Abstract
Obesity is associated with consumption of energy-dense diets and development of systemic inflammation. Gut microbiota play a role in energy harvest and inflammation and can influence the change from lean to obese phenotypes. The nucleus of the solitary tract (NTS) is a brain target for gastrointestinal signals modulating satiety and alterations in gut-brain vagal pathway may promote overeating and obesity. Therefore, we tested the hypothesis that high-fat diet-induced changes in gut microbiota alter vagal gut-brain communication associated with increased body fat accumulation. Sprague-Dawley rats consumed a low energy-dense rodent diet (LFD; 3.1 kcal/g) or high energy-dense diet (HFD, 5.24 kcal/g). Minocycline was used to manipulate gut microbiota composition. 16S Sequencing was used to determine microbiota composition. Immunofluorescence against IB4 and Iba1 was used to determine NTS reorganization and microglia activation. Nodose ganglia from LFD rats were isolated and co-cultured with different bacteria strains to determine neurotoxicity. HFD altered gut microbiota with increases in Firmicutes/Bacteriodetes ratio and in pro-inflammatory Proteobacteria proliferation. HFD triggered reorganization of vagal afferents and microglia activation in the NTS, associated with weight gain. Minocycline-treated HFD rats exhibited microbiota profile comparable to LFD animals. Minocycline suppressed HFD-induced reorganization of vagal afferents and microglia activation in the NTS, and reduced body fat accumulation. Proteobacteria isolated from cecum of HFD rats were toxic to vagal afferent neurons in culture. Our findings show that diet-induced shift in gut microbiome may disrupt vagal gut-brain communication resulting in microglia activation and increased body fat accumulation.
Keywords: microglia, obesity, vagus, bacteria, plasticity
INTRODUCTION
Obesity rate in the United States climbed to nearly 38 percent of adults in 2013–2014, up from 32 percent about a decade earlier. Among contributing factors is an increase in the intake of energy-dense diets leading to body weight gain (Little and Feinle-Bisset 2011, Prentice and Jebb 2003). Obesity is considered to be a state of chronic inflammation (Cox et al. 2015) originating, at least partially, from the gastrointestinal (GI) tract (Cani et al. 2008). There is evidence that the GI microbiota contribute to the development of obesity (Montiel-Castro et al. 2013). Conventionalization of germ-free animals with microbiota from lean or obese donors results in recapitulation of the donor phenotype (Ridaura et al. 2013). The mechanisms and pathways by which GI microbiota may affect regulation of feeding are not well understood. Interestingly, diet composition rather than adiposity affects the microbiota composition (de La Serre et al. 2010, Hildebrandt et al. 2009).
Changes in satiation have been linked to diet composition. Protein induces supercaloric compensation; carbohydrate leads to approximate caloric compensation; and fat generates subcaloric compensation and hence promotes excess energy intake (Rolls 1995, 2009). At first, consumed fat changes meal patterns coherent with stimulation of a short-term satiety. This effect is reduced with long-term exposure to fat in the diet, independently of calorie intake or body weight. This alteration in short-term satiety leads to an increase in meal size and contributes to development of high fat diet-induced obesity (Paulino et al. 2008). Moreover, vagally-mediated effects (decreased response to cholecystokinin) reported in high fat diet-induced obesity are due to the diet composition because they appear before the increased body fat accumulation leading to obesity (Troy et al. 2016).
Meal size is primarily regulated by gut-brain neural signaling. GI signals inform the brain about the quantity and quality of food being consumed to regulate satiety and food intake (Ritter 2004). Information from GI-borne signals is relayed to the brain via vagal afferents (Dockray 2003). The nucleus of the solitary tract (NTS), located in the caudal brainstem is the site at which the vagal afferents make their first central synapses; while GI-projecting motor neurons are located at the dorsal motor nucleus of the vagus (DMV) (Berthoud et al. 1991, Peters et al. 2013). Neurobiological insights into vagal gut-brain crosstalk have revealed that gut-brain signaling is critical for maintaining adequate adiposity and preventing obesity (Berthoud et al. 2011, Ritter 2004).
Vagal afferents can relay microbiota signals to the brain to alter host behavior. Bacteria-driven hippocampal activation and modulation of anxiety are notably abolished by vagotomy (Wang et al. 2002). We have recently shown that a pro-inflammatory bacteria product, lipopolysaccharide (LPS), can activate vagal afferent neurons and impair satiety signaling (de La Serre et al. 2015). Therefore, diet-shifted microbiota may alter vagal satiety signaling to the hindbrain feeding centers to increase energy intake and adiposity (de Lartigue et al. 2011).
The goal of our study was to determine whether HFD-induced (High-fat-diet-induced) changes in gut microbiota composition can drive remodeling of the vagal pathway, to stimulate body fat accumulation.
METHODS
Male Sprague-Dawley rats (~460 g BW, Simonsen Laboratories, CA) were individually housed in a temperature-controlled vivarium with ad libitum access to food and water (12-hour light/dark schedule). Individual housing was necessary for food intake measurements and fecal pellet collection. Animal procedures were approved by the Washington State University Institutional Animal Care and Use Committee and conformed to National Institutes of Health guidelines for the use of vertebrate animals (publication 86-23). Appropriate measures were taken to minimize pain and discomfort of the animals.
Following a seven-days baseline (low energy-dense rodent diet; LFD; Teklad F6; 3.1 kcal/g; 19% fat), animals were assigned to their respective diet groups. LFD rats (n=16) were maintained on LFD for additional 7 (n=8) or 21 days (n=8). High fat diet (HFD) fed animals (n=16) were switched to a high energy-dense diet (Research Diets, D12492; 5.24 kcal/g; 60% fat) for 7 days (n=8) or 21 days (n=8). Beginning at dietary switch and continuing until sacrifice, LFD and HFD rats received daily injections of a broad-spectrum antibiotic, minocycline, (n=8/group; 20 mg/kg i.p.; Sigma-Aldrich) or sterile 0.9% NaCl (n=8/group; 0.5 ml). Body weight and food intake were monitored daily. Fecal pellets were collected before introducing the HFD and 7 and 21 days after dietary switch. Body composition was determined by Dual-Energy X-ray Absorptiometry (DEXA) at baseline and 7 and 21 day after dietary switch. After 21 days, rats were euthanized by CO2 and blood samples were collected. Animals were then transcardially perfused with 0.1 M PBS (pH 7.4) followed by 4% paraformaldehyde and hindbrains were collected. Additionally, the nodose ganglia (NG) from a separate group of LFD rats (n=20) were isolated and co-cultured with different bacterial strains.
Microbiome analysis
Bacterial DNA was extracted from fecal samples using a kit following the recommendation of the manufacturer (Zymo research, CA). Fecal contents were lysed by bead beating and DNA was isolated using fast-spin columns. DNA was filtered to remove humic acids and polyphenols and the eluted DNA was sent to SeqMatic facility (Fremont, CA) for sequencing. A library was generated by targeting the 16S V4 region. Sequencing was performed via Illumina MiSeq and sequences aligned to reference genomes. Illumina BaseSpace software was used for data analysis. Diversity was determined using Shannon-Weiner index. Abundance at the Phylum, Class, Order, Family, Genus and Species levels were calculated and changes in composition from baseline were expressed as Log2 (n-folds change). Principal component analysis (PCA) at the order levels was run using XLSTAT (Addinsoft, NY).
To identify and isolate the cecal bacteria strains from HF rats, the samples were normalized by weight, and serially-diluted lysates were plated on three separate culture media (MHB, Mueller-Hinton Blood agar plate; MRS, deMan, Rogosa and Sharpe agar plate; LB, Luria-Bertani agar plate). Identification of microbial taxa was based on 16S sequencing of colony types that were enriched in the animals given a high fat diet. DNA was extracted from individual colony isolates and PCR of the 16S ribosomal RNA subunit was performed using the 27F primer: 5′-AGAGTTTGATCMTGGCTCAGAACG-3′ and 1435R primer: 5′-CGATTACTAGCGATTCCRRCTTCA-3′, where M=A or C and R=A or G. The primers used for sequencing included the 27F primer, 1435R primer, 533F primer: 5′-GTGCCAGCMGCCGCGGTAA-3′, and 519R primer: 5′-GTATTACCGCGGCTGCTGG-3′. Sequences were trimmed and analyzed by BLAST for identification. The Genus and species classification of an individual isolate was based on 100% sequence identify of >1000 nucleotides.
Quantification of serum LPS
Serum LPS was measured as described previously (de La Serre et al. 2015). LPS was quantified using a Pyrochrome Lysate Mix, a quantitative chromogenic reagent, (Associate of Cape Cod, MA) diluted in Glucashield buffer which inhibits cross-reactivity with (1→3)-β-D-Glucans. Briefly, serum samples were diluted 1:10 in Pyrogen free water (Lonza, Switzerland) and heated for 10 min at 70°C. Samples and reactive solution were incubated at 37°C for 30 min and absorbance was read at 405 nm.
Immunofluorescence
Hindbrains were cryosectioned at 20 μm thickness and stained for selected antigens. After blocking in 10% normal horse serum in Tris-phosphate buffered saline (TPBS, pH 7.4) sections were incubated overnight in a primary antibody against ionized calcium binding adaptor molecule 1 (Iba1, 1:1000; 019-19741, Dako, GA) followed by an Alexa-488 secondary antibody (1:400; A21206, Invitrogen, CA) to visualize microglia activation as previously described (Gallaher et al. 2012). For visualization of vagal afferents, the hindbrain sections were incubated with isolectin B4 biotin-conjugated antibody (IB4, 1:400, cat# B-1205, Vector Laboratories, CA) for 12 h at room temperature (Shehab 2009), followed by ExtrAvidin-CY3 (1:600, E-4142, Sigma-Aldrich) for 2 h. Negative controls were performed by omission of primary antibodies. Sections were mounted in ProLong (Molecular Probes, OR) and examined under Nikon 80-I fluorescent microscope as previously described (Gallaher et al. 2012, Peters et al. 2013).
NG primary cultures
NG were isolated from LFD fed animals 3 hours after light onset under deep anesthesia (Ketamine, 25 mg/100 g; plus Xylazine, 2.5 mg/100 g). Once isolated, NG were digested in Ca2+/Mg2+ free Hank’s Balanced Salt Solution containing 1 mg/mL of Dispase II and Collagenase Type 1A (120 min at 37°C in 95% air/5% CO2). Dispersed cells were plated onto polylysine-coated coverslips and maintained in DMEM+10% FBS (37°C in 95% air/5% CO2). Cultures were infected with one of the following facultative anaerobic bacteria strains identified in HFD rats’ cecal contents: Streptococcus mitis, Proteus mirablis, Lactobacillus animalis or Enterococcus faecalis (n=4 rats/strain) at a concentration of ~5×10(7). Culture medium without bacteria was added to control plates (n=4 rats). Thirty min later all cultures were fixed in paraformaldehyde and stained with beta III-tubulin antibody (1:500, ab 78078, Abcam, UK) to identify neuronal profiles. For neurons quantification, beta-III tubulin-stained neuronal perikarya were counted in 3 randomly assigned frames per culture (frame size 3×3 mm).
Statistics
GraphPad prism (GraphPad, CA) was used for statistical analysis. A two-way ANOVA with a Tukey post hoc test was used to analyze the data. Differences were considered significant if p<0.05.
RESULTS
Minocycline decreased energy intake and body fat accumulation induced by HFD
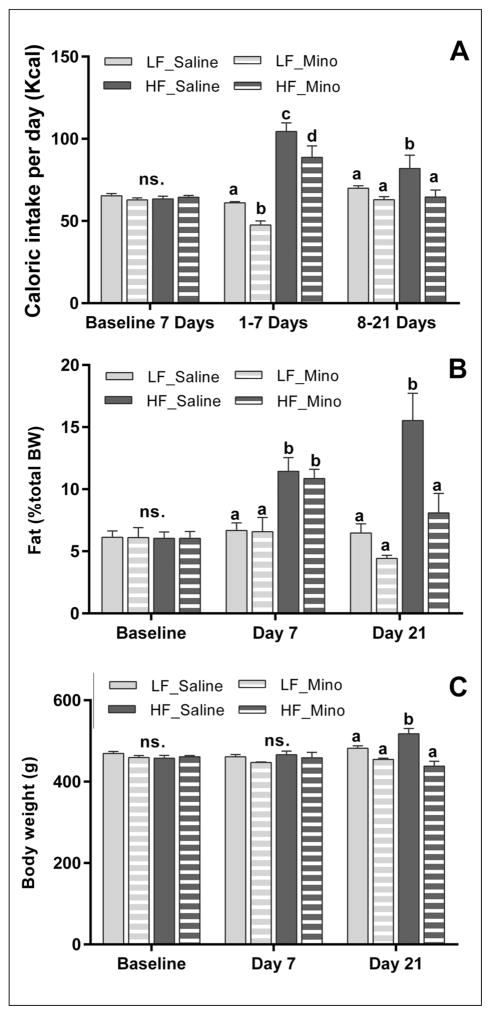
During the baseline we observed no statistically significant differences in energy intake, body fat and body weight between rats (Figs 1A–1C). Seven-day exposure to HFD was sufficient to significantly increase energy intake and body fat accumulation, but not the body weight. After 21 days on HFD rats significantly increased the energy intake, body fat accumulation and body weight (Figs 1A–1C). Minocycline reduced energy intake after one week of treatment and there was no effect of minocycline on body fat or body weight gain at day 7 (Figs 1A–1C). However, after 21 days on HFD minocycline-treated rats exhibited a significant decrease in energy intake, in body fat accumulation and in body weight gain (Figs 1A–1C). Body fat and body weight in rats fed a HFD and treated with minocycline were significantly lower than HF_Saline rats (8.1±1.6% vs. 15.5±2.2%; Fig. 1B and 438.275 g±11.8 g vs. 517.7g±13.2; Fig. 1C) and normalized to the level of control LF_Saline animals (6.5±0.7%; Fig. 1B and 469.087 g±5.0 g; Fig. 1C). Minocycline reduced the caloric intake in LFD rats only in the first week (Fig. 1A). However, between the 8th and 21st day of the experiment, this effect was abolished (Fig. 1A). Minocycline did not affect body fat accumulation and body weight of LFD rats (Figs 1B, 1C).
Fig. 1.
Minocycline treatment decreased energy intake and body fat accumulation induced by HFD. Seven days exposure to HFD (High fat diet) significantly increased energy intake (A, p<0.001; n=8/group) and body fat accumulation (B, p<0.05; n=8/group). After 21 days, when compared to saline-treated animals, HFD minocycline-treated rats exhibited a significant decrease in energy intake (A, p<0.05; n=4/group), body fat accumulation (B, p<0.05; n=4/group) and in body weight (C, p<0.05; n=4/group). Minocycline reduced the caloric intake in LFD rats only in the first week (A). However, between the 8th and 21st day of the experiment, this effect was abolished (A). Minocycline did not affect body fat accumulation and body weight of LFD rats (B, C). Bars represent the average value ±SEM; a, b, c – different letters denote significant differences.
Bacterial composition altered by HFD was improved by minocycline
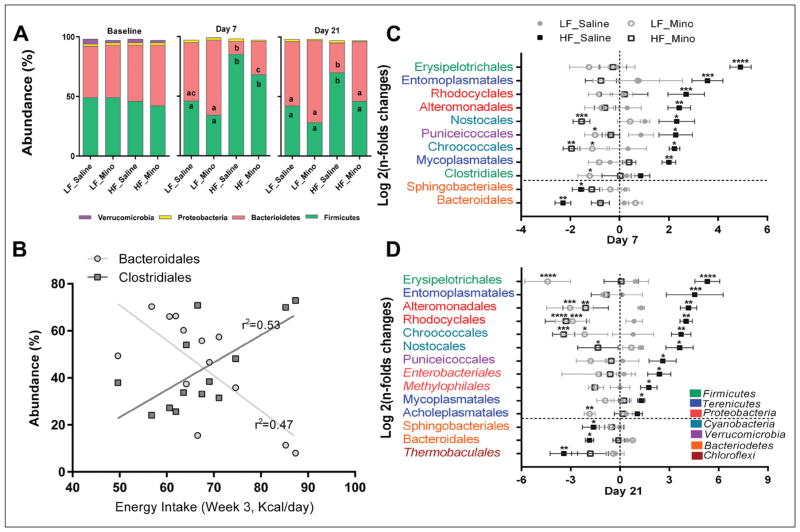
Firmicutes and Bacteroidetes were the most abundant phyla and represented over 85% of the bacteria identified (Fig. 2A). In all groups, there was a significant reduction in Verrucomicrobia abundance at day 7 and day 21. Bacterial composition was changed after 7 days on HFD. HFD consumption led to a significant increase in Firmicutes abundance (LF_Saline 46.8±3.2% vs. HF_Saline 85.5±3.4%) and a reduction in Bacteroidetes (LF_Saline 49.3±3.5% vs. HF_Saline 11.3±3.2%) in the fecal pellets. The same changes were present after 21 days of HFD consumption (LF_Saline Firmicutes 42.2±4.8% vs. HF_Saline 70.7±12.4%; LF_Saline Bacteroidetes 54.4±4.9% vs. HF_Saline 25.4±12.7%) in the fecal pellets.
Fig. 2.
Bacterial composition altered by HFD was improved by minocycline treatment. (A) Bacterial phyla abundance was quantified in fecal samples before dietary switch and treatment (baseline) and after 7 and 21 days on the different diets and treatments regimen. Firmicutes and Bacteriodetes were the most abundant bacterial phyla in all groups and at all time points. In all groups, there was a significant reduction in Verrucomicrobia abundance at day 7 (p<0.001) and day 21 (p<0.0001). Seven days of HFD were sufficient to induce a significant increase in Firmicutes (p<0.0001) and decrease in Bacteriodetes abundances (p<0.0001). Similar changes were observed after 21 days of HFD (A, Firmicutes, p<0.01; Bacteriodetes, p<0.001). Minocycline treatment improved HFD rats’ bacterial phyla profile with a significant increase in Bacteriodetes abundance after 7 days (A, p<0.05) and a significant reduction in Firmicutes (A, p<0.01) and increase in Bacteriodetes abundances (A, p<0.01) after 21 days. After 21 days of treatment, there were no significant differences in bacterial phyla abundance between the HFD /minocycline-treated rats and the LFD animals. Minocycline treatment did not significantly affect bacterial phyla abundance in LFD rats (Carvalho et al. 2012). a, b, c – different letters denote significant differences. (B) Negative and positive correlations were observed between energy intake and bacterial orders depleted (Bacteroidales) and enriched (Clostridiales) by HF feeding. Energy intake during the third week of experiment (14 to 21days) was correlated with order abundance measured at day 21 across diets and treatments. Bacteroidales abundance was negatively correlated with energy intake (r2=0.53, p<0.01) while Clostridiales abundance was positively correlated with intake (r2=0.47, p<0.01). (C and D) Bacterial orders that were significantly enriched or depleted by HFD and/or minocycline treatment (Log 2 fold changes from baseline). * – denotes significant difference from the LF_Saline group: * – p<0.05, ** – p<0.01, *** – p<0.001, **** – p<0.0001. (B) Consumption of HFD for 7 days led to significant increase in abundance of several bacterial orders belonging to the Firmicutes (Erysipelotrichales), Terenicutes (Entomoplasmatales, Mycoplasmatales), Proteobacteria (Rhodocyclales, Altermondales), Cyanobacteria (Nostocales, Chroococcales) and Verrucomicrobia (Puniceicoccales). HFD also led to significant depletion in Bacteriodetes orders Bacteroidales and Sphingobacteriales. C. Similar results were observed after 21 days with additional orders enriched (Enterobacteriales and Methylophilales, Proteobacteria) and depleted (Thermobaculales, Chloroflexi). (B) Minocycline treatment normalized HFD-induced dysbiosis. Seven days of minocycline exposure were sufficient to significantly reduce the HFD-induced proliferation of bacterial orders mentioned above, leading to other normalization of abundance or significant depletion (Nostocales, Chroococcales). Minocycline also prevented HFD-induced depletion in Bacteroidales and Sphingobacteriales. In LFD animals, minocycline alone significantly reduced the abundance of obesity-associated Puniceicoccales, Chroococcales and Clostridiales. (C) Similar results were observed after 21 days of minocycline treatment; minocycline led to normalization or depletion of HFD-associated bacterial orders and restored HFD-depleted orders. Minocycline alone led to a significant reduction in Erysipelotrichales (Firmicutes) and several Proteobacteria and Cyanobacteria orders, such as Rhodocyclales and Chroococcales.
In LFD animals, minocycline (Mino) did not affect the microbiota composition, at the phylum level; however, it significantly improved the microbiome profile of the HFD rats. After 7 days of minocycline, there was a reduction in Firmicutes abundance in the HF_Mino rats compared to the HF_Saline rats but it did not reach significance. There was a significant increase in Bacteriodetes abundance in the HF_Mino groups compared to the HF_Saline rats (HF_Saline 11.3±3.2% vs. HF_Mino 28.4±7.8%). After 21 days of minocycline, Firmicutes and Bacteriodetes abundance levels were normalized in the HFD rats. Firmicutes abundance in the HF_Mino rats (Day 21: 46.9±6.3%) was not different from the LF_Saline group and significantly lower than in the HF_Saline animals. Bacteroidetes abundance in the HF_Mino rats (Day 21:50.9±6.4%) was not different from the LF_Saline group and was significantly higher than in HF_Saline animals. Clostridiales (the main Firmicutes order) and Bacteroidales (the main bacteriodetes order) abundances were positively and negatively correlated with energy intake across diets and treatments (Fig. 2B).
Principal component analysis (PCA) was run to identify the main contributors to variance (at the order level). Log2 fold changes from baseline were calculated to specifically identify bacterial orders that were enriched or depleted by HFD and/or minocycline (Figs 2C, 2D). Interestingly, for the majority of bacterial orders identified by PCA, the relative abundance was significantly positively or negatively correlated with energy intake and/or adiposity (Fig. 2B).
Seven days on HFD led to a significant increase in the abundance of several orders belonging to the Firmicutes (Erysipelotrichales), Terenicutes (Entomoplasmatales, Mycoplasmatales), Proteobacteria (Rhodocyclales, Altermondales), Cyanobacteria (Nostocales, Chroococcales) and Verrucomicrobia (Puniceicoccales). The orders classified as Terenicutes all belong to the Mollicutes class which has previously been classified as Firmicutes. HFD consumption led to a significant depletion in Bacteriodetes, especially Sphingobacteriales and Bacteroidales (Fig. 2C). Similar results were observed after 3 weeks on HFD, additional orders were found to significantly contribute to variance, such as Enterobacteriales and Methylophilales (Proteobacteria), which were enriched on HFD and Thermobaculales (Chloroflexi) was depleted by HFD (Fig. 2D).
Minocycline had the opposite modulatory effects; 7 days of minocycline exposure were sufficient to significantly reduce the HFD-induced proliferation of bacterial orders mentioned above, leading to normalization of abundance or significant depletion (Nostocales, Chroococcales). Minocycline prevented HFD-induced depletion of Bacteroidales and Sphingobacteriales. In LFD animals, minocycline alone significantly reduced the abundance of obesity-associated Puniceicoccales, Chroococcales and Clostridiales. Similar results were observed after 21 days on minocycline. Minocycline led to normalization or depletion of HFD-associated bacterial orders and restored HFD-depleted orders. Minocycline alone led to significant reduction in Erysipelotrichales and several Proteobacteria and Cyanobacteria orders, (Figs 2C, 2D).
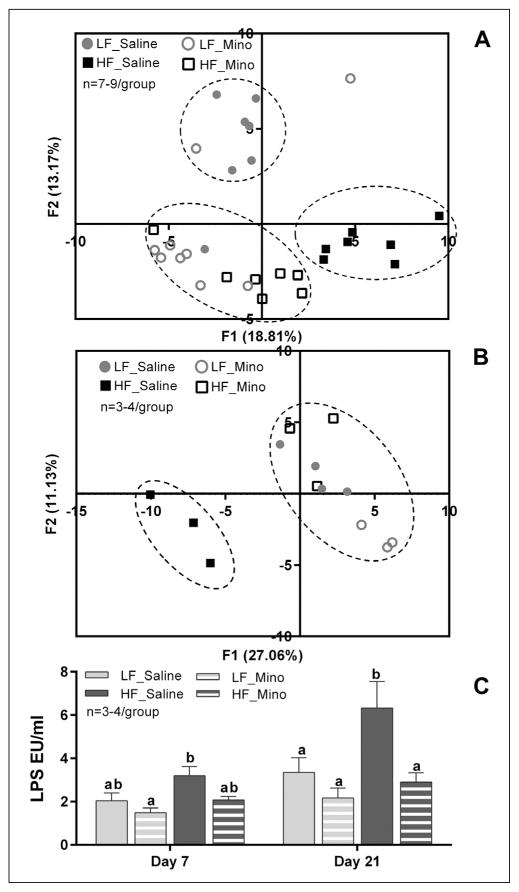
PCA showed that after 7 days, LFD and HFD rats had different microbiota profile; minocycline treatment resulted in a third profile with more variabilities, based on the diet consumed (LFD vs. HFD; Fig. 3A). After 7 days, the HF_Mino microbiota profile showed more similarities with the HF_Saline rats than the LF_Saline rats (Fig. 3A). However, after 21 days, HF_Mino microbiota profile overlap with LF_Saline groups. While minocycline did affect abundance of some specific species it did not result in a different microbiota profile in LFD rats (Fig. 3B).
Fig. 3.
Minocycline normalized HFD rats’ ‘microbiota profile and reduced HFD-induced metabolic endotoxemia. (A and B) PCA at the order level after 7 or 21 days of diet/minocycline treatments A. PCA showed that 7 days of HFD were sufficient to induce dysbiosis with a distinct microbiota profile while minocycline treatment led in a third profile with more variability, based on the diet consumed (LFD vs. HFD). B. HFD-induced dysbiosis was confirmed after 21 days of diet. Minocycline treatment led to normalization of the microbiota profile in HFD rats, with HF_Mino animals clustering with LF_Saline animals. There was no significant effect of minocycline treatment on the LF animals PCA scores. C. Circulating LPS levels in plasma after 7 or 21 days of diet/minocycline treatments. Seven days of HFD a non-significant increase in circulating LPS which became significant after 21 days of HFD (p<0.01). Minocycline treatment reduced circulating LPS in HFD rats and this effect was significant after 21 days (p<0.001). In LFD rats, minocycline treatment did not result in significant changes of LPS plasma levels at any time point. Bars represent the average value ±SEM; a, b – different letters denote significant differences.
HFD-related increases in plasma levels of LPS were suppressed by minocycline
HFD led to an increase in circulating LPS after 7 days when compared to LFD animals, and LPS levels were significantly elevated in HFD rats after 21 days on the diet (6.3 EU±1.2 vs. 3.3 EU±0.7; Fig. 3C). Minocycline reduced circulating LPS in HFD rats. This effect was not significant after 7 days of treatment, however, after 21 days, minocycline significantly decreased LPS levels in HFD rats (6.3 EU±1.2 vs. 2.9 EU±0.4; Fig. 3C). In LFD rats, minocycline did not result in changes of LPS levels at any time point (2.0 EU±0.4 vs. 1.5 EU±0.2 and 3.3 EU±0.7 vs. 2.2 EU±0.5 for 7 and 21 days; Fig. 3C).
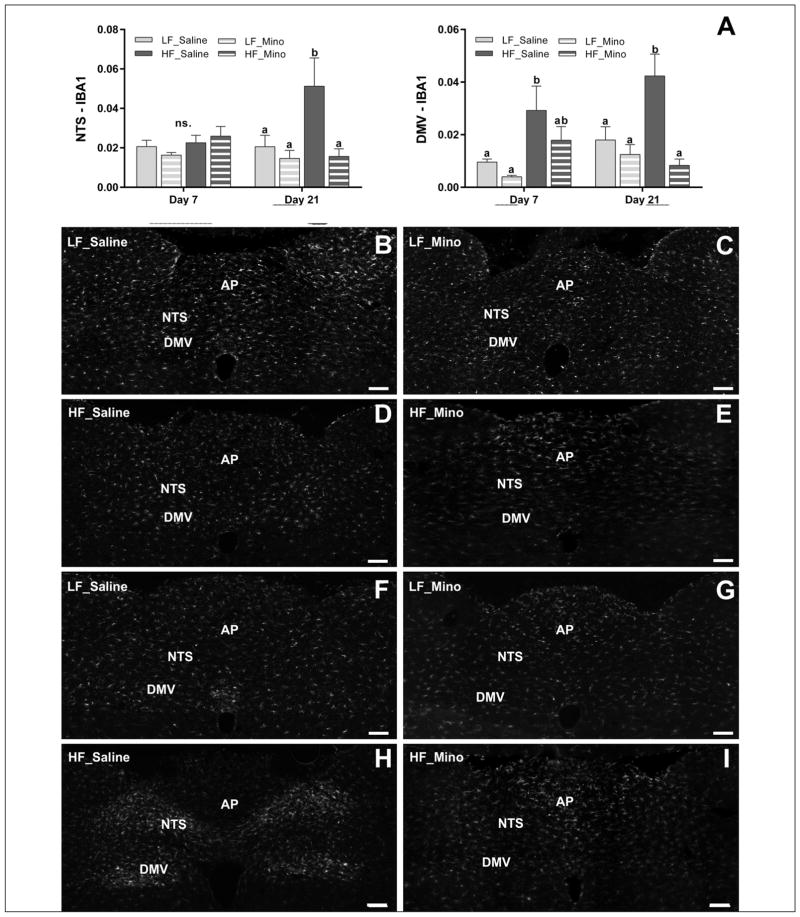
HFD-induced activation of microglia in the NTS and DMV was suppressed by minocycline
Immunostaining against Iba-1 revealed that HFD increased microglia activation in the NTS and the DMV. After 7 days on HFD microglia activation was observed only in the DMV (Figs 4A–4E). The area fraction populated by fluorescent staining against Iba1 in the DMV was increased in HFD when compared to LFD rats (0.0292±0.0092 vs. 0.0096±0.0011). After 21 days on HFD microglia activation was observed in both the DMV and the NTS (Figs 4A, 4F–4I). The area fraction populated by the fluorescent staining against Iba1 in the DMV and the NTS was increased in HFD rats when compared to LFD (0.0423±0.0083 vs. 0.0180±0.005 and 0.0512±0.0143 vs. 0.0206±0.0057 respectively).
Fig. 4.
High-fat diet-induced activation of microglia in the NTS and DMV was suppressed by minocycline treatment. (B–I) Representative images of Iba1 immunoreactivity in the hindbrain between bregma −13.10 and −14.10 mm. Immunostaining against Iba-1 revealed that HFD increased microglia activation in the NTS and the DMV. After 7 days on HFD microglia activation was observed only in the DMV (A, B–E) (p<0.05). After 21 days on HFD microglia activation was observed in both the DMV (p<0.01) and the NTS (p<0.01) (A, F–I). Minocycline treatment attenuated the HFD-induced increases in the microglia activation in both the DMV (p<0.001) and the NTS (p<0.01) at day 21 (A, F–I). Minocycline treatment did not significantly change the microglia activation in the DMV and the NTS in rats fed LFD at any studied time point. Bars represent the average value ±SEM; a, b – different letters denote significant differences. NTS: nucleus of the solitary tract; DMV: dorsal motor nucleus of the vagus; AP: area postrema; scale bar =200 μm.
Minocycline attenuated the HFD-induced increases in the microglia activation in both the DMV and the NTS. The area fraction populated by the fluorescent staining against Iba1 in the DMV was significantly smaller in HF_Mino rats when compared to HF_Saline rats (0.0179±0.0052 vs. 0.0292±0.0092 and 0.0083±0.0024 vs. 0.0423±0.0083 at 7 and 21 days respectively; Fig. 4A). The area fraction populated by Iba1 staining in the NTS was significantly smaller in HF_Mino rats when compared to HF_Saline rats only at day 21 (0.0156±0.0039 vs. 0.0512±0.0143; Fig. 4A). Minocycline did not significantly change the microglia activation in the DMV and the NTS in rats fed LF diet at any time point (Fig. 4A).
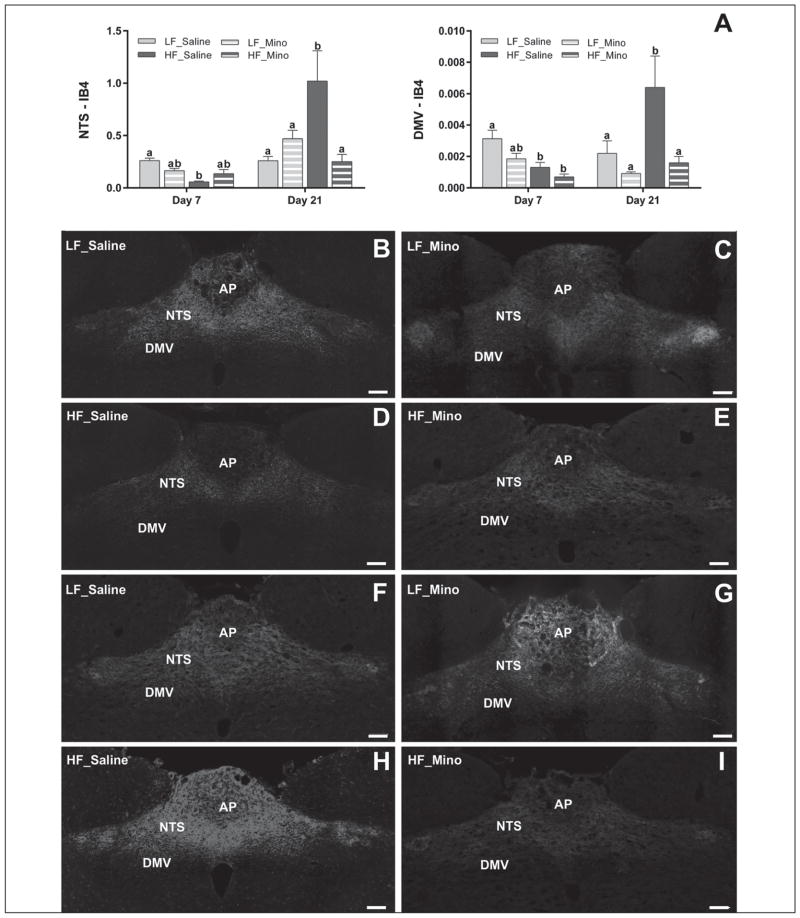
HFD-induced vagal remodeling in the NTS and DMV was suppressed by minocycline
Binary analysis of the area fraction of IB4-labeled vagal afferents in the intermediate NTS and DMV revealed significant differences in the density of labeled vagal terminals between rats fed different diets (Fig. 5). After 7 days the HFD decreased total IB4 labeling in the NTS and the DMV when compared to LFD (0.0566±0.0089 vs. 0.2611±0.0232 for NTS and 0.0013±0.0003±0.0005 vs. 0.0031 for DMV; Figs 5A, 5B–5E). This effect was dampened by minocycline only in the NTS (Figs 5A–5E) but failed to reach significance. At day 21, we observed a significant increase in density of IB4-labeled vagal afferents projecting to the NTS and DMV in HFD rats when compared to LFD rats (1.0187±0.2895 vs. 0.2622±0.0411 for NTS and 0.0064±0.0021 vs. 0.0022±0.0008 for DMV; Figs 5A, 5F–5I). This effect was significantly decreased in both the NTS and DMV by minocycline (Figs 5A, 5F–5I). There were no significant differences between LF_Saline and LF_Mino rats at any time point.
Fig. 5.
HFD-induced vagal remodeling in the NTS and DMV was suppressed by minocycline. Binary analysis of the area fraction of IB4-labeled vagal afferents in the intermediate NTS and DMV revealed significant differences in the density of labeled afferent terminals between rats fed different diets. After 7 days HFD decreased the total IB4 labeling in the NTS (p<0.001) and the DMV (p<0.05) with compare to LFD (A, B–E). This effect was dampened by minocycline treatment only in the NTS but failed to reach significance. At day 21, we observed significant increase in density of IB4-labeled vagal afferents projecting to the NTS (p<0.001) and DMV (p<0.01) in HFD rats with compare to LFD rats (A, F–I). This effect was significantly decreased in both the NTS (p<0.001) and DMV (p<0.01) by minocycline treatment (A, F–I). There were no significant differences between LF_Saline and LF_Mino rats at any studied time point. Bars represent the average value ±SEM; a, b – different letters denote significant differences. NTS: nucleus of the solitary tract; DMV: dorsal motor nucleus of the vagus; AP: area postrema; scale bar =200 μm.
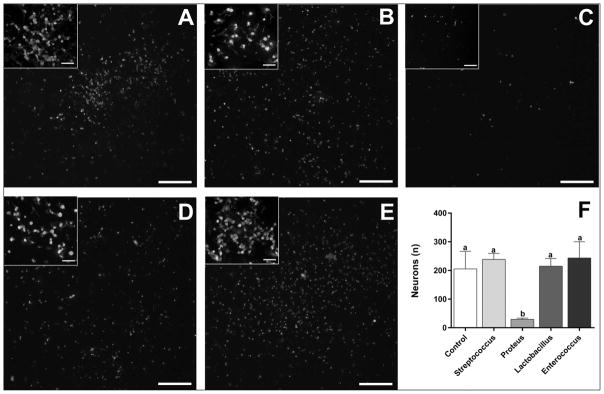
Gram-negative bacteria isolated from HFD rats significantly reduced the number of NG neurons in culture
Co-culture of NG primary sensory neurons isolated from LFD rats with Streptococcus mitis, Lactobacillus animalis or Enterococcus faecalis (Firmicutes, Bacilli, Lactobacillales) isolated from the cecum of HFD rats did not change the number of surviving neurons when compared to NG cultures without the bacteria (238.5±20.5; 214.8±26.8; 243.5±56.6 vs. 205.3±61.5; Fig. 6). Adding Proteus mirablis (Proteobacteria, Gammaproteobacteria, Enterobacteriales) to NG cultures induced a dramatic loss of neurons when compared to NG cultures without the bacteria (29.3±4.2 vs. 205.3±61.5; Fig. 6)
Fig. 6.
Gram-negative bacteria isolated from HFD rats significantly reduced the number of NG neurons in culture. Co-culture of NG primary sensory neurons isolated from LFD rats with Streptococcus mitis, Lactobacillus animalis or Enterococcus faecalis (B, D and E respectively; Firmicutes, Bacilli, Lactobacillales) isolated from HFD rats did not change the number of surviving neurons with compare to NG cultures without the bacteria (A, F). Adding Proteus mirablis (C; Proteobacteria, Gammaproteobacteria, Enterobacteriales), isolated from HFD rats, to NG cultures from LFD rats induced a dramatic loss of primary sensory neurons with compare to NG cultures without the bacteria (A, F, p<0.05). Inserts show high magnification images from the same section demonstrating neuronal morphology. Scale bar =200 μm or 20 μm in inserts. Bars represent the average value ±SEM; a, b – different letters denote significant differences.
DISCUSSION
This study provides evidence for dynamic diet-influenced and bacteria-driven neural plasticity in the NTS and DMV, the first relay stations for nutritionally relevant information from the GI tract (Rogers and McCann 1993). We show that a HFD rapidly and significantly changed gut microbiota composition. HFD led to an increase in circulating pro-inflammatory LPS and was associated with neuronal damage to the NTS and DMV. This damage was reflected by induced activation of microglia and vagal remodeling in the NTS and DMV. Normalization of the microbiota composition in HFD rats using minocycline was protective against HFD-induced neuronal damage and increase in adiposity, showing that gut microbiota dysbiosis is necessary for HFD-driven deleterious effects on gut-brain signaling. Therefore, bacteria-driven brain plasticity in response to HFD may be responsible for increased energy intake, body fat accumulation, and body weight promoting obesity.
Results show that HFD induced an inflammatory response reflected by microglia activation in NTS and DMV. The pro-inflammatory action of the HFD has been also reported in the NG and hypothalamus (Waise et al. 2015, Yi et al. 2012). Taken together, these results suggest that a HFD weakens neuroprotective signaling and promotes inflammation in the brain feeding centers, which may lead to increased food intake and obesity.
We found that a HFD triggered reorganization of vagal afferents in the NTS and DMV. HFD induced transient withdrawal of vagal afferents from the hindbrain feeding centers following by an increase in the density of vagal afferents in NTS and DMV. The increase in the density may reflect a sprouting of vagal inputs into the hindbrain feeding centers. Interestingly, our previous studies revealed that damage to peripheral axons of the vagus (subdiaphragmatic vagotomy) also induced transient decrease in the density of vagal afferents projecting to the NTS (Ballsmider et al. 2015, Peters et al. 2013) followed by vagal inputs sprouting. Vagal damage also resulted in microglia activation in the NTS and DMV (Gallaher et al. 2012). We previously found that vagal afferent remodeling led to alterations in frequency and amplitude of glutamate release in the NTS (Peters et al. 2013). Satiety peptide CCK triggers glutamate release in the NTS while hunger signal ghrelin inhibits glutamate inputs to the NTS. Therefore, HFD driven vagal remodeling may lead to alteration in satiety signaling and overeating.
Dietary fat-induced neuronal remodeling has previously been reported in the hypothalamus (Benani et al. 2012) and obesity has been found to have neurodegenerative effects in this nucleus (McNay et al. 2012). Microglia activation is involved in neuronal circuit remodeling (Kettenmann et al. 2013) and has been found to control HFD-induced hypothalamic inflammation and loss of neuronal function (Valdearcos et al. 2014). Interestingly, HFD-induced hypothalamic inflammation is significantly reduced by vagotomy (Lee et al. 2015, Waise et al. 2015) suggesting that vagal afferents may transfer bacterial-derived inflammatory signals to the brain feeding centers to trigger microglia activation and neural remodeling.
Supporting this hypothesis, we found that microbiota normalization blunted the inflammatory effect of HFD on hindbrain, showing the dysbiosis is necessary for HFD-induced vagal remodeling. While our study did not establish whether gut microbiota dysbiosis is sufficient to promote vagal remodeling, colonization of germ free animals with dysbiotic microbiota has previously been found to induce hypothalamic inflammation (Duca et al. 2014). Taken together, these data point towards a causal role for the microbiota in HFD-driven hindbrain inflammation and neural remodeling. This pathway is supported by previous studies showing that obesity-associated inflammation originates, at least partially, from bacterial endotoxin in the GI tract (de La Serre et al. 2010, Lassenius et al. 2011). LPS is a constituent of the gram-negative bacterial cell wall and circulating LPS levels have been found to be chronically increased in diet-induced obesity (Cani et al. 2007, Moreira et al. 2014). Interestingly, constant infusion of low doses of LPS results in weight gain (Cani et al. 2007). An increase in circulating LPS could be caused by overpopulation of gram-negative bacteria in the gut (Nguyen et al. 2004) and/or an increase in GI permeability (de La Serre et al. 2010).
We found that HFD led to rapid changes in microbiota composition with a bloom in several orders, belonging mostly to the Firmicutes and Proteobacteria phyla; notably Erysipelotrichales and Enterobacteriales. Both orders have previously been linked to obesity (de La Serre et al. 2010) and systemic inflammation (Dinh et al. 2015). We also found positive correlations between the abundance of bacterial orders enriched by HFD and energy intake and adiposity. Conversely, orders that were depleted on HFD were negatively correlated with intake, body fat mass and body weight gain. Erysipelotrichales are gram-positive anaerobic bacteria while Enterobacteriales are gram-negative, potential LPS producer, and facultative anaerobic bacteria. HFD rats exhibited a significant increase in circulating LPS that was normalized by minocycline. LPS, is a potent inducer of inflammation and activator of microglia (Herrera et al. 2000). Dietary-driven microglia activation in the retina is notably dependent on LPS receptor activation (Lee et al. 2015). Interestingly, vagal sensitivity to GI originating signals is altered in LPS treated animals, identifying LPS as a potential trigger in HFD-induced neuronal inflammation and hindbrain remodeling (de La Serre et al. 2015). Neurotoxic effects of bacteria and/or bacterial components were confirmed by culture experiments in our study. Streptococcus mitis, Lactobacillus animalis and Enterococcus faecalis belong to the Lactobacillales order (Firmicutes, Bacilli), are gram-positive bacteria, and there was no difference in Lactobacillales abundance with diet or minocycline treatment. Co-culture of NG neurons with these bacteria did not affect neuron growth. Conversely, co-culture with Proteus mirablis, a gram-negative Enterobacteriales that was enriched in the HFD rats, led to neuronal death. Proteus mirablis is an LPS producing bacteria and its abundance has previously been correlated to metabolic changes induced by HFD (Lecomte et al. 2015). Culture experiments were limited to facultative anaerobic bacteria. We were unable to assess the potential neurotoxicity of anaerobic Erysipelotrichales because it is not possible to culture NG neurons without oxygen. Another limitation of the culture experiments was an isolation of the bacteria from cecum, rather than fecal samples. The rationale for this was the fact that facultative anaerobes represent 25% of total bacteria in the cecum versus only 1% in the fecal samples (Marteau et al., 2001).
While evidence from this study points towards bacteria-driven damage to the hindbrain, we cannot rule out an additional effect of minocycline alone. The anti-inflammatory action of minocycline has notably been reported in the brainstem after damage to the vagus nerve (Gallaher et al. 2012) and minocycline may directly reduce HFD-driven inflammation and body fat accumulation. It has been previously reported that minocycline can suppress body weight gain (Sun et al. 2015). It should be noted the above study minocycline was administered directly into the spinal cord at a dose of 10, 50 or 100 μg/kg. Direct anti-inflammatory effects of minocycline have been reported for doses ranging from 20 to 100 mg/kg (Amin et al. 2015, Gallaher et al. 2012, Kumar and Addepalli 2011). Minocycline may alleviate inflammation via a decrease in bacteria-driven cytokine production. Minocycline has been shown to decrease expression of the LPS receptor, Toll-like receptor 4 (TLR4), in dorsal horn of spinal cord (Nazemi et al. 2015). In our study, minocycline alone, at 20 mg/kg, was not sufficient to induce significant changes in hindbrain inflammation, intake or adiposity in the LFD rats. The absence of significant differences between LF_Saline and LF_Mino animals supports a microbiota-driven pathway. It should be noted that while non-significant, hindbrain inflammation and adiposity were lowered in the LF_Mino rats compared to the LF_Saline animals. However, microbiota analysis revealed that minocycline led to a reduction in obesity-associated bacterial orders, such as Erysipelotrichales and Enterobacteriales in LFD rats. Therefore, the potential effect of minocycline in LFD rats may be related to modulation in microbiota composition rather than a direct anti-inflammatory effect.
Antibiotics-driven modulation of gut microbiota has traditionally been conducted via oral delivery of broad spectrum antibiotics at doses ranging from 40 to 500 mg daily (Membrez et al. 2008, Mikkelsen et al. 2015, Vrieze et al. 2014). These treatments led to acute and dramatic decreases in microbiota diversity and abundance, with Firmicutes and Bacteriodetes not being the most abundant phyla (Carvalho et al. 2012). While effective in promoting weight loss, such treatment did not result in normalization of the HFD-associated microbiota, hence in this study we chose to use a lower dose and a different rout of administration to avoid “wiping out” the gut microbiota. Minocycline has been reported to have potential weight-loss effect (Sun et al. 2015) which may be related to its targeting specific obesity-associated bacterial strains. Minocycline and HFD had opposite modulatory effects on several orders abundance, including Erysipelotrichales and Enterobacteriales and HFD/minocycline rats exhibited normalized microbiota composition, with microbiome profile comparable to the LFD rats.
Our data support a diet-associated and bacteria-driven gut to brain pathway promoting hindbrain inflammation; however, descending brain to gut communication may also modulate the gut microbiota. Vagal efferent neurons located in the DMV send motor information from the brain to the GI tract (de Lartigue et al. 2014). In HFD-rats, we have found that minocycline increased microglia activation in the DMV and vagal remodeling. Vagal efferent fibers modulate gut motility (Chang et al. 2003), and motility has been demonstrated to modify microbiota composition (Kashyap et al. 2013); therefore, we cannot rule out a possible indirect effect of minocycline on microbiota composition in HFD rats via changes in vagal efferent output.
CONCLUSIONS
Taken together, the results show that HFD induces changes in the diversity of the intestinal microbiota, and alters the gut-brain communication resulting in inflammation of the hindbrain feeding centers associated with overeating, overweight and increased in body fat accumulation. Normalization of microbiota composition via minocycline blunted the effects of HFD on hindbrain inflammation, energy intake and adiposity, which let us to conclude that a HFD triggered shift in gut microbiome may disrupt vagal gut-brain communication resulting in microglia activation and increased body fat accumulation leading to obesity.
Acknowledgments
This work was supported by NIH 1R01DC013904 Grant, WSU/CVM Intramural Grants Programs and UGA start-up funds.
References
- Amin B, Hajhashemi V, Hosseinzadeh H. Minocycline potentiates the anti-hyperalgesic effect of ceftriaxone in CCI-induced neuropathic pain in rats. Res Pharm Sci. 2015;10:34–42. [PMC free article] [PubMed] [Google Scholar]
- Ballsmider LA, Vaughn AC, David M, Hajnal A, Di Lorenzo PM, Czaja K. Sleeve gastrectomy and Roux-en-Y gastric bypass alter the gut-brain communication. Neural Plast. 2015;2015:601985. doi: 10.1155/2015/601985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benani A, Hryhorczuk C, Gouaze A, Fioramonti X, Brenachot X, Guissard C, Krezymon A, Duparc T, Colom A, Nedelec E, Rigault C, Lemoine A, Gascuel J, Gerardy-Schahn R, Valet P, Knauf C, Lorsignol A, Penicaud L. Food intake adaptation to dietary fat involves PSA-dependent rewiring of the arcuate melanocortin system in mice. J Neurosci. 2012;32:11970–11979. doi: 10.1523/JNEUROSCI.0624-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200–R207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Shin AC, Zheng H. Obesity surgery and gut-brain communication. Physiol Behav. 2011;105:106–119. doi: 10.1016/j.physbeh.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Cani PD, Delzenne NM, Amar J, Burcelin R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol Biol (Paris) 2008;56:305–309. doi: 10.1016/j.patbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Carvalho BM, Guadagnini D, Tsukumo DM, Schenka AA, Latuf-Filho P, Vassallo J, Dias JC, Kubota LT, Carvalheira JB, Saad MJ. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia. 2012;55:2823–2834. doi: 10.1007/s00125-012-2648-4. [DOI] [PubMed] [Google Scholar]
- Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: what’s new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol. 2003;284:G357–G366. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3:207–215. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav. 2011;105:100–105. doi: 10.1016/j.physbeh.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, Ronveaux CC, Raybould HE. Vagal plasticity the key to obesity. Mol Metab. 2014;3:855–856. doi: 10.1016/j.molmet.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de La Serre CB, de Lartigue G, Raybould HE. Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol Behav. 2015;139:188–194. doi: 10.1016/j.physbeh.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, Wanke CA, Ward HD. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211:19–27. doi: 10.1093/infdis/jiu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ. Luminal sensing in the gut: an overview. J Physiol Pharmacol. 2003;54(Suppl 4):9–17. [PubMed] [Google Scholar]
- Duca FA, Sakar Y, Lepage P, Devime F, Langelier B, Dore J, Covasa M. Replication of obesity and associated signaling pathways through transfer of microbiota from obese-prone rats. Diabetes. 2014;63:1624–1636. doi: 10.2337/db13-1526. [DOI] [PubMed] [Google Scholar]
- Gallaher ZR, Ryu V, Herzog T, Ritter RC, Czaja K. Changes in microglial activation within the hindbrain, nodose ganglia, and the spinal cord following subdiaphragmatic vagotomy. Neurosci Lett. 2012;513:31–36. doi: 10.1016/j.neulet.2012.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera AJ, Castano A, Venero JL, Cano J, Machado A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis. 2000;7:429–447. doi: 10.1006/nbdi.2000.0289. [DOI] [PubMed] [Google Scholar]
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferreyra JA, Higginbottom SK, Million M, Tache Y, Pasricha PJ, Knight R, Farrugia G, Sonnenburg JL. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013;144:967–977. doi: 10.1053/j.gastro.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Kumar BL, Addepalli V. Minocycline with aspirin: an approach to attenuate diabetic nephropathy in rats. Ren Fail. 2011;33:72–78. doi: 10.3109/0886022X.2010.528117. [DOI] [PubMed] [Google Scholar]
- Lassenius MI, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, Porsti I, Rissanen A, Kaprio J, Mustonen J, Groop PH, Lehto M. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34:1809–1815. doi: 10.2337/dc10-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecomte V, Kaakoush NO, Maloney CA, Raipuria M, Huinao KD, Mitchell HM, Morris MJ. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One. 2015;10:e0126931. doi: 10.1371/journal.pone.0126931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Wang PW, Yang IH, Huang HM, Chang CS, Wu CL, Chuang JH. High-fat diet induces toll-like receptor 4-dependent macrophage/microglial cell activation and retinal impairment. Invest Ophthalmol Vis Sci. 2015;56:3041–3050. doi: 10.1167/iovs.15-16504. [DOI] [PubMed] [Google Scholar]
- Little TJ, Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity – oral and gastrointestinal sensory contributions. Physiol Behav. 2011;104:613–620. doi: 10.1016/j.physbeh.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Marteau P, Pochart P, Doré J, Béra-Maillet C, Bernalier A, Corthier G. Comparative study of bacterial groups within the human cecal and fecal microbiota. Appl Environ Microbiol. 2001;67(10):4939–4942. doi: 10.1128/AEM.67.10.4939-4942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay DE, Briancon N, Kokoeva MV, Maratos-Flier E, Flier JS. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest. 2012;122:142–152. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin RG, Corthesy I, Mace K, Chou CJ. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22:2416–2426. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- Mikkelsen KH, Frost M, Bahl MI, Licht TR, Jensen US, Rosenberg J, Pedersen O, Hansen T, Rehfeld JF, Holst JJ, Vilsboll T, Knop FK. Effect of antibiotics on gut microbiota, gut hormones and glucose metabolism. PLoS One. 2015;10:e0142352. doi: 10.1371/journal.pone.0142352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel-Castro AJ, Gonzalez-Cervantes RM, Bravo-Ruiseco G, Pacheco-Lopez G. The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci. 2013;7:70. doi: 10.3389/fnint.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira AP, Teixeira TF, Alves RD, Peluzio MC, Costa NM, Bressan J, Mattes R, Alfenas RC. Effect of a high-fat meal containing conventional or high-oleic peanuts on post-prandial lipopolysaccharide concentrations in overweight/obese men. J Hum Nutr Diet. 2014;29(1):95–104. doi: 10.1111/jhn.12284. [DOI] [PubMed] [Google Scholar]
- Nazemi S, Manaheji H, Noorbakhsh SM, Zaringhalam J, Sadeghi M, Mohammad-Zadeh M, Haghparast A. Inhibition of microglial activity alters spinal wide dynamic range neuron discharge and reduces microglial Toll-like receptor 4 expression in neuropathic rats. Clin Exp Pharmacol Physiol. 2015;42:772–779. doi: 10.1111/1440-1681.12414. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, D’Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino G, Darcel N, Tome D, Raybould H. Adaptation of lipid-induced satiation is not dependent on caloric density in rats. Physiol Behav. 2008;93:930–936. doi: 10.1016/j.physbeh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, Gallaher ZR, Ryu V, Czaja K. Withdrawal and restoration of central vagal afferents within the dorsal vagal complex following subdiaphragmatic vagotomy. J Comp Neurol. 2013;521:3584–3599. doi: 10.1002/cne.23374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice AM, Jebb SA. Fast foods, energy density and obesity: a possible mechanistic link. Obes Rev. 2003;4:187–194. doi: 10.1046/j.1467-789x.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van TW, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav. 2004;81:249–273. doi: 10.1016/j.physbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Rogers RC, McCann MJ. Intramedullary connections of the gastric region in the solitary nucleus: a biocytin histochemical tracing study in the rat. J Auton Nerv Syst. 1993;42:119–130. doi: 10.1016/0165-1838(93)90043-t. [DOI] [PubMed] [Google Scholar]
- Rolls BJ. Carbohydrates, fats, and satiety. Am J Clin Nutr. 1995;61:960S–967S. doi: 10.1093/ajcn/61.4.960S. [DOI] [PubMed] [Google Scholar]
- Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav. 2009;97:609–615. doi: 10.1016/j.physbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehab SA. Acute and chronic sectioning of fifth lumbar spinal nerve has equivalent effects on the primary afferents of sciatic nerve in rat spinal cord. J Comp Neurol. 2009;517:481–492. doi: 10.1002/cne.22163. [DOI] [PubMed] [Google Scholar]
- Sun JS, Yang YJ, Zhang YZ, Huang W, Li ZS, Zhang Y. Minocycline attenuates pain by inhibiting spinal microglia activation in diabetic rats. Mol Med Rep. 2015;12:2677–2682. doi: 10.3892/mmr.2015.3735. [DOI] [PubMed] [Google Scholar]
- Troy AE, Simmonds SS, Stocker SD, Browning KN. High fat diet attenuates glucose-dependent facilitation of 5-HT3-mediated responses in rat gastric vagal afferents. J Physiol. 2016;594:99–114. doi: 10.1113/JP271558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, van NE, Holleman F, Knaapen M, Romijn JA, Soeters MR, Blaak EE, Dallinga-Thie GM, Reijnders D, Ackermans MT, Serlie MJ, Knop FK, Holst JJ, van der Ley C, Kema IP, Zoetendal EG, de Vos WM, Hoekstra JB, Stroes ES, Groen AK, Nieuwdorp M. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60:824–831. doi: 10.1016/j.jhep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- Waise TM, Toshinai K, Naznin F, NamKoong C, Md Moin AS, Sakoda H, Nakazato M. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun. 2015;464:1157–1162. doi: 10.1016/j.bbrc.2015.07.097. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang BR, Zhang XJ, Xu Z, Ding YQ, Ju G. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol. 2002;8:540–545. doi: 10.3748/wjg.v8.i3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, Tschop MH, Woods SC, Hofmann SM. High-fat-diet exposure induces IgG accumulation in hypothalamic microglia. Dis Model Mech. 2012;5:686–690. doi: 10.1242/dmm.009464. [DOI] [PMC free article] [PubMed] [Google Scholar]