Abstract
Purpose
To quantify the long-term success of repeat injections for trigger fingers and to identify predictors of treatment outcomes.
Methods
This retrospective case series analyzed 292 repeat corticosteroid injections for trigger fingers administered by hand surgeons at a single tertiary center between January 2010 and January 2013. One hundred eighty-seven patients (64%) were female, 139 patients (48%) had multiple trigger fingers, and 63 patients (22%) were diabetic. The primary outcome, treatment failure, was defined as receiving a subsequent injection or surgical treatment. Patients without either documented failure or a return office visit in 2015–2016 were surveyed by telephone to determine if they had required subsequent treatment. Kaplan-Meier analyses with log-rank testing assessed the median time to treatment failure and the effect of demographic and disease-specific characteristics on injection success rate while predictors of injection outcome (success vs failure) were assessed with multivariable logistic regression.
Results
Second injections provided long-term treatment success in 39% (111/285) of trigger fingers with 86 receiving an additional injection and 108 ultimately undergoing surgical release. Thirty-nine percent (24/62) of third injections resulted in long-term success, with 22 receiving an additional injection, and 23 ultimately undergoing surgery. Median times-to-failure for second and third injections were 371 and 407 days respectively. Success curves did not differ significantly according to any patient or disease factor. Logistic regression identified that advancing patient age and injection for trigger thumb were associated with success of 2nd injections.
Conclusion
Thirty nine percent of second and third corticosteroid injections for trigger finger yield long-term relief. While most patients ultimately require surgical release, 50% of patients receiving repeat trigger injections realize one year or more of symptomatic relief. Repeat injections of trigger fingers should be considered in patients who prefer non-operative treatment.
Level of Evidence
IV, Therapeutic
Keywords: corticosteroid, injection, repeat, stenosing tenosynovitis, trigger finger
INTRODUCTION
Corticosteroid injections are the definitive treatment for the majority of newly diagnosed trigger fingers.1–12 The response to initial corticosteroid injections is well-studied with the percentage of symptom-free patients gradually declining over the first year after injection before plateauing at 45% treatment success by five years.13,14 Factors impacting the outcome after initial trigger finger injection include symptom duration,2,3 nodule type,8 finger involved,7,9 presence of multiple trigger fingers,2,9,13,14 sex,7,14 age,13 comorbid upper extremity tendonopathies,13 and diabetes.15,16
The chance of long-term success after second and third injections for trigger finger is less precisely understood. Estimates for the chance of symptomatic relief ranges from 23–79% for second injections and from 6–74% for third injections.2,4,5,9,10,13,17 These wide ranges reflect prior studies reporting these outcomes with secondary descriptive statistics as opposed to being evaluated as a primary research focus.
Based on the available literature, surgeons are unable to counsel patients precisely regarding the risk of symptom recurrence after a repeat corticosteroid injection. Furthermore, the pattern of symptom recurrence over time and the factors impacting treatment success remain unclear. This study was designed to quantify the likelihood of long-term success after second and third corticosteroid injections for trigger fingers. The secondary aim was to identify factors predictive of treatment success. Our working hypothesis was that the success of repeat injections would be less than initial injections and that factors associated with the success of initial injections (i.e., multiple trigger fingers, diabetes) would impact the chance of success with repeat injection.
MATERIALS AND METHODS
Participant Selection
This institutional review board-approved this single-center retrospective case series, which analyzed 289 second and third corticosteroid injections in flexor tendon sheaths to quantify the chance of long-term success of repeat corticosteroid injections for trigger finger. Injections were placed into the flexor tendon sheath through the A1 pulley angled 45o from proximal to distal with 1mL of 40mg/mL Depo-Medrol (methylprednisolone acetate) and 0.5–1.0 mL of 1% lidocaine. The primary outcome, treatment failure, was defined as a subsequent injection or surgical release of the A1 pulley of the affected digit.
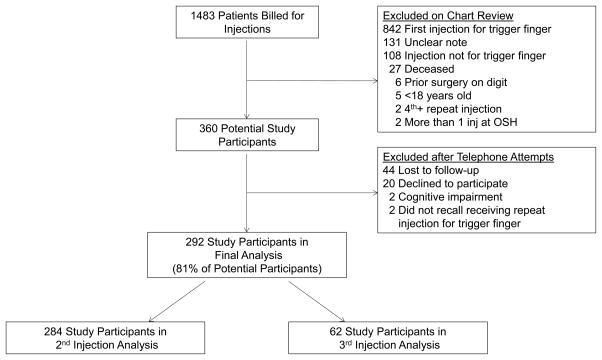
Potential subjects treated for trigger finger by one of 7 fellowship-trained hand orthopedic surgeons at a single tertiary center between January 2010 and December 2013 were identified by a search of departmental billing data. The search was conducted using Current Procedural Terminology (CPT) codes 20550 (injection; tendon sheath, ligament), 20551 (injection; tendon origin/insertion), or 20600 (arthrocentesis, aspiration, or injection) and International Classification of Diseases, Ninth Revision (ICD-9) codes 727.03 (trigger finger) or 727.05 (tenosynovitis; hand, wrist). Inclusion criteria required delivery of a repeat injection for trigger finger during the study period to a patient at least 18 years old. Our database search identified 2176 injections in 1483 patients over the study period.
Medical records were manually reviewed for the patient’s age at first injection, sex, injection date, digit injected, symptoms graded according to the Green classification at each injection (Table 1), presence of multiple trigger fingers, and associated co-morbidities with potential relevance to the trigger finger (i.e., rheumatoid arthritis, hypothyroidism, carpal tunnel syndrome, other upper extremity tendonitis, and diabetes [non-insulin-dependent vs. insulin-dependent]) at time of injection. The presence of multiple trigger fingers was defined as more than one symptomatic trigger finger either at or before the time of repeat injection. For patients with return visits for treatment failure, the time to failure and subsequent treatment were recorded. For patients with repeat injections for multiple digits within the three-year period, only the first repeat injection contributed data to the study to ensure independent observations for statistical analyses. In the case of concurrent bilateral injections, a random number generator was used to pick the studied side. This decision minimized bias introduced by the selection of a digit according to duration, severity, side, or subsequent treatment needed. Subjects were excluded if the medical record notes did not specify the digit injected (n=131).
Table 1.
Green classification of trigger finger grade.
| Grade I | Pain or tenderness at the A1 pulley |
| Grade II | Catching, can actively extend digit |
| Grade III | Locking, requiring passive extension |
| Grade IV | Locked, unable to passively extend, fixed flexion contracture |
Manual chart review identified 360 eligible patients (Figure 1). Eligible subjects without documented failure who had not been seen in our office in June 2015 or later were contacted by telephone using three attempts with voicemails on separate days. Subjects were excluded at time of the telephone phone call if they were unreachable (N=44), declined participation (N=20), had died (N=0), were unable to give study consent in English (N=2), could not recollect receiving a repeat injection for a trigger finger (N=2), or if they stated that they received a later treatment but could not estimate the date within one month (N=0). Thus, 292 completed follow-up and were included in the final analysis. Follow-up data from telephone calls were collected after patients provided verbal consent for study participation. Patients were queried regarding recurrence of symptoms, dates of any necessary subsequent treatment, the presence of other trigger fingers, and current diabetes status. As patients who experience resolution of symptoms do not schedule routine follow-up visits in our practice, telephone follow-up minimized bias toward a lower estimated chance of treatment success associated with analyzing only patients who returned to the office as study subjects.
Figure 1.
Flow diagram of participant selection for study.
Among analyzed patients, 284 patients received second injections and 62 patients received third injections, with 54 patients receiving both during the specified period. The distribution of affected fingers and patient demographic data are detailed in Table 2. Patients with successful injection who contributed data to this study had a minimum follow-up duration of 1.5 years.
Table 2.
Baseline population characteristics (all patients).
| Variables | Participants (N=292) N (%) |
|---|---|
|
| |
| Age at 1st Injection (years), (SD) | 60 (11) |
|
| |
| Sex, Female | 187 (64%) |
|
| |
| Affected Digit | |
| Thumb | 79 (27%) |
| Index | 21 (7%) |
| Middle | 104 (36%) |
| Ring | 79 (27%) |
| Small | 9 (3%) |
|
| |
| Multiple Triggers | 139 (48%) |
|
| |
| Diabetes Status at 1st Injection | |
| None | 229 (78%) |
| NIDDM* | 41 (14%) |
| IDDM** | 22 (8%) |
|
| |
| Comorbidities | |
| Rheumatoid Arthritis | 11 (4%) |
| Hypothyroidism | 40 (14%) |
| Carpal Tunnel Syndrome | 96 (33%) |
| Upper Extremity Tendonitis | 51 (18%) |
NIDDM – non-insulin dependent diabetes
IDDM – insulin dependent diabetes
Statistical Analysis
Descriptive analyses produced frequencies and percentages for categorical variables and mean (standard deviations) or median values for continuous variables. The median time to treatment failure was determined with Kaplan-Meier analysis. The effect of sex, multiple trigger fingers, and diabetes on the pattern of injection failure over time were tested with log-rank testing. Participants without treatment failure were censored at the time point corresponding to their most recent office visit or telephone follow-up. Bivariate analyses using chi-square or Fisher’s exact test for categorical variables and independent samples t-test for continuous variables determined independent predictors of success. Multivariable logistic regression was used to model the relationship between treatment success and hypothesized predictors of success accounting for other patient characteristics. The presence of multiple trigger fingers (included based on documented impact on success of initial trigger injection) and any other factors that approached significance during bivariate analysis were planned for inclusion in the multivariable logistic regression model for associations with the dependent outcome of treatment failure. A forward stepwise selection procedure was used with an alpha of 0.1 for entry. We assessed model explanatory power and fit using the Nagelkerke R2 and Hosmer-Lemeshow lack-of-fit test (confirmed p>0.05). An unadjusted alpha level of 0.05 for significance was used for all tests.
We chose the inclusive study dates to allow adequate sample size for multivariate testing to determine factors associated with success or failure of injections. Assuming at least a 50% chance of injection failure when planning this study, we determined the study interval to include 40 failures after 2nd and 3rd injections respectively to allow for at least 4 predictor variables in our regression modeling. After data collection, we realized that the cohort of patients receiving 3rd injections comprised small numbers of individuals when categorized by demographic and disease characteristics so multivariate testing was limited to the 2nd injection cohort.
RESULTS
Second Injections
Among the 284 patients who received second injections, 183 (64%) were female, with an average age of 60 years (SD 11). One hundred and thirty one patients (46%) had multiple trigger fingers. Fifty-nine patients (21%) were diabetic, of which 39 had NIDDM and 20 had IDDM. The median time between initial and second injection was 214 days in patients without diabetes and was 235 in diabetic patients (366 days for IDDM, 213 days NIDDM). Digits involved, trigger finger grade, and the presence of selected comorbidities are detailed in Table 3.
Table 3.
Second injection characteristics and bivariate contrasts between successes and failures.
| Variables | Participants (N=284) N (%) | Success (N=111) N (%) | Failure (N=173)N (%) | P-value |
|---|---|---|---|---|
|
| ||||
| Age at 1st Injection (years), (SD) | 60 (11) | 61 (10) | 59 (11) | 0.09 |
|
| ||||
| Time Between 1st and 2nd Injection (days), median (range) | 217 (8–5825) | 235 (8–1939) | 210 (21–5825) | <0.05 |
|
| ||||
| Sex, Female | 183 (64%) | 79 (71%) | 104 (60%) | 0.06 |
|
| ||||
| Affected Finger | <0.05 | |||
| Thumb | 78 (28%) | 41 (37%) | 37 (21%) | |
| Index | 20 (7%) | 8 (7%) | 12 (7%) | |
| Middle | 103 (36%) | 29 (26%) | 74 (43%) | |
| Ring | 74 (26%) | 30 (27%) | 44 (25%) | |
| Small | 9 (3%) | 3 (3%) | 6 (4%) | |
|
| ||||
| Multiple Triggers, yes | 131 (46%) | 44 (40%) | 87 (50%) | 0.08 |
|
| ||||
| Diabetes Status at 1st Injection | 0.57 | |||
| None | 225 (79%) | 90 (81%) | 135 (78%) | |
| NIDDM | 39 (14%) | 12 (11%) | 27 (16%) | |
| IDDM | 20 (7%) | 9 (8%) | 11 (6%) | |
|
| ||||
| Comorbidities | ||||
| Rheumatoid Arthritis | 11 (4%) | 4 (4%) | 7 (4%) | 1.00 |
| Hypothyroidism | 37 (13%) | 17 (15%) | 20 (12%) | 0.36 |
| Carpal Tunnel Syndrome | 93 (33%) | 33 (30%) | 60 (35%) | 0.39 |
| Upper Extremity Tendonitis | 48 (17%) | 22 (20%) | 26 (15%) | 0.29 |
|
| ||||
| Green Classification at 2nd Injection (N=242) | 0.94 | |||
| Grade I | 83 (34%) | 33 (34%) | 50 (35%) | |
| Grade II | 147 (61%) | 60 (62%) | 87 (60%) | |
| Grade III | 7 (3%) | 2 (2%) | 5 (3%) | |
| Grade IV | 5 (2%) | 2 (2%) | 3 (2%) | |
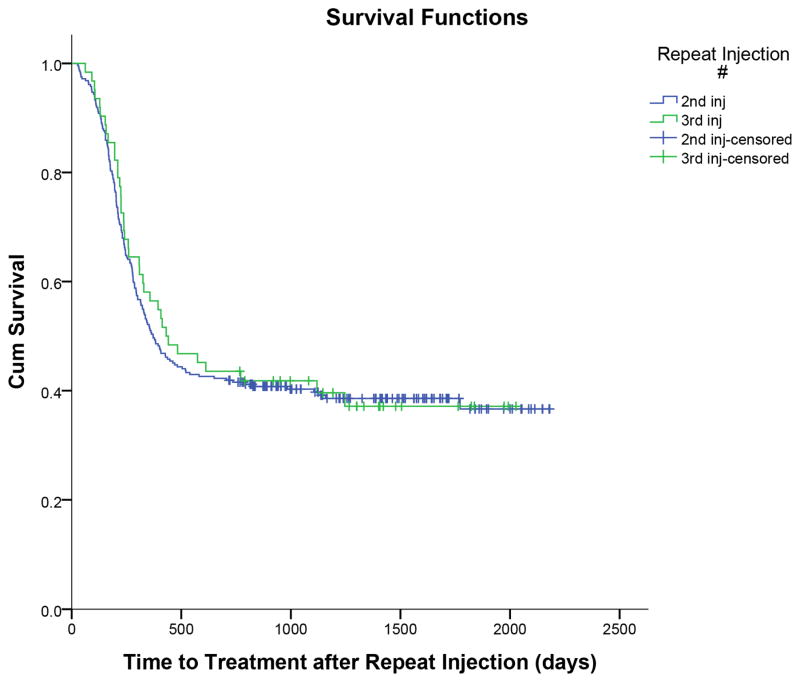
Thirty nine percent of patients (111/284) were successfully treated with a second injection. One hundred and seventy three (61%) patients experienced treatment failure, with 86 patients (50%) receiving a subsequent third injection and 87 patients (50%) undergoing surgical release of the A1 pulley as the next treatment. As demonstrated in Figure 2, the median time to treatment failure was 371 days, 95%CI [290, 452]. One hundred and eight (62%) ultimately required a surgical release even after a subsequent injection for symptomatic relief. Median time from injection to last follow-up, defined as either treatment failure or last follow-up if a success, for second injections was 371 days (range 27–2181days). Median follow up for injections deemed successful without subsequent treatment was 1325 days (range 717–2181 days). Sixty nine percent (100/144) of patients requiring telephone follow-up reported treatment success.
Figure 2.
Kaplan Meier curves for success after 2nd and 3rd injections for trigger fingers.
Third Injections
62 patients were analyzed for third injections during the study period. Thirty-seven (60%) were female, with an average age of 60 years (SD 10). 34 patients (55%) had multiple trigger fingers. 18 patients (29%) were diabetic, of which 12 were NIDDM and 6 IDDM. Table 4 presents the digits involved, trigger finger grade, and presence of selected comorbidities.
Table 4.
Third injection characteristics and bivariate contrasts between successes and failures.
| Variables | Participants (N=62) Mean (SD)/N (%) | Success (N=24) Mean (SD)/N (%) | Failure (N=38) Mean (SD)/N (%) | P-value |
|---|---|---|---|---|
|
| ||||
| Age in years at 1st Injection | 60 (10) | 58 (9) | 61 (10) | 0.30 |
|
| ||||
| Sex, Female | 37 (60%) | 17 (71%) | 20 (53%) | 0.16 |
|
| ||||
| Affected Finger | 0.53 | |||
| Thumb | 16 (26%) | 8 (33%) | 8 (21%) | |
| Index | 7 (11%) | 3 (13%) | 4 (11%) | |
| Middle | 18 (29%) | 6 (25%) | 12 (32%) | |
| Ring | 20 (32%) | 6 (25%) | 14 (37%) | |
| Small | 1 (2%) | 1 (4%) | 0 (0%) | |
|
| ||||
| Multiple Triggers | 34 (55%) | 13 (54%) | 21 (55%) | 0.93 |
|
| ||||
| Diabetes Status at 1st | 0.10 | |||
| Injection | ||||
| None | 44 (71%) | 14 (58%) | 30 (79%) | |
| NIDDM | 12 (19%) | 8 (33%) | 4 (11%) | |
| IDDM | 6 (10%) | 2 (8%) | 4 (11%) | |
|
| ||||
| Comorbidities | ||||
| Rheumatoid Arthritis | 3 (5%) | 1 (4%) | 2 (5%) | 1.00 |
| Hypothyroidism | 8 (13%) | 2 (8%) | 6 (16%) | 0.47 |
| Carpal Tunnel Syndrome | 26 (42%) | 12 (50%) | 14 (37%) | 0.31 |
| Upper Extremity Tendonitis | 9 (15%) | 3 (13%) | 6 (16%) | 1.00 |
|
| ||||
| Green Classification at 3rd Injection (N=43) | 0.89 | |||
| Grade I | 11 (26%) | 5 (29%) | 6 (23%) | |
| Grade II | 24 (56%) | 10 (59%) | 14 (54%) | |
| Grade III | 4 (9%) | 1 (6%) | 3 (12%) | |
| Grade IV | 4 (9%) | 1 (6%) | 3 (12%) | |
Thirty nine percent (24/62) of patients receiving a third injection did not require further treatment. Thirty eight (61%) patients experienced treatment failure, with 22 patients (59%) receiving a fourth subsequent injection and 16 patients (42%) undergoing surgical release of the A1 pulley as their next treatment. In total, 23 patients (61%) ultimately required a surgical release. Median time to treatment failure was 407 days, 95%CI [222,592] (Figure 2). Median time from injection to last follow-up for third injections was 436 days (range 62–2027 days). Median follow up for injections deemed success without subsequent treatment was 1317 days (range 767–2027 days). Of the 20 patients requiring telephone follow-up, 16 (80%) reported treatment success.
Factors Affecting Treatment Success
Bivariate testing demonstrated that sex, trigger finger grade, diabetes status, the presence of multiple trigger fingers and select comorbidities were not associated with success of second or third injections (Tables 3, 4). Log rank analyses did not reveal any differences in success curves for patients receiving second or third injections according to sex, presence of multiple trigger fingers, or diabetes status. Multivariate logistic regression modeling revealed that for second injections, older patient age at the time of initial injection (p<0.05, OR 1.03 95% CI 1.00–1.05) and trigger thumb (p<0.05, OR 2.47 95% CI 1.4–4.3) significantly predicted injection success while accounting for time between first and second injection, diabetes, sex and multiple trigger fingers.
DISCUSSION
Our data confirm that 39% of patients who fail a first or second corticosteroid injection for trigger finger may respond to a subsequent steroid injection. Published literature has suggested that repeat injections for trigger fingers offer some benefit but are less effective than initial injections. Clark et al. found a 55% chance of success after one injection improved to 82% with several injections after an average follow-up of 44 months.4 Reporting similar experiences, Rhoades et al. reported 66% success after one injection and a 72% cumulative chance of success after multiple injections at 25 months3 while Newport et al. reported a 49% chance of success after an initial injection and a combined success percentage of 77% for patients receiving up to 5 injections with an average follow-up of 35 months.2 Despite a wide range of follow up duration and ultimate number of injections provided, multiple investigators have confirmed this finding of additional marginal benefit of repeat steroid injections for trigger fingers.5,6,9,10,11, 13,17,18, Consistent with these publications, our current study’s 39% chance of success after 2nd and 3rd injections yields a hypothetical 82% cumulative success rate after 3 injections when combined with our center’s previously reported initial injection success rate of 45% (reference blinded).
The 39% of patients realizing success after second and third injections in this study fall within the wide range reported in the literature for repeat injections (Table 5). Variability in the chances for success among studies may be attributed to variable duration of follow up and methodologic differences. Studies have inconsistently excluded patients with inflammatory arthritis13 and the corticosteroid type and dose administered varies. Furthermore, the definition of treatment failure is not standard, frequently cited as either symptom recurrence or need for subsequent intervention. Schubert et al., reported a mean duration of relief after second and third injections with 8mg of triamcinolone (300 and 286 days respectively) was completed with an average follow-up of 66 months (range 2–152 months).10 While our study found a longer median time to failure of 371 and 407 days for a second and third injection, it is unclear if this is attributable to differences in patient demographics or the steroid preparation.
Table 5.
Reported success after repeat injections.
| Study | Study Design | Trigger Fingers Analyzed (N) | Definition of Failure | Second Injection Success | Third Injection Success | Duration of Follow-Up¥ |
|---|---|---|---|---|---|---|
| Clark et al.4 | Retrospective | 76 | Return of symptoms | 33% | 50% | 44 months |
| Newport et al.2 | Retrospective | 356 | No improvement in symptoms | 23% | 5%§ | 35 months (11–105 months) |
| Anderson et al.5 | Prospective | 77 | Return of symptoms | 59% | ---- | 4.6 years |
| Benson et al.17 | Retrospective | 109 | Return of symptoms | 36% | 33% | 18 months |
| Rozental et al.13 | Prospective | 124 | Surgical release | 79% | ---- | 12.6 months (12.1–13.2 months) |
| Dala-Ali et al.9 | Retrospective | 90 | Injection or surgery | 44% | 6% | 1 year minimum |
| Schubert et al.10 | Retrospective | 577 | Injection or surgery | 52% | 74% | 66.4 months (2–152 months) |
| Castellanos et al.11 | Prospective | 71 | Return of symptoms | 68% | 8 years (7–8 years) |
Median (range)
5% for 3–5 injections
Studies investigating patient and disease factors associated with success of corticosteroid injections for trigger fingers have focused on patients with initial injections or a mixed group of multiple injections, without specific analysis of repeat injections. Factors documented to affect injection success include duration of symptoms < 4 months3 and >6 months,2 sex,7,14 finger type,7,9 nodular subtype,8 presence of multiple trigger fingers,2,9,13,14 younger patient age,13 and history of upper extremity tendonopathies13. Diabetes has also been proposed as a factor related to ultimate need for surgical release,13,15,16 though this may be partly confounded by an increased presence of multiple trigger fingers. We considered these potential predictors of injection success in our current study. However, our data only indicated that successful 2nd injection is associated with treatment for trigger thumb and that there is a small but significant positive impact of advancing patient age at the time of presentation for injection. We had expected diabetes to predict repeat injection failure. When our data did not substantiate this, we reexamined the time between first and second injection. Median time to second injection in diabetic patients was 235 days (366 days for insulin dependent diabetic patients). This indicates that although diabetes may predict failure of first injections for trigger finger, those diabetic patients receiving repeat injections are a subgroup that realized often prolonged symptom relief before recurrence prompting repeat injection. Presumably, diabetic patients who experienced no initial response, or very brief response, to first injection were more likely to be offered surgery as opposed to repeat injection. This selection bias impacting patients with diabetes may explain why this group experienced relief similar to unaffected patients after second injection. The increased chance of success for injection to the thumb in comparison to other digits was also found in the reports of Marks and Gunther and Castellanos et al..7,11 While the reason for this is unclear, we hypothesize that this difference may be attributable to the isolated flexor pollicis longus with the thumb flexor sheath versus the other digits where both the flexor digitorum superficialis and profundus must both glide within each flexor sheath.11
The relative paucity of published data specifically examining the outcomes after repeat corticosteroid injections for trigger fingers may impact hand surgeon’s practice patterns. While corticosteroid injections remain a physician’s first choice for initial treatment of trigger finger, there is considerable variation as to whether or not providers offer a repeat injection, especially if a patient has already received two injections. A recent online survey of the American Association of Hand Surgeons members (139 respondents) found that only 11% and 13% of physicians reported giving 3 or more injections for trigger thumb and finger, respectively, prior to recommending surgery.19 This was consistent with a national database study assessing 102,778 records for treatments of trigger fingers finding that only 16% of which received at least 3 injections.20 Based on a presumed minimal chance of third injection to prevent surgery, a cost-minimization analysis by Kerrigan et al. determined that the optimal treatment strategy for recurrent trigger finger was two steroid injections followed by surgery.21 This same analysis found that the third injections would have to provide ≥9% of patients with long term relief to be the most cost-effective treatment.21 Given patients’ preference for corticosteroid injections over surgery,22 Kerrigan’s cost-minimization analysis,21 and this study’s 39% success rate for third injections, changes in practice patterns incorporating a three-injection treatment algorithm may be more cost-effective for providers and deliver care in accordance with patient preferences.
Several limitations are inherent to this study’s design. We defined treatment failure as a repeat injection or surgery rather than recurrence of symptoms. This should detect clinically relevant symptoms prompting patients to seek care but presumably underestimates the percentage of patients with mild persistent symptoms. We did not control for adjunctive non-operative management such as splinting, therapy, or NSAIDs. These are not specifically prescribed by our providers but are not prohibited. We designed the study to call all patients without a follow-up in the last 6 months to mitigate selection bias. Chart review alone would have disproportionately selected patients with symptom recurrence seeking treatment biasing the study toward a lower estimate of treatment success. Nevertheless, 68 patients, all of whom only received second injections, were lost to follow-up. Assuming that these patients had the same percentage of success as others contacted by phone, a sensitivity analysis incorporating those lost to follow-up would result in an adjusted chance of treatment success after second injections from 39% to 45%. We chose to include patients who had seen an outside provider for their initial injection as this did not detract from analysis of repeat injections (all performed within our division) and more broadly reflects our practice experience, thereby increasing external validity. We could not assess the outcome of four or more injections for any trigger finger as our practice is to avoid that number of injections secondary to the theoretical risk of injection-related tendon damage. Finally, as some patients contributed data to both the 2nd and 3rd injection groups, we did not perform any analysis that would combine these groups so that all injections remained independent events for statistical contrasts.
Second and third injections for trigger finger each provide sustained relief that may prevent the need for surgery. Although the majority of patients ultimately require surgical release we believe our data support our practice of offering up to 3 injections for persistent or recurrent trigger fingers. We offer surgical intervention after failure of an initial injection but inform patients about the expected chance of success associated with repeat injection and honor the patient’s desire for either continued non-operative or operative treatment.
Acknowledgments
Sources of Funding: Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, sub-award TL1TR000449, from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842, which supported the maintenance and use of REDCap electronic data capture tools, hosted in the Biostatistics Division of Washington University School of Medicine.. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. This funding did not play a direct role in this investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patel MR, Bassini L. Trigger fingers and thumb: when to splint, inject, or operate. J Hand Surg Am. 1992;17(1):110–113. doi: 10.1016/0363-5023(92)90124-8. [DOI] [PubMed] [Google Scholar]
- 2.Newport M, Lane L, Stuchin S. Treatment of trigger finger by steroid injection. J Hand Surg Am. 1990;15:748–750. doi: 10.1016/0363-5023(90)90149-l. [DOI] [PubMed] [Google Scholar]
- 3.Rhoades CE, Gelberman RH, Manjarris JF. Stenosing tenosynovitis of the fingers and thumb. Results of a prospective trial of steroid injection and splinting. Clin Orthop Relat Res. 1984;190:236–238. **Blinded by JHS** [PubMed] [Google Scholar]
- 4.Clark DD, Ricker JH, MacCollum MS. The efficacy of local steroid injection in the treatment of stenosing tenovaginitis. Plast Reconstr Surg. 1973;51(2):179–180. doi: 10.1097/00006534-197302000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Anderson B, Kaye S. Treatment of flexor tenosynovitis of the hand (“trigger finger”) with corticosteroids. A prospective study of the response to local injection. Arch Intern Med. 1991;151(1):153–156. [PubMed] [Google Scholar]
- 6.Faunø P, Andersen HJ, Simonsen O. A long-term follow-up of the effect of repeated corticosteroid injections for stenosing tenovaginitis. J Hand Surg Br. 1989;14(2):242–243. doi: 10.1016/0266-7681_89_90138-1. [DOI] [PubMed] [Google Scholar]
- 7.Marks M, Gunther S. Efficacy of cortisone injection in treatment of trigger fingers and thumbs. J Hand Surg Am. 1989;14(4):722–727. doi: 10.1016/0363-5023(89)90199-8. [DOI] [PubMed] [Google Scholar]
- 8.Freiberg A, Mulholland R, Levine R. Nonoperative treatment of trigger fingers and thumbs. J Hand Surg Am. 1989;14(3):553–558. doi: 10.1016/s0363-5023(89)80024-3. [DOI] [PubMed] [Google Scholar]
- 9.Dala-Ali B, Nakhdjevani A, Lloyd M, Schreuder F. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263–268. doi: 10.4055/cios.2012.4.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schubert C, Hui-Chou HG, See AP, Deune EG. Corticosteroid injection therapy for trigger finger or thumb: a retrospective review of 577 digits. Hand. 2013;8(4):439–444. doi: 10.1007/s11552-013-9541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellanos J, Munoz-Mahamud E, Dominguez E, Del Amo P, Izquierdo O, Fillat P. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121–126. doi: 10.1016/j.jhsa.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Sato E, Gomes dos Santos J, Belloti J, Albertoni W, Faloppa F. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology. 2012;51:93–99. doi: 10.1093/rheumatology/ker315. [DOI] [PubMed] [Google Scholar]
- 13.Rozental T, Zurakowski D, Blazar P. Trigger finger: prognostic indicators of recurrence following corticosteroid injection. J Bone Jt Surg Am. 2008;90(8):1665–1672. doi: 10.2106/JBJS.G.00693. [DOI] [PubMed] [Google Scholar]
- 14.Wojahn R, Foeger N, Gelberman R, Calfee R. Long-term outcomes following a single corticosteroid injection for trigger finger. J Bone Jt Surg Am. 2014;96(22):1849–1854. doi: 10.2106/JBJS.N.00004. **Blinded by JHS** [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheikh E, Peters J, Sayde W, Maltenfort M, Leinberry C. A prospective randomized trial comparing the effectiveness of one versus two (staged) corticosteroid injections for the treatment of stenosing tenosynovitis. Hand. 2014;9(3):340–345. doi: 10.1007/s11552-014-9603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgarten K, Gerlach D, Boyer M. Corticosteroid injection in diabetic patients with trigger finger. A prospective, randomized, controlled double-blinded study. J Bone Jt Surg Am. 2007;89(12):2604–2611. doi: 10.2106/JBJS.G.00230. [DOI] [PubMed] [Google Scholar]
- 17.Benson L, Ptaszek A. Injection versus surgery in the treatment of trigger finger. J Hand Surg Am. 1997;22:138–144. doi: 10.1016/S0363-5023(05)80194-7. [DOI] [PubMed] [Google Scholar]
- 18.Nimigan A, Ross D, Gan B. Steroid injections in the management of trigger fingers. Am J Phys Med Rehabil. 2006;85:36–43. doi: 10.1097/01.phm.0000184236.81774.b5. [DOI] [PubMed] [Google Scholar]
- 19.Pruzansky J, Goljan P, Lundmark D, Shin E, Jacoby S, Osterman A. Treatment preferences for trigger digit by members of the American Association for Hand Surgery. Hand. 2014;9:529–533. doi: 10.1007/s11552-013-9594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sears E, Swiatek P, Chung K. National utilization patterns of steroid injection and operative intervention for treatment of common hand conditions. J Hand Surg Am. 2016;41(3):367–373. doi: 10.1016/j.jhsa.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerrigan CL, Stanwix MG. Using evidence to minimize the cost of trigger finger care. J Hand Surg Am. 2009;34(6):997–1005. doi: 10.1016/j.jhsa.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Doring A, Hageman M, Mulder F, Guitton T, Ring D Science of Variation Group. Trigger finger: assessment of surgeon and patient preferences and priorities for decision making. J Hand Surg Am. 2014;39(11):2208–2213. doi: 10.1016/j.jhsa.2014.08.010. [DOI] [PubMed] [Google Scholar]