Abstract
Objective
Increasing evidence suggests that Bone Morphogenetic Protein (BMP) signaling regulates angiogenesis. Here, we aimed to define the function of BMP receptors in regulating early post-natal angiogenesis by analysis of inducible, endothelial specific deletion of the BMP receptor components Bmpr2, Alk1, Alk2 and Alk3 in mouse retinal vessels.
Approach and Results
Expression analysis of several BMP ligands showed that pro-angiogenic BMP ligands are highly expressed in postnatal retinas. Consistently, BMP receptors are also strongly expressed in retina with a distinct pattern. To assess the function of BMP signaling in retinal angiogenesis, we first generated mice carrying an endothelial-specific inducible deletion of BMP Type 2 receptor (Bmpr2). Postnatal deletion of Bmpr2 in endothelial cells substantially decreased the number of angiogenic sprouts at the vascular front and branchpoints behind the front, leading to attenuated radial expansion. To identify critical BMPR1s associated with BMPR2 in retinal angiogenesis, we generated endothelial-specific inducible deletion of three BMPR1s abundantly expressed in endothelial cells and analyzed the respective phenotypes. Among these, endothelial specific deletion of either Alk2/acvr1 or Alk3/Bmpr1a caused a delay in radial expansion, reminiscent of vascular defects associated with postnatal endothelial specific deletion of BMPR2, suggesting that ALK2/ACVR1 and ALK3/BMPR1A are likely to be the critical BMPR1s necessary for pro-angiogenic BMP signaling in retinal vessels.
Conclusions
Our data identify BMP signaling mediated by coordination of ALK2/ACVR1, ALK3/BMPR1A, and BMPR2 as an essential pro-angiogenic cue for retinal vessels.
Keywords: Angiogenesis, BMP signaling, retina, vertebrate development
INTRODUCTION
Bone Morphogenetic Protein (BMP) signaling has been implicated as a key regulator for angiogenesis1. Depending on the nature of the ligands, BMP signaling can either promote or inhibit angiogenesis2; Pro-angiogenic BMP2/4 augments vessel sprouting in a matrigel plug assay3, and stimulation with BMP2 promotes angiogenic responses such as filopodia extension and migration in human umbilical vein endothelial cells (HUVEC) by inducing the expression of target genes, such as MYOX4. In addition, its zebrafish orthologue, Bmp2b, functions as the predominant angiogenic cue for veins5, 6. Similar pro-angiogenic effects have been demonstrated for BMP64, 7. In contrast, BMP9 and BMP10 induce quiescence of endothelial cells, and therefore function as anti-angiogenic cues8–10. Consistent with the idea that BMP9 and BMP10 modulate the homeostasis of mature vessels, it has been shown that BMP9 can promote differentiation of endothelial progenitors during ischemic neovascularization 11
On the cell membrane of the signal receiving cells, BMP ligands interact with tetra-heteromeric receptor complexes composed of two BMP Type 1 Receptors (BMPR1s) and two BMP Type 2 Receptors (BMPR2s). The signaling specificity of each BMP ligand is determined by the interaction between BMP ligand and its cognate BMPR1, since BMPR2 can only serve as a low affinity receptor12, 13. Therefore, BMPR1s are essential for the outcomes of BMP signaling. In the mammalian genome, four BMPR1s, Alk1/Acvrl1, Alk2/Acvr1, Alk3/Bmpr1a, and Alk6/Bmpr1b, have been annotated14, 15. Previous work showed that endothelial-specific deletion of Alk1 generates exuberant angiogenesis, indicating that ALK1 is likely to mediate anti-angiogenic BMP9/10 signaling in endothelial cells 9, 16, 17. Considering that ALK2, ALK3, and ALK6 bind to BMP2, BMP4, and BMP6 in other circumstances18, these receptors are likely to mediate pro-angiogenic BMP signaling. However, since global deletion of these receptors leads to early embryonic lethality19–22, surprisingly little is known about the individual function of these BMPR1s in endothelial cells. In addition, previous attempts to elucidate the role of each BMPR1 in endothelial cells failed to provide comprehensive analyses due to the lack of suitable endothelial-specific Cre driver lines23, 24. Most importantly, manipulations of BMPR1 in endothelial cells were not performed in the same way in the same vascular bed in previous studies, making it difficult to determine the role of each BMPR1s in endothelial cells23, 24.
In this study, we investigated the function of each BMPRI in angiogenesis using postnatal mouse retinal vessels to better understand the molecular and cellular underpinning of pro-angiogenic BMP signaling. To assess the contribution of BMPs in retinal angiogenesis, we showed BMP signaling reporter activity in the early postnatal retina, and found that BMP6 and BMP7 are the most abundant BMP ligands in that organ. We found that several BMPR1s were expressed in distinct regions of the retinal vasculature. Conditional deletion of Bmpr2 and three highly expressed BMPRIs, Alk1/Acvrl1, Alk2/Acvr1, and Alk3/Bmpr1a, in endothelial cells of postnatal mice showed that mice deficient for Alk2/Acvr1 or Alk3/Bmpr1a partially phenocopied the vascular defects of mice with an endothelial-specific deletion of Bmpr2. Taken together, our data indicate that the pro-angiogenic BMP signaling mediated by ALK2 and ALK3 receptors, likely in conjunction with BMPR2, is essential for proper retinal angiogenesis.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Diverse BMP ligands and receptors are expressed during retinal angiogenesis
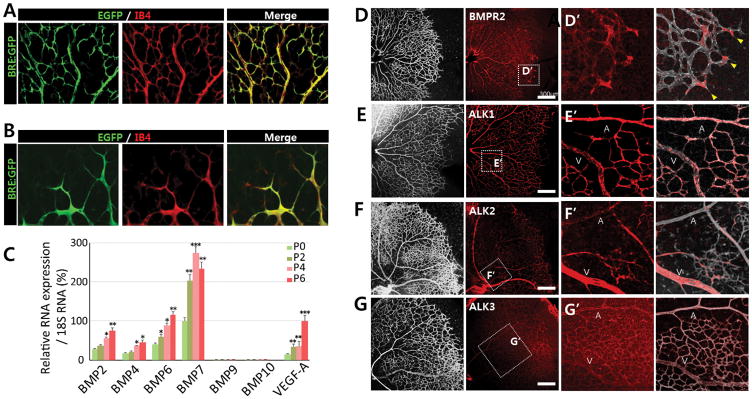
To determine the role of pro-angiogenic BMP signaling, we first examined the expression of GFP in retinal vessels of BRE-GFP mice, where the expression of GFP is regulated by BMP Responsive Elements (BRE) isolated from the Id1 promoter25. As previously reported26, BRE-GFP expression was broadly detected in P5 retinal vessels (Figure 1A). Within the vascular front, BRE-GFP was highly expressed in both tip and stalk cells (Figure 1B). While the expression of BRE-GFP in stalk cells can be attributed to the ALK1-mediated anti-angiogenic BMP signaling activity9, 26, it is not clear how BRE-GFP expression in tip cells is induced.
FIGURE 1. Diverse BMP ligands and receptors are expressed during retinal development.
(A–B) Representative overview of retinal vessels at (A) the retinal center and (B) vascular front shown in higher magnification in postnatal day P5 BRE-gfp transgenic mice. Endothelial cells are shown in red. BRE-GFP is highly expressed in both tip cells and stalk cells. (C) qRT-PCR for Bmp ligands and Vegfa in P0, P2, P4, and P6 retina. Bmp6 and Bmp7 are the most abundant BMP ligands between P0 and P6 (n=5). (D–G) Immunohitochemistry of P5 retina showing BMPR2 (D), ALK1/ACVRL (E), ALK2/ACVR1 (F), and ALK3/BMPR1A (G). While BMPR2, ALK1 and ALK3 are expressed ubiquitously, ALK2 expression is enriched in the veins within the plexus region; scale bar, 300μm. (H–K) Areas within the white rectangles in panels D to G are shown in higher magnification.
To identify which BMP ligands and receptors induce BRE-GFP expression in the retinal vessels, we performed in situ hybridization analyses on mouse retinas and quantitative RT-PCR using cells isolated from postnatal retinas at different developmental stages (Figure 1C and Online Figure I). A number of pro-angiogenic BMP ligands were highly expressed during the stages coincident with extensive retinal angiogenesis (Figure 1C and Online Figure II). In particular, the expression of BMP6 and BMP7, was increased over two folds. In contrast, expression of BMP9 and 10, which are ligands for ALK1 and are produced by liver and heart respectively, were not detected in the retina (Online Figure I). However, given that they are delivered by circulation, it is likely that the mature BMP9 and BMP10 protein are present in the retina. In endothelial cells, only three BMPR1s, ALK1, ALK2, and ALK3, but not ALK6, were highly expressed. Therefore, ALK6 was excluded from further analyses (Online Figure III).
Next, we analyzed the expression of BMPR2 and the three BMPR1s that are abundant in endothelial cells, ALK1/ACVRL, ALK2/ACVR1A, and ALK3/BMPR1A by immunostaining in retinas (Figure 1D–G and data not shown). BMPR2 staining is vascular and appears in all vessels, with heightened expression at the vascular front (Figures 1D and H). Interestingly, each BMPR1 has distinct expression pattern; ALK1 and ALK3 proteins are found in both plexus and vascular front, with ALK1 being enriched in the arteries of the plexus region. ALK2 staining is highly enriched in veins in the plexus region and not associated with the vascular front or arteries (Figures 1E–G and I–K). These findings show that pro-angiogenic BMP ligands and receptors are highly expressed in blood vessels during retinal development, and the receptors have distinct expression patterns. Therefore, it is likely that BRE-GFP reporter activity in tip cells at the vascular front results from the activity of pro-angiogenic BMP ligands.
BMPR2 activity promotes retinal angiogenesis
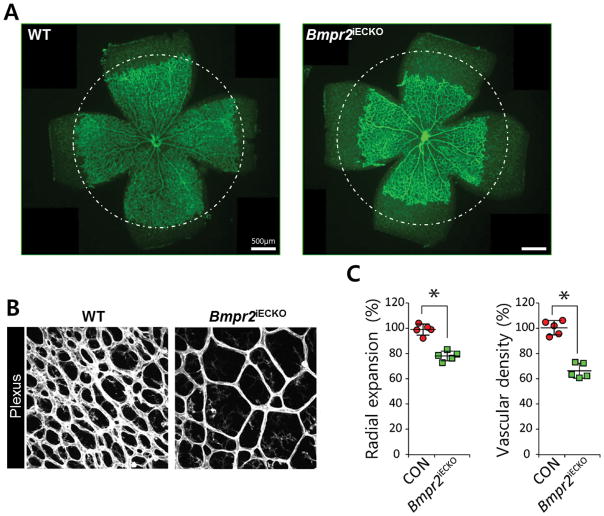
To examine the requirement for pro-angiogenic BMP signaling during retinal angiogenesis, we generated mice carrying an inducible, endothelial-specific Bmpr2 deletion by crossing Bmpr2fl/fl and Cdh5(Pac)CreERT2 mice27. Given that BMPR2 is the predominant binding partner for BMPR1s mediating pro-angiogenic BMP activity, we postulated that deletion of Bmpr2 is likely to attenuate signaling outcomes downstream of most pro-angiogenic BMP ligand engagement during retinal angiogenesis. Consistent with our hypothesis, endothelial cell deletion of Bmpr2 significantly decreased the radial expansion of the retinal vessels (Figures 2A and 2C), indicating that BMP signaling is essential for retinal angiogenesis. Bmpr2fl/fl;Cdh5(Pac)CreERT2 mice also displayed a significant reduction in vascular density (% vascularized area) compared to wild-type littermates (Figures 2B and 2C). This data shows that pro-angiogenic BMP signaling mediated by BMPR2 is a critical positive regulator of retinal angiogenesis.
FIGURE 2. BMPR2 is essential for retinal angiogenesis.
(A) Representative overview of P5 retinal vessels taken from inducible endothelial specific knock out of Bmpr2fl/fl;Cdh5(Pac)CreERT2 (right) and their phenotypic wild-type littermates (left); scale bar, 500μm. Mean radial expansion of the retinal vessels in P5 Bmpr2fl/fl retinas was decreased to 78.9±9.8% of wildtype littermates. (B) Representative overview of plexus region of P5 retina in Bmpr2fl/fl;Cdh5(Pac)CreERT2 (right) and their phenotypic wild-type littermates (left). Mean vascular density in the plexus region in Bmpr2fl/fl retinas was reduced to 65.7±8.3% of wildtype littermates; scale bar, 500μm. Endothelial cells are visualized by anti- Isolectin B4 (IB4) staining. (C) Quantification of radial expansion and vascular density (% vascularized area) in Bmpr2fl/+;Cdh5(Pac)CreERT2 (gray) and Bmpr2fl/fl;Cdh5(Pac)CreERT2 mice injected with 100μg tamoxifen at P1, retinas were assayed P5 (n=5). P<0.05. Statistical significance was assessed using a Student’s unpaired t-test.
Endothelial specific deletion of Alk2/ACVR1A or Alk3/BMPR1A but not Alk1/ACVRL recapitulates vascular defects of endothelial specific deletion of Bmpr2
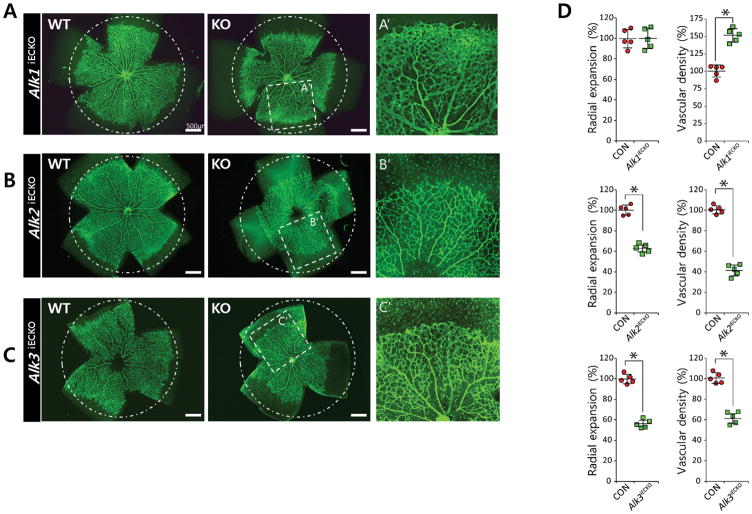
Next, we investigated critical BMPR1 interactions mediating pro-angiogenic BMP signaling during retinal angiogenesis. Endothelial deletion of Alk1, Alk2, or Alk3 during postnatal retinal angiogenesis was induced by crossing homozygous mice carrying a floxed allele of each BMPR1 and Cdh5(Pac)CreERT2 mice (Online Figure IV). As previously reported9, 27, tamoxifen-mediated deletion of Alk1 in endothelial cells at P1 did not exhibit severe defects in radial extension, but caused exuberant angiogenesis in developing retinas compared to phenotypic wild-type littermates and therefore substantially increased vascular density (% vascularized area) (Figure 3A, D). In Alk1fl/fl;Cdh5(Pac)CreERT2 mice, the vascular phenotype was more pronounced in the vascular front than in the plexus region (Figure 3A). Considering the distinct phenotypes between Bmpr2fl/fl;Cdh5(Pac)CreERT2 and Alk1fl/fl;Cdh5(Pac)CreERT2 mice, it is unlikely that ALK1 is involved in pro-angiogenic BMP signaling mediated by BMPR2.
FIGURE 3. ALK2 and ALK3 promotes retinal angiogenesis.
(A–C) Representative overview of retinal vessels taken from inducible endothelial specific knock out of Alk1fl/fl;Cdh5(Pac)CreERT2 (A), Alk2fl/fl;Cdh5(Pac)CreERT2 (B), or Alk3fl/fl;Cdh5(Pac)CreERT2 (C) and their phenotypic wild-type littermates. Mice were injected with 50μg tamoxifen at P1, retinas were assayed P5; scale bar, 500μm. Both radial expansion and vascular density in the plexus region were significantly increased in P5 Alk1fl/fl;Cdh5(Pac)CreERT2 retinas (101±6.7% for radial expansion and 155±6.2% for vascular density compared to wildtype littermates). By contrast, deletion of either Alk2 or Alk3 significantly decreased both radial expansion (63.2±17.1% for Alk2fl/f;Cdh5(Pac)CreERT2 retinas and 56.4±14.2% for Alk3fl/fl;Cdh5(Pac)CreERT2 retinas compared to wildtype littermates) and vascular density in the plexus region (42.3±15.9% for Alk2fl/f;Cdh5(Pac)CreERT2 l retinas, and 61.9±12.8% for Alk3fl/fl;Cdh5(Pac)CreERT2 retinas compared to wildtype littermates). Areas within the white rectangle in middle column are shown in higher magnification (right column). While radial expansion was similarly affected by deletion of either Alk2 or Alk3, vascular density in the plexus region is more severely affected by the deletion of Alk2 than Alk3. Endothelial cells are visualized by anti- Isolectin B4 (IB4) staining. (D) Quantification of radial expansion and vascular density (% vascularized area) in inducible endothelial specific knockout of each BMPR1 (pink) and their littermates (gray) (n=5). P<0.05. Statistical significance was assessed using a Student’s unpaired t-test.
In contrast, tamoxifen-mediated deletion of Alk2 or Alk3 in endothelial cells at P1 led to a substantial reduction in the radial expansion of retinal vessels (Figures 3B–C and Online Figure IV), reminiscent of the defects in Bmpr2fl/fl;Cdh5(Pac)CreERT2 mice (Figure 2A). In addition, vascular density was substantially reduced in tamoxifen-injected Alk2fl/fl;Cdh5(Pac)CreERT2 or Alk3fl/fl;Cdh5(Pac)CreERT2 retinas compared to wild-type littermates (Figure 3D). These findings show that both ALK2 and ALK3 are important for proper retinal vessel morphogenesis, likely in conjunction with BMPR2.
Endothelial specific deletion of Alk3/BMPR1A and Bmpr2 but not Alk2/ACVR1A affect angiogenic sprouts at the vascular front
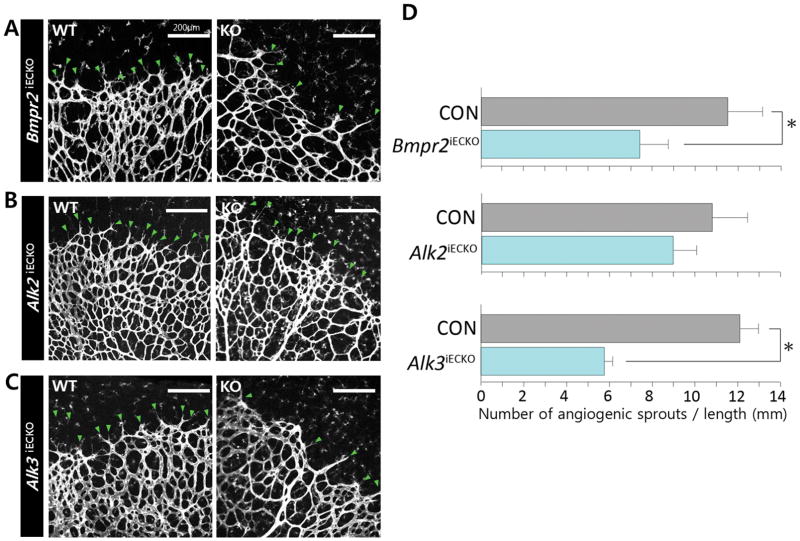
Considering that both Alk2 and Alk3 deletion in endothelial cells significantly delayed radial expansion but only Alk3 is expressed at the vascular front, we hypothesized that ALK2 and ALK3 receptors would differentially affect angiogenic sprouts. To examine this notion, we first examined the phenotype of endothelial deletion of Bmpr2 and found that lack of Bmpr2 significantly decreased the number of angiogenic sprouts (Figure 4A). Endothelial deletion of Alk3/BMPR1A similarly decreased the number of angiogenic sprouts present at the vascular front compared to phenotypic wild-type littermates (Figures 4B to 4C), while deletion of Alk2 did not significantly alter the number of angiogenic sprouts at the vascular front. Considering that the vascular coverage (% vascularized area) was significantly decreased in retinas of mice with deletion of either receptor (Figure 3B–D), it is likely that different BMPR1 receptors mediate pro-angiogenic BMP signaling at the vascular front and behind the front.
FIGURE 4. ALK3/BMPR2 signaling is required for the formation of angiogenic sprouts in the vascular front.
(A) Representative image of vascular front in postnatal day P5 Bmpr2fl/fl;Cdh5(Pac)CreERT2 (A), Alk2fl/fl; Cdh5(Pac)CreERT2 (B), or Alk3fl/fl;Cdh5(Pac)CreERT2 Endothelial cells are visualized by anti- Isolectin B4 (IB4) staining. (C) and their phenotypic wild-type littermates. Green arrows point the angiogenic sprouts in the vascular front (n=5); scale bar, 200μm. (D) Quantification of angiogenic sprouts. The number of angiogenic sprouts in the vascular front was measured by dividing the number of angiogenic sprouts by the total length of vascular front (See Supplemental methods for detail). While the number of angiogenic sprouts in the vascular front was mildly decreased by 20% to in P5 Alk2fl/fl retinas (8.8±0.2 compared to 10.8±1.9 in wildtype littermates), it was decreased by 45% in Bmpr2fl/fl (6.5±1.3 in Bmpr2fl/fl retinas compared to 11.8±1.6 in wildtype littermates), and by 53% in Alk3fl/fl retinas (5.3±0.4 in Alk3fl/fl retinas compared to 11.2±0.9 in wildtype littermates). P<0.05. Statistical significance was assessed using a Student’s unpaired t-test.
DISCUSSION
Our results identify BMPR2/ALK2 and BMPR2/ALK3 as key receptors that mediate pro-angiogenic BMP signaling in the early post-natal retina, and reveal regional differences among BMPR1s by analysis of parallel genetic experiments in a defined vascular bed. Deletion of the common BMPR2 receptor reduced vascular sprouting and density. Deletion of ALK3, which is ubiquitously expressed in retinal endothelial cells, also dramatically reduced vascular sprouting and density, while loss of ALK2, which is enriched behind the vascular front, did not significantly affect sprouting but reduced overall vessel density. Therefore, we propose that spatially regulated BMPR1 expression fine-tunes endothelial cell responses to pro-angiogenic BMP ligands in development. Since expression of BMP ligands selective for ALK2 and ALK3 is elevated during retinal angiogenesis, it is tempting to speculate that BMP6/7-ALK2/3-BMPR2 signaling axis may provide essential input for the developing retina.
Since the phenotype of endothelial-specific deletion of Bmpr2 is quite distinct from the vascular phenotype caused by endothelial-specific deletion of Alk1, it is likely that BMPR2-mediated pro-angiogenic signaling is dominant over interactions with ALK1 in the early postnatal retina. In contrast, ALK2 and ALK3 appear to be essential for BMPR2-mediated pro-angiogenic signaling. These receptors function similarly but non-redundantly behind the vascular front in mediating branching, as deletion of either Type 1 receptor partially phenocopies the phenotype of Bmpr2 deletion and results in reduced branching. This finding suggests that both ALK2/BMPR2 and ALK3/BMPR2 complexes contribute to branching morphogenesis, which is consistent with our previous finding that pro-angiogenic BMP signaling leads to increased branching in vitro and in vivo5, 6. Angiogenic sprouting at the vascular front is likely to selectively utilize ALK3/BMPR2 complexes, since genetic deletion of Alk2 did not significantly affect angiogenic sprouting. This is consistent with the expression patterns of the receptors in the early postnatal retina, as ALK3 but not ALK2 reactivity was found at the vascular front. ALK2 reactivity is enriched in veins, while ALK3 expression is not enriched in larger vessels, suggesting potential differences in ALK2 and ALK3 mediated pro-angiogenic BMP signaling. ALK1, which does not appear to be essential for BMPR2-mediated pro-angiogenic BMP signaling, may provide an additional regulation for vessel remodeling and homeostasis at later stages.
Previously, it has been shown that BMPR1s can promiscuously form heterodimeric complex16, 28–30. For instance, it has been shown that ALK1 and ALK2 can form heterodimers under certain circumstances29, 30. Similarly, ALK2 and ALK3 are proposed to form heterodimers to mediate BMP2 signaling28. Therefore, it is possible that they may influence the signaling property of each other. While we cannot rule of this possibility due to the lack of appropriate reagents, given the distinct expression pattern of BMPR1s, we believe that the vascular phenotype caused by endothelial specific deletion of single BMPR1 is likely to represent the unique signaling property of each receptor.
Our analyses indicate that ALK3/BMPR2 mediated BMP signaling regulates angiogenic sprouting at the vascular front, while both ALK3/BMPR2 and ALK2/BMPR2 regulate branching behind the vascular front. This is consistent with our finding that BMP signaling, as read out by BRE-EGFP, is active throughout the retinal vasculature, and suggests that pro-angiogenic BMP signaling regulates both sprouting and branching. These results are consistent with a previous report describing ubiquitous expression of BRE-EGFP in retinal vessels26.
Our work presents compelling evidence that pro-angiogenic BMP signaling is important for angiogenesis. Here we show that expression of Type 1 receptors, specifically ALK2 and ALK3, may explain the different effects of BMP signaling on developing vessels, and they each likely complex with BMPR2 to mediate pro-angiogenic effects in blood vessels in a non-redundant way. While the molecular underpinning of the phenotypic differences between Alk2fl/fl;Cdh5(Pac)CreERT2 and Alk3fl/fl;Cdh5(Pac)CreERT2 mice needs further analyses, careful phenotypic comparisons between these mice will help elucidate how angiogenesis is regulated in development and disease, and may provide useful information in developing therapeutic strategies.
Supplementary Material
HIGHLIGHTS.
Pro-angiogenic BMP signaling is essential for retinal angiogenesis.
Alk2/Acvr1 and Alk3/Bmpr1a relays distinct aspects of pro-angiogenic effects of BMP.
Acknowledgments
We thank Rita Webber, Nicole Copeland, and Elle Law for excellent animal care, and Drs. Ralf Adams and S. Paul Oh, for providing mice strains.
SOURCE OF FUNDING
This work was supported by NIH grants HL43174 and HL116719 (VLB), EY025979 and HLI125811 (EA), HL090960 and HL114820 (SWJ), Grants from GIST Research Institute (GRI), Cell Logistics Research Center, National Research Foundation of Korea (2016R1A5A1007318) (SWJ), and an AHA Postdoctoral Fellowship 15POST25560114 (RO).
LIST OF NON-STANDARD ABBREVIATIONS AND ACRONYMS
- ALK
Activin receptor-like kinase
- BMP
Bone Morphogenetic Protein
- BMPR1
BMP Type 1 Receptor
- BMPR2
BMP Type 2 Receptor
- BRE
BMP Response Element
- HUVEC
Human Umbilical Vein Endothelial Cell
Footnotes
DISCLOSURE
NONE
References
- 1.Cai J, Pardali E, Sanchez-Duffhues G, ten Dijke P. Bmp signaling in vascular diseases. FEBS letters. 2012;586:1993–2002. doi: 10.1016/j.febslet.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 2.David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine & growth factor reviews. 2009;20:203–212. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Molecular cancer research : MCR. 2004;2:141–149. [PubMed] [Google Scholar]
- 4.Pi X, Ren R, Kelley R, Zhang C, Moser M, Bohil AB, Divito M, Cheney RE, Patterson C. Sequential roles for myosin-x in bmp6-dependent filopodial extension, migration, and activation of bmp receptors. The Journal of cell biology. 2007;179:1569–1582. doi: 10.1083/jcb.200704010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiley DM, Kim JD, Hao J, Hong CC, Bautch VL, Jin SW. Distinct signalling pathways regulate sprouting angiogenesis from the dorsal aorta and the axial vein. Nature cell biology. 2011;13:686–692. doi: 10.1038/ncb2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakayama Y, Fukuhara S, Ando K, Matsuda M, Mochizuki N. Cdc42 mediates bmp-induced sprouting angiogenesis through fmnl3-driven assembly of endothelial filopodia in zebrafish. Developmental cell. 2015;32:109–122. doi: 10.1016/j.devcel.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Ren R, Charles PC, Zhang C, Wu Y, Wang H, Patterson C. Gene expression profiles identify a role for cyclooxygenase 2-dependent prostanoid generation in bmp6-induced angiogenic responses. Blood. 2007;109:2847–2853. doi: 10.1182/blood-2006-08-039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of bmp9 and bmp10 as functional activators of the orphan activin receptor-like kinase 1 (alk1) in endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 9.Larrivee B, Prahst C, Gordon E, del Toro R, Mathivet T, Duarte A, Simons M, Eichmann A. Alk1 signaling inhibits angiogenesis by cooperating with the notch pathway. Developmental cell. 2012;22:489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S. Bmp9 and bmp10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119:6162–6171. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Kim M, Jeong Y, Lee WB, Park H, Kwon JY, Kim YM, Hwang D, Kwon YG. Bmp9 induces cord blood-derived endothelial progenitor cell differentiation and ischemic neovascularization via alk1. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:2020–2031. doi: 10.1161/ATVBAHA.115.306142. [DOI] [PubMed] [Google Scholar]
- 12.Pouliot F, Blais A, Labrie C. Overexpression of a dominant negative type ii bone morphogenetic protein receptor inhibits the growth of human breast cancer cells. Cancer research. 2003;63:277–281. [PubMed] [Google Scholar]
- 13.Townson SA, Martinez-Hackert E, Greppi C, Lowden P, Sako D, Liu J, Ucran JA, Liharska K, Underwood KW, Seehra J, Kumar R, Grinberg AV. Specificity and structure of a high affinity activin receptor-like kinase 1 (alk1) signaling complex. The Journal of biological chemistry. 2012;287:27313–27325. doi: 10.1074/jbc.M112.377960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 15.Mazerbourg S, Sangkuhl K, Luo CW, Sudo S, Klein C, Hsueh AJ. Identification of receptors and signaling pathways for orphan bone morphogenetic protein/growth differentiation factor ligands based on genomic analyses. The Journal of biological chemistry. 2005;280:32122–32132. doi: 10.1074/jbc.M504629200. [DOI] [PubMed] [Google Scholar]
- 16.Aspalter IM, Gordon E, Dubrac A, Ragab A, Narloch J, Vizan P, Geudens I, Collins RT, Franco CA, Abrahams CL, Thurston G, Fruttiger M, Rosewell I, Eichmann A, Gerhardt H. Alk1 and alk5 inhibition by nrp1 controls vascular sprouting downstream of notch. Nature communications. 2015;6:7264. doi: 10.1038/ncomms8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Q, Kim YH, Wang D, Oh SP, Luo K. Snon facilitates alk1-smad1/5 signaling during embryonic angiogenesis. The Journal of cell biology. 2013;202:937–950. doi: 10.1083/jcb.201208113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type i receptors for osteogenic protein-1 and bone morphogenetic protein-4. The Journal of biological chemistry. 1994;269:16985–16988. [PubMed] [Google Scholar]
- 19.Komatsu Y, Scott G, Nagy A, Kaartinen V, Mishina Y. Bmp type i receptor alk2 is essential for proper patterning at late gastrulation during mouse embryogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236:512–517. doi: 10.1002/dvdy.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijnden-van Raaij J, Donahoe PK, Li E. The type i serine/threonine kinase receptor actria (alk2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551–2561. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- 21.Mishina Y, Crombie R, Bradley A, Behringer RR. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Developmental biology. 1999;213:314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- 22.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type i bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes & development. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 23.Song L, Fassler R, Mishina Y, Jiao K, Baldwin HS. Essential functions of alk3 during av cushion morphogenesis in mouse embryonic hearts. Developmental biology. 2007;301:276–286. doi: 10.1016/j.ydbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by alk2 in the developing mouse heart. Developmental biology. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monteiro RM, de Sousa Lopes SM, Bialecka M, de Boer S, Zwijsen A, Mummery CL. Real time monitoring of bmp smads transcriptional activity during mouse development. Genesis. 2008;46:335–346. doi: 10.1002/dvg.20402. [DOI] [PubMed] [Google Scholar]
- 26.Moya IM, Umans L, Maas E, Pereira PN, Beets K, Francis A, Sents W, Robertson EJ, Mummery CL, Huylebroeck D, Zwijsen A. Stalk cell phenotype depends on integration of notch and smad1/5 signaling cascades. Developmental cell. 2012;22:501–514. doi: 10.1016/j.devcel.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands dll4 and jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type i receptor complexes to pattern the dorsoventral axis. Nature cell biology. 2009;11:637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo J, Tang M, Huang J, He BC, Gao JL, Chen L, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, Bi Y, Luo X, Jiang W, Su Y, Shen J, Kim SH, Huang E, Gao Y, Zhou JZ, Yang K, Luu HH, Pan X, Haydon RC, Deng ZL, He TC. Tgfbeta/bmp type i receptors alk1 and alk2 are essential for bmp9-induced osteogenic signaling in mesenchymal stem cells. The Journal of biological chemistry. 2010;285:29588–29598. doi: 10.1074/jbc.M110.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Lowik CW, ten Dijke P. Bmp-9 signals via alk1 and inhibits bfgf-induced endothelial cell proliferation and vegf-stimulated angiogenesis. Journal of cell science. 2007;120:964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.