which enamel was considered as a stiff sponge from which liquid was expelled in compression and drawn in again when the load is released.
The most organic substances are protein content, which changes dramatically during normal development ranging from about 20% protein by weight during the secretory stage to 7% at the beginning of the maturation stages. Ultimately, the ameloblasts remove almost the entire original matrix as mineralization progresses. As a result, fully developed normal human enamel contains only ∼1% protein by weight, which is the remnant component of the development matrix proteins [71]. The organic matrix in mature enamel is a multi-component protein/peptide mix, which is lying between crystallites clearly and has the function of gluing hydroxyapatite crystallites together, thereby maintaining the hierarchical structure of enamel.
Human enamel consists of ∼5 μm diameter rods encapsulated by ∼1 μm thick protein rich sheathes that are arranged parallel in a direction perpendicular to the dentino-enamel junction from dentin to the outer enamel surface. Crystallite plates in the central part of the rod are parallel to the rod axis while those near the edge of the rod usually have an angle near 15–45° to the longitudinal axis of the rods [72]. The rod unit is the most important level in understanding the microstructure and function of enamel.
2.2. Mechanical behavior of natural enamel
As the outer cover of teeth, enamel must retain its shape as well as resist fracture and wear during load-bearing function for the life of the individual. Understanding fracture properties and crack propagation procedure of enamel is important for both clinicians and material scientists.
Anisotropic microstructure of the enamel, such as rod orientation, and organic components, controlled the anti-fracture ability of enamel. The dominant rods are primarily oriented so as to approach the outer tooth surface in an approximately perpendicular orientation. This is in order to increase hardness and reduce wear. Interconnections between rod and interrod, and complex cleavage planes limit critical crack size and uncontrolled crack propagation that would otherwise lead to premature fracture [73]. The amount of anisotropy may not only reflect the balance between wear and fracture resistance, but may also reflect a balance between differing vectors of functional stress as well as the transfer of occlusal loads to the resilient supporting dentin [74]. Connections between adjacent rods via the interrod region and the presence of interrod crystallites oriented in a plane different from the main rod direction have been discerned in cross-sectional and long-sectional scanning electron micrographs [75], [76]. The variation of crystal directions is the result of bio-fabrication process during the maturation of enamel, which is essential in shielding the cracks. Rasmussen et al. [77] illustrated that fracture in enamel is anisotropic with respect to the orientation of the enamel rods, with the work of fracture for fracture parallel to the rods being 13 J/m2 but of the order of 200 J/m2 for fracture perpendicular to the rods; fractographs of enamel showed that the enamel rods behaved as integral units during controlled fracture. Xu et al. [78] illustrated that the cracks in the enamel axial section were significantly longer in the direction perpendicular to the occlusal surface than parallel. The cracks propagating toward the dentino-enamel junction were always arrested and unable to penetrate dentin. The fracture toughness of enamel was not single-valued but varied by a factor of three as a function of enamel rod orientation. White et al. [76] found that enamel was approximately three times tougher than geologic hydroxyapatite demonstrating the critical importance of biological manufacturing. What is more, they suggested that enamel is a composite ceramic with the crystallites oriented in a complex three-dimensional continuum. Zhou and Hsiung [79] found that enamel demonstrated better resistance to penetration. They indicated that the minor organic matrix does regulate the mechanical behavior of enamel significantly.
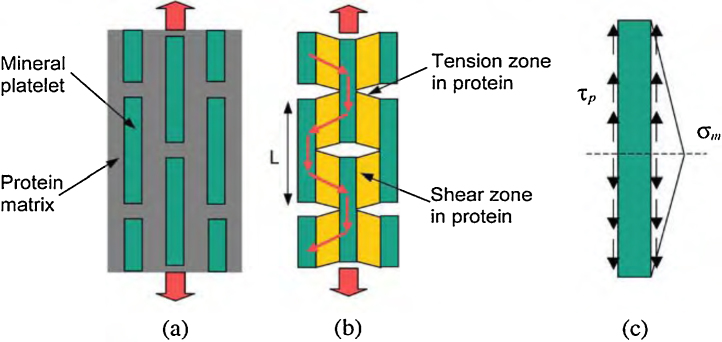
Although most of the enamel organic matrix is removed during mineralization and maturation, some protein, notably ameloblastin, is retained, primarily at the incisal edges and proximal sides of rod boundaries defining a rod sheath [75]. This prevents cracks from advancing straight through enamel to cause catastrophic macro-mechanical failure, but instead spreads the damage laterally and hence energy absorbed over a larger volume. Also, the presence of minute quantities of protein remnants could allow limited differential movement between adjacent rods. Limited slippage could reduce stresses without crack growth. The minor components of enamel, protein remnants and water, have a profound plasticizing effect. As mentioned previously, the protein matrix behaves like a soft wrap around the mineral platelets and protects them from the peak stresses caused by the external load and homogenizes stress distribution within the composite structure. At the most elementary structure level, natural biocomposites exhibit a generic microstructure consisting of staggered mineral bricks. It was proposed that under an applied tensile stress, the mineral platelets carry the tensile load while the protein matrix transfers the load between mineral crystals via shear [80]. The strength of the protein phase in a biological material is amplified by the large aspect ratio of mineral platelets. Besides, the larger volume concentration of protein significantly reduces impact damage to the protein–mineral interface (Fig. 1).
Figure 1.
Models of biocomposites. (a) Perfectly staggered mineral inclusions embedded in protein matrix. (b) A tension–shear chain model of biocomposites in which the tensile regions of protein are eliminated to emphasize the load transfer within the composite structure. (c) The free body diagram of a mineral crystal.
From Ji and Gao [80].
By comparison with dense hydroxyapatite material, White et al. [76] found that enamel was approximately three times tougher than geologic hydroxyapatite, which only demonstrates the critical importance of biological manufacturing. The inorganic substances have been reported to vary from the outer enamel surface to dentino-enamel junction. Many investigators reported that the mineral content [64], [81] and the density [66] were decreased toward the dentino-enamel junction. Some studies on the mechanical properties of human enamel are presented in Table 1.
Table 1.
Some studies on the mechanical properties of the human dental enamel.
| Author(s) | Surface and site | Hardness (GPa) | Elastic modulus (GPa) |
|---|---|---|---|
| Stanford et al. [82] | Variable (cusp) | – | 47.5 |
| Cross section (side) | 30.3 | ||
| Top surface (occlusal) | 8.96 | ||
| Stanford et al. [83] | Canine: | – | |
| Variable (cusp) | 47.5 ± 5.5 | ||
| Cross section (side) | 33 ± 2.1 | ||
| Variable (cusp) | 20 ± 6.2 | ||
| Molar: | |||
| Variable (cusp) | 46.2 ± 4.8 | ||
| Cross section (side) | 32.4 ± 4.1 | ||
| Top surface (side) | 9.65 ± 3.45 | ||
| Craig et al. [84] | Top surface | – | 84.1 ± 6.2 |
| Cross section | 77.9 ± 54.8 | ||
| Tyldesley [85] | – | – | 131 ± 16 |
| Reich et al. [86] | Top surface | – | 76.5 |
| Staines et al. [87] | Top surface | – | 83 ± 8 |
| Xu et al. [78] | Top surface | 3.23 ± 0.38 | – |
| Cross section | 3.03 ± 0.09 | ||
| Cuy et al. [88] | Cross section: | 2.7–6.4 | 47–120 |
| Outer enamel | >6 | >115 | |
| EDJ | <3 | <70 | |
| Zhou et al. [89] | Top surface | 5.7–3.6 | 104–70 |
| Ge et al. [90] | Top surface: | 4.3 ± 0.8 | 83.4 ± 7.1 |
| Rod | 1.1 ± 0.3 | 39.5 ± 4.1 | |
| Interrod | |||
| Mahoney et al. [91] | Cross section (primary molar) | 4.9 ± 0.4 | 80.4 ± 7.7 |
| Marshall et al. [92] | Cross section (EDJ area) | 3.51 ± 0.13 | 63.55 ± 1.46 |
| Fong et al. [93] | Top surface | 4.78 ± 0.36 | 98.3 ± 5.9 |
| Cross section | 4.53 ± 0.26 | 95.6 ± 4.9 | |
| Habelitz et al. [94] | Top surface | 3.8 ± 0.31 | 87.5 ± 2.1 |
| Cross section | 3.3 ± 0.35 | 72.7 ± 4.4 | |
| Head of rod | 4.3 ± 0.4 | 88.0 ± 8.6 | |
| Tail of rod | 3.7 ± 0.4 | 80.3 ± 7.2 | |
| Interrod | 3.9 ± 0.4 | 86.4 ± 11.7 | |
| Habelitz et al. [95] | Cross section | 3.2 ± 0.4 | 74 ± 4 |
| 3.7 ± 0.5 | 80 ± 9.1 | ||
| Barbour et al. [96] | Top surface | 4.81 ± 0.15 | 99.6 ± 1.8 |
| 4.77 ± 0.13 | 101.9 ± 1.6 | ||
| 4.75 ± 0.12 | 105.2 ± 1.3 | ||
3. Microstructure and behavior of dentino-enamel junction
Natural teeth are composed by layered structures, dentin and enamel, that are bonded by a functionally graded dentino-enamel junction (DEJ) layer [97], [98], [99]. Marshall et al. [92] stated the DEJ acts as a bridge between the hard brittle enamel (E ∼ 70 GPa) and the softer durable dentin layer (E ∼ 20 GPa), allowing a smooth Young's modulus transition between the two structures (Fig. 2). Huang et al. [51] studied the microstructure of the DEJ and they reported that collagen fibrils from the dentin gather into coarse bundles and penetrate across the junction, anchoring into the enamel. The hydroxyapatite is continuous across the junction. The interface is not smooth, but instead is a series of linked semi-circles, or scallops, that increase contact area, and thus the adhesion when DEJ serves as the bonding between dentin and enamel. It also resists cracks that originate in enamel from penetrating into the dentin. Lin and Douglas [99] noticed that there was an extensive plastic deformation, 8%, collateral to the fracture process in the DEJ. Correspondingly, microscopic analysis revealed clear evidence of crack-tip blunting and crack deflection. The parallel-oriented coarse collagen bundles at the DEJ may play a significant role in resisting the crack. Likewise, White et al. [100] investigated the DEJ failure mechanisms by performing micro-indentation tests across the DEJ. Their results exhibited that DEJ does not undergo catastrophic interfacial delamination and the damage was distributed over a broad zone instead.
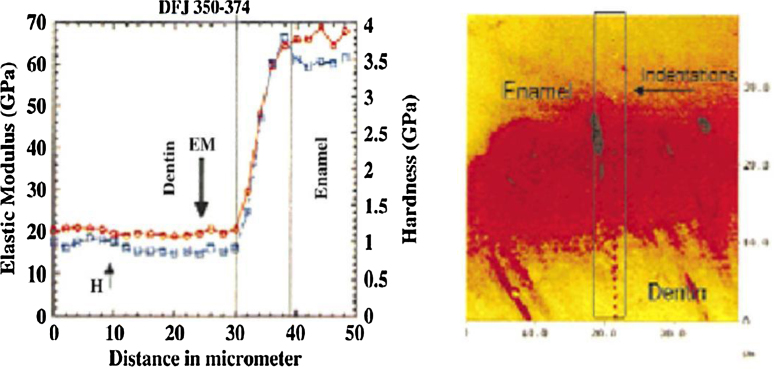
Figure 2.
Elastic modulus distribution in natural dentino-enamel junction.
Marshall et al. [92] and Fong et al. [93] used nanoindentation tests to measure the Young's modulus of the natural DEJ area. Their results showed that, within the DEJ region, the Young's modulus varies from ∼70 GPa for enamel to ∼20 GPa for dentin. The fracture results [85] once again demonstrated that it is extremely difficult to initiate cracks in dentin at the DEJ, or to propagate cracks from enamel to dentin across the DEJ. Featherstone et al. [102] and Meredith et al. [103] reported that hardness and modulus of elasticity were the highest at the outer surface of the enamel and decreases toward the DEJ. He and Swain [104] reported that inner enamel has lower stiffness and hardness but higher creep and stress redistribution abilities than their outer counterpart. They attributed this observation to the gradual compositional change throughout the enamel from the outer region near the occlusal surface to the inner region near EDJ. The gradients in the elastic modulus of tooth have been attributed to the distribution of the mineral phase, while different toughening mechanisms in the natural tooth have been attributed to collagen microstructure and water content. They suggested that enamel can be regarded as a functionally graded natural biocomposite. The natural tooth is a remarkable example of nature's ability to design a complex and functional composite.
In order to replace the mechanical function of tooth from a restorative perspective, it is not only important to study its localized tissue properties but also its bulk structural behavior. Nonetheless, more research is necessary to comprehend the mechanisms by which tooth structures resist functional forces in the mouth. Thus, the mechanical properties and microstructural features of dental enamel are important to understanding stress dissipation in the tooth, for developing biomimetic restorative materials and for the execution of clinical dental preparations.
4. Bioinspired functionally graded approach
Learning from nature, materials scientists increasingly aim to engineer graded materials that are more damage-resistant than their conventional homogeneous counterparts. This is particularly important at surfaces or at interfaces between dissimilar materials, where contact failure commonly occurs. Therefore, many engineered materials are graded in some manner, but functionally graded materials (FGMs) are often characterized by a gradient purposefully formed using compositional or microstructural design. FGM is a material with engineered gradients of composition, structure and/or specific properties aiming to become superior over homogeneous material composed of same or similar constituents [105], [106], [107], [108]. The aim of producing FGM is to obtain a material with two different characteristics in its two opposite faces, Fig. 3. The properties of this innovative FGM can mimic the natural gradients occur, including a graded elastic modulus in hard tissues such as the human enamel and dentin–enamel junction [109]. This novel technology is designed to improve the performance of the materials [110], [111], [112], [113].
Figure 3.
Morphology of the graded zone. (a) Schematic of graded structure. (b) Section view of graded zone of glass-infiltrated yttria stabilized zirconia.
From Zhang et al. [108].
Although the efficacy of FGMs has been recognized since the early 1970s [114], the field of FGM did not take off until the mid-1980s, probably due to a lack of suitable fabrication methods until that time. This concept has been later expanded for different application such as coatings, packing, optical, biomedical, etc. In the biomedical field, several approaches have been used to develop functionally graded biomaterials for implants [115], [116], [117], [118], [119], [120].
With established methods currently available to synthesize and process materials, gradations in composition, structure, and properties could be engineered over a wide range of length scales ranging from nanometers to meters. There are a wide range of process technologies that are now available for fabrication FGMs such as powder metallurgical process [121], layer stacking [56], glass infiltration [122], centrifugation [123], electrophoretic deposition [124], plasma spray [125], direct-write assembly [126] and rapid prototype color ink-jet printing [127].
5. Dental prostheses mimic the dentino-enamel junction behavior
Among the previously mentioned processing methods, the glass infiltration technology is particularly suitable for the fabrication of all-ceramic restorations [128]. It combines an esthetic, low modulus, and low hardness glass “veneer” with a high strength ceramic “core”, without a sharp interface between the materials (Fig. 4). The lack of interface due to grading improves interfacial bond strengths, reduces residual stresses, and eliminates delaminations. The processing of these structures is simple and straightforward, and can be readily adapted to CAD/CAM technology [128], [130], [131].
Figure 4.
Schematic of the conventional sharp restoration and the new graded approach.
From Henriques [129].
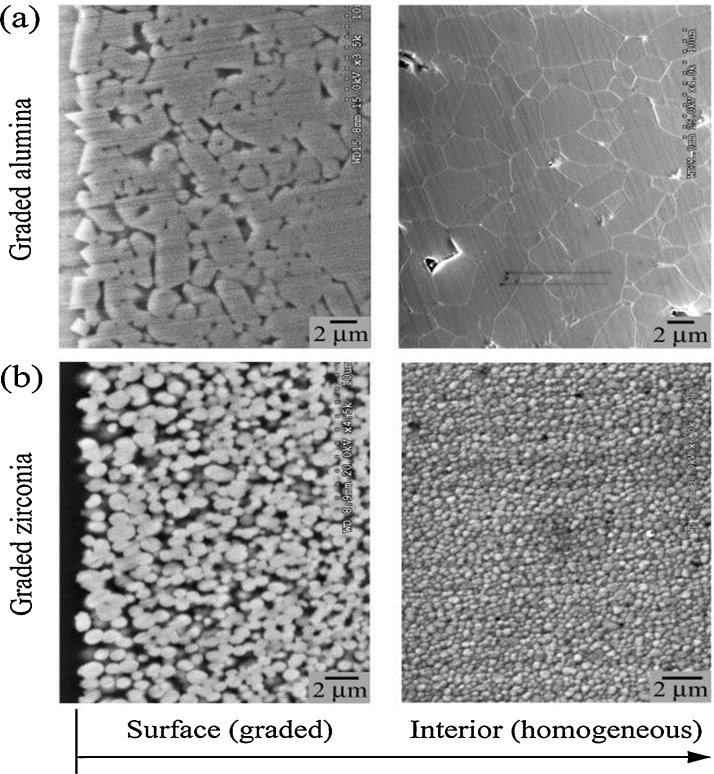
5.1. Graded glass-zirconia structures
Glass-zirconia structures with gradual elastic modulus may be created by using infiltration method [128]. Comparing to the sintering temperature of zirconia, zirconia templates with somewhat low heat-treatment temperature are used for combining glass infiltration and zirconia densification into a single process [128], [132]. This way the glass infiltration depth can be tailored by manipulating the porosity of the zirconia templates. Therefore, the grain growth and/or destabilizing of the tetragonal zirconia phase [133] associated with the post-sintering heat-treatment can be prevented. As coefficient of thermal expansion and Poisson's ratio of the infiltrating glass and zirconia (3Y-TZP) are relatively the same, no significant long-range thermal stresses are developed in the graded structure [134]. The resultant structure consists of a thin, outer surface residual glass layer followed by a graded glass-zirconia layer at both the top and bottom surfaces (Fig. 5).
Figure 5.
Cross-sectional view of a graded glass-alumina in (a) and graded glass-zirconia structure (b), respectively.
From Zhang et al. [137].
5.2. Graded glass-alumina structures
Glass-alumina graded structures may be produced by infiltrating dense alumina surfaces with silica-based glasses [130], [134], [135]. Following a power-law relationship, the transition of elastic modulus from the graded glass-alumina surface to the alumina core is continuous [136], [137]. The resultant structure consists of a thin, outer surface residual glass layer followed by a graded glass-alumina layer, sandwiching a dense alumina core (Fig. 5).
Inspired by the microstructure and mechanical properties of natural teeth, synthetic functionally graded materials were proposed to mimic the DEJ. Francis et al. [62] described a procedure to produce a DEJ-like interface and enamel coating involved depositing slurries of oxide or glass powder by a draw-down blade method, drying at then higher temperature heating. They used alumina-glass or alumina-polymer composite to mimic the dentin and a calcium phosphate-based coating to mimic the enamel. Bonding between the two materials was accomplished by a eutectic melt in the CaO–Al2O3–SiO2 system. The interpenetration in this DEJ-like interface originates from a solidified melt phase penetrating into the dentin. Huang et al. [51] added bioinspired FGM layer between the dental ceramic and the dental cement and investigated the effects of the functionally graded layer on the stress in the crown and its surrounding structures. From their results, the functionally graded layer was shown to promote significant stress reduction and improvements in the critical crack size. From their study, they concluded that the low stress concentrations were associated with the graded distributions in the DEJ. This provided new insights into the design of functionally graded crown architecture that can increase the durability of future dental restorations. Rahbar and Soboyejo [54] used computational and experimental effort to develop crack-resistant multilayered crowns that are inspired by the functionally graded DEJ structure. The computed stress distributions showed that the highest stress was concentrated at the ceramic outer layer of crown and reduced significantly toward the DEJ when bioinspired functionally graded architecture was used. They reported that the bioinspired functionally graded layers were also shown to promote improvements in the critical crack size. Suresh [122] established that controlled gradients in mechanical properties offer unprecedented opportunities for the design of surfaces with resistance to contact deformation and damage that cannot be realized in conventional homogeneous materials.
Graded dental restorations have been shown to display improved features relative to conventional ones, namely higher resistance to contact and sliding [122], [138], [139]; higher adhesion of porcelain to the substructure (metal or ceramic) [140], [141], [142]; improved esthetical properties and improved behavior under fatigue conditions [142]. Another important point to which the FGM design can address is the reduction of thermal residual stresses that remains at the metal–ceramic interface during the cooling cycles after the porcelain firing. These stresses are further magnified when there is a significant difference between the thermal expansion behavior of the metal and the porcelain. Depending on the thermal residual stress level that remains in the crown and together with those arising from occlusal loads, a catastrophic failure of the restoration can occur. FGMs have been shown to decrease significantly the thermal residual stresses formed at the interface between metals and ceramics in other fields of applications [143]. Some studies demonstrated that when the contact surface of alumina or silicon nitride was infiltrated with aluminosilicate or oxynitride glass, respectively, they noticed that the graded glass/ceramic surfaces produced in this manner offered much better resistance to contact damage with and without a sliding action than either constituent ceramic or glass [136], [144], [145].
A number of the studies investigated the effects of increasing elasticity as a function of depth from the surface on the resistance to contact damage. They demonstrated that veneer failure and bulk fracture may be substantially mitigated by controlled gradients of elastic modulus within the restoration layer. Such graded structures exhibit significantly higher resistance to fatigue sliding-contact and flexural damage relative to veneered and monolithic core ceramics. This is because the gradient diminishes the intensity of tensile stresses and simultaneously transfers these stresses from the layer surface into the interior, away from the source of failure-inducing surface flaws [128], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141].
6. Clinical implications
In clinical applications, these graded alumina materials can be used as monolithic crowns and bridges. Although the graded alumina has limited translucency, the external glass layer and the graded glass-alumina layer provide necessary shade options. In addition, color stains can be applied to the surface of the external glass layer using powdered glass slurry that has similar composition to the infiltrated glass. This staining technique has been used on the Empress system to improve the esthetic outcome of a single color pressed block of glass ceramic and is well established in esthetic dentistry [146], [147], [148]. Also, the cementation surface of graded restorations can be etched with hydrofluoric acid and silanized to facilitate a resin–cement bond.
Use of zirconia in crowns and bridges has increased over recent years, owing to esthetic and biocompatibility demands. However, the fact remains that porcelain-veneered zirconia restorations suffer unexpectedly high chipping rates, regardless of the manufacturer [149], [150], [151], [152], [153]. Additionally, dental crowns generate over $2 billion in revenues each year, with 20% of crowns being all ceramic units [39]. Also, aging populations will drive the demand for all types of dental restorations even higher [34]. If these chipping rates could be reduced, zirconia-based all-ceramic prostheses would become more widely used, addressing a quality of life issue [154]. A great demand for the development of improved dental crowns has been stimulated by the large and ever growing market of the dental crowns [155].
Graded glass-zirconia structures offer a simple remedy. Zirconia cores are, however, only a portion of the all-ceramic restoration. Alternative monolithic graded glass-zirconia restorations are recommended without porcelain veneer, which could be successfully and economically used in posterior applications. These restorations are suggested to eliminate the vulnerable porcelain veneer, while providing superior strength and esthetics. The color characterization of these graded glass-zirconia restorations is achieved by external residual glass and subsequent staining. Therefore, many studies developed a straightforward protocol for fabricating anatomically correct zirconia crowns and bridges with graded surfaces [136], [137], [138], [139], [140], [141], [142], [143], [144]. These findings found that restorations made from graded glass-zirconia are orders of magnitude more resistant to sliding-contact damage than the current porcelain-veneered zirconia systems. The graded layer also enhances the flexural fracture resistance of zirconia, allowing the utilization of thinner restorations for highly conservative restorative protocols that preserve tooth structure. Additionally, the cementation surface of graded restorations can be etched with hydrofluoric acid and silanized to facilitate a resin–cement bond.
7. Conclusions
In order to replace the mechanical function of tooth from a restorative perspective, it is not only important to study its localized tissue properties but also its bulk structural behavior. Therefore, the functionally graded dental prostheses inspired by the DEJ have been reviewed. These prostheses such as “graded glass-zirconia and graded glass-alumina structures” offer better resistance to immediate flexural damage, better esthetics, and potentially better veneering and cementation properties over homogeneous ceramics materials. The further development of the grading technology could potentially lead to superior long-term clinical performance for dental prostheses.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Lawn B.R., Deng Y., Thompson V.P. Use of contact testing in the characterization and design of all-ceramic crown like layer structures: a review. J Prosthet Dent. 2001;86:495–510. doi: 10.1067/mpr.2001.119581. [DOI] [PubMed] [Google Scholar]
- 2.Studart A.R., Filser F., Kocher P., Gauckler L.J. In vitro lifetime of dental ceramics under cyclic loading in water. Biomaterials. 2007;28:2695–2705. doi: 10.1016/j.biomaterials.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 3.Rahaman M.N., Li Y., Bal B.S., Huang W. Functionally graded bioactive glass coating on magnesia partially stabilized zirconia (Mg-PSZ) for enhanced biocompatibility. J Mater Sci Mater Med. 2008;19:2325–2333. doi: 10.1007/s10856-007-3328-7. [DOI] [PubMed] [Google Scholar]
- 4.Akagi M., Nakamura T., Matsusue Y., Ueo T., Nishijyo K., Ohnishi E. The bisurface total knee replacement: a unique design for flexion. Four-to-nine-year follow-up study. J Bone Joint Surg Am. 2000;82A:1626–1633. doi: 10.2106/00004623-200011000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Garino J.P. Modern ceramic-on-ceramic total hip systems in the United States: early results. Clin Orthop Relat Res. 2000:41–47. doi: 10.1097/00003086-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hannouche D., Hamadouche M., Nizard R., Bizot P., Meunier A., Sedel L. Ceramics in total hip replacement. Clin Orthop Relat Res. 2005:62–71. doi: 10.1097/01.blo.0000149996.91974.83. [DOI] [PubMed] [Google Scholar]
- 7.Jazrawi L.M., Kummer F.J., Di Cesare P.E. Hard bearing surfaces in total hip arthroplasty. Am J Orthop. 1998;27:283–292. [PubMed] [Google Scholar]
- 8.Yasuda K., Miyagi N., Kaneda K. Low friction total knee arthroplasty with the alumina ceramic condylar prosthesis. Bull Hosp Jt Dis. 1993;53:15–21. [PubMed] [Google Scholar]
- 9.Burke F.J., Fleming G.J., Nathanson D., Marquis P.M. Are adhesive technologies needed to support ceramics? An assessment of the current evidence. J Adhes Dent. 2002;4:7–22. [PubMed] [Google Scholar]
- 10.Kelly J.R. Dental ceramics: current thinking and trends. Dent Clin North Am. 2004;48:513–530. doi: 10.1016/j.cden.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Kelly J.R., Denry I. Stabilized zirconia as a structural ceramic: an overview. Dent Mater. 2008;24:289–298. doi: 10.1016/j.dental.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Jarrett C.A., Ranawat A.S., Bruzzone M., Blum Y.C., Rodriguez J.A., Ranawat C.S. The squeaking hip: a phenomenon of ceramic-on-ceramic total hip arthroplasty. J Bone Joint Surg Am. 2009;91:1344–1349. doi: 10.2106/JBJS.F.00970. [DOI] [PubMed] [Google Scholar]
- 13.Kelly J.R. Clinically relevant approach to failure testing of all-ceramic restorations. J Prosthet Dent. 1999;81:652–661. doi: 10.1016/s0022-3913(99)70103-4. [DOI] [PubMed] [Google Scholar]
- 14.Lawn B., Bhowmick S., Bush M.B., Qasim T., Rekow E.D., Zhang Y. Failure modes in ceramic-based layer structures: a basis for materials design of dental crowns. J Am Ceram Soc. 2007;90:1671–1683. [Google Scholar]
- 15.Willmann G. Ceramics for total hip replacement – what a surgeon should know. Orthopedics. 1998;21:173–177. doi: 10.3928/0147-7447-19980201-11. [DOI] [PubMed] [Google Scholar]
- 16.Yesil Z.D., Karaoglanoglu S., Akyıl M.S., Seven N. Evaluation of the bond strength of different bonding agents to porcelain and metal alloy. Int J Adhes Adhes. 2009;29:32–35. [Google Scholar]
- 17.Özcan M. Fracture reasons in ceramic-fused-to metal restorations. J Oral Rehabil. 2003;30:265–269. doi: 10.1046/j.1365-2842.2003.01038.x. [DOI] [PubMed] [Google Scholar]
- 18.Anusavice K.J. Standardizing failure, success, and survival decisions in clinical studies of ceramic and metal–ceramic fixed dental prostheses. Dent Mater. 2012;28:102–111. doi: 10.1016/j.dental.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swain M.V. Unstable cracking (chipping) of veneering porcelain on all-ceramic dental crowns and fixed partial dentures. Acta Biomater. 2009;5:1668–1677. doi: 10.1016/j.actbio.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Rizkalla A.S., Jones D.W. Indentation fracture toughness and dynamic elastic moduli for commercial feldspathic dental porcelain materials. Dent Mater. 2004;20:198–206. doi: 10.1016/s0109-5641(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 21.Lawn B.R., Deng Y., Lloyd I.K., Janal M.N., Rekow E.D., Thompson V.P. Material design ceramic based layer structures for crown. J Dent Res. 2002;81:433–438. doi: 10.1177/154405910208100615. [DOI] [PubMed] [Google Scholar]
- 22.Soboyejo W.O., Wang R., Katsube N., Seghi R., Pagedas C., Skraba P. Contact damage of model dental multilayers: experiments and finite element simulations. Key Eng Mater. 2001;198–199:135–178. [Google Scholar]
- 23.Lang B.R., Bernier S.H., Giday Z., Asgar K. Tarnish and corrosion of noble metal alloys. J Prosthet Dent. 1982;48:245–252. doi: 10.1016/0022-3913(82)90003-8. [DOI] [PubMed] [Google Scholar]
- 24.Prochazkova J., Podzimek S., Tomka M., Kucerova H., Mihaljevic M., Hana K. Metal alloys in the oral cavity as a cause of oral discomfort in sensitive patients. Neuro Endocrinol Lett. 2006;27:53–58. [PubMed] [Google Scholar]
- 25.Khamaysi Z., Bergman R., Weltfriend S. Positive patch test reactions to allergens of the dental series and the relation to the clinical presentations. Contact Dermatitis. 2006;55:216–218. doi: 10.1111/j.1600-0536.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 26.Valentine-Thon E., Muller K., Guzzi G., Kreisel S., Ohnsorge P., Sandkamp M. LTT-MELISA is clinically relevant for detecting and monitoring metal sensitivity. Neuro Endocrinol Lett. 2006;27:17–24. [PubMed] [Google Scholar]
- 27.Venclikova Z., Benada O., Bartova J., Joska L., Mrklas L., Prochazkova J. In vivo effects of dental casting alloys. Neuro Endocrinol Lett. 2006;27:61–68. [PubMed] [Google Scholar]
- 28.Venclikova Z., Benada O., Bartova J., Joska L., Mrklas L. Metallic pigmentation of human teeth and gingiva: morphological and immunological aspects. Dent Mater J. 2007;26:96–104. [PubMed] [Google Scholar]
- 29.Blatz M.B., Sadan A., Kern M. Resin–ceramic bonding: a review of the literature. J Prosthet Dent. 2003;89:268–274. doi: 10.1067/mpr.2003.50. [DOI] [PubMed] [Google Scholar]
- 30.Kern M., Wegner S.M. Bonding to zirconia ceramic: adhesion methods and their durability. Dent Mater. 1998;14:64–71. doi: 10.1016/s0109-5641(98)00011-6. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Lawn B.R., Malament K.A., Van Thompson P., Rekow E.D. Damage accumulation and fatigue life of particle-abraded ceramics. Int J Prosthodont. 2006;19:442–448. [PubMed] [Google Scholar]
- 32.Zhang Y., Lawn B.R., Rekow E.D., Thompson V.P. Effect of sandblasting on the long-term performance of dental ceramics. J Biomed Mater Res B: Appl Biomater. 2004;71:381–386. doi: 10.1002/jbm.b.30097. [DOI] [PubMed] [Google Scholar]
- 33.Barrack R.L., Burak C., Skinner H.B. Concerns about ceramics in THA. Clin Orthop Relat Res. 2004;429:73–79. doi: 10.1097/01.blo.0000150132.11142.d2. [DOI] [PubMed] [Google Scholar]
- 34.Chevalier J. What future for zirconia as a biomaterials. Biomaterials. 2006;27:534–543. doi: 10.1016/j.biomaterials.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 35.Tinschert J., Schulze K.A., Natt G., Latzke P., Heussen N., Spiekermann H. Clinical behavior of zirconia-based fixed partial dentures made of DC-Zirkon: 3-year results. Int J Prosthodont. 2008;21:217–222. [PubMed] [Google Scholar]
- 36.Sailer I., Pjetursson B.E., Zwahlen M., Hämmerle C.H.F. A systematic review of the survival and complication rates of all-ceramic and metal–ceramic reconstructions after an observation period of at least 3 years. Part II: Fixed dental prostheses. Clin Oral Implants Res. 2007;18:86–96. doi: 10.1111/j.1600-0501.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 37.Sailer I., Feher A., Filser F., Gauckler L.J., Luthy H., Hammerle C.H. Five-year clinical results of zirconia frameworks for posterior fixed partial dentures. Int J Prosthodont. 2007;20:383–388. [PubMed] [Google Scholar]
- 38.Sailer I., Holderegger C., Jung R.E., Suter A., Thievent B., Pietrobon N. Clinical study of the color stability of veneering ceramics for zirconia frameworks. Int J Prosthodont. 2007;20:263–269. [PubMed] [Google Scholar]
- 39.Rekow D., Thompson V.P. Engineering long term clinical success of advanced ceramic prostheses. J Mater Sci Mater Med. 2007;18:47–56. doi: 10.1007/s10856-006-0661-1. [DOI] [PubMed] [Google Scholar]
- 40.Kelly J.R. Ceramics in restorative and prosthetic dentistry. Annu Rev Mater Sci. 1997;27:443–468. [Google Scholar]
- 41.Lawn B.R., Deng Y., Miranda P., Pajares A., Chai H., Kim D.K. Overview: damage in brittle layer structures from concentrated loads. J Mater Res. 2002;17:3019–3036. [Google Scholar]
- 42.Huang M., Wang R., Thompson V., Rekow D., Soboyejo W.O. Bioinspired design of dental multilayers. J Mater Sci Mater Med. 2007;18:57–64. doi: 10.1007/s10856-006-0662-0. [DOI] [PubMed] [Google Scholar]
- 43.Taskonak B., Mecholsky J.J., Jr., Anusavice K.J. Residual stresses in bilayer dental ceramics. Biomaterials. 2005;26:3235–3241. doi: 10.1016/j.biomaterials.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 44.Aboushelib M.N., Feilzer A.J., de Jager N., Kleverlaan C.J. Prestresses in bilayered all-ceramic restorations. J Biomed Mater Res B: Appl Biomater. 2008;87:139–145. doi: 10.1002/jbm.b.31083. [DOI] [PubMed] [Google Scholar]
- 45.Watari F., Yokoyama A., Omori M., Hirai T., Kondo H., Uo M. Biocompatibility of materials and development to functionally graded implant for biomedical application. Compos Sci Technol. 2004;64:893–908. [Google Scholar]
- 46.Hsueh C.H., Luttrelll C.R., Becher P.F. Analyses of multilayered dental ceramics subjected to biaxial flexure tests. Dent Mater. 2006;22:460–469. doi: 10.1016/j.dental.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 47.Hsueh C.H., Luttrelll C.R., Becher P.F. Modeling of multilayered disks subjected to biaxial flexure tests. Int J Solids Struct. 2006;43:6014–6025. [Google Scholar]
- 48.Hedia H.S., Mahmoud N.A. Design optimization of functionally graded dental implant. Biomed Mater Eng. 2004;14:133–143. [PubMed] [Google Scholar]
- 49.Hedia H.S. Design of functionally graded dental implant in the presence of cancellous bone. J Biomed Mater Res B: Appl Biomater. 2005;75:74–80. doi: 10.1002/jbm.b.30275. [DOI] [PubMed] [Google Scholar]
- 50.Hedia H.S. Effect of cancellous bone on the functionally graded dental implant concept. Biomed Mater Eng. 2005;15:199–209. [PubMed] [Google Scholar]
- 51.Huang M., Rahbar N., Wang R., Thompson V., Rekow D., Soboyejo W.O. Bioinspired design of dental multilayers. Mater Sci Eng A. 2007;464:315–320. doi: 10.1007/s10856-006-0662-0. [DOI] [PubMed] [Google Scholar]
- 52.Wang F., Lee H.P., Lu C. Thermal–mechanical study of functionally graded dental implants with the finite element method. J Biomed Mater Res A. 2007;80:146–158. doi: 10.1002/jbm.a.30855. [DOI] [PubMed] [Google Scholar]
- 53.Yang J., Xiang H.-J. Three-dimensional finite element study on the biomechanical behavior of an FGBM dental implant in surrounding bone. J Biomech. 2007;40:2377–2385. doi: 10.1016/j.jbiomech.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Rahbar N., Soboyejo W.O. Design of functionally graded dental multilayers. Fatigue Fract Eng Mater Struct. 2011;34:887–897. [Google Scholar]
- 55.Niu X., Rahbar N., Farias S., Soboyejo W. Bio-inspired design of dental multilayers: experiments and model. J Mech Behav Biomed Mater. 2009;2:596–602. doi: 10.1016/j.jmbbm.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Madfa A.A. University Malaya; 2011. Development of functionally graded composite for fabrication of dental post. [Ph.D. thesis] [Google Scholar]
- 57.Kim J.W., Liu L., Zhang Y. Improving the resistance to sliding contact damage of zirconia using elastic gradients. J Biomed Mater Res B: Appl Biomater. 2010;84:347–352. doi: 10.1002/jbm.b.31657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erdogan F., Kaya A.C., Joseph P.F. The crack problem in bonded nonhomogeneous materials. J Appl Mech. 1991;58:400. [Google Scholar]
- 59.Erdogan F. Fracture mechanics of functionally graded materials. Comp Eng. 1995;5:753. [Google Scholar]
- 60.Kim A.S., Suresh S., Shih C.F. Plasticity effects on fracture normal to interfaces with homogenous and graded compositions. Int J Solids Struct. 1997;34:3415. [Google Scholar]
- 61.Kim A.S., Besson J., Pineau A. Global and local approaches to fracture normal to interfaces. Int J Solids Struct. 1999;36:1845. [Google Scholar]
- 62.Francis L.F., Vaidya K.J., Huang H.Y., Wolf W.D. Design and processing of ceramic-based analogs to the dental crown. Mater Sci Eng C. 1995;3:63–74. [Google Scholar]
- 63.Simmelink J.W. Histology of enamel. In: Avery J.K., editor. Oral development and histology. Williams and Wilkins; Baltimore: 1987. pp. 140–151. [Google Scholar]
- 64.Robinson C., Weatherell J.A., Hallsworth A.S. Variation in composition of dental enamel within thin ground tooth sections. Caries Res. 1971;5:44–57. doi: 10.1159/000259731. [DOI] [PubMed] [Google Scholar]
- 65.Dibdin G.H., Poole D. Surface area and pore size analysis for human enamel and dentin by water vapour sorption. Arch Oral Biol. 1982;27:235–241. doi: 10.1016/0003-9969(82)90057-7. [DOI] [PubMed] [Google Scholar]
- 66.Dibdin G.H. The water in human dental enamel and its diffusional exchange measured by clearance of tritiated water from enamel slabs of varying thickness. Caries Res. 1993;27:81–86. doi: 10.1159/000261522. [DOI] [PubMed] [Google Scholar]
- 67.Elliott J.C. Structure, crystal chemistry and density of enamel apatites. In: Chadwick D.J., Cardew G., editors. Dental enamel. Wiley; Chichester: 1997. pp. 54–72. [PubMed] [Google Scholar]
- 68.Nalla R.K., Kinney J.H., Tomsia A.P., Ritchie R.O. Role of alcohol in the fracture resistance of teeth. J Dent Res. 2006;85:1022–1026. doi: 10.1177/154405910608501109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pashley D.H., Agee K.A., Carvalho R.M., Lee K.W., Tay F.R., Callison T.E. Effects of water and water-free polar solvents on the tensile properties of demineralized dentin. Dent Mater. 2003;19:347–352. doi: 10.1016/s0109-5641(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 70.Fox P. The toughness of tooth enamel, a natural fibrous composite. J Mater Sci. 1980;15:3113–3121. [Google Scholar]
- 71.Robinson C., Shore R.C., Wood S.R., Brookes S.J., Smith D.A., Wright J.T. Subunit structures in hydroxyapatite crystal development in enamel: implications for Amelogenesis imperfecta. Connet Tissue Res. 2003;44:65–71. [PubMed] [Google Scholar]
- 72.Poole D.F., Brooks A. The arrangement of crystallites in enamel prisms. Arch Oral Biol. 1961;5:14–26. doi: 10.1016/0003-9969(61)90110-8. [DOI] [PubMed] [Google Scholar]
- 73.Kishen A. Mechanisms and risk factors for fracture predilection in endodontically treated teeth. Endod Topics. 2006;13:57–83. [Google Scholar]
- 74.Spears I.R., van Noort R., Crompton R.H., Cardew G.E., Howard I.C. The effects of enamel anisotropy on the distribution of stress in a tooth. J Dent Res. 1993;72:1526–1531. doi: 10.1177/00220345930720111101. [DOI] [PubMed] [Google Scholar]
- 75.Hu C., Fukae M., Uchida T. Sheathalin: cloning, cDNA polypeptide sequences, and immunolocalization of porcine enamel sheath proteins. J Dent Res. 1997;76:648–657. doi: 10.1177/00220345970760020501. [DOI] [PubMed] [Google Scholar]
- 76.White S.N., Luo W., Paine M.L., Fong H., Sarikaya M., Snead M.L. Biological organization of hydroxyapatite crystallites into a fibrous continuum toughens and controls anistropy in human enamel. J Dent Res. 2001;80:321–327. doi: 10.1177/00220345010800010501. [DOI] [PubMed] [Google Scholar]
- 77.Rasmussen S.T., Patchin R.E., Scott D.B. Fracture properties of human enamel and dentin. J Dent Res. 1976;55:154–164. doi: 10.1177/00220345760550010901. [DOI] [PubMed] [Google Scholar]
- 78.Xu H.H., Smith D.T., Jahanmir S., Romberg E., Kelly J.R., Thompson V.P. Indentation damage and mechanical properties of human enamel and dentin. J Dent Res. 1998;77:472–480. doi: 10.1177/00220345980770030601. [DOI] [PubMed] [Google Scholar]
- 79.Zhou J., Hsiung L.L. Biomolecular origin of the rate-dependent deformation of prismatic enamel. Appl Phys Lett. 2006;89:051904. [Google Scholar]
- 80.Ji B., Gao H. Mechanical properties of nanostructure of biological materials. J Mech Phys Solids. 2004;52:1963–1990. [Google Scholar]
- 81.Robinson C., Weatherell J.A., Hallsworth A.S. Distribution of magnesium in mature human enamel. Caries Res. 1981;15:70–77. doi: 10.1159/000260502. [DOI] [PubMed] [Google Scholar]
- 82.Stanford J.W., Paffenberger G.C., Kampula J.W., Sweeney A.B. Determination of some compressive properties of human enamel and dentin. J Am Dent Assoc. 1958;57:487–495. doi: 10.14219/jada.archive.1958.0194. [DOI] [PubMed] [Google Scholar]
- 83.Stanford J.W., Weigel K.V., Paffenberger G.C., Sweeney W.T. Compressive properties of hard tooth tissues and some restorative materials. J Am Dent Assoc. 1960;60:746–751. doi: 10.14219/jada.archive.1960.0258. [DOI] [PubMed] [Google Scholar]
- 84.Craig R.G., Peyton F.A., Johnson W. Compressive properties of enamel, dental cements, and gold. J Dent Res. 1961;40:936–943. [Google Scholar]
- 85.Tyldeslsy W.R. Mechanical properties of human dental enamel and dentin. Br Dent J. 1959;106:269–278. [Google Scholar]
- 86.Reich F.R., Brenden B.B., Porter N.S. Batelle Memorial Institute; Washington: 1967. Ultrasonic imaging of teeth. [Google Scholar]
- 87.Staines M., Robinson W.H., Hood J.A.A. Spherical indentation of tooth enamel. J Mater Sci. 1981;16:2551–2556. [Google Scholar]
- 88.Cuy J.L., Manna A.B., Livi K.J., Teaford M.F., Weihs T.P. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch Oral Biol. 2002;47:281–291. doi: 10.1016/s0003-9969(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 89.Zhou J., Hsiung L.L. Depth-dependent mechanical properties of enamel by nanoindentation. J Biomed Mater Res. 2007;81A:66–74. doi: 10.1002/jbm.a.31012. [DOI] [PubMed] [Google Scholar]
- 90.Ge J., Cui F.Z., Wang X.M., Feng H.L. Property variations in the prism and the organic sheath within enamel by nanoindentation. Biomaterials. 2005;26:3333–3339. doi: 10.1016/j.biomaterials.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 91.Mahoney E., Holt A., Swain M., Kilpatrick N. The hardness and modulus of elasticity of primary molar teeth: an ultra-micro-indentation study. J Dent. 2000;28:589–594. doi: 10.1016/s0300-5712(00)00043-9. [DOI] [PubMed] [Google Scholar]
- 92.Marshall G.W., Balooch M., Gallagher R.R., Gansky S.A., Marshal S.J. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. J Biomed Mater Res. 2001;54:87–95. doi: 10.1002/1097-4636(200101)54:1<87::aid-jbm10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 93.Fong H., Sarikaya M., White S.N., Snead M.L. Nano-mechanical properties profiles across dentin–enamel junction of human incisor teeth. Mater Sci Eng. 2000;7:119–128. [Google Scholar]
- 94.Habelitz S., Marshall S.J., Marshall G.W., Jr., Balooch M. Mechanical properties of human dental enamel on the nanometre scale. Arch Oral Biol. 2001;46:173–183. doi: 10.1016/s0003-9969(00)00089-3. [DOI] [PubMed] [Google Scholar]
- 95.Habelitz S., Marshall G.W., Baloochb M., Marshalla S.J. Nanoindentation and storage of teeth. J Biomech. 2002;35:995–998. doi: 10.1016/s0021-9290(02)00039-8. [DOI] [PubMed] [Google Scholar]
- 96.Barbour M.E., Parker D.M., Jandt K.D. Enamel dissolution as a function of solution degree of saturation with respect to hydroxyapatite: a nanoindentation study. J Colloid Interface Sci. 2003;265:9–14. doi: 10.1016/s0021-9797(03)00087-0. [DOI] [PubMed] [Google Scholar]
- 97.Francis L.F., Vaidya K.J., Huang H.Y., Wolf W.D. Design and processing of ceramic-based analogs to the dental crown. Mater Sci Eng C: Mater Biol Appl. 1995;3:63–74. [Google Scholar]
- 98.Lin C.P., Douglas W.H., Erlandsen S.L. Scanning electron microscopy of type I collagen at the dentin–enamel junction of human teeth. J Histochem Cytochem. 1993;41:381–388. doi: 10.1177/41.3.8429200. [DOI] [PubMed] [Google Scholar]
- 99.Lin C.P., Douglas W.H. Structure–property relations and crack resistance at the bovine dentin–enamel junction. J Dent Res. 1994;73:1072–1078. doi: 10.1177/00220345940730050901. [DOI] [PubMed] [Google Scholar]
- 100.White S.N., Miklus V.G., Chang P.P., Caputo A.A., Fong H., Sarikaya M. Controlled failure mechanisms toughen the dentino-enamel junction zone. J Prosthet Dent. 2005;94:330–335. doi: 10.1016/j.prosdent.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 102.Featherstone J.D., Ten Cate J.M., Shariati M., Arends J. Composition of artificial caries-like lesion by quantitative microradiography and microhardness profiles. Caries Res. 1983;17:385–391. doi: 10.1159/000260692. [DOI] [PubMed] [Google Scholar]
- 103.Meredith N., Sheriff M., Setchell D.J., Swanson S.A. Measurement of the microhardness and Young's modulus of human enamel and dentin using an indentation technique. Arch Oral Biol. 1996;41:539–545. doi: 10.1016/0003-9969(96)00020-9. [DOI] [PubMed] [Google Scholar]
- 104.He L.H., Swain M.V. Enamel – a functionally graded natural coating. J Dent. 2009;37:596–603. doi: 10.1016/j.jdent.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 105.Erdogan F. Fracture-mechanics of functionally graded materials. Compos Eng. 1995;5:753–770. [Google Scholar]
- 106.Khor K.A., Gu Y.W., Quek C.H., Cheang L. Plasma spraying of functionally graded hydroxyapatite/Ti–6Al–4V coatings. Surf Coat Technol. 2003;168:195–201. [Google Scholar]
- 107.Grandfield K., Chattah N.L., Djomehri S., Eidelmann N., Eichmiller F.C., Webb S. The narwhal (Monodon monoceros) cementum-dentin junction: a functionally graded biointerphase. Proc Inst Mech Eng H. 2014;228:754–767. doi: 10.1177/0954411914547553. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Y., Chai H., Lawn B.R. Graded structures for all-ceramic restorations. J Dent Res. 2010;89:417–421. doi: 10.1177/0022034510363245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tesch W., Eidelman N., Roschger P., Goldenberg F., Klaushofer K., Fratzl P. Graded microstructure and mechanical properties of human crown dentin. Calcif Tissue Int. 2001;69:147–157. doi: 10.1007/s00223-001-2012-z. [DOI] [PubMed] [Google Scholar]
- 110.Lannuttia J.J. Functionally graded materials: properties, potential and design guidelines. Comp Eng. 1994;4:81–94. [Google Scholar]
- 111.Koizumi M. FGM activities in Japan. Compos Part B: Eng. 1997;28:1–2. [Google Scholar]
- 112.Koizumi M., Niino M. Overview of FGM research in Japan. MRS Bull. 1995;20:19–21. [Google Scholar]
- 113.Narayan R.J., Hobbs L.W., Jin C., Rabiei A. The use of functionally gradient materials in medicine. JOM J Miner Met Mater Soc. 2006;58:52–56. [Google Scholar]
- 114.Bever M.B., Duwez P.E. Gradients in composite materials. Mater Sci Eng. 1972;10:1–8. [Google Scholar]
- 115.Kon M., Ishikawa K., Miyamoto Y., Asaoka K. Development of calcium phosphate based functional gradient bioceramic. Biomaterials. 1995;16:709–714. doi: 10.1016/0142-9612(95)99699-m. [DOI] [PubMed] [Google Scholar]
- 116.Chu C.L., Wang S.D., Lin P.H., Yin Z.D., Zhu J.C. Characterization and optimized design of HA–Ti/Ti/Ha–Ti symmetrical functionally graded biomaterial. Mater Sci Eng A. 2001;316:205–210. [Google Scholar]
- 117.Miyao R., Yokoyama A., Watari F., Kawasaki T. Properties of titanium/hydroxyapatite functionally graded implants by spark plasma sintering and their biocompatibility. J Dent Mater. 2001;20:344–355. [Google Scholar]
- 118.Lima Y.M., Parkb Y.J., Yunc Y.H., Hwang K.S. Functionally graded Ti/HAP coatings on Ti–6Al–4V obtained by chemical solution deposition. Ceram Int. 2002;28:37–41. [Google Scholar]
- 119.Zhu J.C., Chu C.L., Yin Z.D. Bonding strength of hydroxyapatite/Ti FGM implant to bone. Rare Met Mater Eng. 2003;32:432–435. [Google Scholar]
- 120.Boss J.N., Ganesh V.K. Fabrication and properties of graded composite rods for biomedical applications. Compos Struct. 2006;74:289–293. [Google Scholar]
- 121.Kawasaki A., Watanabe R. Concept and P/M fabrication of functionally gradient materials. Ceram Int. 1997;23:73–83. [Google Scholar]
- 122.Suresh S. Graded materials for resistance to contact deformation and damage. Science. 2001;292:2447–2451. doi: 10.1126/science.1059716. [DOI] [PubMed] [Google Scholar]
- 123.Moon R.J., Bowman K.P., Trumble K.P., Rödel J. Fracture resistance curve behavior of multi-layered alumina–zirconia composites produced by centrifugation. Acta Mater. 2001;49:995–1003. [Google Scholar]
- 124.Put S., Vleugels J., Anné G., Van der Biest O. Functionally graded ceramic and ceramic–metal composites shaped by electrophoretic deposition. Colloids Surf A: Physicochem Eng Asp. 2003;222:223–232. [Google Scholar]
- 125.Widjaja S., Limarga A.M., Yip T.H. Oxidation behavior of a plasma-sprayed functionally graded ZrO2/Al2O3 thermal barrier coating. Mater Lett. 2002;57:628–634. [Google Scholar]
- 126.Jennifer A.L. Direct-write assembly of ceramics from colloidal inks. Curr Opin Solid State Mater Sci. 2002;6:245–250. [Google Scholar]
- 127.Wang J.W., Shaw L.L. Fabrication of functionally graded materials via inkjet color printing. J Am Ceram Soc. 2006;89:3285–3289. [Google Scholar]
- 128.Zhang Y., Kim J.W. Graded structures for damage resistant and aesthetic all-ceramic restorations. Dent Mater. 2009;25:781–790. doi: 10.1016/j.dental.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Henriques B. Universidade do Minho; 2012. Bond strength enhancement of metal–ceramic dental restorations by FGM design. [Google Scholar]
- 130.Ren L., Janal M.N., Zhang Y. Sliding contact fatigue of graded zirconia with external esthetic glass. J Dent Res. 2011;90:1116–1121. doi: 10.1177/0022034511412075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Y., Kim J.W. Graded zirconia glass for resistance to veneer fracture. J Dent Res. 2010;89:1057–1062. doi: 10.1177/0022034510375289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y., Ma L. Optimization of ceramic strength using elastic gradients. Acta Mater. 2009;57:2721–2729. doi: 10.1016/j.actamat.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Piascik J.R., Thompson J.Y., Bower C.A., Stoner B.R. Stress evolution as a function of substrate bias in rf magnetron sputtered yttria-stabilized zirconia films. J Vac Sci Technol A. 2006;24:1091–1095. [Google Scholar]
- 134.Cannillo V., Manfredini T., Montorsi M., Siligardi C., Sola A. Microstructure-based modelling and experimental investigation of crack propagation in glass-alumina functionally graded materials. J Eur Ceram Soc. 2006;26:3067–3073. [Google Scholar]
- 135.Dorthé E., Zhang Y. Load-bearing increase in alumina evoked by introduction of a functional glass gradient. J Eur Ceram Soc. 2012;32:1213–1220. doi: 10.1016/j.jeurceramsoc.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jitcharoen J., Padture N.P., Giannakopoulos A.E., Suresh S. Hertzian-crack suppression in ceramics with elastic-modulus-graded surfaces. J Am Ceram Soc. 1998;81:2301–2308. [Google Scholar]
- 137.Zhang Y., Sun M.-j., Zhang D. Designing functionally graded materials with superior load-bearing properties. Acta Biomater. 2012;8:1101–1108. doi: 10.1016/j.actbio.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ren L., Zhang Y. Sliding contact fracture of dental ceramics: principles and validation. Acta Biomater. 2014;10:3243–3253. doi: 10.1016/j.actbio.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Y. Overview: damage resistance of graded ceramic restorative materials. J Eur Ceram Soc. 2012;32:2623–2632. doi: 10.1016/j.jeurceramsoc.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Henriques B., Soares D., Silva F. Optimization of bond strength between gold alloy and porcelain through a composite interlayer obtained by powder metallurgy. Mater Sci Eng A: Struct Mater. 2011;528:1415–1420. [Google Scholar]
- 141.Henriques B., Gasik M., Soares D., Silva F.S. Experimental evaluation of the bond strength between a CoCrMo dental alloy and porcelain through a composite metal–ceramic graded transition interlayer. J Mech Behav Biomed Mater. 2012;13:206–214. doi: 10.1016/j.jmbbm.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 142.Henriques B., Felix S., Soares D., Silva F.S. Shear bond strength comparison between conventional porcelain fused to metal and new functionally graded dental restorations after thermal–mechanical cycling. J Mech Behav Biomed Mater. 2012;13:194–205. doi: 10.1016/j.jmbbm.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 143.Gasik M. Micromechanical modeling of functionally graded materials. Comp Mater Sci. 1998;13:42–55. [Google Scholar]
- 144.Pender D.C., Padture N.P., Giannakopoulos A.E., Suresh S. Gradients in elastic modulus for improved contact-damage resistance. Part I: the silicon nitride–oxynitride glass system. Acta Mater. 2001;49:3255–3262. [Google Scholar]
- 145.Suresh S., Olsson M., Giannakopoulos A.E., Padture N.P., Jitcharoen J. Engineering the resistance to sliding-contact damage through controlled gradients in elastic properties at contact surfaces. Acta Mater. 1999;47:3915–3926. [Google Scholar]
- 146.Dong J.K., Luthy H., Wohlwend A., Scharer P. Heat-pressed ceramics: technology and strength. Int J Prosthodont. 1992;5:9–16. [PubMed] [Google Scholar]
- 147.Luthy H., Dong J.K., Wohlwend A., Scharer P. Effects of veneering and glazing on the strength of heat-pressed ceramics. Schweiz Monatsschr Zahnmed. 1993;103:1257–1260. [PubMed] [Google Scholar]
- 148.Kim J.W., Bhowmick S., Hermann I., Lawn B.R. Transverse fracture of brittle bilayers: relevance to failure of all-ceramic dental crowns. J Biomed Mater Res B: Appl Biomater. 2006;79:58–65. doi: 10.1002/jbm.b.30511. [DOI] [PubMed] [Google Scholar]
- 149.Ortorp A., Kihl M.L., Carlsson G.E. A 3-year retrospective and clinical follow-up study of zirconia single crowns performed in a private practice. J Dent. 2009;37:731–736. doi: 10.1016/j.jdent.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 150.Vult von Steyern P., Carlson P., Nilner K. All-ceramic fixed partial dentures designed according to the DC-Zirkon technique. A 2-year clinical study. J Oral Rehabil. 2005;32:180–187. doi: 10.1111/j.1365-2842.2004.01437.x. [DOI] [PubMed] [Google Scholar]
- 151.Crisp R.J., Cowan A.J., Lamb J., Thompson O., Tulloch N., Burke F.J. A clinical evaluation of all ceramic bridges placed in UK general dental practices: first-year results. Br Dent J. 2008;205:477–482. doi: 10.1038/sj.bdj.2008.937. [DOI] [PubMed] [Google Scholar]
- 152.Edelhoff D., Florian B., Florian W., Johnen C. HIP zirconia fixed partial dentures – clinical results after 3 years of clinical service. Quintessence Int. 2008;39:459–471. [PubMed] [Google Scholar]
- 153.Larsson C., Vult von Steyern P., Nilner K. A prospective study of implant-supported full-arch yttria-stabilized tetragonal zirconia polycrystal mandibular fixed dental prostheses: three-year results. Int J Prosthodont. 2010;23:364–369. [PubMed] [Google Scholar]
- 154.Daou E.E. The zirconia ceramic: strengths and weaknesses. Open Dent J. 2014;8:33–42. doi: 10.2174/1874210601408010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rekow E.D., Silva N.R., Coelho P.G., Zhang Y., Guess P., Thompson V.P. Performance of dental ceramics: challenges for improvements. Dent Res. 2011;90:937–939. doi: 10.1177/0022034510391795. [DOI] [PMC free article] [PubMed] [Google Scholar]