Abstract
To support translation of microneedle patches from pre-clinical development into clinical trials, this study examined the effect of microneedle patch application on local skin reactions, reliability of use and acceptability to patients. Placebo patches containing dissolving microneedles were administered to fifteen human participants. Microneedle patches were well tolerated in the skin with no pain or swelling and only mild erythema localized to the site of patch administration that resolved fully within seven days. Microneedle patches could be administered by hand without the need of an applicator and delivery efficiencies were similar for investigator-administration and self-administration. Microneedle patch administration was not considered painful and the large majority of subjects were somewhat or fully confident that they self-administered patches correctly. Microneedle patches were overwhelmingly preferred over conventional needle and syringe injection. Altogether, these results demonstrate that dissolving microneedle patches were well tolerated, easily usable and strongly accepted by human subjects, which will facilitate further clinical translation of this technology.
Keywords: Dissolvable microneedle patch, human study, skin vaccination, acceptability, usability, tolerability, drug delivery
1. Introduction
Microneedle patches contain hundreds of microneedles less than one millimeter long to deliver drugs and vaccines into skin. The patch contains an array of microneedles attached to an adhesive backing to facilitate application to the skin. In a dissolving microneedle patch, the microneedles dissolve in the skin within minutes, thereby delivering the drug contained in them and not generating sharps waste. Microneedle patches are being developed as an alternative to conventional needle-and-syringe injections due to the expectation of ease of administration, good tolerability in the skin and strong patient acceptance. This study is designed to assess these expectations.
Microneedle patches have previously been studied for delivery of a number of drugs and vaccines in pre-clinical studies, but limited information is available about the use of dissolving microneedle patches in human subjects [1-7]. Microneedle patches are typically designed either as coated microneedle patches made of solid metal, silicon or polymer microneedles coated with drug that is released upon dissolution of the coating in the skin or as dissolving microneedle patches containing solid, dissolving microneedles made of water-soluble materials that encapsulate the drug and release it when the microneedles dissolve in the skin.
Coated microneedle patches are being evaluated in clinical trials for delivery of parathyroid hormone to treat osteoporosis [8], glucagon to treat hypoglycemia [9] and zolmitriptan to treat migraine [10]. Dissolving microneedle patches are being evaluated in clinical trials for delivery of parathyroid hormone as well as for influenza vaccination [11].
Previous clinical trials involving microneedle patches have used high velocity insertion devices, which can improve reliability of microneedle penetration into skin, but add additional bulk and cost to the microneedle device. In this study, we are examining the usability of dissolving microneedle patches without the use of an applicator in human subjects; the microneedle patches are applied with thumb pressure only. To our knowledge, no other study has evaluated the puncture and delivery efficiencies of dissolving microneedle patches in humans or the acceptability preferences regarding vaccination using dissolving microneedle patches. As dissolving microneedle patches continue being developed for clinical translation in the coming years, it is important to fully characterize the insertion and dissolution of microneedles in humans.
The goal of this study is to evaluate skin tolerability, usability and acceptability of dissolving microneedle patches in human subjects to further clinical translation of microneedle patches for delivery of drugs and vaccines. This work was specifically designed to prepare for a phase 1 clinical trial of influenza vaccination using microneedle patches of a similar design [12]. We therefore developed placebo dissolving microneedle patches (i.e., not containing vaccine) and conducted a human study to assess reactions in the skin after microneedle patch application, determine microneedle patch delivery efficiency in investigator-administration and self-administration, and carry out a survey about participants' preferences concerning microneedle patch administration.
2. Materials and methods
2.1 Fabrication of dissolving microneedle patch
The microneedle fabrication process has been described previously [13]. In this study, microneedle patches contained 100 conical microneedles measuring 650 μm in height and 200 μm in base diameter positioned in a ∼1 cm2 area that were adhered to a medical-grade tape (3M, St. Paul, MN) that incorporated a force-feedback indicator that made a clicking sound when a force greater than 10 lbf is applied. The microneedles were composed of 50% (w/w) polyvinyl alcohol (molecular weight 31 kDa) and 50% (w/w) sucrose. Microneedle patches were stored for 2 to 4 weeks in a sealed foil pouch with silica gel desiccant (5 g of desiccant) at room temperature (20 – 25 °C) and humidity (30 – 60% rh) until used at the time of study. Each patch was visually inspected under a microscope before and after storage to verify the integrity of the patch and the microneedles.
2.2 Study approval and study subjects
This study was approved by the Georgia Institute of Technology Institutional Review Board and informed consent was obtained from all participants. To be eligible, participants had to be healthy non-pregnant adults with normal skin, no known problems with pain perception and no known allergies to the materials used in the study. Participants could not have previously seen or worked with microneedle patches to be eligible for the study. Fifteen subjects (seven females and eight males), ages 18 - 57 were recruited from the Georgia Institute of Technology and other sites in Atlanta, GA.
2.3 Experimental design
Participants received three microneedle patches – one self-administered and two investigator-administered. Participants were provided a brief overview of the study and watched a short instructional audiovisual presentation on self-administration of microneedle patches. An outline of the microneedle patch administration process is shown in Figure 1.
Figure 1.
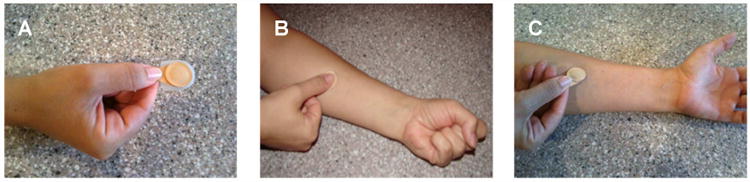
Procedure to apply a microneedle patch to the skin. (A) The subject or investigator picks up the patch and removes the protective cap. (B) The subject forms a fist in the other hand and then the subject or investigator places the patch on the forearm. The subject or investigator pushes on the patch with the thumb and continues to apply force until hearing a ‘click’ sound, indicating that enough force has been applied. (C) The subject leaves the patch on the skin for 20 min, after which the subject or investigator peels the patch off the skin and the investigator saves the patch for additional analysis.
Each participant first self-administered a microneedle patch to his or her forearm without assistance from the investigator. After the patch removal, the investigator stained this skin site (see below). The investigator then applied two microneedle patches to the participant, one on each forearm. Only one of these skin sites was stained by the investigator. The site not stained was used to make measurements of skin tolerability (see below). The two stained sites were used for usability measurements (see below).
Participants then answered a survey about microneedle patch administration to assess acceptability of the microneedle patches. Participants returned to the study site 1, 2, 3, 4 and 7 days after microneedle patch administration for skin tolerability measurements.
2.4 Skin tolerability measurements
Skin tolerability was measured using the skin scoring scale listed in Table S.1 (see Supplementary Material). The scale was adapted for microneedle patches using established guidelines for vaccine clinical trials and clinical testing of transdermal patches [14, 15]. The skin site was scored for pain, tenderness, erythema (size and intensity) and swelling on a grading scale of 0 to 4. Pain and tenderness were scored based on the participant's response whereas erythema and swelling were measured by the investigator.
Participants were asked if they felt any pain at the skin site after microneedle patch administration was complete. This pain was assessed separately from the pain during microneedle patch application, which is addressed in usability measurements. Tenderness was defined as any pain felt at the skin site when the investigator gently touched the skin site. Erythema size was measured using a ruler scale and intensity by visual observation of the skin site. Since there was no erythema scale for microneedle patches already in place, the investigator was trained on erythema measurements using guidelines and training available for Psoriasis Area Severity Index (PASI) scores [16]. Swelling was measured by the investigator by gently moving the thumb over the skin site to notice any raised surfaces on the skin. The investigator recorded a numerical score for each of these criteria and photographically imaged the skin at each time point.
2.5 Skin staining and microscopy to measure usability
Usability was measured in terms of microneedle puncture efficiency by skin staining (percentage of microneedles that penetrated the skin surface) and delivery efficiency by microscopy (percentage volume of microneedles that dissolved after administration).
To measure puncture efficiency, skin was stained using gentian violet 1% solution (Humco, Texarkana, TX). Immediately after microneedle patch administration, gentian violet was pooled on the skin site, dabbed dry with gauze after 1 min and cleaned with alcohol after 10 min. The stained skin site was imaged and microneedle puncture efficiency was measured by counting the number of stained skin punctures, which appeared as blue dots. It has been previously shown that the number of stained skin punctures visible after microneedle patch insertion is correlated with skin puncture by measuring trans-epidermal water loss [17].
To measure delivery efficiency, microneedle patches were imaged using brightfield microscopy (SZX12 Olympus, Center Valley, PA) before and after administration, and the microneedle dimensions were measured to calculate the volume dissolved after microneedle patch administration. Since placebo microneedle patches (i.e., containing no drug or other active substance) were used in this study, it was not possible to assay the microneedle patches for delivery efficiency of a drug or other active, and therefore this method of usability from staining and microscopy was used.
2.6 Survey about microneedle patch administration to measure acceptability
Participants answered a short questionnaire to solicit information about the acceptability of microneedle patches for delivery of drugs or vaccines. We surveyed the subjects about pain during microneedle patch application, confidence during self-administration and subject preferences regarding microneedle patches, conventional intramuscular injection and conventional oral delivery using pills.
Pain during microneedle patch administration was reported by participants using a visual analog scale from 0 (no pain) to 10 (worst pain ever). Participants were also asked to score their confidence during self-administration of microneedle patches on a score of 1 to 5 using the following scale: (1) I'm confident that I applied the patch incorrectly, (2) I'm somewhat confident that I applied the patch incorrectly, (3) I do not know if I applied the patch correctly or incorrectly, (4) I'm somewhat confident that I applied the patch correctly, (5) I'm confident that I applied the patch correctly.
Subjects were then asked their preferences regarding obtaining medications by microneedle patches versus hypodermic needles and microneedle patches versus conventional oral delivery by pill.
2.7 Statistical methods
Statistical analysis was carried out using Prism software version 5 (Graphpad, La Jolla, CA). P values < 0.05 were considered significant. Average values of delivery efficiency by microscopy were analyzed using a paired t test.
3. Results
3.1 Skin tolerability
We studied skin tolerability of microneedle patch administration to identify and quantify local reactions in the skin that can occur. These reactions may be associated with the patch administration process and the patch excipients left in the skin after microneedle dissolution. This study did not assess the possible additional effects of delivery of a drug or other active to the skin. The investigator administered one patch onto the subject's skin and the skin site was monitored, imaged and scored once per day for 7 days.
Figure 2 shows a series of images from a representative subject after microneedle patch application. Immediately after microneedle patch application on Day 0, a rectangular area corresponding to the size of the microneedle array exhibited mild erythema. Faint redness was also seen in a circular area around the rectangle, corresponding to the area where the patch adhesive backing contacted the skin. On Day 1, redness from the adhesive backing was fully resolved and the rectangular area decreased in redness and size. For the subject in Figure 2, all erythema resolved by Day 4. There were no other skin reactions or adverse effects noted, and overall the microneedle patch was well tolerated.
Figure 2.
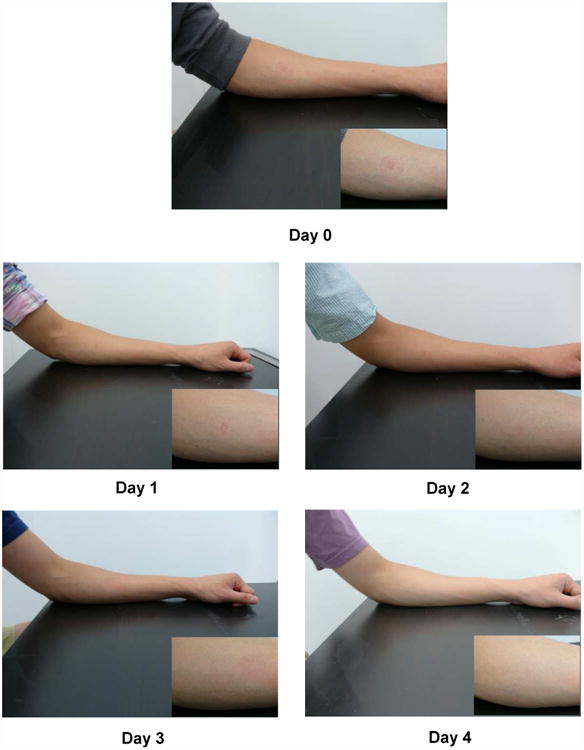
Representative images of the site of microneedle patch application on the skin over time. Inset shows magnified images of the skin site. Day 0 is immediately after patch application and removal. These images are all from the same subject.
We quantified skin reactions noted after microneedle patch application over time in Figure 3. Subjects were asked if they felt pain or tenderness at the skin site. Pain in this case was assessed after microneedle patch application was complete, which differs from possible pain experienced during microneedle patch insertion, which is addressed in the context of the usability analysis below. None of the subjects reported pain at the skin site after microneedle patch application on Day 0 through Day 7. Only one subject (out of 15) reported tenderness at the skin site on Day 0 which was fully resolved by Day 1. This tenderness was reported of Grade 1 (i.e., mild discomfort to touch, on a scale of 0 to 4). None of the other subjects reported tenderness at the skin site at any time.
Figure 3.
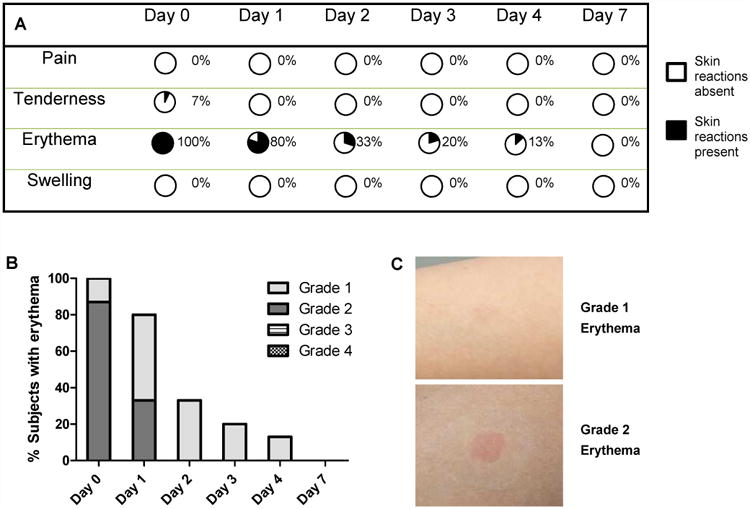
Skin tolerability after microneedle patch application. Skin sites were monitored and scored over a period of one week. (A) Summary of the prevalence of skin reactions at different time points. Percentages indicate the presence of skin reactions. (B) Intensity of erythema in the skin after microneedle patch application. Column bars show the percentage of subjects with different grades of erythema. None of the subjects had Grade 3 or 4 erythema scores. (C) Representative images of skin showing Grade 1 and Grade 2 erythema.
The investigator also scored the skin site for erythema and swelling. Erythema was scored based on size and intensity. Based on size, erythema, when present, was always of Grade 0.5, which corresponds to a size equal to or less than the patch. As noted in Figure 3A, erythema was present in 100% of subjects on Day 0 and 80% of subjects on Day 1. Erythema continued to subside over time with 13% of subjects having erythema on Day 4 and, by Day 7, erythema in all subjects was fully resolved.
Figure 3B charts the score of erythema intensity on a scale of 0 - 4 over time. Representative examples of Grade 1 and 2 erythema seen in the study are shown in Figure 3C. On Day 0, 13% (2 out of 15) subjects had very slight erythema (Grade 1) while 87% (13 out of 15) subjects had mild erythema (Grade 2). On Day 1, 47% (7 out of 15) subjects showed Grade 1 erythema and 33% (5 out of 15) subjects showed Grade 2 erythema. From Day 2 onwards, erythema, when present, was only Grade 1. None of the subjects showed Grade 3 or Grade 4 erythema at any point in the study.
In addition, none of the subjects showed swelling at the skin site at any time during the study. Overall the patches were tolerated very well in the skin with only mild transient erythema that resolved fully by Day 7, almost no tenderness and no pain or swelling.
3.2 Usability
We next determined if microneedle patches could be inserted and dissolved in the skin in a reliable manner. We also determined if subjects could self-administer microneedle patches after only brief training.
Figure 4A charts the puncture efficiency as measured by the percentage of microneedles in a given patch that inserted into the skin after investigator-administration and self-administration. Figure 4B shows an example of the subject's skin stained with gentian violet after microneedle patch application. The blue dots show the number of sites that were punctured in the skin during microneedle patch application. The mean puncture efficiency by skin staining was 99±1% after investigator-administration and 98±2% after self-administration, which indicates that microneedle insertion efficiency was excellent, independent of who administered the patches.
Figure 4.
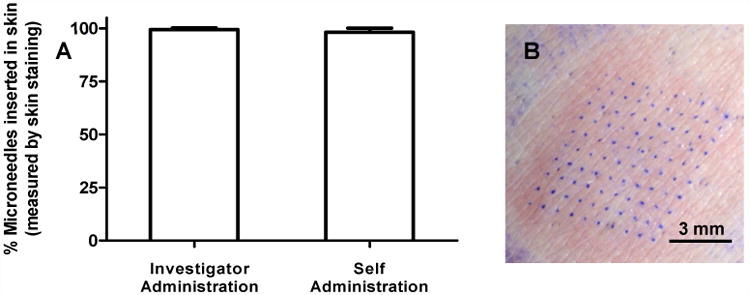
Puncture efficiency of microneedle patch application as determined by the percentage of microneedles that punctured into skin. Skin sites were stained with gentian violet dye and the number of dots were counted to measure the number of microneedles that punctured into the skin. (A) Puncture efficiency of microneedle patches after investigator-administration and self-administration. Column bars represent the average percentage of microneedles that inserted into skin with standard deviation error bars shown. (B) Representative magnified image of a stained skin site showing a 10 × 10 array where the microneedles punctured into skin.
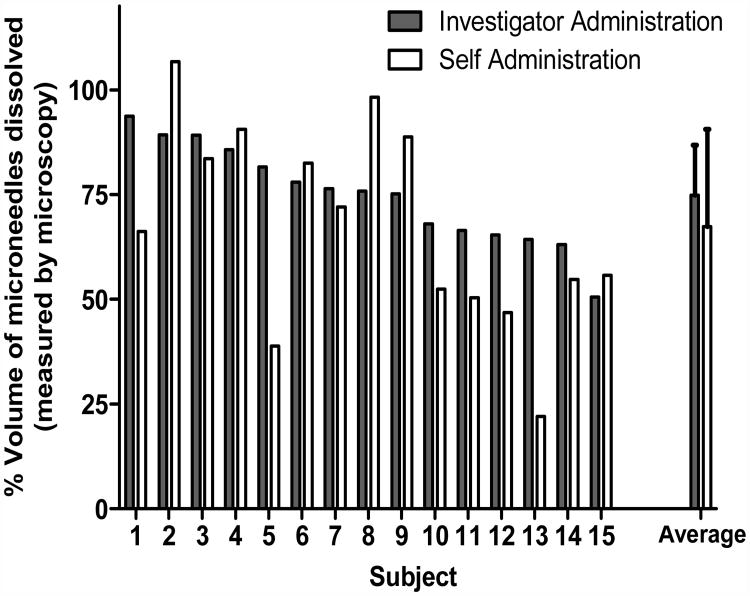
Figure 5 charts the delivery efficiency as measured by the percentage volume of microneedles in a given patch that dissolved after patch administration. The mean delivery efficiency by microscopy was 74±11 % after investigator-administration and 67±23 % after self- administration (Figure 5a). These two values were not statistically different from each other (p = 0.16).
Figure 5.
Delivery efficiency as determined by the percentage of the volume of microneedles that dissolved during microneedle patch application to the skin. Microneedle patches were imaged by brightfield microscopy before and after insertion into the skin and image analysis was used to determine the volume of microneedles dissolved. (a) Volume of microneedles dissolved is interpreted as a measure of the dose delivered, i.e., if a drug or vaccine had been incorporated into the full volume of the microneedles. Each bar represents the result from an individual subject. The data are presented in descending order of investigator-administered delivery efficiency. The ‘average’ bars represent the averages of the 15 individual bars, with standard deviation error bars shown. (b) Percent of subjects that delivered more than 50% of the volume of the microneedles.
Often, microneedles are not filled with drug, but have drug preferentially toward the microneedle tip. If the upper 50% of the microneedle volume toward the microneedle tip (i.e., the upper 516 μm of the 650 μm-long microneedle) contained drug, then 100% of investigator-administered patches and 87% of self-administered patches would have delivered their full dose. Together, this suggests that overall usability was similar between investigator-administration and self-administration after brief training.
3.3 Acceptability
We studied acceptability of microneedle patches by surveying the subjects about pain during microneedle patch application, confidence during self-administration and subject preferences regarding microneedle patches compared to conventional intramuscular injection and oral delivery by pills.
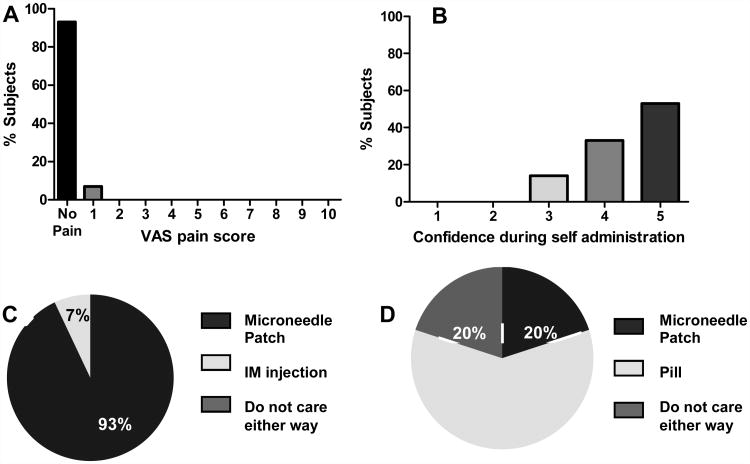
Figure 6A shows the pain score reported by subjects during microneedle patch administration. Fourteen out of the 15 subjects reported a pain score of 0 or ‘no pain’ during microneedle patch administration. One subject reported a pain score of 1 (on a scale of 0 to 10). This indicates that microneedle patch administration was not considered painful.
Figure 6.
Acceptability survey results from subjects concerning microneedle patch administration. (A) Assessment of pain during microneedle patch administration scored by subjects on a visual analog scale (VAS) of 0 (No pain) to 10 (Worst pain ever). (B) Confidence of subjects during self-administration of microneedle patches scored by subjects on a scale of 1 (least confident) to 5 (most confident); see Methods for details. (C) Preference of subjects for application of microneedle patch as compared to intramuscular injection for delivery of medications. (D) Preference of subjects for microneedle patch administration as compared to oral administration by conventional pill for delivery of medications.
Figure 6B shows the confidence score reported by subjects during self-administration of microneedle patches. Among the subjects, 53% (8 out of 15) reported that they were confident that they had applied the microneedle patch correctly (score of 5), and 33% (5 out of 15) subjects reported that they were somewhat confident that they applied the microneedle patch correctly (score of 4). Only 14% (2 out of 15) subjects reported that they did not know if they applied the microneedle patch correctly or incorrectly (score of 3). None of the subjects reported that they thought that they applied the patch incorrectly (score of 1 or 2). Therefore, 86% of the subjects were somewhat or fully confident that they applied the microneedle patch correctly (confidence score of 4 or 5).
Figures 6C and 6D compare the preferences reported by subjects concerning microneedle patches versus conventional delivery methods. Figure 6C shows that 93% (14 out of 15) of subjects would prefer to obtain their medication by a microneedle patch as compared to a conventional intramuscular injection. Only one subject reported a preference for intramuscular injection over microneedle patch. That subject stated that the longer wear time of the microneedle patch as compared to intramuscular injection was the primary reason for preferring intramuscular injection. This indicates that microneedle patches were overwhelmingly preferred over intramuscular injections.
Figure 6D shows that 20% (3 out of 15) subjects would prefer a microneedle patch over conventional oral delivery by pill for obtaining medication, while another 20% reported that they did not care either way. The remaining 60% (9 out of 15) subjects reported that they would prefer obtaining their medication by a pill over a microneedle patch. This indicates that although oral delivery was preferred, a significant fraction of subjects found the microneedle patch to be similar or better than oral administration.
4. Discussion
The goal of the study was to evaluate skin tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects to further clinical translation of dissolving microneedle patches.
In this study, microneedle patches were very well tolerated in the skin with only mild, transient erythema that resolved within 7 days. This excellent tolerability may be due to microneedle formulation using biocompatible materials (i.e., polyvinyl alcohol and sucrose) and the minimally invasive nature of microneedle patches. It is, however, important to note that the microneedle patches used in this study were placebos and did not contain any drug or vaccine. The presence of drug or vaccine within the microneedle patches could change the incidence or intensity of skin reactions. Tolerability in the skin should also be dependent upon the materials used for microneedle patch fabrication and the properties of these materials upon dissolution in the skin. For example, a prior study reported significantly greater erythema associated with application of dissolving microneedle patches composed of hyaluronic acid and containing influenza vaccine in human subjects [11].
This study also showed that 98% – 99% of microneedles in a given patch punctured the skin's surface based on data from skin staining, which leaves little room for improvement. Based on microscopy analysis, about 70% of the volume of microneedles was dissolved after microneedle patch administration. Because the microneedles were made of highly water-soluble materials and there was a patch wear time of 20 min, we believe that the portions of the microneedles that penetrated the skin were fully dissolved in the skin. We therefore expect that the incomplete dissolution of the microneedles in this study was due to incomplete penetration of microneedles to their full length into skin, which is consistent with prior observations in animal skin in vivo and ex vivo [11, 13, 18]. Further optimization of microneedle patch geometry, materials that comprise the microneedle patch, the force of microneedle patch application, patch wear time and other factors, could lead to greater delivery efficiency. Alternatively, because drug encapsulation in the microneedle need not be uniformly distributed, drug encapsulated in microneedles could be located preferentially toward the microneedle tip, thereby enabling complete drug delivery even with incomplete microneedle dissolution (e.g., by localizing all of the drug in the upper 70% of the microneedles, a 70% microneedle dissolution would correspond to 100% drug delivered).
For most subjects, investigator-administration and self-administration delivery efficiencies were similar to each other with no statistically significant difference overall. However, there were certain subjects (e.g., subjects 5 and 13) where self-administration delivery was much lower than investigator-administration. That being said, there were also subjects for whom self-administration yielded more efficient delivery than investigator-administration. Future studies should expand upon these proof-of-concept results to assess self-administration protocols in larger populations and using microneedle designs that are further optimized for simple, reliable administration.
Microneedle patches were well accepted in this study, with no pain of insertion reported by almost all subjects, and were overwhelmingly preferred over intramuscular injection. This is consistent with previous studies reporting less pain from microneedle administration compared to injection [17, 19, 20] and reporting overall preference of microneedle patches over drug or vaccine delivery by injection [19, 21-23].
The large majority of participants were at least somewhat confident that they self-administered the microneedle patch correctly, and none reported that they thought they had applied the patch incorrectly. Almost all participants preferred microneedle patch administration to intramuscular injection and some preferred the patch to oral administration. This indicates that offering microneedle patch administration for medications that otherwise require injection could be a strategy to increase patient compliance with these therapies.
5. Conclusions
This study evaluated skin tolerability, usability and acceptability of dissolving microneedle patch administration in humans. The microneedle patches were well tolerated in the skin with mild erythema that resolved fully within seven days and caused no pain or swelling. Microneedle patches were administered by hand without the need of an applicator, and delivery efficiencies were similar between investigator-administration and self-administration. Microneedle patch administration was not painful, and the large majority of subjects were somewhat or fully confident that they self-administered the patch correctly. Microneedle patch administration was overwhelmingly preferred over conventional needle and syringe injection for delivery of medications.
Altogether the results of this study show the feasibility of using dissolving microneedle patches for investigator-administration as well as self-administration for future applications in drug and vaccine delivery in a well-tolerated and preferred manner that offers an attractive alternative to conventional hypodermic needles.
Supplementary Material
Acknowledgments
The authors would like to thank Donna Bondy for administrative support; Danny Pardo for help with microscopy assay development, microscopy measurements and participant recruitment; and Matt Mistilis and other colleagues at Georgia Tech for help with preliminary clinical assessment of microneedle patches. This work was supported by a grant from the National Institutes of Health.
SH, DVM and MRP are inventors of patents that have been or may be licensed to companies developing microneedle-based products; MRP is a paid advisor to companies developing microneedle-based products; SH, DVM and MRP are founders/shareholders of companies developing microneedle-based products, including Micron Biomedical; and SH, WPP and DVM are currently employees of Micron Biomedical. These potential conflicts of interest have been disclosed and are being managed by Georgia Tech and/or Emory University.
Footnotes
These potential conflicts of interest have been disclosed and are being managed by Georgia Tech and/or Emory University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arya J, Prausnitz MR. Microneedle patches for vaccination in developing countries. Journal of Controlled Release. doi: 10.1016/j.jconrel.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Advanced Drug Delivery Reviews. 2012;64(14):1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall S, Sahm LJ, Moore AC. The success of microneedle-mediated vaccine delivery into skin. Hum Vaccin Immunother. 2016;12(11):2975–2983. doi: 10.1080/21645515.2016.1171440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caffarel-Salvador E, Donnelly RF. Transdermal Drug Delivery Mediated by Microneedle Arrays: Innovations and Barriers to Success. Curr Pharm Des. 2016;22(9):1105–17. doi: 10.2174/1381612822666151216145645. [DOI] [PubMed] [Google Scholar]
- 5.Suh H, Shin J, Kim YC. Microneedle patches for vaccine delivery. Clin Exp Vaccine Res. 2014;3(1):42–9. doi: 10.7774/cevr.2014.3.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koutsonanos DG, Compans RW, Skountzou I. Targeting the skin for microneedle delivery of influenza vaccine. Adv Exp Med Biol. 2013;785:121–32. doi: 10.1007/978-1-4614-6217-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–55. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Daddona PE, et al. Parathyroid hormone (1-34)-coated microneedle patch system: clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm Res. 2011;28(1):159–65. doi: 10.1007/s11095-010-0192-9. [DOI] [PubMed] [Google Scholar]
- 9.Safety and Efficacy of ZP-Glucagon to Injectable Glucagon for Hypoglycemia. [cited 2016 December]; Available from: www.clinicaltrials.gov/ct2/show/NCT02459938.
- 10.Safety and Efficacy of ZP-Zolmitriptan Intracutaneous Microneedle Systems for the Acute Treatment of Migraine (Zotrip) [cited 2016 December]; Available from: https://clinicaltrials.gov/ct2/show/NCT02745392.
- 11.Hirobe S, et al. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials. 2015;57:50–8. doi: 10.1016/j.biomaterials.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Inactivated Influenza Vaccine Delivered by Microneedle Patch or by Hypodermic Needle. [cited 2016 December]; Available from: https://clinicaltrials.gov/ct2/show/NCT02438423.
- 13.Vassilieva EV, et al. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature. Drug Deliv Transl Res. 2015;5(4):360–71. doi: 10.1007/s13346-015-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FDA. Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. 2016 Feb 7; doi: 10.1016/j.vaccine.2023.07.072. 2007. Available from: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm074775.htm. [DOI] [PubMed]
- 15.FDA. Guidance for Industrv: Skin Irritation and Sensitization Testing of Generic Transdermal Drug Products. 2016 Feb 7; 1999. Available from: http://www.fda.gov/ohrms/dockets/98fr/990236Gd.pdf.
- 16.PASI. PASI Score Training: How to Calculate the PASI. 2016 Feb 7; Available from: http://www.pasitraining.com/pasi_score/
- 17.Haq MI, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices. 2009;11(1):35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan SP, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–20. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norman JJ, et al. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32(16):1856–62. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill HS, et al. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24(7):585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birchall JC, et al. Microneedles in clinical practice--an exploratory study into the opinions of healthcare professionals and the public. Pharm Res. 2011;28(1):95–106. doi: 10.1007/s11095-010-0101-2. [DOI] [PubMed] [Google Scholar]
- 22.Mooney K, McElnay JC, Donnelly RF. Paediatricians' opinions of microneedle-mediated monitoring: a key stage in the translation of microneedle technology from laboratory into clinical practice. Drug Deliv Transl Res. 2015;5(4):346–59. doi: 10.1007/s13346-015-0223-5. [DOI] [PubMed] [Google Scholar]
- 23.Marshall S, Sahm LJ, Moore AC. Microneedle technology for immunisation: Perception, acceptability and suitability for paediatric use. Vaccine. 2016;34(6):723–34. doi: 10.1016/j.vaccine.2015.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.