Abstract
A mouse and human brain-enriched micro-RNA-146a (miRNA-146a) is known to be important in modulating the innate immune response and inflammatory signaling in certain immunological and brain cell types. In this study we examined miRNA-146a levels in early-, moderate- and late-stage Alzheimer’s disease (AD) neocortex and hippocampus, in several human primary brain and retinal cell lines, and in 5 different transgenic mouse models of AD including Tg2576, TgCRND8, PSAPP, 3xTg-AD and 5xFAD. Inducible expression of miRNA-146a was found to be significantly up-regulated in a primary co-culture of human neuronal–glial (HNG) cells stressed using interleukin1-beta (IL-1β), and this up-regulation was quenched using specific NF-κB inhibitors including curcumin. Expression of miRNA-146a correlated with senile plaque density and synaptic pathology in Tg2576 and in 5xFAD transgenic mouse models used in the study of this common neurodegenerative disorder.
Keywords: 5xFAD, Alzheimer’s disease, CAY10512, Curcumin, miRNA-146a, Neurodegeneration, NF-κB, PDTC, Tg2576, Transgenic models of Alzheimer’s disease
Micro-RNAs (miRNAs) constitute a novel class of small, evolution-arily conserved non-coding regulatory RNAs that interact with the 3′ un-translated region (3′-UTR) of specific messenger RNA (mRNA) populations, and in doing so function in mRNA processing, inhibition, or termination of that mRNA’s expression [22,11,13,14]. A number of miRNAs, such as the brain-enriched micro-RNA-146a (miRNA-146a) have been strongly implicated in regulation of innate immune, viral, and inflammatory responses in neurodegenerative disorders, including Alzheimer’s disease (AD) [11,13,14,16,24,23,5,20]. In this report we have studied miRNA-146a abundance in the superior temporal lobe neocortex and hippocampal CA1 region in the early, middle and late stages of AD, in several distinct types of human brain and retinal cells in primary culture (Figs. 1 and 2), and in 5 different transgenic mouse models of AD (Tg-AD; Table 1). The expression of miRNA-146a was found to be significantly expressed in the human neocortex and the limbic system and significantly increased as the severity of AD advanced (Fig. 1A). In primary human neuronal–glial (HNG) cell co-cultures miRNA-146a was found to be induced by IL-1β, a pro-inflammatory cytokine known to be elevated in AD brain (Fig. 1B) [12]. Interestingly, the levels of miRNA-146a were found to correlate with the senile plaque density and the appearance of synaptic pathology in the brain cortex of certain Tg-AD mouse models (Fig. 2, Table 1) [19]. Transfection of HNG cells with a miRNA-146a luciferase reporter (construct A547), and the inclusion of 3 different classes of NF-κB inhibitors, indicated that in IL-1β-stressed HNG cells, as in other cell types, miRNA-146a is under transcriptional regulatory control by NF-κB. This suggests an important, potentially pathogenic, interplay between a pro-inflammatory transcription factor and an inducible miRNA under NF-κB regulatory control in specific types of human brain cells, tissues, and in amyloid over-expressing Tg-AD models (Figs. 2 and 3) (2–6,13).
Fig. 1.
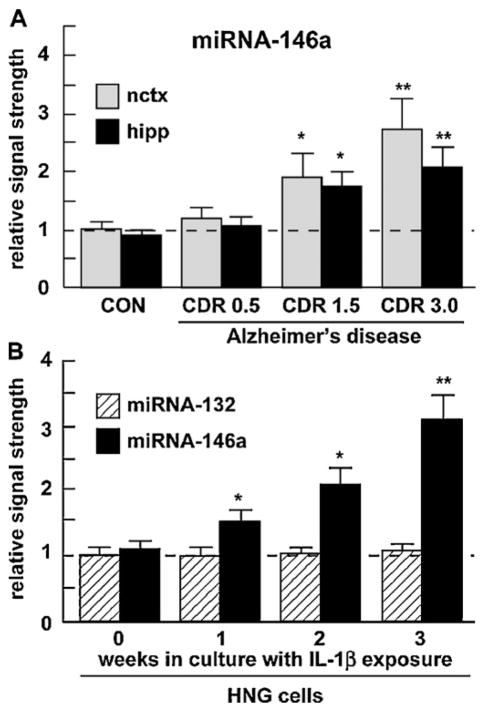
Up-regulation of an inducible miRNA-146a (A) in various stages of AD and (B) in IL-1β-stressed HNG cells. (A) Abundance of miRNA-146a in superior temporal lobe neocortex (nctx) and hippocampus (hipp) compared to 5S RNA in the same sample and to age-matched controls; number of samples used CON = 6, CDR 0.5 = 6, CDR 1.5 = 6, CDR 3.0 = 6 (3 males and 3 females in each group; see text), and (B) miRNA-146a abundance in stressed HNG cells compared to miRNA-132 in the same sample and to age-matched controls [16]. For ease of comparison control miRNA-146a levels in the neocortex arbitrarily set to 1.0; dashed horizontal line. In (B) HNG cells were treated with human recombinant IL-1β (10 ng/ml cell culture medium) and were cultured for 0–3 weeks, representing inflammatory cytokine IL-1β-mediated stress for up to one half of their in vitro lifetime of 6 weeks. The results indicate a specific up-regulation of miRNA-146a; a related miRNA-132 showed no change in either AD or in stressed HNG cells. Hence miRNA-146a is up-regulated in HNG cells stressed with inflammatory cytokines known to be abundant in AD brain. For ease of comparison control miRNA-132 levels arbitrarily set to 1.0; dashed horizontal line; N = 3–5; *p < 0.05, **p < 0.01 (ANOVA).
Fig. 2.
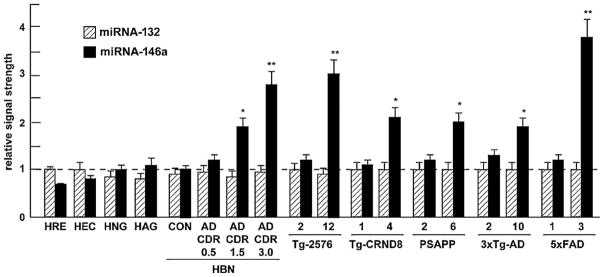
Relative signal strength of miRNA-146a in comparison to a lower abundance miRNA-132 in various human primary brain and retinal primary cells, control (N = 6) and Alzheimer-affected (N = 6 for each CDR rating) neocortex, and in 5 different transgenic mouse models of Alzheimer’s disease (Tg-AD). In the Tg-AD models, two ages of animals are given, the first is a younger animal with no neuropathology and the second is after the onset of neuropathology (Table 1). Only the Tg-2576 and 5xFAD Tg-AD models showed miRNA-146a induction of 3-fold or greater over controls. HRE, human retina, HEC, human brain microvessel endothelial cells, HAG, human astroglia, HBN, human brain neocortex; CON, control; AD, Alzheimer’s disease, CDR, clinical dementia rating; see Table 1 for further description of the Tg-AD; time in months (mo); N = 3–6 animals for each determination; for ease of comparison control (CON) miRNA-146a levels arbitrarily set to 1.0; dashed horizontal line;*p < 0.05, **p < 0.01 (ANOVA).
Table 1.
Various neuropathological characteristics of transgenic AD (Tg-AD) mouse models derived from previous studies and unpublished data. Genotype and phenotype are further described in detail in the original ‘source’ references provided for these Tg-AD models.
| Alzheimer transgenic model | Age of onset (senile plaques) | Tg-AD promoter/transgene | Senile plaque density | CNS-specific expression of amyloid | Synaptic pathology | Source reference |
|---|---|---|---|---|---|---|
| Tg2576 | 10 | Hamster prion promoter/human APP695 cDNA with KM670/671NL | ++++ | ++ | ++++ | [7,9] |
| Tg-CRND8 | 3 | Hamster prion promoter/APPSwe/Ind (KM670/671NL + V717F) | ++ | + | − | [2] |
| PSAPP | 6 | Tg2576 × PSEN1M146L | ++ | + | [6,10,27] | |
| 3xTg-AD | 6 | Thy1 promoter/APP695-Swedish/Tau isoform 4R0N(P301L mutation)/PSEN1M146L | ++ | ++++ | + | [18] |
| 5xFAD | 2 | Thy1 promoter/B6SJL-Tg(APPSwFlLon,PSEN1M146L*L286V)6799Vas/J | ++++ | ++++ | ++++ | [17] |
Symptomatic Tg-AD models exhibiting the highest senile plaque density (as seen anywhere within the CNS in aged Tg-AD models), and synaptic neuropathology (as determined by microscopic and molecular abundance measures; see text for further descriptions, unpublished observations) also displayed the highest levels of the inducible, inflammation-associated miRNA-146a species; + detected, ++ moderate abundance, ++++ extensive phenotype; see also Refs. [19,1,29,4,26,3,28,30]. The scoring system adapted from Ref. [19], also took into consideration additional Tg-AD characteristics listed at the Tg-AD website www.alzforum.org/res/com/tra/app/default.asp, in addition to consideration of deficits in the abundance of several important synaptic and cytoarchitectural support proteins, including synapsin-2, spectrin, syntenin, synaptophysin and neurofilament light chain (NF-L) protein assayed in these Tg-AD models (data not shown), and from observations made in the original source and supporting references for each Tg-AD type (rightmost column and text).
Fig. 3.
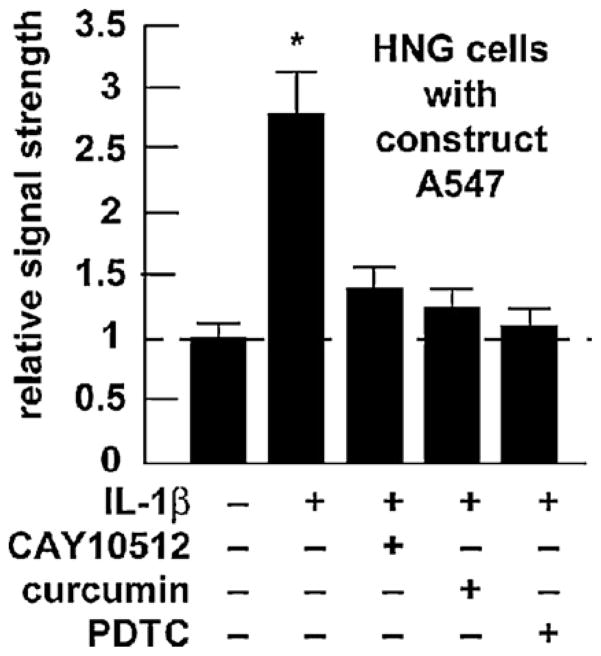
Transfection of pre-miRNA-146a promoter-luciferase reporter pGL3 expression A547 construct into HNG cells—inhibition with the NF-(B inhibitors CAY10512, a resveratrol analog, curcumin and pyrrolidine dithiocarbamate (PDTC) [14,16]. Two-week-old HNG cells were transfected with the pre-miRNA-146a promoter and luciferase reporter vector (construct A547; Addgene, Cambridge, MA) as described [16]. Cells were untreated or treated with CAY10512 (0.5 uM), curcumin (5 uM) or PDTC (20 uM) or just prior to [IL-1β]-induced stress; 24 h post-transfection treated cells and controls were processed using a luciferase reporter assay kit (Dual Luciferase System, Promega) as previously described [16]. For ease of comparison control (untreated) HNG cell A547 expression levels arbitrarily set to 1.0, dashed horizontal line; N = 3–5 for each experiment; significance over control *p < 0.01 (ANOVA).
Reagents used in these experiments were obtained from commercial suppliers and were used without further purification. RNAse-free plasticware and RNAse-free isolation reagents including diethyl pyrocarbonate (DEPC; Ambion, Austin TX; Invitrogen, Carlsbad CA; Sigma–Aldrich, St Louis, MO) were used as previously described [13,14,16,24,12]. The NF-κB inhibitors curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione diferuloylmethane; purity >98.5%, Axxora, San Diego, CA), dissolved in dimethylsulfoxide as a 100 mM stock solution, was used at 5 uM ambient concentration in HNG cell medium; the metal chelator, anti-oxidant and NF-κB translocation inhibitor pyrrolidine dithiocarbamate (PTDC; P8765; Sigma, St Louis, MO) was used at 20 uM; and the polyphenolic trans-stilbene resveratrol analog CAY10512 (10009536; Cayman Chemical, Ann Arbor, MI) was used at 0.5 uM as previously described [16]. Primary HNG cells were cultured as previously described [14,16,24,15].
Stringent selection parameters were used in choosing control and AD superior temporal lobe neocortex and hippocampal CA1 tissues employed in this study [16,24]. As post-mortem interval (PMI; the time of death to brain freezing at −81 °C) is a factor that affects total RNA quality [24,15,21], all total RNA fractions were derived from tissues having a PMI of 3 h or less. CERAD/NIH criteria were used to categorize AD tissues in accordance with established guidelines [15]. AD brain tissues were obtained from patients with a clinical dementia rating (CDR; an index of cognitive impairment) ranging from 0.5 to 3.0, indicating very mild- to severe-AD [15,21,8]. Brain tissues were used in accordance with the institutional review board (IRB)/ethical guidelines at the Louisiana State University Health Sciences Center and the donor institutions [15,21]. All total RNA isolation, micro-RNA enrichment, quality control, quantitation and characterization using Northern dot blot miRNA analysis for 5S rRNA, miRNA-132, and miRNA146a, and bioinformatics analysis were performed as previously described [13,14,16,24,5,20,12,15,21]. All cell and tissue total RNA samples showed 28S/18S ratios larger than 1.45 indicating high spectral quality RNA [16,24]. Parameters involving control and AD superior temporal lobe neocortical tissues and detailed analysis of RNA spectral purity have been previously described, and most of these tissue samples have been previously subjected to wide spectrum gene expression analysis using DNA array technologies [12,15]. Samples of control and AD tissues used in these experiments exhibited no significant differences in age (69.0 ± 1.8 vs 70.3 ± 3.3 years, p < 0.87), PMI (mean 2.1 ± 0.7 vs 2.0 ± 0.7 h, p < 0.96), RNA A260/280 indices (2.09 ± 0.2 vs 2.09 ± 0.1, p < 0.97), control versus AD, respectively.
Samples of age-matched control and AD tissues, cultured brain and retinal cells, and the transgenic animals Tg2576, TgCRND8, PSAPP, 3xTg-AD and 5xFAD were analyzed for 5S RNA, miRNA-132 and miRNA-146a abundance using miRNA array and/or Northern dot blot hybridization as previously described by our group [13,14,16,24,5,20,12]. Human 5S ribosomal RNA (5S rRNA), a relatively abundant RNA polymerase III transcribed 107 ribonucleotide marker, was used as an internal control for miRNA determinations in brain and retinal cell and tissue samples as described [14,16,24]. DNA array and Northern hybridizations were quantified using data-acquisition/statistical-analysis software provided with a GS250 molecular imager (Bio-Rad, Hercules, CA). Data analyses and graphic presentations were performed using Excel algorithms (Microsoft, Seattle, WA) and Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA). Relevance of miRNA-regulated gene expression to brain aging and neurodegeneration and GenBank nucleic acid links for specific miRNAs were searched using the Medline nucleotide search website access at www.ncbi.nlm.nih.gov. Statistical significance was analyzed using a two-way factorial analysis of variance (p, ANOVA; Statistical Analysis System; SAS Institute, Cary, NC).
Higher miRNA-146a levels were consistently found in the superior temporal lobe neocortex when compared to the hippocampal CA1 region. As the CDR increased from CDR = 0.5 (mild cognitive impairment) to CDR = 3.0 (severe cognitive impairment; Fig. 1A) miRNA-146a levels in AD versus age-matched control brain neo-cortex increased 1.3-fold over control at CDR 0.5 and increased to 2.7-fold over control at a CDR = 3.0, exhibiting a positive correlation coefficient of CDR to miRNA146a abundance of r2 = 0.90, p < 0.001 [15,8]. Similarly, in comparison to a related brain-enriched miRNA-132 in the same sample which did not change, miRNA-146a increased 1.45-fold after 1 week in culture to over 3-fold over 0 time controls in IL-1β-stressed HNG cells (Fig. 1B). We further note relatively unchanging miRNA-132 levels in several human retinal and neural cells and tissues and transgenic mouse brain cortex, and relatively high miRNA-146a levels in moderate and late stage AD, and in transgenic tissues after the onset of AD-type neuropathology, especially in 12-month-old Tg-2576 and 3-month-old 5xFAD animals (Fig. 2). In IL-1β-stressed HNG cells the NF-κB inhibitors CAY10512, curcumin and PDTC were found to reduce expression of the A547 construct containing the miRNA-146a promoter with luciferase reporter to 0.51-, 0.47- and 0.39-fold of untreated controls, respectively (Fig. 3), suggesting a novel, genetic regulatory circuit between NF-κB and miRNA-146a expression. Interesting both NF-κB and miRNA-146a are known to participate in regulating innate immune signaling and the inflammatory response in several human immune and brain cell types [11,13,14,16,24,15].
Of the ~835 human miRNAs so far identified, only specific subsets are detectable or abundant in specific human cells and tissues of the central nervous system (CNS) [22,11,13,14,16,24,23,5,20,12]. The 22 nucleotide A + U-rich human miRNA-146a (5′-UGAGAACUGAAUUCCAUGGGUU-3′), also abundant in murine brain cells where it was originally described, is encoded from a single copy gene on human chromosome 5q34 [11,13,14,16,24,23,5,20,25]. Stressors that induce miRNA-146a include neurotropic viruses such as HSV-1, microbial endotoxins, pro-inflammatory cytokines such as IL-1β, the 42 amino acid amyloid-beta (Aβ42) peptide and oxidative-stress induced in vivo by pathological generation of neurotoxic metals, reactive oxygen species or in vitro by hydrogen peroxide [5,20,25].
In this study we have further assayed for miRNA-146a abundance in several different human brain and retinal cell types and tissues, and in 5 Tg-AD mouse models developed between 1996 and 2006 (Fig. 2; Table 1). Each of the Tg-AD mouse models analyzed here was specifically developed using the insertion of one or more human pathological transgenes, and involved incorporation of mutated human genes encoding the beta amyloid precursor protein (βAPP), presenilin or tau proteins [25,7,9,2,6,10,27,18,17]. These transgenic animals have been propagated to study specific genetic and pathological aspects of AD against a background of aging (Table 1). Importantly, while no transgenic mouse model of AD recapitulates every aspect of the in vivo human brain condition, all transgenic models used in the current studies employed specific βAPP, PS1 and/or tau gene mutations that result in transgenic animals exhibiting age-related changes in the accumulation of amyloid-containing senile plaques and other AD-related pathology [15,7,9,2,6,10,27,18]. In fact, each of the transgenic AD models studied have variable ages when senile plaques first appear, exhibit varying senile plaque densities as they age, show varying degrees of CNS-specific expression of amyloid, display variable presence or absence of synaptic pathology, and exhibit other interrelated pathological features [19,25,7,9,2,6,10,27,18,17,1,29,4].
The various neuropathological characteristics observed in different Tg-AD animal models (Table 1) were graded using (1) a scoring system adapted from Philipson et al., [19], (2) genotypic and phenotypic characteristics listed at the βAPP Tg-AD model database (www.alzforum.org/res/com/tra/app/default.asp), (3) specific source references describing the initial neuropathological findings in aged Tg-AD animals [7,9,2,6,10,27,18,17], and (4) consideration of deficits in the abundance of several important synaptic and cytoarchitectural support proteins, including synapsin, spectrin, syntenin, synaptophysin and neurofilament light (NFL) chain protein assayed in the brains of the various Tg-AD models studied (Table 1; data not shown). While our multifaceted scoring system for Tg-AD neuropathology is supported by several independent lines of evidence, it may need to be further refined. However, the CNS abundance and location of amyloid, senile plaque accumulation and density, synaptic pathology, synaptic loss, and abnormal synapse morphology in aged, symptomatic Tg2576 and 5xFAD mice in Table 1 is further reiterated in additional neuropathology reports from aged Tg-AD animals [1,29,4,26,3,28,30]. We observe the greatest up-regulation in miRNA-146a levels in Tg2576 and 5xFAD transgenic animals, exceeding 3-fold or more over controls. Interestingly, symptomatic Tg2576 and 5xFAD mice also appear to exhibit the greatest deviation from controls in senile plaque density and synaptic pathology, the later considered to hold the strongest correlation to cognitive changes observed in AD affected brain (Table 1, Fig. 2) [13,29,4].
The work in this study also showed that IL-1β-induced transcription of the pre-miRNA-146a gene was significantly attenuated by three different types of NF-κB activation inhibitors; CAY10512, the potent trans-stilbene analog of the anti-inflammatory antioxidant resveratrol; curcumin, the principal anti-tumor and anti-viral phytochemical curcuminoid of the Indian spice turmeric; and PDTC, an antioxidant, metal ion-chelator and inhibitor of NF-κB nuclear translocation (Fig. 3) [16]. The efficacy and variation among these 3 NF-κB inhibitors have both research and therapeutic significance—with PDTC being consistently the most effective miRNA-146a expression inhibitor at the dosage used in our stressed HNG cell test system [16]. The observation that miRNA-146a is an NF-κB-regulated gene in monocytes, neurons and astrocytes further underscores a significant pro-inflammatory transcription factor-miRNA interplay in certain immune and neural cell types [11,13,14,16,25]. Related studies have shown miRNA-146a to base-pair with sequences in the 3′-UTR of the TNF receptor-associated factor 6 (TRAF6), the IL-1 receptor-associated kinase 1 (IRAK1) and complement factor H (CFH) mRNAs, and to function in the control of Toll-like receptor (TLR) and cytokine signaling through a complex negative feedback regulation loop involving down-regulation of TRAF6, IRAK1 and CFH (5–10,23). In AD, NF-κB-mediated miRNA-146a and TLRs may provide a critical link between immune stimulants, such as Aβ42 peptides, and the initiation of host innate immune defenses and further activation of TLR-NF-κB signaling, which further modulates the release of pro-inflammatory cytokines [11,16,25]. It will be interesting to further compare the degree of TLR and NF-κB activation within primary human astroglial cells, which are key mediators of the brain’s innate immune and inflammatory response, and in the brains of various Tg-AD models to understand how miRNA-146a levels change in response to the onset of neuropathology against a background of brain cell and tissue aging.
In brief, transcription of miRNA-146a is significantly induced by pathophysiological stress factors, such as the pro-inflammatory cytokine IL-1β. Relatively high levels of miRNA-146a, but not of other miRNAs, in AD superior temporal lobe neocortex and hippocampus, in stressed human brain cells, and in Tg-AD animal models underscore the potential importance of this NF-κB-regulated, brain-enriched miRNA species in neurodegenerative disease. In Tg-AD studies, those aged models that exhibited higher senile plaque density and synaptic pathology also exhibited higher miRNA-146a levels. Specific murine Tg-AD models, such as Tg2576 and 5xFAD may prove superior platforms to study the miRNA-146a-mediated innate immune response, inflammatory and synaptic pathobiology, and the manipulation of physiological stressors and environmental factors which drive the AD process.
Acknowledgments
Thanks are extended to Darlene Guillot and Aileen Pogue for expert technical assistance, and to Drs. F. Culicchia. W. Poon, G. Tejada and T. Saing for Tg-AD mouse and human brain and retinal tissues or extracts. Some of the brain tissues used in this project was provided by the Institute for Memory Impairments and Neurological Disorders and the University of California at Irvine Alzheimer's Disease Research Center (UCI-ADRC); funding for the UCI-ADRC was provided by NIH/NIA Grant P50 AG16573. The authors' work were further supported in part through Translational Research Initiative (TRI) Grants from LSU Health Sciences Center New Orleans (JMH and WJL); an Alzheimer Association Investigator-Initiated Research Grant IIRG-09-131729 (WJL); NIH NIA AG18031 (WJL), NIH EY06311 (JMH); an unrestricted grant to the LSU Eye Center from Research to Prevent Blindness (RPB); the Louisiana Eye Lions and Lions International Foundation.
References
- 1.Ashe KH, Zahs KR. Probing the biology of Alzheimer’s disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 3.Dong H, Martin MV, Chambers S, Csernansky JG. Spatial relationship between synapse loss and beta-amyloid deposition in Tg2576 mice. J Comp Neurol. 2007;500:311–321. doi: 10.1002/cne.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Intraneuronal β-amyloid accumulation and synapse pathology in Alzheimer’s disease. Acta Neuropathol. 2010;119:523–541. doi: 10.1007/s00401-010-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill JM, Zhao Y, Clement C, Neumann DM, Lukiw WJ. HSV-1 infection of human brain cells induces miRNA-146a and Alzheimer-type inflammatory signaling. Neuroreport. 2009;20:1500–1505. doi: 10.1097/WNR.0b013e3283329c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 7.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 8.Jellinger KA. Clinicopathological analysis of dementia disorders in the elderly. J Alzheimers Dis. 2006;9:61–70. doi: 10.3233/jad-2006-9s308. [DOI] [PubMed] [Google Scholar]
- 9.King DL, Arendash GW. Maintained synaptophysin immunoreactivity in Tg2576 transgenic mice during aging: correlations with cognitive impairment. Brain Res. 2002;926:58–68. doi: 10.1016/s0006-8993(01)03294-2. [DOI] [PubMed] [Google Scholar]
- 10.Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D, Van Broeckhoven CC. Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am J Pathol. 2005;167:527–543. doi: 10.1016/S0002-9440(10)62995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Chen XP, Li YJ. MiRNA-146a and human disease. Scand J Immunol. 2010;71:227–231. doi: 10.1111/j.1365-3083.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 12.Lukiw WJ. Gene expression profiling in fetal, aged, and Alzheimer hippocampus: a continuum of stress-related signaling. Neurochem Res. 2004;29:1287–1297. doi: 10.1023/b:nere.0000023615.89699.63. [DOI] [PubMed] [Google Scholar]
- 13.Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport. 2007;18:297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- 14.Lukiw WJ, Pogue AI. Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J Inorg Biochem. 2007;101:1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukiw WJ, Zhao Y, Cui JG. An NF-κB-sensitive miRNA-146a-mediated inflammatory circuit in AD and in stressed human brain cells. J Biol Chem. 2008;283:31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 19.Philipson O, Lord A, Gumucio A, O’Callaghan P, Lannfelt L, Nilsson LN. Animal models of amyloid-beta-related pathologies in Alzheimer’s disease. FEBS J. 2010;277:1389–1409. doi: 10.1111/j.1742-4658.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- 20.Pogue AI, Li YY, Cui JG, Zhao Y, Kruck TP, Percy ME, Tarr MA, Lukiw WJ. Characterization of an NF-kB-regulated miRNA-146a in metal-sulfate-stressed human brain cells. J Inorg Biochem. 2009;11:156–164. doi: 10.1016/j.jinorgbio.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Pogue AI, Cui JG, Li YY, Zhao Y, Culicchia F, Lukiw WJ. Micro RNA-125b (miRNA-125b) function in astrogliosis and glial cell proliferation. Neurosci Lett. 2010;476:18–22. doi: 10.1016/j.neulet.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 22.Provost P. MicroRNAs as a molecular basis for mental retardation, Alzheimer’s and prion diseases. Brain Res. 2010;1338:58–66. doi: 10.1016/j.brainres.2010.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saba R, Goodman CD, Huzarewich RL, Robertson C, Booth SA. A miRNA signature of prion induced neurodegeneration. PLoS One. 2008;3:e3652. doi: 10.1371/journal.pone.0003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sethi P, Lukiw WJ. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci Lett. 2009;459:100–104. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 25.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of miRNA-146a, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeuchi A, Irizarry MC, Duff K, Saido TC, Hsiao Ashe K, Hasegawa M, Mann DM, Hyman BT, Iwatsubo T. Age-related amyloid beta deposition in transgenic mice over-expressing both Alzheimer mutant presenilin 1 and amyloid beta precursor protein Swedish mutant is not associated with global neuronal loss. Am J Pathol. 2000;157:331–339. doi: 10.1016/s0002-9440(10)64544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Townsend M, Qu Y, Gray A, Wu Z, Seto T, Hutton M, Shearman MS, Middleton RE. Oral treatment with a gamma-secretase inhibitor in a mouse model of Alzheimer’s disease. J Pharmacol Exp Ther. 2010;333:110–119. doi: 10.1124/jpet.109.163691. [DOI] [PubMed] [Google Scholar]
- 29.van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep. 2010;10:207–214. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XM, Cai Y, Xiong K, Cai H, Luo XG, Feng JC, Clough RW, Struble RG, Patrylo PR, Yan XX. Beta-secretase-1 elevation in transgenic mouse models of Alzheimer’s disease is associated with synaptic/axonal pathology and amyloidogenesis: implications for neuritic plaque development. Eur J Neurosci. 2009;30:2271–2283. doi: 10.1111/j.1460-9568.2009.07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]