Summary
During tooth development, ameloblasts differentiate from inner enamel epithelial cells to enamel-forming cells by modulating the signal pathways mediating epithelial–mesenchymal interaction and a cell-autonomous gene network. The differentiation process of epithelial cells is characterized by marked changes in their morphology and polarity, accompanied by dynamic cytoskeletal reorganization and changes in cell–cell and cell–matrix adhesion over time. Functional ameloblasts are tall, columnar, polarized cells that synthesize and secrete enamel-specific proteins. After deposition of the full thickness of enamel matrix, ameloblasts become smaller and regulate enamel maturation. Recent significant advances in the fields of molecular biology and genetics have improved our understanding of the regulatory mechanism of the ameloblast cell life cycle, mediated by the Rho family of small GTPases. They act as intracellular molecular switch that transduce signals from extracellular stimuli to the actin cytoskeleton and the nucleus. In our review, we summarize studies that provide current evidence for Rho GTPases and their involvement in ameloblast differentiation. In addition to the Rho GTPases themselves, their downstream effectors and upstream regulators have also been implicated in ameloblast differentiation.
Keywords: Tooth, Ameloblasts, Rho GTPase, RhoA, Rac1, ROCK
1. Introduction
Development of teeth as epithelial appendages is a complex process regulated by inductive interaction between the epithelium and the underlying mesenchymal cells. The earliest event of tooth development is the thickening of the epithelium (the primary dental lamina), followed by condensation of the mesenchymal cells [1], [2]. The development of the tooth crown advances through various stages defined by the morphology of the epithelium (bud, cap, and bell) and is followed by the formation of the root. The transition from the bud to the cap stage is a critical step in tooth morphogenesis. Signals from the enamel knot, an early epithelial signaling center, regulate growth and determine the site of epithelial folds that correspond directly with the cusp pattern of the mature tooth [3]. During the cap and bell stages, the size and shape of the tooth crown become apparent by the differentiation of cells into ameloblasts and odontoblasts that secrete the mineralizing matrices of the enamel and dentin, respectively. In the bell stage, the dental epithelium (enamel organ) segregates into four distinct cell types: inner enamel epithelial cells (IEEs), outer dental epithelial cells (OEEs), stratum intermedium (SI), and stellate reticulum (SR). The IEEs eventually differentiate into ameloblasts [4]. In the subsequent transitional stage from crown to root formation, the central core of the epithelium (SI and SR) disappears, leaving only a double layer of IEEs and OEEs called Hertwig's epithelial root sheath (HERS). It directs root growth and gives rise to a fenestrated network of epithelial cells which covers the root, known as the epithelial cell rests of Malassesz (ERM) [4].
The differentiation of epithelial cells into functional ameloblasts comprises several steps of morphological and functional changes. In the proliferation stage, the low columnar IEEs actively proliferate to form the basic shape of the tooth. Then, in differentiation stage, IEEs grow into columnar cells (preameloblasts) with more protein synthesizing organelles. The distal ends of the preameloblasts are flat, and the enamel matrix secreted is called rodless enamel matrix. In the secretory stage, the cells (secretory ameloblasts) lengthen, polarize, and form conical projections called Tome's process and deposit enamel in the form of rods. In transitional stage, when enamel reaches its full thickness, the height of ameloblasts decrease and protein synthesizing organelles are drastically reduced (transitional stage ameloblasts). The number of the ameloblasts is reduced by apoptosis in this stage. In the maturation stage, the ameloblasts modulate and transport specific ions necessary for the simultaneous deposition of minerals, and at the same time they also degrade enamel proteins and resorb the degraded proteins and water. The ameloblasts initiate a series of repetitive morphological change at the enamel surface, in which tight junction and deep membrane infoldings periodically appear (ruffle-ended ameloblasts [RA]), then disappear for short intervals (smooth ended ameloblasts [SA]) from distal end of the cells. In the regressive stage, the ameloblasts (reduced enamel epithelium) lose their differentiation and become short cuboidal cell, which is indistinguishable from other layers of the enamel organ. Reduced enamel epithelium remains on the surface of formed enamel until the tooth erupts. After crown morphogenesis, the boundary where IEEs and OEEs meet, referred to as the cervical loop, ceases to differentiate into ameloblasts and forms HERS with OEEs to induce root formation [4], [5], [6], [7].
Amelogenesis is a complicated process, as described above, and for the last several decades, various animal and human studies have used molecular genetics to identify a number of signaling molecules and gene networks that act at specific stages of the ameloblast life cycle and regulate its patterning and differentiation processes. Rho GTPases, including RhoA, Rac1, and Cdc42, have been identified as the regulatory mechanism for cellular events such as migration, polarization, cytokinesis, cell–cell adhesion, cell cycle, and gene expression in many cell types [8], [9], [10]. Until recently, Rho GTPases were believed to be involved primarily in the regulation of cytoskeletal organization in response to extracellular molecules. However, recent studies have demonstrated that they play crucial roles in many cellular events such as transcriptional activation, cell proliferation, cell polarity, cell–cell adhesion, membrane trafficking, muscle contraction, ion channel activity, endothelial permeability, reactive oxygen species production, phospholipid metabolism, and embryonic development [9], [11]. Further, they are involved in osteoclastogenesis as well as in hematopoiesis and hemopathies [12], [13]. Recently, evidence has emerged showing the involvement of Rho signaling in tooth development [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. In this article, we summarize some interesting recent findings that provide molecular insights into how signaling by Rho GTPases results in tooth development, focusing on ameloblast differentiation in particular.
2. General aspects of Rho GTPases
2.1. Rho GTPases
Rho GTPases form a subgroup of the Ras superfamily of 20–30 kDa GTP-binding proteins that have been shown to regulate a wide spectrum of cellular functions. The Ras gene was first discovered as the v-Ras oncogene of the Rous sarcoma virus [25]. The Ras superfamily contains more than 130 members that belong to the Ras, Rho, Arf/Sar1, and Rab/Ran subfamilies [25], [26]. A comparison of the amino acid sequences of Rho proteins from various species has revealed that they preserve their primary structure and are 50–55% homologous to one another. The Rho gene was discovered as a homolog of the ras gene in Aplysia, and the Rho homologs RhoA, RhoB, and RhoC were discovered in mammalian cells [27], [28]. Other members of the Rho-subfamily including Cdc42, Rac1, and Rac2 were identified and found to be distinct in function from the other Rho proteins [28], [29], [30], [31]. Among the Rho GTPases, Rho, Rac, and Cdc42 [32] are the most frequently studied members. In the literature, the Ras, Arf/Sar1, and Rab/Ran-subfamilies have received a smattering of attention but overall remain relatively unclear and therefore, will not be discussed in this review.
2.2. Regulating Rho GTPases activity
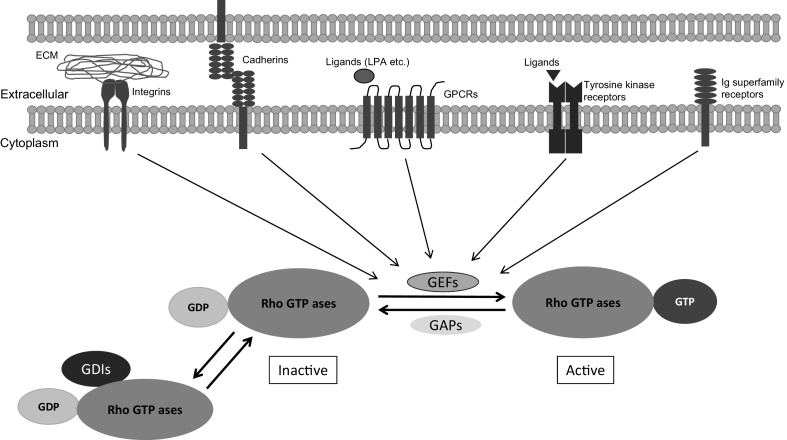
Similar to all members of the Ras superfamily, Rho GTPases function as molecular switches, cycling between an inactive GDP-bound state and an active GTP-bound state. Upstream signals as described below stimulate dissociation and the binding of GTP. This leads to conformational changes in the effector-binding region of the GTPase, leading to interaction of this region with downstream effectors. The GTP-bound form is then converted to the GDP-bound form by the intrinsic GTPase activity that releases bound effectors [32], [33]. The ratio of the two forms is regulated by the opposing effects of guanine nucleotide exchange factors (GEFs) that boost the exchange of bound GDP for GTP and the GTPase-activating proteins (GAPs) that enhance the intrinsic rate of hydrolysis of bound GTP. The Rho GTPases are also regulated by guanine nucleotide dissociation inhibitors (GDIs) that inhibit the exchange of GTP and the hydrolysis of bound GTP [34] (Fig. 1). Dominant active mutants such as Cdc42V12, Rac V12, and Rho V14 correspond to the permanent GTP-bound state, while dominant negative mutants such as Cdc42N17, Rac N17, and Rho N19 bind tighter with GEFs than the wild-type (WT) GTPases but do not bind to effector proteins [35]. In addition, recent findings showed additional levels of regulation through ubiquitination and subsequent targeting of the proteasome [36]. Rho GDIs have recently been shown to protect Rho GTPases from degradation [37].
Figure 1.
Regulation of Rho GTPase activity. Rho-GDP dissociation inhibitors (Rho GDI) sequester inactive GDP-bound Rho GTPases in the cytoplasm. When released from Rho-GDIs, transmembrane receptors and cell adhesion molecules activate GDP-bound Rho GTPases through GEF, which in turn catalyze the exchange of GDP for GTP. Once GTP-bound, Rho GTPases can bind to a variety of downstream effectors and elicit diverse responses. Inactivation of Rho GTPases is mediated by GAPs that promote GTP hydrolysis to GDP.
Previous articles have described the upstream signaling from the cell surface to Rho proteins. Lysophosphatidic acid (LPA) was identified as a Rho activator, while bombesin was shown to independently activate Rho and Rac, both of which are ligands for G protein-coupled receptors (GPCRs) [29], [31] (Fig. 1). For example, LPA has seven transmembrane receptors that couple with heterotrimeric G proteins. The signal to Rho is mediated by the activation of Gα12/13 subunits, which in turn bind to the RGS domain of particular Rho GEFs, bringing them to the membrane and activating them [38], [39]. Rho GEFs containing an RGS domain are p115Rho GEF, PDZ-Rho GEF, and leukemia-associated RhoGEF, and they are all activated by heterotrimeric G proteins [40]. Rho GEFs can also be activated by the α subunit of Gq (Gαq), which normally couples with phospholipase C [41], [42], [43], [44]. For Rac1, activation of GPCRs that couples with Gαi (N-formyl-methionyl-leucyl-phenylalanine bombesin, endothelin-1, LPA, etc.) or receptor tyrosine kinase in turn activates Rho GEFs. These receptors stimulate phosphatidylinositol 3-kinase activity and increase formation of phosphatidylinositol-3, 4, 5-(PO4) 3 that can activate Rac GEFs such as Tiam 1, P-Rex1, and others [9], [45]. Additionally, other growth factors such as platelet-derived growth factor, epidermal growth factor, and insulin binding tyrosine kinase receptors were also seen to stimulate Rac, leading to a subsequent Rho activation (Fig. 1), while protein kinase C agonists such as phorbol myristate acetate were found to activate Rac without activating Rho.
Rho GTPases also activate downstream signaling pathways that are initiated by the cell adhesion molecules. Several classes of cell adhesion molecules including integrins [46], cadherins [47], and Ig superfamily members [48] have been shown to affect Rho, Rac, or Cdc42 activity (Fig. 1). Integrin-mediated spreading and focal adhesion maturation develop as a result of biphasic reactions associated with the relative activities of RhoA and Rac1 [49], [50], [51], [52], [53], [54]. Early adhesion, characterized by the presence of small and nascent adhesions that form just behind the leading edge of spreading or migrating cell [55], [56], is dependent on Rac1 activation and a concomitant suppression of RhoA activity. In contrast, late adhesion and mature focal adhesion formation rely on elevated RhoA activity and Rac1 inhibition. Moreover, there is an elevation in the activities of Rac and Cdc42 (Fig. 1) upon cadherin engagement, which may enhance cadherin function by positive feedback. However, cadherin engagement drastically suppresses Rho activity, partly by increasing p190Rho GAP activity [57].
2.3. Downstream effectors of RhoA
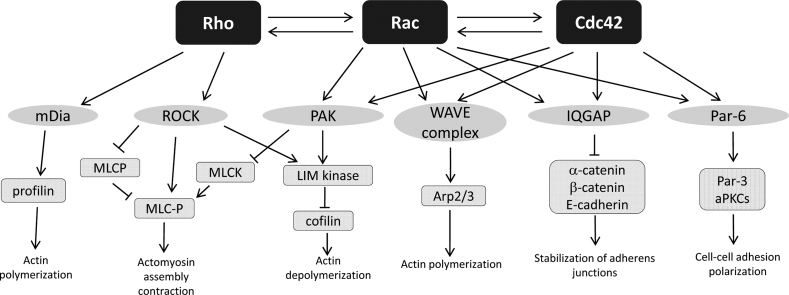
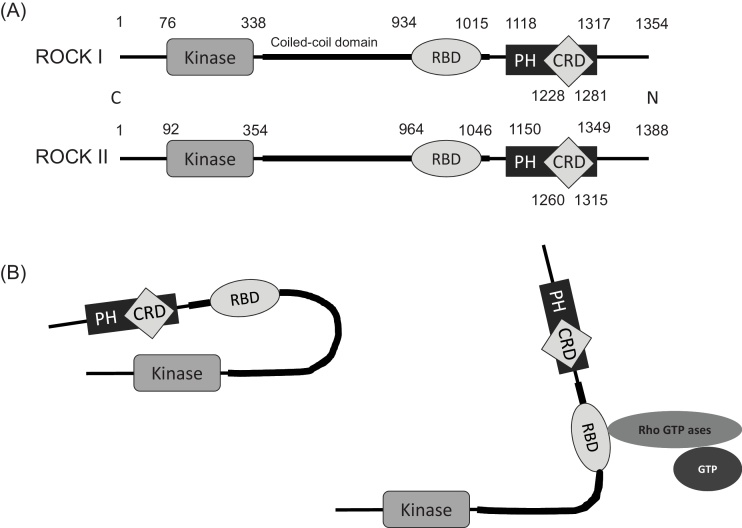
The effects of Rho GTPases on a wide variety of cellular events are mediated by the stimulation of downstream effector kinases by activated Rho GTPases. Among RhoA effectors, the most well-known proteins are Rho kinase (ROCK) and mammalian diaphanous (mDia) (Fig. 2). ROCK is a serine/threonine kinase with a molecular mass of approximately 160 kDa. Two distinct genes encode two isoforms: ROCK I (p160-ROCK, ROKβ) and ROCK II (Rho kinase and ROKα) [58], [59], [60] (Fig. 3). The human ROCK I and ROCK II genes are located on chromosome 18 (18q11.1) and chromosome 2 (2P24), respectively. These isoforms are highly homologous, with an overall amino acid sequence identity of 65%. Their homology reaches 90% in the N-terminal serine/threonine kinase domains, with lower identity in their C-terminal [60]. Both isoforms consist of N-terminal kinase domains, a central coiled-coil region containing the Rho binding domain (RBD), and a C-terminal PH domain with a Cys-rich region. The C-terminus, including the RBD and PH domains, is an auto-inhibitory region that inhibits kinase activity under basal conditions via intramolecular association with the kinase domain [61], [62]. Rho proteins bind to the ROCK RBD domain in their active GTP-charged state, which enhances ROCK catalytic activity through induction of conformational changes that diminish C-terminal-mediated auto-inhibition via exposure of the kinase domain [59], [62], [63], [64] (Fig. 3).
Figure 2.
Effector of Rho GTPases. Activated Rho GTPases bind to and activate protein kinases such as members of the PAK or ROCK families or to scaffolding proteins. These effector proteins interact with several other proteins and have distinct effects on cytoskeletal organization, cell–cell adhesion, and polarization. The cross talk between the Rho GTPases is also indicated.
Figure 3.
The molecular structure of ROCK. ROCK sequences include a kinase domain located at the amino terminus of the protein, a coiled-coil region containing RBD, and a PH domain with a cysteine-rich domain (CRD). ROCK I and ROCK II are highly homologous, with an overall amino acid sequence identity of 65% (A). Intramolecular association of the C-terminal region and the kinase domain. Rho binding induces conformational changes that inhibit its ability to promote kinase activation (B).
In addition to Rho GTPase, lipids such as archidonic acid and sphingosine, caspase-3, and granzyme B can also activate ROCK [65], [66], [67], [68]. The ROCK phosphorylates an abundant array of downstream targets, which in turn modify the ultrastructural assemblies of stress fibers and induce focal adhesions that are important for the regulation of cell contractility, motility, and morphology. For these functions, ROCK phosphorylates a variety of substrates such as myosin light chain (MLC), MLC phosphatase, Lin-11ISL-1 Mec-3 (LIM) kinase (LIMK) (Fig. 2), ERM, and intermediate filament proteins [69], [70], [71], [72], [73], [74]. Another Rho effector, mDia, mediates actin polymerization through a profilin-dependent mechanism, as well as stabilization of microtubule plus ends in cell migration [75], [76] (Fig. 2).
2.4. Downstream effectors of Rac and Cdc42
Numerous effectors of Rac and Cdc42 that mediate cellular activities such as formation of lamellipodia and filopodia, membrane raffling for cell migration, cell–cell adhesion, and polarization have been identified. The members of the WASP/SCAR/WAVE family of scaffold proteins are key nucleation-promoting factors that activate the Arp2/3 complex, crucial for localized assembly of actin networks within filopodia [77]. p21-activated kinase (PAK) family is another downstream effector of Rac and Cdc42 that plays a role in cytoskeletal arrangement and membrane ruffling. Several PAK substrates or binding partners, including actin-binding protein filamin [78], LIMK [79], myosin light chain kinase [80], Paxillin/Pix/PKL complex [81], and the adaptor protein Nck [82], have been implicated in the effects of PAK.
The scaffolding proteins IQGAP and Par-6, both of which can be activated by either Rac or Cdc42, promote cell polarization and contribute to cell–cell adhesion [83], [84], [85], [86]. Agitation of these molecules, and subsequently cell–cell contacts, suggests that they may promote motility through disruption of the normal organization of neighboring cells (Fig. 2).
3. Implication of Rho GTPases signaling in ameloblasts
3.1. Expression of RhoA and effector molecules in ameloblasts
Studies that investigated tooth developments based on their protein- or gene-expression profiles showed that RhoA and ROCK are highly correlated with ameloblast differentiation. During initiation and morphogenesis of tooth germ, RhoA is uniformly distributed in enamel organ. Nonpolarized cells in the inner enamel epithelium of the molar tooth germ of 3-day-old rats showed weak staining for RhoA, which differed from that seen in polarizing cells. RhoA is evenly distributed throughout the cytoplasm [14]. mRNA expression for RhoA, ROCK I, and ROCK II is low in embryonic and newborn molar tooth germs but increases until postnatal day (PN) 5 in rat molars [14].
In a mouse incisor, the expression of ROCK I and ROCK II gradually increases during ameloblast differentiation. Both isoforms are strongly expressed in highly polarized secretory ameloblasts and the underlying SI. In contrast, low expression levels are observed in basal dental epithelial cells, IEEs, OEEs and preameloblasts, suggesting a strong correlation between ROCK and ameloblast differentiation [15].
3.2. Functional role of RhoA-ROCK signaling in ameloblast differentiation
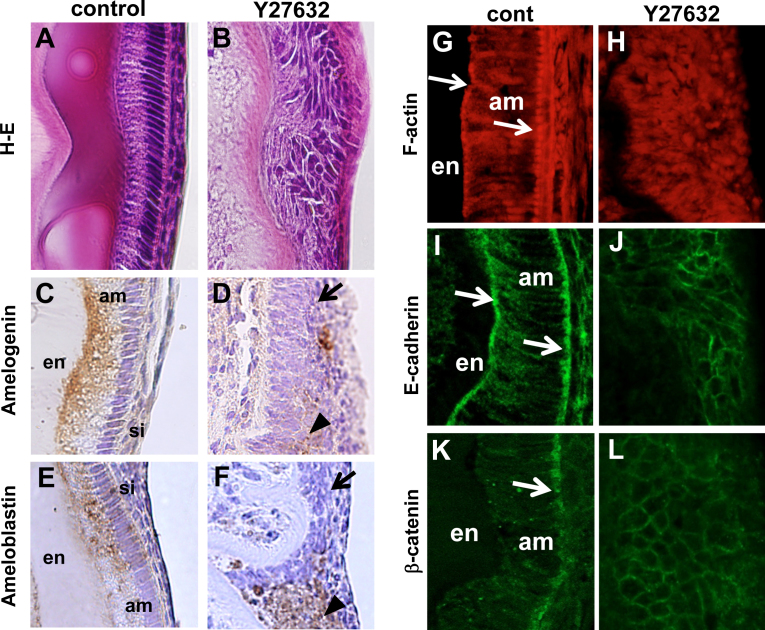
Several organ cultures of tooth germs and transgenic (Tg) approaches have been developed to evaluate the function of RhoA-ROCK signal cascade in ameloblast differentiation and enamel formation. The inhibition of all Rho GTPase by Clostridium difficile toxin A and specific inhibition of ROCK decrease amelogenin expression in tooth germs cultured in an anterior eye chamber [14]. ROCK inhibitors also disrupt ameloblast polarity and enamel formation, and amelogenins and ameloblastins are no longer directionally secreted in cultured incisors [15], [16] (Fig. 4). ROCK inhibitors were seen to markedly affect actin, E-cadherin, and β-catenin localization in the same samples and dental epithelial cell cultures (Fig. 4). Furthermore, knockdown of ROCK expressions by siRNA in cultured dental epithelial cells affects actin organization and cell–cell adhesion and reduces the expression of E-cadherin and β-catenin mRNA [15]. Inhibition of ROCK also accelerates proliferation of dental epithelial cells [15].
Figure 4.
Effects of a ROCK inhibitor on ameloblasts. H–E staining of ROCK inhibitor (Y27632)-treated mouse ameloblasts shows disruption of polarity and enamel formation (A and B). Immunostaining for amelogenin and ameloblastin (C–F) shows that these proteins are no longer directionally secreted. Arrows indicate cells that do not express enamel matrix proteins. Arrowheads indicate cells that secrete enamel matrix proteins in all directions. Staining of F-actin, E-cadherin and β-catenin shows the polarized distribution of those molecules in differentiated ameloblasts (G, I and K arrows), whereas ROCK inhibition disrupts the polarized distribution (H, J and L). The figures were reproduced from the study by Otsu et al. [15]. am, ameloblasts; en, enamel; si, stratum intermedium.
The transgenic mice in which dominant-negative T19N RhoA was expressed under the control of the amelogenin regulatory sequences showed enamel hypoplasia and surface defects in the molar cusps [17], [18]. In the Tg molar, amelogenin, E-cadherin, and Ki67 (proliferation marker) were reduced, and both canonical and noncanonical Wnt signaling pathways were activated [17], [19]. Sodium fluoride treatment (NaF) elevated filament actin (F-actin) through the RhoA pathway in ameloblasts [18], [20]. NaF can also activate both canonical and noncanonical Wnt pathways [19]. Cultured molars from the Tg strain showed lower F-actin fluorescence after NaF treatment, when compared with WT mice [17]. Furthermore, the elevation of F-actin by NaF is diminished in the presence of ROCK inhibitors in tooth organ cultures [18], [20]. Myosin, the other downstream target of ROCK, is abundant at the junction of secretory ameloblasts [21]. However, the regulatory mechanism of Myosin by Rho signaling during ameloblasts differentiation has not been elucidated.
RhoA protein is formed during differentiation of murine ameloblasts, and the levels of the Rho inhibitor, Rho GDI, normally decrease in ameloblasts as enamel protein expression begins, presumably to direct RhoA activation. In contrast, the double knockout for amelogenin and ameloblastin exhibits increase expression of Rho GDI, reinforcing the idea that Rho pathways are stimulated in a potentially positive feedback loop once ameloblasts begin enamel secretion [22].
These results indicate that RhoA-ROCK signaling may be a crucial molecular indicator for the structural integrity of ameloblasts, and the establishment of spatiotemporally appropriate modifications of cell–cell adhesion and cytoskeleton, corresponding to the degree of cell differentiation, is required for proper ameloblast differentiation.
3.3. Expression of Rac1 and Cdc42 in ameloblasts
It has been reported that Rac1 and Cdc42 express during ameloblast differentiation. The expression pattern of Rac1 protein in rat molar is similar to that of RhoA during initiation and morphogenesis. However, its expression becomes intense in the ameloblasts during cytodifferentiation. Rac1 strongly expresses in polarizing ameloblasts with a punctate appearance and is concentrated in the distal pole. Pak3, one of Rac1 effector, shows the same expression pattern as Rac1. The expression of Rac1 and Pak3 mRNA increases from PN3 to PN5. Cdc42 is uniformly distributed during the proliferation and cytodifferentiation phases [14].
3.4. Functional role of Rac1 and Cdc42 in ameloblast differentiation
The role of Rac1 in cell–matrix interaction, and subsequent matrix biomineralization, during enamel formation has been determined using Rac1 conditional knockout mice in which the cytokeratin 14 (K14) promoters bring about Cre expression in dental epithelial cells [23]. In the Tg mice, the Tomes’ processes lose contact with the forming enamel matrix in unerupted incisors, and the quantity of amelogenin and ameloblastin is reduced in the ameloblasts. After eruption, the enamel of the Tg mice shows severe structural defects, with complete loss of enamel [23]. These results suggest the involvement of Rac1 in cell–matrix interaction and matrix biomineralization. Further, Fukumoto et al. demonstrated that Rac1 and Cdc42 regulate laminin-10/-11, interact with integrin α6β4, and mediate cell polarity, spreading, and filopodia formation of the dental epithelium [24].
4. Rho GTPases in ameloblastomas
Interestingly, it appears that Rho GTPase plays a crucial role not only in normal ameloblasts differentiation but also in pathologies such as ameloblastomas. A study using immunohistochemistry to analyze the expression and distribution of Rho GTPase in solid and unicystic amemoblastomas reported that RhoA and Rho B were observed in a high number of cells and also had greater intensity in nonpolarized cells in all follicular, plexiform, and unicystic ameloblastomas. Comparison of differences in solid and unicystic variations was significant with the unicystic variant showing a higher number of positive cells [87]. Similarly, Cdc42 expresses stronger in nonpolarized cells than in basal polarized cells in all types of ameloblastomas. The unicystic subtype showed a higher number of positive cells compared with the solid ameloblastomas [87]. These results suggest that Rho GTPase plays a role in the determination of epithetical cell phenotypes, variants, and subtypes in ameloblastomas.
5. Concluding remarks
In this review, we have outlined the general aspects of Rho GTPase and its involvement with amelogenesis. The findings referred to in this article provide evidence for essential functions performed by Rho GTPase in cytoskeletal rearrangement, cell–cell or cell–matrix adhesion, cell proliferation, and gene transcription in ameloblasts. While our understanding of the biological mechanisms that control ameloblast differentiation is continually advancing, there is much left to be revealed about the specific role of each Rho GTPase protein in the process. Finally, future studies may establish a novel strategy for therapeutic interventions in which Rho GTPases will be targeted to prevent dental abnormalities such as amelogenesis imperfecta and odontogenic tumors including ameloblastomas. They may also help elucidate the molecular and cellular mechanisms of tooth regeneration.
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers 20890208 and 22791774 to KO, 19390466 to HH, and Open Research Project Grant (2007–2011 to KO and HH) from MEXT.
References
- 1.Stock D.W. Zebrafish dentition in comparative context. J Exp Zool B: Mol Dev Evol. 2007;308(5):523–549. doi: 10.1002/jez.b.21187. [DOI] [PubMed] [Google Scholar]
- 2.Smith M.M. Vertebrate dentitions at the origin of jaws: when and how pattern evolved. Evol Dev. 2003;5(4):394–413. doi: 10.1046/j.1525-142x.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- 3.Jernvall J., Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92(1):19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- 4.Nanci A., editor. Ten Cate's oral histology. 7th ed. Elsevier; 2008. [Google Scholar]
- 5.Fujiwara N., Kagiya T., Ishizeki K., Harada H. Molecular mechanisms regulating transition from crown to root formation in the development of mouse molars. J Oral Biosci. 2008;50(3):154–159. [Google Scholar]
- 6.Yokohama-Tamaki T., Ohshima H., Fujiwara N., Takada Y., Ichimori Y., Wakisaka S. Cessation of Fgf10 signaling, resulting in a defective dental epithelial stem cell compartment, leads to the transition from crown to root formation. Development. 2006;133(7):1359–1366. doi: 10.1242/dev.02307. [DOI] [PubMed] [Google Scholar]
- 7.Bei M. Molecular genetics of ameloblast cell lineage. J Exp Zool B: Mol Dev Evol. 2009;312B(5):437–444. doi: 10.1002/jez.b.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Aelst L., Symons M. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev. 2002;16(9):1032–1054. doi: 10.1101/gad.978802. [DOI] [PubMed] [Google Scholar]
- 9.Burridge K., Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz A.A., Govek E.-E., Böttner B., Van Aelst L. Rho GTPases: signaling, migration, and invasion. Exp Cell Res. 2000;261(1):1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz M. Rho signalling at a glance. J Cell Sci. 2004;117(23):5457–5458. doi: 10.1242/jcs.01582. [DOI] [PubMed] [Google Scholar]
- 12.Mulloy J.C., Cancelas J.A., Filippi M.-D., Kalfa T.A., Guo F., Zheng Y. Rho GTPases in hematopoiesis and hemopathies. Blood. 2010;115(5):936–947. doi: 10.1182/blood-2009-09-198127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung R., Glogauer M. Springer; 2012. Rho GTPase techniques in osteoclastogenesis. Rho GTPases; pp. 167–179. [DOI] [PubMed] [Google Scholar]
- 14.Biz M., Marques M., Crema V., Moriscot A., dos Santos M. GTPases RhoA and Rac1 are important for amelogenin and DSPP expression during differentiation of ameloblasts and odontoblasts. Cell Tissue Res. 2010;340(3):459–470. doi: 10.1007/s00441-010-0961-0. [DOI] [PubMed] [Google Scholar]
- 15.Otsu K., Kishigami R., Fujiwara N., Ishizeki K., Harada H. Functional role of rho-kinase in ameloblast differentiation. J Cell Physiol. 2011;226(10):2527–2534. doi: 10.1002/jcp.22597. [DOI] [PubMed] [Google Scholar]
- 16.Otsu K., Sakano M., Masuda T., Fujiwara N., Harada H. The role of Rho-kinases in ameloblast differentiation. J Oral Biosci. 2013;55(4):159–164. [Google Scholar]
- 17.Xue H., Li Y., Everett E.T., Ryan K., Peng L., Porecha R. Ameloblasts require active RhoA to generate normal dental enamel. Eur J Oral Sci. 2013;121(4):293–302. doi: 10.1111/eos.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y., Pugach M.K., Kuehl M.A., Peng L., Bouchard J., Hwang S.Y. Dental enamel structure is altered by expression of dominant negative RhoA in ameloblasts. Cells Tissues Organs. 2011;194(2–4):227. doi: 10.1159/000324559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng L., Li Y., Shusterman K., Kuehl M., Gibson C.W. Wnt-RhoA signaling is involved in dental enamel development. Eur J Oral Sci. 2011;119(s1):41–49. doi: 10.1111/j.1600-0722.2011.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., Decker S., Yuan Z.A., Denbesten P.K., Aragon M.A., Jordan-Sciutto K. Effects of sodium fluoride on the actin cytoskeleton of murine ameloblasts. Arch Oral Biol. 2005;50(8):681–688. doi: 10.1016/j.archoralbio.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Nishikawa S., Fujiwara K., Kitamura H. Formation of the tooth enamel rod pattern and the cytoskeletal organization in secretory ameloblasts of the rat incisor. Eur J Cell Biol. 1988;47(2):222–232. [PubMed] [Google Scholar]
- 22.Hatakeyama J., Fukumoto S., Nakamura T., Haruyama N., Suzuki S., Hatakeyama Y. Synergistic roles of amelogenin and ameloblastin. J Dent Res. 2009;88(4):318–322. doi: 10.1177/0022034509334749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z., Kim J., Lacruz R.S., Bringas P., Jr., Glogauer M., Bromage T.G. Epithelial-specific knockout of the Rac1 gene leads to enamel defects. Eur J Oral Sci. 2011;119:168–176. doi: 10.1111/j.1600-0722.2011.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukumoto S., Miner J.H., Ida H., Fukumoto E., Yuasa K., Miyazaki H. Laminin α5 is required for dental epithelium growth and polarity and the development of tooth bud and shape. J Biol Chem. 2006;281(8):5008–5016. doi: 10.1074/jbc.M509295200. [DOI] [PubMed] [Google Scholar]
- 25.Takai Y., Sasaki T., Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 26.Rojas A.M., Fuentes G., Rausell A., Valencia A. The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol. 2012;196(2):189–201. doi: 10.1083/jcb.201103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madaule P., Axel R. A novel ras-related gene family. Cell. 1985;41(1):31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- 28.Ridley A.J. Springer; 2012. Historical overview of Rho GTPases. Rho GTPases; pp. 3–12. [DOI] [PubMed] [Google Scholar]
- 29.Ridley A.J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 30.Shinjo K., Koland J.G., Hart M.J., Narasimhan V., Johnson D.I., Evans T. Molecular cloning of the gene for the human placental GTP-binding protein Gp (G25K): identification of this GTP-binding protein as the human homolog of the yeast cell-division-cycle protein CDC42. Proc Natl Acad Sci U S A. 1990;87(24):9853–9857. doi: 10.1073/pnas.87.24.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridley A.J., Paterson H.F., Johnston C.L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 32.Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 33.Khalil B.D., El-Sibai M. Rho GTPases in primary brain tumor malignancy and invasion. J Neurooncol. 2012;108(3):333–339. doi: 10.1007/s11060-012-0866-8. [DOI] [PubMed] [Google Scholar]
- 34.Jaffe A.B., Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 35.Feig L.A. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat Cell Biol. 1999;1(2):E25–E27. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- 36.Doye A., Mettouchi A., Lemichez E. Springer; 2012. Assessing ubiquitylation of Rho GTPases in mammalian cells. Rho GTPases; pp. 77–86. [DOI] [PubMed] [Google Scholar]
- 37.Boulter E., Garcia-Mata R., Guilluy C., Dubash A., Rossi G., Brennwald P.J. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12(5):477–483. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hart M.J., Jiang X., Kozasa T., Roscoe W., Singer W.D., Gilman A.G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα13. Science. 1998;280(5372):2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 39.Kozasa T., Jiang X., Hart M.J., Sternweis P.M., Singer W.D., Gilman A.G. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science. 1998;280(5372):2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 40.Fukuhara S., Chikumi H., Gutkind J.S. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene. 2001;20(13):1661. doi: 10.1038/sj.onc.1204182. [DOI] [PubMed] [Google Scholar]
- 41.Chikumi H., Vázquez-Prado J., Servitja J.-M., Miyazaki H., Gutkind J.S. Potent activation of RhoA by Gαq and Gq-coupled receptors. J Biol Chem. 2002;277(30):27130–27134. doi: 10.1074/jbc.M204715200. [DOI] [PubMed] [Google Scholar]
- 42.Booden M.A., Siderovski D.P., Der C.J. Leukemia-associated Rho guanine nucleotide exchange factor promotes Gαq-coupled activation of RhoA. Mol Cell Biol. 2002;22(12):4053–4061. doi: 10.1128/MCB.22.12.4053-4061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogt S., Grosse R., Schultz G., Offermanns S. Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11. J Biol Chem. 2003;278(31):28743–28749. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]
- 44.Lutz S., Freichel-Blomquist A., Yang Y., Rümenapp U., Jakobs K.H., Schmidt M. The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J Biol Chem. 2005;280(12):11134–11139. doi: 10.1074/jbc.M411322200. [DOI] [PubMed] [Google Scholar]
- 45.Welch H.C., Coadwell W.J., Stephens L.R., Hawkins P.T. Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 2003;546(1):93–97. doi: 10.1016/s0014-5793(03)00454-x. [DOI] [PubMed] [Google Scholar]
- 46.DeMali K.A., Burridge K. Coupling membrane protrusion and cell adhesion. J Cell Sci. 2003;116(12):2389–2397. doi: 10.1242/jcs.00605. [DOI] [PubMed] [Google Scholar]
- 47.Braga V.M. Cell–cell adhesion and signalling. Curr Opin Cell Biol. 2002;14(5):546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 48.Thompson P.W., Randi A.M., Ridley A.J. Intercellular adhesion molecule (ICAM)-1, but not ICAM-2, activates RhoA and stimulates c-fos and rhoA transcription in endothelial cells. J Immunol. 2002;169(2):1007–1013. doi: 10.4049/jimmunol.169.2.1007. [DOI] [PubMed] [Google Scholar]
- 49.Arthur W.T., Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12(9):2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arthur W.T., Petch L.A., Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10(12):719–722. doi: 10.1016/s0960-9822(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 51.Huveneers S., Danen E.H.J. Adhesion signalingcrosstalk between integrins, Src and Rho. J Cell Sci. 2009;122(8):1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 52.Guilluy C., Garcia-Mata R., Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21(12):718–726. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price L.S., Leng J., Schwartz M.A., Bokoch G.M. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9(7):1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ren X.D., Kiosses W.B., Alexander Schwartz M. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18(3):578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alexandrova A.Y., Arnold K., Schaub S., Vasiliev J.M., Meister J.-J., Bershadsky A.D. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE. 2008;3(9):e3234. doi: 10.1371/journal.pone.0003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi C.K., Vicente-Manzanares M., Zareno J., Whitmore L.A., Mogilner A., Horwitz A.R. Actin and [alpha]-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10(9):1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noren N.K., Arthur W.T., Burridge K. Cadherin engagement inhibits RhoA via p190RhoGAP. J Biol Chem. 2003;278(16):13615–13618. doi: 10.1074/jbc.C200657200. [DOI] [PubMed] [Google Scholar]
- 58.Leung T., Manser E., Tan L., Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270(49):29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- 59.Matsui T., Amano M., Yamamoto T., Chihara K., Nakafuku M., Ito M. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15(9):2208. [PMC free article] [PubMed] [Google Scholar]
- 60.Nakagawa O., Fujisawa K., Ishizaki T., Saito Y., Nakao K., Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392(2):189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 61.Amano M., Chihara K., Nakamura N., Kaneko T., Matsuura Y., Kaibuchi K. The COOH terminus of Rho-kinase negatively regulates rho-kinase activity. J Biol Chem. 1999;274(45):32418–32424. doi: 10.1074/jbc.274.45.32418. [DOI] [PubMed] [Google Scholar]
- 62.Ishizaki T., Maekawa M., Fujisawa K., Okawa K., Iwamatsu A., Fujita A. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15(8):1885. [PMC free article] [PubMed] [Google Scholar]
- 63.Fujisawa K., Madaule P., Ishizaki T., Watanabe G., Bito H., Saito Y. Different regions of Rho determine Rho-selective binding of different classes of Rho target molecules. J Biol Chem. 1998;273(30):18943–18949. doi: 10.1074/jbc.273.30.18943. [DOI] [PubMed] [Google Scholar]
- 64.Conway A.-M., James A., O’kane E., Rakhit S., Morris B. Regulation of myosin light chain phosphorylation by RhoB in neuronal cells. Exp Cell Res. 2004;300(1):35–42. doi: 10.1016/j.yexcr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 65.Shirao S., Kashiwagi S., Sato M., Miwa S., Nakao F., Kurokawa T. Sphingosylphosphorylcholine is a novel messenger for Rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery. Unimportant role for protein kinase C. Circ Res. 2002;91(2):112–119. doi: 10.1161/01.res.0000026057.13161.42. [DOI] [PubMed] [Google Scholar]
- 66.Sebbagh M., Hamelin J., Bertoglio J., Solary E., Breard J. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J Exp Med. 2005;201(3):465–471. doi: 10.1084/jem.20031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng J., Ito M., Kureishi Y., Ichikawa K., Amano M., Isaka N. Rho-associated kinase of chicken gizzard smooth muscle. J Biol Chem. 1999;274(6):3744–3752. doi: 10.1074/jbc.274.6.3744. [DOI] [PubMed] [Google Scholar]
- 68.Sebbagh M., Renvoize C., Hamelin J., Riche N., Bertoglio J., Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3(4):346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 69.Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273(5272):245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 70.Maekawa M., Ishizaki T., Boku S., Watanabe N., Fujita A., Iwamatsu A. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285(5429):895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 71.Matsui T., Maeda M., Doi Y., Yonemura S., Amano M., Kaibuchi K. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140(3):647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inada H., Togashi H., Nakamura Y., Kaibuchi K., Nagata K-i, Inagaki M. Balance between activities of Rho kinase and type 1 protein phosphatase modulates turnover of phosphorylation and dynamics of desmin/vimentin filaments. J Biol Chem. 1999;274(49):34932–34939. doi: 10.1074/jbc.274.49.34932. [DOI] [PubMed] [Google Scholar]
- 73.Inada H., Goto H., Tanabe K., Nishi Y., Kaibuchi K., Inagaki M. Rho-associated kinase phosphorylates desmin, the myogenic intermediate filament protein, at unique amino-terminal sites. Biochem Biophys Res Commun. 1998;253(1):21–25. doi: 10.1006/bbrc.1998.9732. [DOI] [PubMed] [Google Scholar]
- 74.Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271(34):20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 75.Palazzo A.F., Cook T.A., Alberts A.S., Gundersen G.G. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat Cell Biol. 2001;3(8):723–729. doi: 10.1038/35087035. [DOI] [PubMed] [Google Scholar]
- 76.Watanabe N., Kato T., Fujita A., Ishizaki T., Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1(3):136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 77.Bishop A., Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- 78.Vadlamudi R.K., Li F., Adam L., Nguyen D., Ohta Y., Stossel T.P. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4(9):681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- 79.Edwards D.C., Sanders L.C., Bokoch G.M., Gill G.N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1(5):253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 80.Sanders L.C., Matsumura F., Bokoch G.M., de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283(5410):2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 81.Brown M.C., West K.A., Turner C.E. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell. 2002;13(5):1550–1565. doi: 10.1091/mbc.02-02-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bokoch G.M. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72(1):743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 83.Kuroda S., Fukata M., Kobayashi K., Nakafuku M., Nomura N., Iwamatsu A. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem. 1996;271(38):23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- 84.Erickson J.W., Cerione R.A., Hart M.J. Identification of an actin cytoskeletal complex that includes IQGAP and the Cdc42 GTPase. J Biol Chem. 1997;272(39):24443–24447. doi: 10.1074/jbc.272.39.24443. [DOI] [PubMed] [Google Scholar]
- 85.Kuroda S., Fukata M., Nakagawa M., Fujii K., Nakamura T., Ookubo T. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell–cell adhesion. Science. 1998;281(5378):832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 86.Fukata M., Kuroda S., Fujii K., Nakamura T., Shoji I., Matsuura Y. Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J Biol Chem. 1997;272(47):29579–29583. doi: 10.1074/jbc.272.47.29579. [DOI] [PubMed] [Google Scholar]
- 87.Modolo F., Biz M.T., de Sousa S.M., Fachinelli RdL, Crema V.O. Immunohistochemical expression of Rho GTPases in ameloblastomas. J Oral Pathol Med. 2012;41(5):400–407. doi: 10.1111/j.1600-0714.2011.01108.x. [DOI] [PubMed] [Google Scholar]