Summary
Nitric oxide (NO) is a free radical which is produced from a wide variety of cells and tissues in the human body. NO is involved in the regulation of many physiological processes, such as vascular relaxation, neurotransmission, immune regulation, and cell death. NO is generated by nitric oxide synthase (NOS), which has three identified isoforms: neuronal type NOS (nNOS), endothelial type NOS (eNOS), and inducible type NOS (iNOS). Different isoforms are expressed depending on the organs, tissues, and cells, and investigation of the types and functions of enzymes expressed in various tissues is underway. The oral cavity is a space in which marked changes have been detected in NO levels, and each tissue is constantly influenced by NO. NO is a component of saliva and is produced by oral bacteria in the oral cavity and released by NOS expressed in oral mucosa. NOS isoforms expressed under normal conditions differ among the oral organs. In addition, the overexpression of NOS was involved in carcinogenesis and tumor growth progression. This review summarized the expression of NOS and functions of NO in oral cavity organs, and their roles in diseases and the influences of treatments.
Keywords: Nitric oxide, Nitric oxide synthase, NOS, Oral cavity, Physiological role
1. Introduction
Nitric oxide (NO) is a short-lived free radical with an unpaired electron. Although a factor released by vascular endothelial cells that relaxes the surrounding smooth muscle cells was initially termed endothelium-derived relaxing factor (EDRF), Murad F, Ignarro LJ, and Furchgott RF discovered in 1987 that EDRF was NO. NO is a tasteless and odorless gas that is capable of penetrating the cell membrane, and exerts its effects without the use of a receptor, thereby representing a new signaling pathway for intercellular communication. The Nobel Prize was awarded to Murad F, Ignarro LJ, and Furchgott RF for the discovery that NO is a signaling molecule (1998). Since NO is a gas, difficulties were associated with determining its localization and production in the body; however, the discovery of nitric oxide synthase (NOS), which plays a role in the synthesis of NO from l-arginine, accelerated understanding on the expression and function of NOS in the body. Using purification and cloning methods, 3 NOS isoforms have been identified to date: nNOS, iNOS, and eNOS [1]. NO is actively synthesized in the brain, and the neuronal NOS isoform (nNOS or NOS1) was initially cloned from the rat brain [2], [3]. The inducible NOS isoform (iNOS or NOS2) induced by LPS and various cytokines (IL-1, TNF-α, and IFN-γ) was cloned from macrophages [4], while the endothelial NOS isoform (eNOS or NOS3) was cloned from vascular endothelial cells [5].
The component proteins of the body are nitrosylated by NO [6], [7], and the biotin-switch assay is used as a simple method to measure nitrosylated protein levels [8]. The production and functions of NO have been extensively examined using morphological investigations of NOS and the biotin-switch assay, and the presence of NOS-expressing cells in various tissues and organs has been confirmed, thereby demonstrating the diversity of the functions of NO. NO has also been implicated in various diseases. The physiological and pathological roles of NO are known to be involved include vasodilatation, platelet aggregation, immune responses, cell migration, neurotransmission, apoptosis, wound healing, and carcinogenesis. The functions of NO may differ depending on the state of cells. For example, NO produced by nNOS, which is expressed by nerve cells, has been shown to promote nerve cell death in Parkinson's disease [9]. In contrast, NO has been suggested to induce the expression of brain-derived neurotrophic factor (BDNF) under normal conditions and promote nerve cell survival [10]. NO is also involved in apoptosis in the developmental stage, but inhibits cell death by suppressing the expression of Fas [11], [12], thereby showing the dual functions of NO in cells. The NOS isoform expressed differs depending on the cellular condition. Previous studies using NOS KO mice demonstrated that the isoform not normally expressed is compensatory expressed in KO mice [13].
The expression of NOS has been detected in all types of cells, and the diverse functions of the NO produced have been investigated using various methods. The recently reported expression of NOS and functions of NO in oral cavity organs, and their roles in diseases and the influences of treatments, were herein reviewed.
2. NO in dental pulp
2.1. NOS expression in dental pulp
Regarding NOS in human dental pulp, nNOS and eNOS are known to be expressed in odontoblasts, whereas iNOS is not under normal conditions [14], [15], [16]. The function of NO produced by nNOS and eNOS expressed in odontoblasts was examined, and its involvement in the transmission of pain stimulation has been suggested [14]. In addition to odontoblasts, eNOS was also found to be expressed in vascular endothelial cells and fibroblasts (Fig. 1) [14], [15], [16]. NO produced by eNOS in vascular endothelial cells was suggested to be involved in vascular dilation, which is similar to that in many other tissues [16], [17], [18]. In previous studies using cats, the expression of NOS was detected in nerve fibers, suggesting that the NO is involved in sensation of the dental pulp and regulation of blood flow, and the regulation of blood flow by NO has been demonstrated [19].
Figure 1.
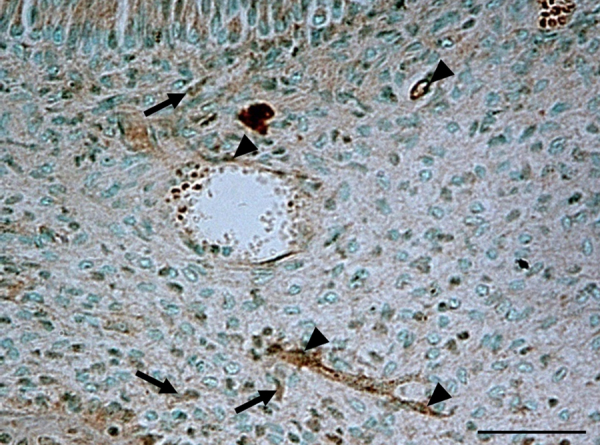
Immunostaining of the mouse dental pulp of lower first molar at 10 weeks of age with anti-eNOS antibody. Some fibroblasts (arrow) and the blood vessels (arrowhead) are positive in the dental pulp. Bar, 50 μm.
2.2. Disease and treatment of dental pulp and NO
Although absent in healthy dental pulp, RT-PCR and Western blotting revealed the expression of iNOS at the mRNA and protein levels with acute inflammation in dental pulp [15]. This iNOS is considered to be derived from white blood cells that migrate from blood vessels in response to pulpitis [15]. Fan et al. [20] exposed dental pulp in order to induce chronic tooth inflammation in rats, examined the role of NOS in the trigeminal ganglion and trigeminal nucleus caudalis, and found that NO and NOS were crucially involved in both peripheral and central nociceptive information.
Although slightly different from its expression in dental pulp diseases, the expression of NO was shown to be induced by tooth preparation in the treatment of dental caries [21], [22], [23]; a large amount of NO was released by odontoblasts and dental pulp cells below the cavity early after tooth preparation [23]. This NO was produced by iNOS [21], [22], [23]. The large amount of NO produced by this iNOS was immediately released after tooth preparation and mainly induced apoptosis in odontoblasts, and the involvement of NO in regulating the subsequent differentiation of odontoblasts was suggested [23]. Odontoblasts then form a tertiary dentin, and the involvement of NO in this mineralization process has also been suggested [23]. Therefore, NO produced in the dental pulp plays roles in diverse events, such as the induction of apoptosis in odontoblasts, promotion of proliferation and differentiation of undifferentiated mesenchymal cells, and mineralization of tertiary dentin by newly differentiated odontoblasts.eNOS is expressed in normal dental pulp, while iNOS is expressed in diseased and treated dental pulp (the level and range of expression vary depending on the disease and content of the treatment). These differences in the expression of NOS prompted the condition of dental pulp to be evaluated based on NOS expression. For example, the influences of light-emitting diode (LED) irradiation [24] and a pulp-capping material, mineral trioxide aggregate (MTA) [25], on dental pulp have been reported.
3. NO in periodontal tissue
3.1. NOS expression in periodontal tissue
The expression of NOS in periodontal tissue has been investigated using laboratory animals (mainly rats) [26], [27], [28], but not humans. These studies demonstrated that NOS was expressed in fibroblasts, the epithelial cell rests of Malassez, macrophages, osteoclasts, and vascular endothelial cells in the rat periodontal ligament [26], [27], [28]. The epithelial cell rests of Malassez have been examined extensively, with the expression of nNOS and eNOS being detected, whereas that of iNOS varied [28]. An in vitro study reported that the epithelial cell rests of Malassez of humans was expressed iNOS [29]. NO produced by nNOS and eNOS expressed in the epithelial cell rests of Malassez has been suggested to promote cell proliferation, differentiation, and apoptosis [28], whereas that produced by fibroblasts may play a role in the differentiation of osteoblasts via the mitogen-activated protein kinase and nuclear factor-kappaB pathways present in human periodontal ligament fibroblasts in vitro [30]. Reactive oxygen, a kind of free radical, has been reported to be associated with differentiation of osteoblasts as well as NO [31]. NO produced by eNOS in vascular endothelial cells has been suggested to be involved in vascular dilation, similar to that produced by vascular endothelial cells in various tissues [18].
On the other hand, the gingival expression of NOS has been reported in the basal cells of the gingival epithelium, fibroblasts, and vascular endothelial cells of the gingival lamina propria [13]. Although few studies have examined the role of NO in normal gingiva, the expression of NOS and production of NO have been suggested. It has also been reported that no abnormality existed in the phenotype of nNOS knockout animals, and the expression of eNOS and iNOS was enhanced in the gingival lamina propria, resulting in no changes to NO levels [13]. NOS expressed in vascular endothelial cells have been shown to regulate blood flow [18].
3.2. Periodontal disease and NO
A large number of studies have examined the various diseases of periodontal tissue. iNOS expression levels were previously reported to be markedly higher in human periodontal disease tissue than in healthy periodontal tissue [32]. This iNOS was considered to be derived from white blood cells that migrated to the lesion; however, studies using human periodontal ligament fibroblasts showed that the addition of LPS enhanced the expression of iNOS in fibroblasts and increased NO production [29]. Therefore, in addition to migrating white blood cells, fibroblasts influenced by various cytokines were suggested to be involved in the periodontal disease-induced enhancements observed in the expression of iNOS and production of NO [29].
Alveolar bone resorption occurs with the progression of periodontal disease (marginal periodontitis). It has also been reported in the root apex in apical periodontitis, and the involvement of NO in these bone resorption processes has been reported [33], [34]. Alveolar bone resorption in marginal periodontitis was prevented in rats by inhibiting the expression of NOS [33], and the induction of multinucleated TRAP-positive cells through the production of NO has been reported in apical periodontitis [34]. Previous studies on the relationship between osteoclasts and NO in bone tissue suggested that NO played an important role in osteoclast differentiation [35], [36]. Regarding the mechanism underlying periodontal disease-induced alveolar bone resorption, osteoclast differentiation may have been promoted by the NO produced by migrating white blood cells and fibroblasts, and these osteoclasts may have resorbed bone.
Periodontal disease reduces occlusal force in patients who lose a tooth and also in the elderly. The dynamics of NOS were examined in relation to the expression of NOS in a hypofunctional periodontal ligament and during its functional recovery process; when occlusal force decreased, NOS expression decreased in the periodontal ligament [37]. This reduction in reactivity was attributed to changes in the NOS-positive region in the periodontal ligament, rather than reductions in the number of NOS-positive cells. In addition, the expression of NOS was enhanced with the recovery of occlusal force. Furthermore, the expression of NOS was found to be enhanced when occlusal force was higher than that in the normal state. These findings suggested that NO was important for the structural changes to the periodontal ligament induced by an increase in occlusal force [37].
4. NOS expression induced by orthodontic treatments
Previous studies have extensively examined orthodontic tooth movement-induced NOS expression, and this may have been based on the reported findings [38], [39], [40], [41], [42] that many cells in periodontal tissue, mainly the periodontal ligament, express NOS under normal conditions, and NO is involved in various functions, as described above.
Rat molars have been used in many studies that morphologically investigated the relationship between orthodontic treatments and NOS [38], [39], [40]. These studies demonstrated that the expression of NOS differed between tension and compression areas (pressure side). The expression of NOS in bone tissue has been attracting attention. When an orthodontic force (10 cN for 120 h) was loaded on the tension area (side) of alveolar bone, the number of eNOS-positive osteocytes started to increase after 24 h, and a small number of iNOS-positive osteocytes were also found to be present. In contrast, the number of iNOS-positive osteocytes in the compression area (pressure side) started to increase 6 h after loading the force, and that of eNOS-positive osteocytes also increased from 24 h. These findings suggested that osteocytes functioned as a regulator in tension and compression areas (pressure side), i.e., eNOS expressed by osteocytes in the tension area (side) acted as a regulator of bone formation while iNOS expressed in the compression area (pressure side) acted as a regulator of inflammation-induced bone resorption [39]. Another study [40] demonstrated that these actions as a mechanosensor in the tension (side) and compression (pressure side) areas were performed by periodontal ligament fibroblasts, not osteocytes.
Orthodontic treatment-induced NOS expression in the gingiva [41] and dental pulp [42] has also been reported, and the expression of eNOS and iNOS in both these tissues was shown to be significantly higher than that under normal conditions.
5. NOS expression in odontogenesis
Few studies have investigated the role of NOS expression in odontogenesis. A neonatal mouse tooth germ was cultured and the influence of NO was investigated, and the findings obtained suggested that a NO signaling pathway was partially involved in the amelogenin production process in ameloblasts [43]. Our group also investigated the function and expression of NOS in ameloblasts at the protein and mRNA levels using a mouse tooth germ. We confirmed the mRNA expression of iNOS and eNOS in ameloblasts in the maturation stage using in situ hybridization, showing that NO was produced by iNOS and eNOS in maturation-stage ameloblasts (Fig. 2). The function of this NO is now being investigated. We assume that it is involved in secondary calcification and protein removal by ameloblasts.
Figure 2.
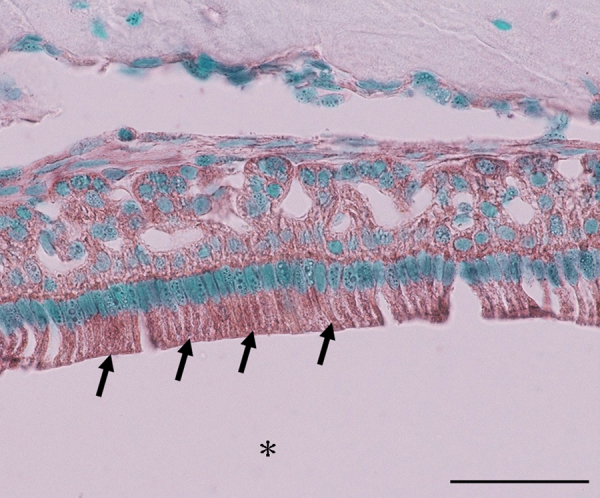
Expression of eNOS mRNA in the mouse lower incisor at 8 weeks of age by in situ hybridization. eNOS signals are detected in ameloblasts (arrow) at the maturation stage (asterisk: enamel). Bar, 50 μm.
6. NOS expression in the tongue
A limited number of studies have investigated the expression of NOS in the tongue; however, eNOS was previously shown to be expressed in blood vessels distributed in the tongue, while NO regulated blood flow [18].
The gustatory receptors, taste buds, are present in the lingual mucosa. The expression of NOS has been reported in these taste buds [44]: the expression of nNOS was detected in taste cells and the surrounding nerve fibers, suggesting that NO is involved in the transmission of gustatory signals. However, this study only examined Amphibia, not humans.
7. NO in the salivary gland
7.1. NOS expression in the salivary gland
The expression of nNOS has been reported in nerve fibers distributed in the acini, and these nNOS-positive nerve fibers were found to be synaptically connected to acinar cells. Many previous studies reported these findings, suggesting the production of NO in the acini [45], [46]. The function of this NO has been examined from various viewpoints. Acinar cells are double-innervated by autonomic sympathetic and parasympathetic nerves. The expression of nNOS has been detected in both sympathetic and parasympathetic nerves. In parasympathetic nerves, NO produced by nNOS enhances the release of H2O and ions through muscarinic receptors on acinar cells [47], [48]. NO has also been reported to act on acinar cells and induce amylase secretion in sympathetic nerves [48], [49].
NO produced by nNOS is known to act on ion channels of acinar cells, and, is crucially involved in Ca2+ release [47]. The production of NO in the acini is considered to occur in nerve fibers distributed to acinar cells and contributes to saliva secretions including ions, as described above; however, NO has recently been suggested to be involved in protein synthesis in and the division of acinar cells [49]. On the other hand, the expression of NOS has also been observed in the ductal system, other than the acini. Our group confirmed the expression of nNOS in the ductal system (Fig. 3). Although the function of NOS in the ductal system currently remains unclear, it has been suggested to play a role in regulating saliva secretions and the direct secretion of NO into saliva [45], [50].
Figure 3.
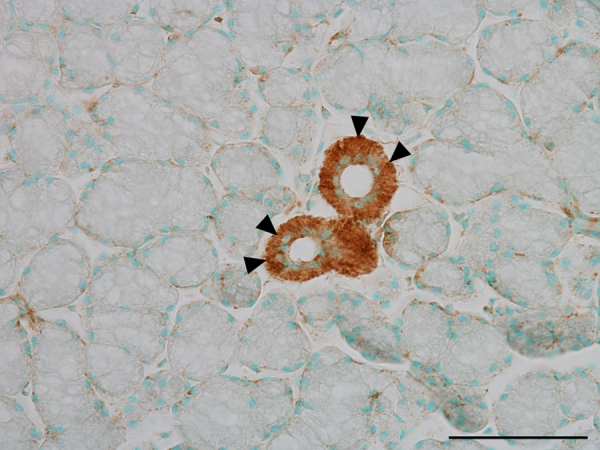
Immunostaining of the mouse sublingual gland at 10 weeks of age with anti-nNOS antibody. nNOS-positive reactions appears in the striated ducts (arrowhead), but the acini are negative. Bar, 50 μm.
The studies on the expression and functions of NOS in the salivary gland described above used the rat parotid gland [46], [47], [48]. Salivary gland tissue is known to markedly vary among animal species, with its histological structure and composition of secretions being diverse, and functions also differing. Species differences have also been reported in the expression of NOS. Previous studies [45] compared the salivary gland between humans and rats, and found that the number of nNOS-positive nerves was lower while the number of ductal cells expressing nNOS was higher in the rat salivary gland than in the human salivary gland. Functional differences due to differences in the region of the salivary gland expressing NOS between humans and rats remain to be investigated in future studies.
7.2. Salivary gland disease and NO
The excess production of NO in the salivary gland has been implicated in the development of various diseases. NO-related diseases of the salivary gland are characterized by the overexpression of NOS in acinar cells, in which it is not expressed under normal conditions, unlike other tissues. Sjögren's syndrome is a representative NO-related disease, and the relationship between its pathology and the expression of NO and NOS has been closely investigated [51], [52], [53]. Sjögren's syndrome is an autoimmune disease, in which T-cells secrete various cytokines that damage or induce apoptosis in acinar cells, leading to the symptoms of the disease, such as acinar atrophy and hypofunction of the salivary gland. The involvement of NO in this acinar cell hypofunction has been reported. NO is not secreted by inflammatory cells, such as T-cells (not necessarily indicating the complete absence of secretion), and acinar cells produce NO by themselves, i.e., acinar cells express iNOS and produce NO, which then nitrosylates various signaling pathways in acinar cells, resulting in acinar cell hypofunction. This NO-mediated acinar cell hypofunction has been suggested to be a cause of Sjögren's syndrome [51], [52], [53].
7.3. NO in saliva
Nitrate is secreted into the oral cavity as a saliva component in humans. This nitrate is converted to NO rapidly by oral bacteria and more slowly by salivary peroxidase [54]. Dental plaque deposition has been shown to increase NO levels in the oral cavity, and this has been attributed to the release of NO by the host defense mechanism in an attempt to prevent the proliferation of bacteria in dental plaques [55]. Elevations in NO levels in the oral cavity and saliva due to various causes have been associated with oral diseases. A previous study [56] using the saliva of oral cancer patients showed that salivary NO levels were significantly higher in the saliva of patients than in that of healthy subjects, suggesting that the measurement of salivary NO levels is applicable for the diagnosis and treatment of oral cancer. A high salivary NO level has also been reported in children with rampant caries and early childhood caries [57]. The relationship between salivary NO levels and various diseases needs to be investigated in more detail in future studies.
8. NOS expression in oral cancer
Although NO is known to play a role in carcinogenesis and tumor growth progression [58], the mechanisms underlying its involvement and the pathology associated with NO have not yet been elucidated in detail. This may be because cancers arise from various tissues through different mechanisms, and exogenous factors affecting each tissue are different among tissues. Studies on the relationship between cancers arising in the oral cavity and NO have been actively performed because of many mechanical stimuli, the presence of high levels of NO in saliva [58], [59], [60], [61], [62]. Oral squamous cell carcinomas (OSCC) [60], [61], [62] have been investigated in many of these studies, in which the expression of iNOS was not detected in the oral epithelium under normal conditions, but was markedly higher at the protein and mRNA levels in OSCC [60]. The relationship between the expression of this iNOS and oral epithelial transformation as well as tumor formation and development has been suggested in a large number of previous studies [60], [61], [62]. The expression of eNOS and VEGF has also been observed in OSCC, suggesting that the production of NO is involved in angiogenesis [63], [64]. NOS has been implicated in the development of OSCC. OSCC is induced by NO produced by iNOS, and NO produced by eNOS is involved in cancer growth through angiogenesis.
However, a previous study overexpressed iNOS in vitro, and showed that NO caused the accumulation of p53 and induced apoptosis in cancer cells [65]. In another study using RNAi (tongue squamous cell carcinoma cells), the inhibition of iNOS gene expression was found to suppress cell proliferation and induced apoptosis [66]. The inhibition of tumorigenicity through the overexpression of iNOS has also been reported [67], but only in vitro. This tumor-inhibitory effect of NO has not yet been demonstrated in vivo.
The expression of iNOS has also been reported in oral dysplasia [60] and oral lichen planus [68]. When the expression of iNOS was compared, it was found to be higher in the 2 diseases than in normal tissue, but lower than that in OSCC in a previously reported study [60], [68]. The expression of iNOS leads to the production of NO, which, in turn, induces the development of oral dysplasia and oral lichen planus. If the expression of iNOS increases thereafter, DNA damage by NO increases and may induce the development of oral cancer [60], [68]. The relationship between ameloblastomas and the expression of NOS has also been investigated, and an association between the activation of iNOS and malignant potential has been suggested [69].
9. Conclusion
As described above, NOS expressed under normal conditions differs among the oral organs, and the NO produced has diverse functions. However, the expression and functions of NOS, including those in odontogenesis and tongue and periodontal tissues, have not yet been sufficiently investigated. Furthermore, the relationship between the expression of NOS and NO in various oral diseases remains unclear, and the production of and pathology associated with NO have only been partially elucidated. The application of measuring salivary NO levels for diagnoses and treatments is currently being investigated. NO is a component of saliva and is produced by oral bacteria in the oral cavity. It is also produced and released by NOS expressed in oral mucosa, such as gingiva. The oral cavity is a space in which marked changes have been detected in NO levels, and each tissue is constantly influenced by NO. NO is closely associated with the oral cavity, and the progression of its analysis through the application of various research methods is expected.
Conflict of interest
There are no conflicts of interest associated with this review.
References
- 1.Nathan C., Xie Q.W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 2.Bredt D.S., Hwang P.M., Glatt C.E., Lowenstein C., Reed R.R., Snyder S.H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- 3.Lorenc-Koci E., Czarnecka A. Role of nitric oxide in the regulation of motor function. An overview of behavioral, biochemical and histological studies in animal models. Pharmacol Rep. 2013;65(5):1043–1055. doi: 10.1016/s1734-1140(13)71464-6. [DOI] [PubMed] [Google Scholar]
- 4.Xie Q.W., Cho H.J., Calaycay J., Mumford R.A., Swiderek K.M., Lee T.D. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 5.Lamas S., Marsden P.A., Li G.K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamler J.S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78(6):931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 7.Hess D.T., Matsumoto A., Kim S.O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 8.Jaffrey S.R., Erdjument-Bromage H., Ferris C.D., Tempst P., Snyder S.H. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3(2):193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 9.Tsang A.H., Chung K.K. Oxidative and nitrosative stress in Parkinson's disease. Biochem Biophys Acta. 2009;1792(7):645–650. doi: 10.1016/j.bbadis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Kurauchi Y., Hisatsune A., Isohama Y., Sawa T., Akaike T., Shudo K. Midbrain dopaminergic neurons utilize nitric oxide/cyclic GMP signaling to recruit ERK that links retinoic acid receptor stimulation to up-regulation of BDNF. J Neurochem. 2011;116(3):323–333. doi: 10.1111/j.1471-4159.2010.06916.x. [DOI] [PubMed] [Google Scholar]
- 11.Mannick J.B., Miao X.Q., Stamler J.S. Nitric oxide inhibits Fas-induced apoptosis. J Biol Chem. 1997;272(39):24125–24128. doi: 10.1074/jbc.272.39.24125. [DOI] [PubMed] [Google Scholar]
- 12.Brüne B., von Knethen A., Sandau K.B. Nitric oxide (NO): an effector of apoptosis. Cell Death Differ. 1999;6(10):969–975. doi: 10.1038/sj.cdd.4400582. [DOI] [PubMed] [Google Scholar]
- 13.Ishioka M., Ishizuka Y., Shintani S., Yanagisawa T., Inoue T., Sasaki J. Expression profiles of NOS isoforms in gingiva of nNOS knockout mice. Tissue Cell. 2014;46(2):122–126. doi: 10.1016/j.tice.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Ishizuka Y., Watanabe W., Ishioka M., Yanagisawa T., Sasaki J. Expression of NOS isoforms in dental pulp in NOS1 KO mice. Free Radic Res Int. 2006;13:191–194. [Google Scholar]
- 15.Di Nardo Di Maio F., Lohinai Z., D’Arcangelo C., De Fazio P.E., Speranza L., De Lutiis M.A. Nitric oxide synthase in healthy and inflamed human dental pulp. J Dent Res. 2004;83(4):312–316. doi: 10.1177/154405910408300408. [DOI] [PubMed] [Google Scholar]
- 16.Felaco M., Di Maio F.D., De Fazio P., D’Arcangelo C., De Lutiis M.A., Varvara G. Localization of the e-NOS enzyme in endothelial cells and odontoblasts of healthy human dental pulp. Life Sci. 2000;68(3):297–306. doi: 10.1016/s0024-3205(00)00935-8. [DOI] [PubMed] [Google Scholar]
- 17.Lohinai Z., Balla I., Marczis J., Vass Z., Kovách A.G. Evidence for the role of nitric oxide in the circulation of the dental pulp. J Dent Res. 1995;74(8):1501–1506. doi: 10.1177/00220345950740081101. [DOI] [PubMed] [Google Scholar]
- 18.Toda N., Ayajiki K., Okamura T. Neurogenic and endothelial nitric oxide regulates blood circulation in lingual and other oral tissues. J Cardiovasc Pharmacol. 2012;60(1):100–108. doi: 10.1097/FJC.0b013e318252452a. [DOI] [PubMed] [Google Scholar]
- 19.Lohinai Z., Székely A.D., Benedek P., Csillag A. Nitric oxide synthase containing nerves in the cat and dog dental pulp and gingiva. Neurosci Lett. 1977;227(2):91–94. doi: 10.1016/s0304-3940(97)00319-4. [DOI] [PubMed] [Google Scholar]
- 20.Fan W., Huang F., Li C., Qu H., Gao Z., Leng S. Involvement of NOS/NO in the development of chronic dental inflammatory pain in rats. Brain Res Rev. 2009;59(2):324–332. doi: 10.1016/j.brainresrev.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Law A.S., Baumgardner K.R., Meller S.T., Gebhart G.F. Localization and changes in NADPH-diaphorase reactivity and nitric oxide synthase immunoreactivity in rat pulp following tooth preparation. J Dent Res. 1999;78(10):1585–1595. doi: 10.1177/00220345990780100301. [DOI] [PubMed] [Google Scholar]
- 22.Yasuhara R., Suzawa T., Miyamoto Y., Wang X., Takami M., Yamada A. Nitric oxide in pulp cell growth, differentiation, and mineralization. J Dent Res. 2007;86(2):163–168. doi: 10.1177/154405910708600211. [DOI] [PubMed] [Google Scholar]
- 23.Mei Y.F., Yamaza T., Atsuta I., Danjo A., Kido M.A., Goto M. Sequential expression of endothelial nitric oxide synthase, inducible nitric oxide synthase, and nitrotyrosine in odontblasts and pulp cells during dentin repair after tooth preparation in rat molars. Cell Tissue Res. 2007;328(1):117–127. doi: 10.1007/s00441-005-0003-5. [DOI] [PubMed] [Google Scholar]
- 24.Holder M.J., Milward M.R., Palin W.M., Hadis M.A., Cooper P.R. Effects of red light-emitting diode irradiation on dental pulp cells. J Dent Res. 2012;91(10):961–966. doi: 10.1177/0022034512456040. [DOI] [PubMed] [Google Scholar]
- 25.D’Arcangelo C., Di Nardo-Di Maio F., Patrono C., Caputi S. NOS evaluations in human dental pulp-capping with MTA and calcium-hydroxide. Int J Immunopathol Pharmacol. 2007;20(1):27–32. doi: 10.1177/039463200702001s07. [DOI] [PubMed] [Google Scholar]
- 26.Warita H., Watarai H., Soma K. Nitric oxide synthase expression is increased by occlusal force in rat periodontal ligament. Orthod Craniofac Res. 2004;7(2):122–126. doi: 10.1111/j.1601-6343.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- 27.Korkmaz Y., Bloch W., Addicks K., Schneider K., Baumann M.A., Raab W.H. The Basal phosphorylation sites of endothelial nitric oxide synthase at serine (Ser)1177, Ser116, and threonine (Thr)495 in rat molar epithelial rests of Malassez. J Periodontol. 2005;76(9):1513–1519. doi: 10.1902/jop.2005.76.9.1513. [DOI] [PubMed] [Google Scholar]
- 28.Korkmaz Y., Bloch W., Behrends S., Schröder H., Addicks K., Baumann M.A. NO-cGMP signaling molecules in the rat epithelial rests of Malassez. Eur J Oral Sci. 2004;112(1):55–60. doi: 10.1111/j.0909-8836.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y.S., Pi S.H., Lee Y.M., Lee S.I., Kim E.C. The anti-inflammatory role of heme oxygenase-1 in lipopolysaccharide and cytokine-stimulated inducible nitric oxide synthase and nitric oxide production in human periodontal ligament cells. J Periodontol. 2009;80(12):2045–2055. doi: 10.1902/jop.2009.090145. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.K., Choi H.I., Yang Y.S., Jeong G.S., Hwang J.H., Lee S.I. Nitric oxide modulates osteoblastic differentiation with heme oxygenase-1 via the mitogen activated protein kinase and nuclear factor-kappaB pathways in human periodontal ligament cells. Biol Pharm Bull. 2009;32(8):1328–1334. doi: 10.1248/bpb.32.1328. [DOI] [PubMed] [Google Scholar]
- 31.Ambe K., Watanabe H., Takahashi S., Nakagawa T. Immunohistochemical localization of Nox1, Nox4 and Mn-SOD in mouse femur during endochondral ossification. Tissue Cell. 2014;46(6):433–438. doi: 10.1016/j.tice.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Batista A.C., Silva T.A., Chun J.H., Lara V.S. Nitric oxide synthesis and severity of human periodontal disease. Oral Dis. 2002;8(5):254–260. doi: 10.1034/j.1601-0825.2002.02852.x. [DOI] [PubMed] [Google Scholar]
- 33.Leitão R.F., Ribeiro R.A., Chaves H.V., Rocha F.A., Lima V., Brito G.A. Nitric oxide synthase inhibition prevents alveolar bone resorption in experimental periodontitis in rats. J Periodontol. 2005;76(6):956–963. doi: 10.1902/jop.2005.76.6.956. [DOI] [PubMed] [Google Scholar]
- 34.Silva M.J., Sousa L.M., Lara V.P., Cardoso F.P., Júnior G.M., Totola A.H. The role of iNOS and PHOX in periapical bone resorption. J Dent Res. 2011;90(4):495–500. doi: 10.1177/0022034510391792. [DOI] [PubMed] [Google Scholar]
- 35.Brandi M.L., Hukkanen M., Umeda T., Moradi-Bidhendi N., Bianchi S., Gross S.S. Bidirectional regulation of osteoclast function by nitric oxide synthase isoforms. Proc Natl Acad Sci U S A. 1995;92(7):2954–2958. doi: 10.1073/pnas.92.7.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hukkanen M.V., Platts L.A., Fernandez De Marticorena I., O'Shaughnessy M., MacIntyre I., Polak J.M. Developmental regulation of nitric oxide synthase expression in rat skeletal bone. J Bone Miner Res. 1999;14(6):868–877. doi: 10.1359/jbmr.1999.14.6.868. [DOI] [PubMed] [Google Scholar]
- 37.Watarai H., Warita H., Soma K. Effect of nitric oxide on the recovery of the hypofunctional periodontal ligament. J Dent Res. 2004;83(4):338–342. doi: 10.1177/154405910408300413. [DOI] [PubMed] [Google Scholar]
- 38.Shirazi M., Nilforoushan D., Alghasi H., Dehpour A.R. The role of nitric oxide in orthodontic tooth movement in rats. Angle Orthod. 2002;72(3):211–215. doi: 10.1043/0003-3219(2002)072<0211:TRONOI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 39.Tan S.D., Xie R., Klein-Nulend J., van Rheden R.E., Bronckers A.L., Kuijpers-Jagtman A.M. Orthodontic force stimulates eNOS and iNOS in rat osteocytes. J Dent Res. 2009;88(3):255–260. doi: 10.1177/0022034508330861. [DOI] [PubMed] [Google Scholar]
- 40.Nilforoushan D., Manolson M.F. Expression of nitric oxide synthases in orthodontic tooth movement. Angle Orthod. 2009;79(3):502–508. doi: 10.2319/050808-252.1. [DOI] [PubMed] [Google Scholar]
- 41.D’Attillio M., Di Maio F., D’Arcangela C., Filippi M.R., Felaco M., Lohinai Z. Gingival endothelial and inducible nitric oxide synthase levels during orthodontic treatment: a cross-sectional study. Angle Orthod. 2004;74(6):851–858. doi: 10.1043/0003-3219(2004)074<0851:GEAINO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Leone A., Mauro A., Spatola G.F., Provenzano S., Caradonna C., Gerbino A. MMP-2, MMP-9, and iNOS expression in human dental pulp subjected to orthodontic traction. Angle Orthod. 2009;79(6):1119–1125. doi: 10.2319/110308-557R.1. [DOI] [PubMed] [Google Scholar]
- 43.Iacob S., Veis A. Identification of the functional activity of the [A-4] amelogenin gene splice product in newborn mouse ameloblasts. Bone. 2008;42(6):1072–1079. doi: 10.1016/j.bone.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaccone G., Crescimanno C., Lo Cascio P., Mauceri A., Fasulo S., Sbarbati A. Immunohistochemical investigation of the nitrergic system in the taste organ of the frog, Rana esculenta. Chem Senses. 2002;27(9):825–830. doi: 10.1093/chemse/27.9.825. [DOI] [PubMed] [Google Scholar]
- 45.Soinila J., Nuorva K., Soinila S. Nitric oxide synthase in human salivary glands. Histochem Cell Biol. 2006;125(6):717–723. doi: 10.1007/s00418-005-0123-8. [DOI] [PubMed] [Google Scholar]
- 46.Mitsui Y., Furuyama S. Characterization of nitric oxide synthase in the rat parotid gland. Arch Oral Biol. 2000;45(7):531–536. doi: 10.1016/s0003-9969(00)00029-7. [DOI] [PubMed] [Google Scholar]
- 47.Looms D.K., Tritsaris K., Nauntofte B., Dissing S. Nitric oxide and cGMP activate Ca2+-release processes in rat parotid acinar cells. Biochem J. 2001;355:87–95. doi: 10.1042/0264-6021:3550087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosignoli F., Pérez Leirós C. Activation of nitric oxide synthase through muscarinic receptors in rat parotid gland. Eur J Pharmacol. 2002;439(1–3):27–33. doi: 10.1016/s0014-2999(02)01375-4. [DOI] [PubMed] [Google Scholar]
- 49.Sayardoust S., Ekström J. Parasympathetic nerve-evoked protein synthesis, mitotic activity and salivary secretion in the rat parotid gland and the dependence on NO-generation. Arch Oral Biol. 2006;51(3):189–197. doi: 10.1016/j.archoralbio.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Lomniczi A., Suburo A.M., Elverdin J.C., Mastronardi C.A., Diaz S., Rettori V. Role of nitric oxide in salivary secretion. Neuroimmunomodulation. 1998;5(5):226–233. doi: 10.1159/000026342. [DOI] [PubMed] [Google Scholar]
- 51.Konttinen Y.T., Platts L.A., Tuominen S., Eklund K.K., Santavirta N., Törnwall J. Role of nitric oxide in Sjögren's syndrome. Arthritis Rheum. 1997;40(5):875–883. doi: 10.1002/art.1780400515. [DOI] [PubMed] [Google Scholar]
- 52.Correia P.N., Carpenter G.H., Paterson K.L., Proctor G.B. Inducible nitric oxide synthase increases secretion from inflamed salivary glands. Rheumatology. 2010;49(1):48–56. doi: 10.1093/rheumatology/kep313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caulfield V.L., Balmer C., Dawson L.J., Smith P.M. A role for nitric oxide-mediated glandular hypofunction in a non-apoptotic model for Sjogren's syndrome. Rheumatology. 2009;48(7):727–733. doi: 10.1093/rheumatology/kep100. [DOI] [PubMed] [Google Scholar]
- 54.Takahama U., Hirota S., Takayuki O. Detection of nitric oxide and its derivatives in human mixed saliva and acidified saliva. Methods Enzymol. 2008;440:381–396. doi: 10.1016/S0076-6879(07)00824-5. [DOI] [PubMed] [Google Scholar]
- 55.Carossa S., Pera P., Doglio P., Lombardo S., Colagrande P., Brussino L. Oral nitric oxide during plaque deposition. Eur J Clin Invest. 2001;31(10):876–879. doi: 10.1046/j.1365-2362.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 56.Bahar G., Feinmesser R., Shpitzer T., Popovtzer A., Nagler R.M. Salivary analysis in oral cancer patients: DNA and protein oxidation, reactive nitrogen species, and antioxidant profile. Cancer. 2007;109(1):54–59. doi: 10.1002/cncr.22386. [DOI] [PubMed] [Google Scholar]
- 57.Hegde A.M., Neekhra V., Shetty S. Evaluation of levels of nitric oxide in saliva of children with rampant caries and early childhood caries: a comparative study. J Clin Pediatr Dent. 2008;32(4):283–286. doi: 10.17796/jcpd.32.4.4010kl5262687528. [DOI] [PubMed] [Google Scholar]
- 58.Choudhari S.K., Chaudhary M., Bagde S., Gadbail A.R., Joshi V. Nitric oxide and cancer: a review. World J Surg Oncol. 2013;11:118. doi: 10.1186/1477-7819-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avci A., Tüzüner-Oncül A.M., Gökcan M.K., Namuslu M., Oztürk A., Durak I. Nitric oxide metabolism in cancerous and non-cancerous oral gingivomucosal tissues: possible implications of nitric oxide in cancer process. J Oral Pathol Med. 2009;38(3):304–306. doi: 10.1111/j.1600-0714.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- 60.Connelly S.T., Macabeo-Ong M., Dekker N., Jordan R.C., Schmidt B.L. Increased nitric oxide levels and iNOS over-expression in oral squamous cell carcinoma. Oral Oncol. 2005;41(3):261–267. doi: 10.1016/j.oraloncology.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Varghese S.S., Sunil P.M., Madhavan R.N. Expression of inducible nitric oxide synthase (iNOS) in oral precancer and oral squamous cell carcinoma: an immunohistochemical study. Cancer Biomark. 2010–2011;8(3):155–160. doi: 10.3233/CBM-2011-0207. [DOI] [PubMed] [Google Scholar]
- 62.Morelatto R., Itoiz M.E., Guiñazú N., Piccini D., Gea S., López-de Blanc S. Nitric oxide synthase 2 (NOS2) expression in histologically normal margins of oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2014;19(3):242–247. doi: 10.4317/medoral.19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shang Z.J., Li J.R. Expression of endothelial nitric oxide synthase and vascular endothelial growth factor in oral squamous cell carcinoma: its correlation with angiogenesis and disease progression. J Oral Pathol Med. 2005;34(3):134–139. doi: 10.1111/j.1600-0714.2004.00259.x. [DOI] [PubMed] [Google Scholar]
- 64.Sappayatosok K., Maneerat Y., Swasdison S., Viriyavejakul P., Dhanuthai K., Zwang J. Expression of pro-inflammatory protein, iNOS, VEGF and COX-2 in oral squamous cell carcinoma (OSCC), relationship with angiogenesis and their clinico-pathological correlation. Med Oral Patol Oral Cir Bucal. 2009;14(7):319–324. [PubMed] [Google Scholar]
- 65.Zhao S.F., Tong X.Y., Zhu F.D. Nitric oxide induces oral squamous cell carcinoma cells apoptosis with p53 accumulation. Oral Oncol. 2005;41(8):785–790. doi: 10.1016/j.oraloncology.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Chen W.L., Yang L., Zeng S.G., Wang Y.J. Effect of using RNA interference to alter iNOS gene expression on the proliferation of tongue squamous cell carcinoma cell line Tca8113. Br J Oral Maxillofac Surg. 2008;46(6):435–438. doi: 10.1016/j.bjoms.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Harada K., Supriatno Kawaguchi S., Tomitaro O., Yoshida H., Sato M. Overexpression of iNOS gene suppresses the tumorigenicity and metastasis of oral cancer cells. In Vivo. 2004;18(4):449–455. [PubMed] [Google Scholar]
- 68.Chaiyarit P., Ma N., Hiraku Y., Pinlaor S., Yongvanit P., Jintakanon D. Nitrative and oxidative DNA damage in oral lichen planus in relation to human oral carcinogenesis. Cancer Sci. 2005;96(9):553–559. doi: 10.1111/j.1349-7006.2005.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumamoto H., Suzuki T., Ooya K. Immunohistochemical analysis of inducible nitric oxide synthase (iNOS) and heat shock proteins (HSPs) in ameloblastomas. J Oral Pathol Med. 2002;31(10):605–611. doi: 10.1034/j.1600-0714.2002.00014.x. [DOI] [PubMed] [Google Scholar]


